ABSTRACT
Activated carbons with diverse physical and chemical properties were produced from four agriculture residues, including raw barley husk, biotreated barley husk, rice husk, and pistachio shell. Results showed that with adequate steam activation (30–90 min, 50% H2O(g)/50% N2), activated carbons with surface areas between 360 and 950 m2 g−1 were developed. Further increases in the activation time destroyed the pore structure of activated carbons, which resulted in a decrease in the surface area and pore volume. Biotreated agricultural residues were found to be suitable precursors for producing mesoporous activated carbons. The oxygen content of activated carbons increased with increasing activation time. Results from X-ray photoelectron spectroscopy examination further suggested that H2O molecules react with the carbon surface, enhancing the deconvoluted peak area of carbonyl and carboxyl groups. Equilibrium adsorption of toluene indicated that the adsorption capacities increased with an increase in the inlet toluene concentration and a decrease in temperature. The adsorption isotherms were successfully fitted with Freundlich, Langmuir, and Dubinin– Radushkevich equations. Activated carbons derived from agricultural residues appear to be more applicable to adsorb volatile organic compounds at a low concentration and high-temperature environment.
This paper presents data on the preparation of activated carbons from agricultural residues, especially the waste from biohydrogen generation. Experimental results indicated that with proper carbonization and steam activation, activated carbons with diverse characteristics can be produced from various agricultural residues. The resulting activated carbons effectively adsorb toluene. This work provides useful information for reutilization of these agricultural residues, helping in decreasing the cost of biological waste treatment and providing a cost-effective alternative to conventional adsorbent production and application.
INTRODUCTION
Activated carbon is a versatile adsorbent because of its distinguished properties: large surface area and pore volume, diverse pore structure, extensive adsorption capacity, and high degree of surface reactivity. Applications relating to the use of activated carbon include the removal of color, odor, and taste from water and wastewater; the recovery of natural gas; air purification in inhabited spaces such as chemical industries; and as catalysts and catalyst supports.Citation1,Citation2 Activated carbon can be developed from various carbonaceous source materials. The structural, chemical, adsorptive, and catalytic properties of activated carbon were determined not only by the inherent nature of source materials but also by the methods and conditions used during production.Citation1 The production of activated carbon from carbonaceous precursors involves a series of processes. Carbonization and activation are the most critical steps for activated carbon production because these two processes determine the main surface properties and porous structures. Carbonization removes non-carbon and volatile carbon species while producing a fixed carbon mass with a rudimentary pore structure.Citation1 The carbonized product is a disordered elementary graphitic crystallite with a poorly developed porous structure. The process is usually carried out at temperatures below 800 °C without oxidants existing in the gaseous environment. The rate of heating, the final temperature, the processing time at the final temperature, and the nature and physical state of the carbonaceous precursor are the parameters determining the quality and the yield of the carbonized product.
Activation increases the pore volume, enlarges the width of the pores formed during carbonization, and develops new pores of carbonized materials. Physical and chemical activation are conventional processes to manufacture carbonaceous adsorbents. Physical activation is typically carried out at temperatures between 800 and 1000 °C in the presence of oxidizing gases such as steam, carbon dioxide (CO2), air, or a mixture of these gases.Citation3–6 A major portion of the commercial activated carbons are produced by steam activation because it is cost-effective. However, a study showed that CO2 developed narrow micropores in the early stage of activation, whereas steam widened the initial microporosity from the beginning of the activation, the final result being an activated carbon with lower micropore volume and larger meso- and macropore volumes.Citation4
Chemical activation involves carbonization and activation in a single step, in which the raw materials impregnated with chemical agents are thermally decomposed at temperatures between 300 and 800 °C. The reagents most commonly used are zinc chloride (ZnCl2), phosphoric acid (H3PO4), sulfuric acid (H2SO4), and alkaline salts such as potassium hydroxide (KOH) and sodium hydroxide (NaOH).Citation1 The reagents act as dehydrating agents and as oxidants so that carbonization and activation can take place simultaneously. Activated carbons with marked surface area (>2000 m2 g−1) have been reported via KOH(aq) and NaOH(aq) activation.Citation7–9 Sequential application of chemical activation (using H3PO4, ZnCl2, or KOH) and physical activation (using CO2) has also been shown to develop activated carbon with very high surface area and pore volume.Citation9–12 A study also indicated that activated carbon derived from corncob with pore volume up to 1.533 cm3 g−1 and surface area up to 2844 m2 g−1 can be obtained by chemical activation with KOH at a KOH/ char ratio of 4, followed by 30-min CO2 gasification.Citation9 An important advantage of chemical activation is that it normally proceeds at a lower temperature and takes less time than that required in physical activation. However, a need of subsequent treatment for the leftover chemical reagent is considered as the deficiency of chemical activation.
