ABSTRACT
Propylene and butylene are highly reactive volatile organic compounds (HRVOCs) in terms of ground-level ozone formation. This study examined the effectiveness of biofiltration in removing propylene and butylene as separate compounds. Specific objectives were (1) to measure maximum removal efficiencies for propylene and butylene and the corresponding microbial acclimation times, which will be useful in the design of future biofilters for removal of these compounds; (2) to compare removal efficiencies of propylene and butylene for different ratios of compost/hard wood-chip media; and (3) to identify the microorganisms responsible for propylene and butylene degradation. Two laboratory-scale polyvinyl chloride biofilter columns were filled with 28 in. of biofilter media (compost/wood-chip mixtures of 80:20 and 50:50 ratios). Close to 100% removal efficiency was obtained for propylene for inlet concentrations ranging from 2.9 × 104 to 6.3 × 104 parts per million (ppm) (232–602 g/m3-hr) and for butylene for inlet concentrations ranging from 91 to 643 ppm (1.7–13.6 g/m3-hr). The microbial acclimation period to attain 100% removal efficiency was 12–13 weeks for both compounds. The lack of similar microbial species in the fresh and used media likely accounts for the long acclimation time required. Both ratios of compost/wood chips (80:20 and 50:50) gave similar results. During the testing, media pH increased slightly from 7.1 to 7.5–7.7. None of the species in the used media that treated butylene were the same as those in the used media that treated propylene, indicating that different microbes are adept at degrading the two compounds.
This study is the first, to the authors’ knowledge, to demonstrate the effectiveness of a compost/wood-chip biofilter for propylene and butylene removal. Propylene and butylene are HRVOCs that are very efficient at forming ground-level ozone. This research determined removal efficiency values, microbial acclimation times, and microbial species responsible for compound degradation that will all be useful in the design of future biofilters for the removal of these compounds.
INTRODUCTION
Control of volatile organic compounds (VOCs) remains an important air pollution issue from various standpoints:
VOCs react with nitrogen oxides (NOx) via a complex set of reactions to form ground-level ozone (O3), many VOCs are hazardous air pollutants and odor-causing compounds, and VOCs are important precursors to fine particle formation. Across the United States, 116 regions have been designated as nonattainment areas for the 85 parts per billion (ppb) 8-hr O3 standard.Citation1 Various researchers have developed scales to rank VOCs according to their O3 formation potential.Citation2,Citation3 Alkenes, two of which are the focus of this study, tend to rank high on these scales. Alkenes are very reactive because of rapid addition of the hydroxyl radical to C-C double bonds. Highly reactive VOCs (HRVOCs) play key roles in O3 formation in many regions. For example, alkenes have been found to play an important role in O3 production in Shanghai, accounting for only 7% of VOC concentrations but 34% of the total O3 production.Citation4 Propylene is one of four VOCs found to have the highest O3 formation potential and reactivity in southern Taiwan.Citation5 Alkenes have also been found to play important roles in O3 formation in the state of Texas in the United States, as discussed in the Motivation and Objectives section below.