Agricultural residues are excellent source materials for production of carbonaceous adsorbents. In Taiwan, the amount of agricultural residues was approximately 6 million t/yr. Incineration and landfill were the primary strategies for disposal of these agricultural residues, only approximately 30% of the total was further utilized, mainly as compost and animal feed. In the past years, microbial conversion of agricultural residue biomass into biohydrogen using fermentative bacteria has been considered a novel and environmentally friendly process for clean energy generation.Citation13–15 However, it was estimated that only less than 1 wt % of total biomass can be consumed during the bioconversion. Subsequent treatments such as incineration or landfill are still needed for disposal of the biomass leftovers. Thus, more proper methods of disposal and utilization of agricultural residues must be developed.
Activated carbon of approximately 8000 t is produced annually in Taiwan. To satisfy the elevating demand, extra activated carbon of more than 1000 t yr−1 is im ported from China, Indonesia, Japan, Malaysia, and the United States. Production of activated carbon with desirable surface area and porous structure from agricultural residues seems feasible to fulfill the increasing needs of Taiwan's carbon market and to achieve the goal of resource recovery of undesirable waste materials. The objective of the work presented here was to study the possibility of producing activated carbons with diverse physical and chemical properties from agricultural residues, including raw barley husk (BH), biotreated barley husk (BTBH), rice husk (RH), and pistachio shell (PS). This approach could add value to these agricultural residues, help decreasing the cost of waste treatment, and provide a potentially cost-effective alternative to existing commercial activated carbons. Notably, the BTBH sample was acquired from an anaerobic fermentation process for bio-hydrogen generation.Citation15 As far as is currently known, this is the first study to examine the feasibility of producing activated carbon from a biotreated carbonaceous precursor. Volatile organic compounds (VOCs) have received great attention in the field of environmental control because of their ubiquity in the environment and risk to human health. Using activated carbons to control emissions of VOCs has been referred to as one of the best available control technologies.Citation16–23 In this study, toluene was selected as the representative VOC for adsorption tests because it is a hazardous air pollutant and is extensively used as solvent. The effects of temperature, the inlet concentration of toluene, and the physical and chemical properties of resulting samples on adsorption equilibrium were then examined.
MATERIALS AND METHODS
Activated Carbon Precursors
Four agricultural residues, including raw BH, BTBH, RH, and PS, were selected for producing activated carbons. The chemical composition of these precursors is given in . It is noteworthy that the BTBH was an anaerobic fermentation leftover from a 100-L pilot-scale biohydrogen generation reactor.Citation15 This biohydrogen generation reactor was operated at a quasi-hydraulic retention time of 3 days and at 40 °C. RH has been reported as a precursor of activated carbon and is an abundant agricultural waste in Taiwan easily obtained from processing mills.Citation7 Pistachio is not cultivated in Taiwan. The PS tested here were the leftover of commercially imported products. They have also been shown to be a great precursor for producing activated carbon.Citation6 The purpose of choosing PS as one of the starting materials was to compare the resulting properties of activated carbons derived from grass husks to those from lignocellulosic nutshells.
Table 1. Elemental analysis of agricultural residue precursors (wt % on dry basis; n = 3)
Activated carbon adsorbents were prepared in a 5-cm inner diameter, 120-cm long quartz-tubular reactor (Lindberg/Blue M, model STF55346C). Dry agricultural residues of 1.5 g were placed in a 5-cm long, 1-cm wide ceramic boat and heated in the tubular reactor at 400 ± 10 °C for 1 hr to carbonize the samples and form char. Ultrahigh purity (UHP) N2 continuously flowed (0.4 NL min−1) through the furnace to maintain an oxygen-free environment. After carbonization, the temperature of the reactor was increased to 800 ± 10 °C at a rate of 3.5 °C min−1. The samples were then activated with a 50% H2O/50% N2 steam at 0.4 NL min−1. A microperistaltic pump (Eyela, model MP-1000) and a UHP N2 gas cylinder controlled the flow rate and the volume ratio of H2O and N2. To develop activated carbons with diverse physical and chemical properties, the activation time was 30–180 min for raw BH, 10–90 min for BTBH, 10–240 min for RH, and 10–120 min for PS. After steam activation, the samples were cooled to room temperature with UHP N2 continuously passing through the reactor tube.
Commercial activated carbon (CAC) tested in this study is a coal-based activated carbon imported from China that is used for controlling the emission of dioxins, furans, and other toxic materials from combustion flue gas streams. The CAC sample had a total surface area of 905.2 m2 g−1, a micropore area of 353.4 m2 g−1, a total pore volume of 0.485 cm3 g−1, a micropore volume of 0.162 cm3 g−1, and an ash content of 11 wt %. The processing condition for manufacture of the CAC is confidential.
Activated carbon samples were initially crushed in a 1-L ceramic mortar into particles with a 1- to 5-mm diameter and then ground to decrease the particle diameter to less than 1 mm using a vibratory micromill. Samples were subsequently sieved with U.S standard sieves (Tyler Standard Screen) to obtain −140 + 270 mesh samples. The final activated carbon products are designated X-Y (e.g., BH-30 represents the raw barely husk was steam-activated for 30 min).
Physical and Chemical Characterization of Agricultural Residues and Resulting Activated Carbons
The burnoff (i.e., the percentage of mass loss after carbonization and steam activation) was given by
The total surface area, total pore volume, micropore (pore width < 2 nm) surface area, micropore volume, and pore size distribution of raw agricultural residues and resulting activated carbon adsorbents were determined by N2 adsorption at 77 K (Beckman Coulter, model SA3100). Samples were degassed at 10–20 Torr vacuum and 150 °C for 24 hr before the N2 adsorption measurements occurred between 10−3 and 1 atm. Total surface areas were calculated by the Brunauer, Emmett, and Teller (BET) equation based on the American Society for Testing and Materials D4820-96a method. Micropore surface areas and volumes were calculated from t-plot analyses using the Jura–Harkins equation: t = [13.99/(0.0340 - log(p/ p 0)]0.5.Citation24 The range of relative pressures used to determine micropore surface areas and volumes were based on thickness t values between 0.45 and 0.8 nm. Micropore size distributions were determined by the 3D model.Citation25 Meso-pore (pore width between 2 and 50 nm) size distributions were determined by the Barret-Joyner-Halenda method.Citation26 All of the resulting activated carbon samples had pore widths less than 50 nm, indicating that macropore (pore width ≥50 nm) surface areas and volumes were not observed based on the N2 adsorption examinations.
The chemical composition, including the mass concentrations of C, H, N, and S of agricultural residue precursors and the resulting activated carbons, was determined with an elemental analyzer (Elementar Vario, model EL III) according to the Taiwan National Institute of Environmental Analysis (NIEA) method R409.21C. Ash contents of samples were carried out following NIEA method R204.00T by burning away the samples at 800 °C for 3 hr or until total elimination of fixed carbon and volatile matter. The oxygen content of the resulting samples was determined by difference on the basis of the data from elemental analysis. X-ray photoelectron spectroscopy (XPS) was carried out to determine the surface functional group of the resulting activated carbon samples. The samples were dried at 150 °C for 30–45 min and then analyzed under a 10−8- to 10−10-Torr ultrahigh vacuum circumstance. Alκα radiation at 1486.6 eV was used as the X-ray source. Surface functional groups, including oxygenated groups, were deconvoluted between 280 and 294 eV on the basis of their characteristic binding energies.