Potential control options for HRVOCs include incineration/flaring, adsorption, and biofiltration. Incineration/flaring requires a continuous fuel supply, which can be costly and can generate combustion byproducts. Adsorption does not destroy contaminants, but it transfers them to a solid media that must then be regenerated. Biofiltration uses microorganisms fixed to porous media to break down pollutants present in an airstream.Citation6–9 Hydrocarbons are often completely converted via microbial metabolism to carbon dioxide and water. The microorganisms grow in a biofilm on the surface of the media or are suspended in the water phase surrounding the media. The filter-bed media consists of relatively inert substances (e.g., compost, wood chips, soil, peat), which ensures large surface attachment areas and additional nutrient supply. As the air passes through the bed, the contaminants in the air phases sorb into the biofilm, where they are biodegraded. Advantages of well-designed biofiltration systems include high removal efficiencies and low operating costs.Citation10
Motivation and Objectives
This study thus focuses on removal of propylene and butylene as individual compounds via biofiltration. The Texas Commission on Environmental Quality (TCEQ) has developed a special regulatory status for HRVOCs, which include propylene, butylene, ethylene, and 1,3-butadiene. Recent Houston/Galveston/Brazoria (HGB) field studies have found that the HRVOCs play a critical role in O3 formation. Localized regions with high concentrations of HRVOCs have been found to be frequently associated with rapid O3 formation, leading to exceedances of the O3 standard.Citation11,Citation12 TCEQ has accordingly adopted regulations to control HRVOC emissions in HGB; these regulations impact petroleum refineries and chemical manufacturing facilities.Citation13 According to TCEQ and U.S. Environmental Protection Agency (EPA) data, propylene and butylene rank second and fifth, respectively, among HRVOCs released by Texas’ three O3 nonattainment areas—HGB, Beaumont-Port Arthur, and Dallas-Fort Worth.Citation14,Citation15
Of the 493 chemicals included in the 2008 EPA Toxic Release Inventory, propylene ranked 33rd in the United States in total on- and off-site disposal or other releases.Citation14 Propylene is used for the production of polypropylene, acrylonitrile, acrylic acid, acrolein, plasticizer oxo alcohols, propylene oxide, cumene, and acetone. From 2000 to 2008, annual propylene production remained at approximately 35 million t in Europe and North America, but it has been increasing in East Asia, most notably Singapore and China.Citation16,Citation17 Propylene is also produced as a byproduct in petroleum refining, olefin plant steam crackers, and combustion of organic matter (e.g., biomass burning, motor vehicle exhaust, and tobacco smoke). Butylene can act as a monomer in the formation of polymers and is primarily used as a feedstock to produce industrial chemicals, gasoline blending components, and synthetic rubber.
Only one previous study has been conducted regarding removal of propylene via biofiltration. Reij et al.Citation18 demonstrated the feasibility of propylene removal at concentrations ranging from 10 to 1000 parts per million (ppm) using a microporous hydrophobic membrane inoculated with the bacterial strain Xanthobacter Py2. However, no studies have been conducted that attempt to remove propylene using a typical compost biofilter. To the authors’ knowledge, no previous studies of biofiltration of butylene have been conducted. The work presented here is the first, to the authors’ knowledge, to demonstrate the effectiveness of a compost/wood-chip biofilter for propylene and butylene removal.
The most successful uses of biofilters are for highly soluble organic compounds with low molecular weights. Compared with compounds for which biofilters have been used successfully (e.g., butanol, acetone, and toluene), propylene and butylene have low molecular weights (42 and 56, respectively), which would indicate good potential for biodegradability. However, propylene and butylene also have low Henry's law constants (4.8 × 10−3 and 4 × 10−3 M/atm at 298 K, respectively), indicating low solubility.Citation10,Citation19 The alkanes methane and pentane have some biodegradability, and hexane has moderate biodegradability, although their Henry's law constants are lower than those for propylene and butylene.Citation20 Thus it was deemed worthwhile to test these two alkenes for biofilter removal.
The overall goal of the study was therefore to determine whether propylene and butylene could be effectively treated in a compost/wood-chip biofilter. Specific objectives of the research were
| • | To measure the maximum removal efficiencies for propylene and butylene and the corresponding microbial acclimation time | ||||
| • | To compare the removal efficiencies of propylene and butylene for different ratios of compost/hard wood-chip media | ||||
| • | To identify the microorganisms responsible for propylene and butylene degradation. | ||||
Measuring Maximum Removal Efficiencies for Propylene and Butylene and the Corresponding Microbial Acclimation Time
In designing a biofilter, an engineer needs to know the maximum removal efficiency and the time needed to achieve that removal efficiency. By knowing the maximum removal efficiency over the concentrations tested, the engineer will be able to assess whether the biofilter will provide sufficient pollutant removal to meet regulatory standards; if not, another control option must be considered. If a long acclimation time were needed for the microorganisms to achieve high removal efficiency, the engineer would need to plan for a control alternative during the acclimation period or consider inoculation of the biofilter with microbes adept at degrading the compound of interest. The maximum elimination capacity, or maximum mass of pollutant degradable per unit time per volume of bed, is also needed for sizing the biofilter bed.
Comparing Removal Efficiencies for Various Ratios of Compost/Hard Wood-Chip Media
Different biofilter media can result in differing removal efficiencies for the same pollutant because of differing nutrient contents, carbon contents, moisture retention properties, or mixtures of indigenous microbes. In general, biofilters use organic materials such as peat, compost, and wood products as biofilm growth support media. Although synthetic materials are also available, natural materials are often chosen because they are inexpensive and readily available. In addition, natural media provide a reservoir of nutrients to support microbial growth.Citation21
Compost media possesses a large diversity and density of microorganisms, good water retention properties, neutral pH, and a suitable organic content.Citation20 In addition, it is commonly used in practice as a biofilter bed material. Wood chips are often added to compost in various proportions as bulking agents to prevent bed compaction and allow for homogeneous airflow. Wood chips also constitute a reservoir of water that may in some cases attenuates fluctuations in packing moisture content because of poor reactor control or excessive heat generation.Citation20 Thus, compost mixed with wood chips was chosen as the media to evaluate in this research.
Identifying the Microorganisms Responsible for Propylene and Butylene Degradation
Identifying microorganisms likely responsible for pollutant degradation is useful in the event that the biofilter designer wants to reduce microbial acclimation time by initially inoculating the biofilter with an appropriate microbial species.
METHODS
Experimental Setup
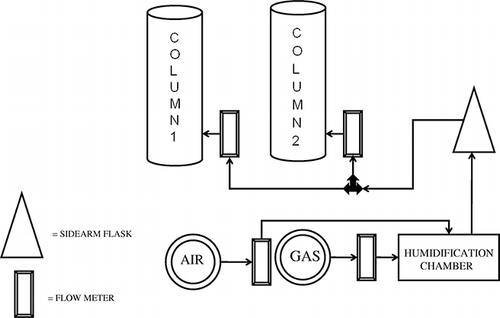
The experimental setup is shown in The two biofilter columns, made of transparent polyvinyl chloride (PVC), were 0.91 m long with an inner diameter of 10.8 cm. The columns were closed on the bottom by 20.3- by 20.3-cm (8- by 8-in.) square plates with an O-ring fitted in a groove. A hole in the center of each plate allowed Teflon tubing carrying a mixture of pollutant gas and air to enter. To facilitate uniform spreading of the pollutant gas/air mixture across the biofilter cross section, a perforated PVC plate was used, 1.3 cm thick and 10.8 cm in diameter with holes 2.4 mm in diameter (0.5 in. thick, 4.25 in. in diameter with holes 3/32 in. in diameter). The perforated plate was situated approximately 13 cm (5 in.) above the bottom end of the column so that the air and alkene were allowed to mix before entering the biofilter media. The air with pollutant was continuously fed through the biofilter. Sampling ports were fixed at the inlet and outlet of each column.
Compost and wood chips were used as biofilter media. The compost was from University of Texas at Arlington landscaping, and wood chips were from Good Times Wood Products, Inc. Media characteristics are given in . The bed depth was 0.71 m (28 in.). The two biofilter columns were filled with 80:20 and 50:50 ratios of compost/wood chips. In previous research, an 80:20 ratio of compost/wood chips produced a high removal efficiency for carbonyl sulfide.Citation22 Replicates with the same media ratio were not used in this study. Previous research showed that replicate columns of compost/hard wood-chip media gave similar results.Citation22,Citation23
Table 1. Biofilter media characteristics
Butylene and propylene gas were ordered from Matheson Tri Gas. Each gas was mixed separately with air from the fume hood supply for dilution and was fed separately to the biofilter (not as a mixture). The biofilter inlet butylene and propylene concentrations during and after the acclimation period were varied between 91 and 1017 ppm (1.7 and 19.8 g/m3-hr) and 887 and 64,541 ppm (9 and 1228 g/m3-hr), respectively. Inlet concentrations remained constant for 2–3 days before being changed. The testing could represent biofilter treating variable loadings of propylene and butylene. The concentrations of butylene tested were lower than those for propylene because of technical difficulties with the butylene cylinder.
A combined flow rate (air and alkene) of 0.4–1 L/min was used. Empty-bed residence times (EBRTs) ranged from 6.5 to 16 min (depending on flow rate) and were thus longer than typical values of 0.5–2 min.Citation20 The EBRT values were varied during the acclimation period and after removal had reached 100%. Such long EBRTs would require very large biofilters, which would increase capital costs. Future research should study the potential for decreasing EBRTs once microbial acclimation has been achieved.