Adsorption Characteristics
One of the main focuses of this study is to determine the equilibrium adsorption capacities of agricultural residue-derived activated carbons (ARACs) for VOCs and then compare the results with those obtained from a commercially obtained coal-based activated carbon. The toluene-containing gas stream was prepared by mixing a certified compressed toluene cylinder at the concentration of 600 ± 50 parts per million by volume (ppmv) with UHP N2. Mass flow controllers (Brooks, model 5850E and 5850E-Kr) were used to dilute and regulate the flow rate of gas mixture entering the testing system. The testing system, having a similar design with a previous study,Citation20 consisted of an electronic, gravimetric balance (Thermo Cahn, model TG-2121) with a capacity up to 1.5 g and a weighing range of 150 mg. The prepared gas stream with a concentration in the range of 50–600 ppmv passed a mixture coil and then passed the activated carbon sample suspended in a thermogravimetric balance at a 90-NmL min−1 flow rate with adsorption temperatures controlled between 40 and 80 °C. As the sample adsorbed the toluene, its mass increased until adsorption equilibrium was reached; namely, no further detectable change in the mass of the sample was observed. The difference between the initial and final masses of the adsorbent represented the total mass of toluene adsorbed at a given concentration and temperature. The mass of toluene adsorbed divided by the initial dry mass of the adsorbent provided the equilibrium adsorption capacity of the adsorbent (milligram toluene per gram activated carbon; mg g−1).
The Freundlich, Langmuir, and Dubinin–Radushkevich (DR) equations were applied to the toluene adsorption isotherms obtained at various inlet toluene concentrations and adsorption temperatures. The Freundlich equation is an empirical model that assumes that the equilibrium adsorption capacity is linear with a change in adsorbate concentration on a log-log scale at a given temperatureCitation27:
The theoretical Langmuir equation is the best known of all isotherms, assuming monolayer adsorption onto a surface with a finite number of identical sits, and is represented as follows:
The DR model was developed to determine physisorption in microporous adsorbents. A detailed description about the development and application of DR model can be found elsewhere.Citation28 A brief statement is presented here for clarity. The DR equation is given by
RESULTS AND DISCUSSION
Physical Properties of ARACs
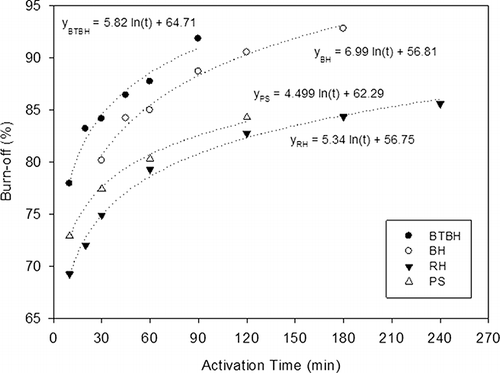
The burnoff of ARACs ranged from 69.3 to 92.8%, mainly depending on steam activation time (). The carbonization process (400 °C, 1 hr) showed a smaller effect on the burnoff of raw BH and BTBH (33.8% and 33.2%, respectively). For RH and PS, burnoff up to 59.7 and 67.5%, respectively, can be achieved during carbonization. These results suggest that a larger amount of volatile matter exists in the RH and PS than in BH samples.
shows that the burnoff increased as an increase in the activation time. Notably, it was also found that the burnoff rates (expressed as d[%] dt −1 ) for raw BH-and BTBH-derived activated carbons (series BH and BTBH) were greater than those for RH- and PS-derived samples (series RH and PS). It was also important to note that the burnoff rate of BTBH was greater than that of BH at activation time between 10 and 60 min. clearly indicates that the raw BH contained greater amounts of carbon and hydrogen than BTBH, suggesting that a portion of biomass was converted into CO2, H2, and various organic acids during the biohydrogen generation process.Citation15 Namely, the microorganism consumes the carbon matrix of BHs during the hydrogen generation processes, simultaneously loosening the carbon structure of BH. Consequently, water molecules react more effectively with the loosening carbon matrix of BTBH, causing a larger burnoff rate during the early stage of BTBH development.