The gas stream containing propylene or butylene was passed through a humidification chamber to increase its relative humidity (RH). The humidification chamber was the same size as the biofilter columns. The alkene/air mixture traveled through a long glass tube to the bottom of the chamber, where it was released. The mixture was humidified as it bubbled upward through the water.
During all experiments, the temperature was maintained at room temperature (22.8 °C [73 °F]).
Analytical Methods
Media pH was measured using an Accumet AR 50 Fisher Scientific pH meter. Duplicate samples of compost or wood chips were taken from the top, middle, and/or bottom of the column. Approximately 30 g of media was mixed with 100 mL of water (pH 8.2) to make slurry, and the probe of the pH meter was inserted into the slurry to obtain a reading according to the procedure recommended by Devinny et al.Citation20
The moisture content of the media was measured by heating a wet media sample to 105 °C in an oven for 24 hr and weighing the wet and dry media samples using an analytical balance. Gas stream humidity was measured using a Testo 605-H1 humidity stick.
To measure alkene concentrations, an SRI 8610 gas chromatograph with a flame-ionization detector (FID) and 60-m capillary column was used. Helium was used as the carrier gas, and hydrogen was used to provide the flame for the FID. The initial and final oven temperatures were 40 and 150 °C, respectively, with a 10 °C/min ramp; the detector temperature was set at 200 °C. Three replicate readings of alkene concentration at the column inlet (after the humidification chamber) and outlet points were taken every 24 hr.
Biomass produced and carbon dioxide released were not measured to enable a mass balance calculation. This could be done in future studies.
Media samples were sent to Microcheck, Inc., for determining the types of microbes present according to the following methodology. Each sample (5 ± 0.5 g) was aseptically added to a sterile capped container. Forty-five milliliters of sterile phosphate-buffered water with Tween 80 (PBWT) was added and the contents were shaken at 150 revolutions per minute for 15 min. Aliquots (0.1 mL) of the shaken samples were then serially diluted 10-fold to isolate the microbes so that they could be enumerated and identified. From each sample, 0.1 mL was aseptically transferred to a sterile 100- by 16-mM test tube containing 0.9 mL of sterile PBWT. This first dilution tube was vortexed and 0.1 mL was transferred to a second serial dilution tube containing 0.9 mL of sterile PBWT. The process was repeated through the nine serial dilution tubes with the final 0.1 mL thrown away. The 0.9-mL aliquots from the nine serial dilution tubes were poured onto separate plates of tryptic soy agar (TSA), which is a general-purpose medium on which bacteria, actinomycetes, yeasts, and filamentous fungi will grow. Of the submitted sample, 0.9 mL was also added to a TSA plate. After aerobic incubation at 28 °C, the plates were examined and the apparently different colony-forming types of microbes were enumerated and identified. The individual colonies from the TSA plates were prepared and analyzed using DNA sequencing. The automated 16S and LSU D2 gene sequencing was capable of identifying aerobic and anaerobic bacteria, actinomycetes, yeast, and fungi.
Limitations of the viable heterotrophic plate count technique described above should be mentioned. This technique only counts microbes that can grow on the agar used; a large fraction of microbes present in the biofilter may not have been culturable.Citation20 In addition, species that were dormant in the biofilter may grow well in the agar, leading to an overstating of its importance in the biofilter.Citation20 The technique also gives no information about the role the microbes are playing in a biofilter. Nevertheless, the technique still provides valuable basic knowledge about the general number of microbes in a biofilter.Citation20 Ideally, many samples should be taken over the life of the biofilter project. In the case of this research, funding restrictions limited the number of samples taken.
RESULTS AND DISCUSSION
Removal Efficiencies, Acclimation Times, and Elimination Capacities
For propylene and butylene, the removal efficiency approached 100% at approximately day 90, or after more than 12 weeks of biofilter operation. The initial propylene removal efficiency was 59% for the 80:20 and 50:50 compost/wood-chip media ratios. The initial butylene removal efficiencies were 16 and 18% for the 80:20 and 50:50 compost/wood-chip media ratios, respectively. Removal efficiency was calculated as (inlet concentration - outlet concentration)/inlet concentration. Removal efficiencies for propylene and butylene for both ratios of media (80:20 and 50:50 compost/wood chips) were similar throughout the tests. The media ratio did not affect removal efficiency or the time to achieve it at the concentrations tested, likely because the amount of compost media was sufficient in all cases to provide a diversity of microbes and sufficient supply of water and nutrients. However, it must be noted that the maximum elimination capacity was not reached, as discussed later.
The 12-week time to achieve maximum removal efficiency was longer than that for many compounds, which is approximately 2 weeks. However, acclimation periods may vary from a few minutes to a year.Citation20 A microbe presented with a new substrate must undergo biochemical changes to begin using the new substance as food (cellular acclimation).Citation20 Degrading organisms must then become abundant enough to transform the pollutant at a high rate. If they were not distributed well initially, then it may take time for them to colonize the entire media (ecosystem acclimation).Citation20 The relatively long acclimation period required for propylene and butylene was most likely due to a long time needed for ecosystem acclimation, as will be discussed later.
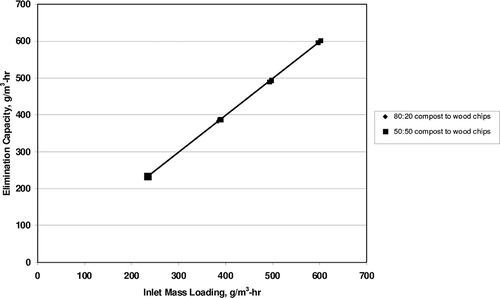
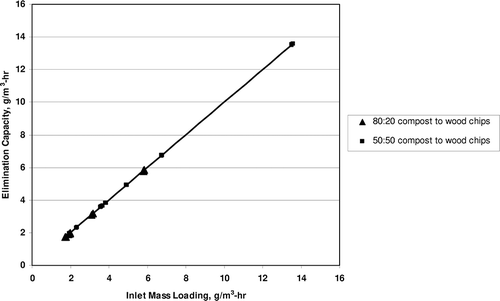
Once the removal efficiency approached 100%, the inlet loading for propylene was varied. A propylene removal efficiency of close to 100% was sustained for inlet loadings (and thus elimination capacities) varying from 232 to 602 g/m3-hr. Elimination capacity versus propylene inlet mass loading is plotted in A butylene removal efficiency of close to 100% was sustained for inlet loadings (and thus elimination capacities) varying from 1.7 to 13.6 g/m3-hr. The elimination capacity versus butylene inlet mass loading is plotted in To determine maximum elimination capacities, propylene and butylene loadings would have needed to be increased further. This is recommended as an item for future research.
Media pH, Media Moisture Content, and Gas Stream RH
Samples were taken from the bottoms of the biofilter columns to compare the pH of the media at the experiment's beginning and after 3 months. The initial pH for all columns was 7.1. The final pH for the column with 80:20 compost/wood-chip media treating propylene was 7.5; for the column with 50:50 compost/wood-chip media treating butylene it was 7.8, and for the other two columns it was 7.7. This indicates that formation of acidic byproducts would not pose a problem requiring mitigation. The reason for the pH increase is unknown.
The moisture content of the used compost/wood-chip mixture was 20.4 and 22.6% after treatment of propylene and butylene, respectively. Although these values are higher than the moisture content of the fresh compost and wood chips (17.4 and 11.6%), they are lower than the moisture content reported as desirable for organic media (40–80%).Citation20 A typical advantage of wood chips is that they can serve as a moisture reservoirCitation20; their comparatively low moisture content is thus somewhat surprising. Gas stream RH during the experiments ranged from 50 to 80%, which is lower than the 95–100% typically recommended for biofilter operation.Citation20 However, despite the low media moisture content and RH, 100% removal efficiency was still achieved. This indicates that providing a high media moisture content and gas stream RH may not be necessary for achieving good removal of propylene and butylene via biofiltration. Whether the microbes would have acclimated more quickly to degrading propylene and butylene if they had been given higher media moisture content is a question for future research.