The regression equations of burnoff (y) versus activation time (t) for each series of ARAC production are also presented in Good correlations (R Citation2 > 0.973) between burnoff and activation time were established. These equations are critical for determining the proper activation time during activated carbon production if a minimum production yield (i.e., maximum burnoff) and surface area are preset as requirements. These equations also provide useful information in prospective scale-up carbon production design.
Carbonization had a small effect on the surface area and pore volume of ARACs ( and ). For example, raw BH and BTBH had a BET surface area (S BET) of 6.5 and 5.2 m2 g−1, respectively. After carbonization, the S BET increased to 8.4 and 5.9 m2 g−1, respectively. Increasing activation time markedly enhanced S BET, micropore area (S micro), total pore volume (V t), and micropore volume (V micro). Among the four activated carbon series, BH and PS activated carbons had the greatest S BET, up to 950 m2 g−1, which was comparable to that of the coal-based CAC (905.2 m2 g−1). These experimental results suggest that BH and PS are excellent starting materials for producing ARACs with a large S BET. However, for BTBH and RH activated carbons, the maximum S BET values were only 600.2 and 362.3 m2 g−1, respectively. It is also important to note that the burnoff of BH activated carbons approached 100% at an activation time of 180 min, which is the longest duration that steam activation can be applied before complete gasification of carbon matrix. These results indicate that activated carbons with diverse physical properties can be developed with a proper control of the activation time.
Table 2. Physical properties of raw sample, char, and activated carbons from raw BH and BTBH (n = 3)
Table 3. Physical properties of raw sample, char, and activated carbons from rice husk and pistachio shell (n = 3)
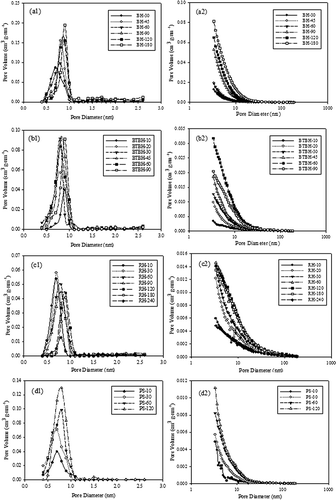
The data of pore area/volume versus activation time, listed in and , also provide an insight into the difference in pore developing characteristics for grass husk and nutshell. The S micro of all three grass husks, including BH, BTBH, and RH, reached their local maxima at activation times of 90 min or less, indicating a rapid development in microporous structure under the tested steam activation conditions. RH showed the fastest development in microporous structure: the local maxima of S micro (248.1 m2 g−1) and V micro (0.111 cm3 g−1) were achieved at 20-min activation (). It was also observed that the S micro and V micro then decreased as the activation time increased. The decrease in S micro and V micro was due to overgasification that caused the collapse of micropore structure by surplus steam (e.g., RH shown in ) or due to the conversion of micropores into mesopores via pore widening (e.g., raw BH and BTBH shown in ). Importantly, both phenomena can cause the declining trend in S micro/S BET and V micro/V t ratios presented in and . On the contrary, PS carbons were highly microporous, possessing a great portion of micropores (S micro/S BET > 78.9% and V micro/V t > 65.7%). Additionally, the collapse of micropore structure caused by overgasification was not observed for PS within the entire activation range. As a consequence, PS is a better precursor to produce microporous activated carbon with a large surface area. On the other hand, mesoporous activated carbons are easily developed from grass husk precursors (e.g., raw BH and BTBH) via steam activation.
Examinations of micropore and mesopore size distribution of ARACs also confirmed the occurrence of pore development during steam activation. It was also observed that ARACs possessed a uniform micropore size distribution. Taking BTBH as an example, the largest V micro was achieved at an activation time of 60 min with a peak at 0.7–0.9 nm (, b1). At the same activation time, the largest mesopore volume was simultaneously achieved (, b2). Other ARACs showed a similar trend in the development of pore size distribution as BTBH.