Microbes in the Media
Compost typically brings with it an initial inoculum of thousands of species.Citation20 These species thrive or fail based on their ability to find a niche in the biofilter ecosystem. For complex pollutants, multiple species may be required to carry out different metabolic steps to transform the pollutant ultimately to carbon dioxide and water.Citation20 Species consuming the same substrate (original pollutant or metabolite) will often compete fiercely, with the less-capable species eventually dying off; however, multiple species consuming the same substrate may also coexist in different locations in the biofilter (e.g., pores of the media where water is abundant vs. surfaces of the media).Citation20
Bacteria and fungi are the two dominant microorganism groups in biofilters. Their small size gives a high surface-to-volume ratio, which means quick pollutant up-take. Bacteria will generally dominate under favorable conditions in a biofilter with high water content treating easily degraded substrate at near-neutral pH.Citation20 Fungi grow more slowly but can degrade a greater variety of pollutants and can withstand harsher conditions (drier or more acidic).Citation20 In most biofilters, oxygen is abundant, especially at the biofilm surface, fostering growth of aerobic microbes.
According to ecologists, most ecosystems developing in a new habitat pass through a succession of states before reaching a climax community with fairly stable species populations.Citation20 Webster et al.Citation24 found that a biofilter microbial ecosystem required hundreds of days to reach a climax community.
The types of microbes present in the fresh compost and fresh wood chips are given in and , respectively; microbes present in the used compost and wood chips treating propylene and butylene are shown in and , respectively. Microbes identified were predominantly aerobic bacterium, along with a few species of fungus and actinomycetes. No anaerobes or facultative anaerobes were identified, indicating that predominantly aerobic conditions were present in the biofilter.
Table 2. Microorganisms in fresh compost media
Table 3. Microorganisms in fresh wood-chip media
Table 4. Microorganisms in used compost and wood-chip media treating propylene
Table 5. Microorganisms in used compost and wood-chip media treating butylene
More colony-forming units (CFUs) were identified in the fresh wood-chip (4.8 × 108 CFUs) media than in the fresh compost (1.1 × 108 CFUs). Four of the eight species identified in the fresh compost were of the genus Bacillus, representing 91.5% of the CFUs; Bacillus mojavensis (Bacillus licheniformis) alone represented 84.5% of the CFUs. Four of the six species identified in the fresh wood chips were of the genus Bacillus, but they represented only 0.8% of the CFUs combined. Enterococcus durans aerobic bacteria and Nocardia brasiliensis actinomycetes represented 79.2 and 20% of the CFUs in the fresh wood chips, respectively.
None of the species originally present in the compost or wood chips ended up in the used media through which propylene was passed. The long acclimation time required to achieve close to 100% removal of propylene was thus likely because of the time required for ecosystem acclimation, or for degrading organisms to become abundant enough to transform the pollutant at a high rate. In the compost/wood-chip media that had been treating propylene, three species of aerobic bacteria—Brachybacterium nesterenkovii (44.7%), Sphingobacterium thalpophilum (23.4%), and Sphingobacterium multivorum (23.4%)—together accounted for 91.5% of the CFUs. Fungi species represented only 0.49% of the CFUs in the media that had been treating propylene. The predominance of aerobic bacteria species indicates that the conditions in the biofilter were favorable.
In the compost/wood-chip media that had been treating butylene, Bacillus megaterium and Rhodococcus koreensis aerobic bacteria each represented 34.8% of the CFUs, for a total of 69.6%. B. megaterium was present originally in the wood chips media, but constituted only 0.0002% of the CFUs. Bacillus barbaricus, originally present as 0.7% of the CFUs in the compost media, increased to 7% of the CFUs in the media that had been treating butylene. The time that was required for these and other species to increase in numbers likely accounted for the long acclimation time required for butylene. Four fungi species together accounted for 7.7% of the CFUs in the compost/ wood-chip media that had been treating butylene.
None of the species in the used media that had been treating butylene were the same as those in the used media that had been treating propylene, indicating that different microbes are adept at degrading the two compounds. Of the species that were abundant in the used media, the particular microorganism(s) responsible for the propylene and butylene degradation is not known. The microbe could be identified in future research by testing biofilters inoculated individually with each species found to be abundant in the used media.
The microbes identified in the used media were those present at the end of 12–13 weeks of testing, or 80–90 days. Because a climax community in a biofilter may take several hundred days to form, the species identified may not have yet been representative of the climax community.