Chemical Properties of ARACs
Steam activation in general reduced the amount of carbon for all ARACs () because of the interaction between water molecules and the carbon matrix followed by an increase in the oxygen content and the release of carbon as gaseous carbon monoxide (CO(g)) and CO2(g). The effects of steam activation on hydrogen and nitrogen contents were difficult to clarify because of their small amounts. The surface functionality of BTBH and RH, such as carbonyl and carboxylic groups, was examined using XPS (). Five major groups including graphitic C-C bonding (284.5–284.6 eV), hydroxyl (286 – 286.2 eV), carbonyl (287.9 –288.1 eV), carboxyl (289.1–289.3 eV), and π-electron resonance (292.7–293 eV) were deconvoluted from the C1s peak between 280 and 294 eV. XPS results indicated that increasing activation duration caused a decrease in the percent deconvoluted area of graphitic peaks and an increase in those of carbonyl and carboxyl peaks. These observed results corresponded with the data obtained from the elemental analysis.
Table 4. Elemental analysis of ARACs (moisture-ash-free basis; wt %)
Table 5. Percent area of deconvoluted surface functional groups from the XPS C1s peak between 280 and 294 eV for activated carbons from BTBH and RH
Adsorption Behaviors of Toluene with ARACs and CAC
A comparison of the equilibrium adsorption of toluene for ARACs and CAC was made using toluene as an adsorbate. BTBH-60 was selected as the representative ARAC to compare with CAC because both samples were mesoporous and had similar S micro, whereas CAC had a larger S BET. In addition, few studies investigated the adsorption behaviors of activated carbon derived from biotreated agricultural residues.
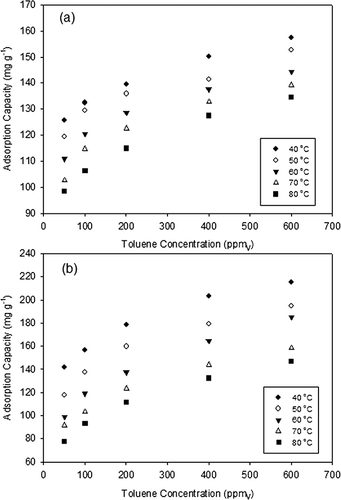
The isotherms (i.e., the equilibrium adsorption capacities as a function of inlet toluene concentration at given temperatures for BTBH-60 and CAC carbons) are illustrated in The expression of correlations; the modeled Freundlich, Langmuir, and DR parameters; and the R 2 values are shown in . As expected, the adsorption capacity enhanced with increasing toluene concentration and reduced with increasing adsorption temperature, confirming that physisorption is the main mechanism causing the partitioning of the vapor between the gas and adsorbed phases.Citation21 In addition, correlations for toluene adsorption capacities of BTBH-60 and CAC showed good agreement from Freundlich (R 2 > 0.970), Langmuir (R 2 > 0.993), and DR (R 2 > 0.965) simulations (). These results reveal that the theoretical models can be operated with a high degree of confidence to predict the equilibrium adsorption of ARACs and CAC within the tested concentration and temperature ranges. These results also suggest that monolayer adsorption may occur on the surface of activated carbon because the adsorption data showed the best agreement with the Langmuir model. It is also important to note that the adsorption characteristic energy (E 0) for CAC to adsorb toluene, within 15.81–18.00 kJ mol−1, was smaller than that for BTBH-60 (22.97–26.09 kJ mol−1). These calculations imply that toluene is easier to adsorb onto the CAC according to the volume filling of micropore theory.Citation29
Table 6. Freundlich, Langmuir, and DR parameters for toluene adsorption with BTBH-60 and CAC
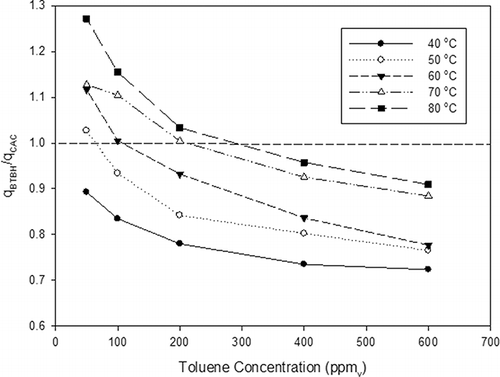