SUMMARY
| • | Biofilters are capable of removing 100% propylene and butylene at concentrations ranging from 2.9 × 104 to 6.3 × 104 ppm (232 to 602 g/m3-hr) for propylene and 91 to 643 ppm (1.7 to 13.6 g/m3-hr) for butylene. | ||||
| • | Maximum elimination capacities of biofilters for propylene and butylene are at least 600 and 13.6 g/m3-hr, respectively. Additional biofilter testing should be conducted at higher inlet loadings to try to determine the maximum elimination capacity. | ||||
| • | Compost/wood-chip mixtures of 50:50 and 80:20 ratios by volume produced similar removal efficiencies for propylene and butylene at the concentrations tested, although the maximum elimination capacity was not reached. | ||||
| • | The acclimation period for propylene and butylene was 12–13 weeks, which is longer than that for many compounds. The lack of similar microbial species in the fresh and used media likely accounts for the long acclimation time required. The acclimation period could potentially be decreased by inoculating the media with microbes identified in this research as proliferating when exposed to propylene and butylene. Whether the microbes would have acclimated more quickly if the media moisture content had been higher is a question for future research. | ||||
| • | Biofilter pH increased only slightly during 13 weeks of operation, indicating that formation of acidic byproducts would not pose a problem. | ||||
| • | None of the species in the used media that had been treating butylene were the same as those in the used media that had been treating propylene, indicating that different microbes are adept at degrading the two compounds. | ||||
ACKNOWLEDGMENTS
The authors gratefully acknowledge John Darling for providing the compost and Paul Shover for his assistance in assembling the laboratory-scale biofilter system.
REFERENCES
- Area Designations for 1997 Ground-Level Ozone Standards; U.S. Environmental Protection Agency; 2009 http://www.epa.gov/air/ozonepollution/designations/1997standards/index.htm (http://www.epa.gov/air/ozonepollution/designations/1997standards/index.htm) (Accessed: 2011 ).
- Carter , W.P.L. 1991 . Development of Ozone Reactivity Scales for Volatile Organic Compounds , NC : Research Triangle Park . EPA 600/3-91-050; U.S. Environmental Protection Agency
- Russell , A.G. , Milford , J. , Bergin , M.S. , McBride , S. , McNair , L. , Yang , Y. , Stockwell , W.R. and Croes , B. 1995 . Urban Ozone Control and Atmospheric Reactivity of Organic Gases . Science , 269 : 491 – 495 .
- Geng , F. , Tie , X. , Xu , J. , Zhou , G. , Peng , L. , Gao , W. , Tang , X. and Zhao , C. 2008 . Characterizations of Ozone, NOx, and VOCs Measured in Shanghai, China . Atmos. Environ. , 42 : 6873 – 6883 .
- Chang , C.-C. , Chen , T.-Y. , Lin , C.-Y. , Yuan , C.-S. and Liu , S.-C. 2005 . Effects of Reactive Hydrocarbons on Ozone Formation in Southern Taiwan . Atmos. Environ. , 39 : 2867 – 2878 .
- Chen , L. , Hoff , S. , Cai , L. , Koziel , J. and Zelle , B. 2009 . Evaluation of Wood Chip-Based Biofilters to Reduce Odor, Hydrogen Sulfide, and Ammonia from Swine Barn Ventilation Air . Journal of the Air & Waste Management Association , 59 : 520 – 530 . doi: 10.3155/1047-3289.59.5.520
- Goncalves , J. and Govind , R. 2009 . Analysis of Biofilters Using Synthetic Macroporous Foam Media . Journal of the Air & Waste Management Association , 59 : 834 – 844 . doi: 10.3155/1047-3289.59.7.834
- Chou , M.S. , Chang , Y.F. and Peng , H.T. 2008 . Treatment of Propylene Glycol Monomethyl Ether Acetate in Air Streams by a Biofilter Packed with Fern Chips . Journal of the Air & Waste Management Association , 58 : 1590 – 1597 . doi: 10.3155/1047-3289.58.12.1590
- Sawvel , R.A. , Kim , B. and Alvarez , P.J. 2008 . Removal of Volatile Organic Compounds at Extreme Shock-Loading Using a Scaled-Up Pilot Rotating Drum Biofilter . Journal of the Air & Waste Management Association , 58 : 1407 – 1414 . doi: 10.3155/1047-3289.58.11.1407
- Cooper , C.D. and Alley , F.C. 2002 . Air Pollution Control: a Design Approach , Long Grove , IL : Waveland .