A detailed comparison of the toluene adsorption capacities for BTBH-60 and CAC demonstrates the advantage of using ARACs in adsorption of VOCs under a low-concentration, high-temperature circumstance (). In , q BTBH/q CAC is the ratio of adsorption capacity for BTBH-60 to that for CAC. Results showed that CAC had greater adsorption capacities than BTBH-60 (i.e.,q BTBH/q CAC < 1) at lower temperature (<50 °C) and large inlet toluene concentration (>200 ppmv). These results are expected because CAC had a greater S BET (905 m2 g−1) than BTBH-60 (600 m2 g−1). Notably, when the temperature was higher than 50 °C, BTBH-60 had a larger adsorption capacity than CAC within the lower toluene concentration range (i.e., between 50 and 200 ppmv) ( and ). Consequently, activated carbon derived from agricultural residues appears to be more applicable to adsorb VOCs at lower concentration and higher temperature. Although the detailed mechanisms causing the observed phenomena are not well understood, the larger VOC adsorption capacity of BTBH-60 at lower concentration and higher temperature may be due to the difference in the porous structure and surface functionality of BTBH-60 and CAC (e.g., oxygenated functional groups) that may result in a polar carbon surface. It is also known that the average micropore diameter (L av) of the slit-shaped micro-pores is reversely proportional to E 0.Citation30 The smaller L av may cause the BTBH-60 activated carbon to be more applicable in a thermodynamically unfavorable environment as compared with the CAC sample. These results are encouraging because the production of ARACs is more cost-effective as compared with those for typical CACs. In addition, ARAC's enhanced adsorption capacities at lower vapor concentrations are valuable because several air quality control applications that utilize physical adsorption as the main mechanism to remove vapors from gas streams are designed for low-concentration, high-temperature operations.
CONCLUSIONS
In this study, agricultural residues including raw BH, BTBH, RH, and PS were chosen to produce activated carbons. With adequate carbonization (400 °C for 1 hr) and 50% H2O(g)/50% N2 steam activation (800 °C for 30–90 min), activated carbons with diverse characteristics can be developed. Mesoporous activated carbons are easily produced from grass husk precursors, including the BTBH, by increasing the activation time, confirming the feasibility of resource recovery of this biohydrogen generation waste. The results from adsorption tests also showed that ARACs are more applicable to adsorb toluene at low concentration (<200 ppmv) and high temperature (>50 °C). The obtained data suggested that the tested ARAC appeared to be more applicable for VOC adsorption in a thermodynamically unfavorable environment as compared with the CAC sample. The adsorption tests also suggested that monolayer adsorption may occur on the surface of the ARAC because the adsorption data showed the best agreement with the Langmuir equation.
ACKNOWLEDGMENTS
The authors thank the National Science Council and Environmental Protection Agency of Taiwan, Republic of China (contracts NSC93-EPA-Z-327-002 and EPA-94-U1U4-04-007) for funding support. The opinions expressed in this paper are not necessarily those of the sponsors.
REFERENCES
- Bansal , R.C. , Donnet , J.B. and Stoeckli , F. 1988 . Active Carbon , Dekker : New York .
- Marsh , H. and Menendez , R. 1989 . Introduction to Carbon Science , Butterworths : London, United Kingdom .
- Gonzalez , M.T. , Molina-Sabio , M. and Rodriguez-Reinoso , F. 1994 . Steam Activation of Olive Stone Chars, Development of Porosity . Carbon , 32 : 1407 – 1413 .
- Molina-Sabio , M. , González , M.T. , Rodríguez-Reinoso , F. and Sepulveda-Escribano , A. 1996 . Effect of Steam and Carbon Dioxide Activation in the Micropore Size Distribution of Activated Carbon . Carbon , 34 : 500 – 509 .