- Kleinman , L.I. , Daum , P.H.D. , Imre , L.Y.-N. , Nunnermacker , L.J. , Springston , S.R. , Weinstein-Lloyd , J. and Rudolph , J. 2002 . Ozone Production Rate and Hydrocarbon Reactivity in 5 Urban Areas: a Cause of High Ozone Concentration in Houston . Geophys. Res. Lett. , 29 doi: 10.1029/2001GL014569
- Ryerson , T.B. , Trainer , M.W. , Angevine , M.C. , Brock , A.R. , Dissly , W.F. , Fehsenfeld , C. , Frost , G.J. , Goldan , P.D. , Holloway , J.S. , Hu , G. , Jakoubek , R.O. , Kuster , W.C. , Neuman , J.A. , Nicks , D.K. Jr. , Parrish , D.D. , Roberts , J.M. , Sueper , D.T. , Atlas , E.L. , Donnelly , S.G. , Flocke , F. , Fried , A. , Potter , W.T. , Schauffler , S. , Stroud , V. , Weinheimer , A.J. , Wert , B.P. , Wiedinmyer , C. , Alvarez , R.J. , Banta , R.M. , Darby , L.S. and Senff , C.J. 2003 . Effect of Petrochemical Industrial Emissions of Reactive Alkenes and NOx on Tropospheric Ozone Formation in Houston, Texas . J. Geophys. Res. , 108 doi: 10.1029/2002JD003070
- Controlling Highly Reactive Volatile Organic Compounds (HRVOCs); Texas Commission on Environmental Quality: Austin, TX, 2009 http://www.tceq.state.tx.us/implementation/air/sip/hrvoc.html (http://www.tceq.state.tx.us/implementation/air/sip/hrvoc.html) (Accessed: 2011 ).
- Toxic Release Inventory (TRI) Explorer; U.S. Environmental Protection Agency http://www.epa.gov/triexplorer/chemical.htm (http://www.epa.gov/triexplorer/chemical.htm) (Accessed: 2011 ).
- Point Source Emissions Inventory; Texas Commission on Environmental Quality; 2007 http://www.tceq.state.tx.us/implementation/air/industei/psei/psei.html (http://www.tceq.state.tx.us/implementation/air/industei/psei/psei.html) (Accessed: 2011 ).
- Propylene; Association of Petrochemicals Producers in Europe; 2008 http://www.petrochemistry.net (http://www.petrochemistry.net) (Accessed: 2011 ).
- McMurry , J. 2005 . Organic Chemistry , Pacific Grove , CA : Brooks/Cole .
- Reij , M.W. , Hamann , E.K. and Hartmans , S. 1997 . Biofiltration of Air Containing Low Concentrations of Propene Using a Membrane Bioreactor . Biotechnol. Prog. , 13 : 380 – 386 .
- Sander, R. Compilation of Henry's Law Constants for Inorganic and Organic Species of Potential Importance in Environmental Chemistry; 1999 http://www.mpch-mainz.mpg.de/~sander/res/henry.html (http://www.mpch-mainz.mpg.de/~sander/res/henry.html) (Accessed: 2011 ).
- Devinny , J.S. , Deshusses , M.A. and Webster , T.S. 1999 . Biofiltration for Air Pollution Control , New York : Lewis .
- Yang , Y. , Togna , A.P. , Gaines , F.R. , Smith , S.C. and Dunn , W.G. Control of Odorous Air Emissions at a Municipal Wastewater Treatment Plant Using a Biofilter . Proceedings of the 92nd Annual Meeting & Exhibition of A&WMA . Pittsburgh , PA . pp. 99 – 105 . A&WMA .
- Nawal , C.P. 2004 . M.S. Thesis , Arlington , TX : University of Texas at Arlington .
- Garrapalli , D.R. 2005 . M.S. Thesis , Arlington , TX : University of Texas at Arlington .
- Webster , T.S. , Devinny , J.S. , Torres , E.M. and Basrai , S.S. 1996 . Biofiltration of Odors, Toxics and Volatile Organic Compounds from Publicly Owned Treatment Works . Environ. Prog. , 15 : 141 – 147 .