- Gonzalez , M.T. , Rodriguez-Reinoso , F. , Garcia , A.N. and Marcilla , A. 1997 . CO2 Activation of Olive Stones Carbonized under Different Experimental Conditions . Carbon , 35 : 159 – 165 .
- Yang , T. and Lua , A.C. 2003 . Characteristics of Activated Carbons Prepared from Pistachio-Nut Shells by Physical Activation . J. Colloid Interface Sci. , 267 : 408 – 417 .
- Guo , Y. , Yang , S. , Yu , K. , Zhao , J. , Wang , Z. and Xu , H. 2002 . The Preparation and Mechanism Studies of Rice Husk Based Porous Carbon . Mater. Chem. Phys. , 74 : 320 – 323 .
- Lillo-Ródenas , M.A. , Cazorla-Amorós , D. and Linares-Solano , A. 2003 . Understanding Chemical Reactions between Carbons and NaOH and KOH: an Insight into the Chemical Activation Mechanism . Carbon , 41 : 267 – 275 .
- Tseng , R.L. , Tseng , S.K. and Wu , F.C. 2006 . Preparation of High Surface Area Carbons from Corncob with KOH Etching Plus CO2 Gasification for the Adsorption of Dyes and Phenols from Water . Colloids Surf. A , 279 : 69 – 78 .
- Molina-Sabio , M. , Rodríguez-Reinoso , F. , Caturla , F. and Sellés , M.J. 1996 . Development of Porosity in Combined Phosphoric Acid-Carbon Dioxide Activation . Carbon , 34 : 457 – 462 .
- Hu , Z. and Srinivasan , M.P. 2001 . Mesoporous High-Surface-Area Activated Carbon . Micropor. Mesopor. Mater. , 43 : 267 – 275 .
- Hu , Z. , Guo , H. , Srinivasan , M.P. and Yaming , N. 2003 . A Simple Method for Developing Mesoporosity in Activated Carbon . Sep. Purif. Technol. , 31 : 47 – 52 .
- Lay , J.J. , Fan , K.S. , Chang , J.I. and Ku , C.H. 2005 . Influence of Chemical Nature of Organic Wastes on Their Conversion to Hydrogen by Heat-Shock Digested Sludge . Int. J. Hydrogen Energy , 28 : 1361 – 1367 .
- Zhang , M.L. , Fan , Y.T. , Xing , Y. , Pan , C.M. , Zhang , G.S. and Lay , J.J. 2007 . Enhanced Biohydrogen Production from Cornstalk Wastes with Acid-ification Pretreatment by Mixed Anaerobic Cultures . Biomass Bioenergy , 31 : 250 – 254 .
- Chou , C.H. , Wang , C.W. , Huang , C.C. and Lay , J.J. 2008 . Pilot Study of the Influence of Stirring and pH on Anaerobes Converting High-Solid Organic Wastes to Hydrogen . Int. J. Hydrogen Energy , 33 : 1550 – 1558 .
- Chiang , Y.C. , Chiang , P.C. and Huang , C.P. 2001 . Effects of Pore Structure and Temperature on VOC Adsorption on Activated Carbon . Carbon , 39 : 523 – 534 .
- Chiang , H.L. , Chiang , P.C. and Huang , C.P. 2002 . Ozonation of Activated Carbon and its Effects on the Adsorption of VOCs Exemplified by Methylethylketone and Benzene . Chemosphere , 47 : 267 – 275 .
- Huang , Z.H. , Kang , F. , Zheng , Y.P. , Yang , J.B. and Liang , K.M. 2002 . Adsorption Characteristics of Trace Volatile Organic Compounds on Activated Carbon Fibers at Room Temperature . Adsorption Sci. Technol. , 20 : 495 – 500 .
- Huang , Z.H. , Kang , F. , Liang , K.M. and Hao , J. 2003 . Breakthrough of Methylethylketone and Benzene Vapors in Activated Carbon Fiber Beds . J. Hazard. Mater. , 98 : 107 – 115 .
- Ramirez , D. , Sullivan , P.D. , Rood , M.J. and Hay , J.K. 2004 . Equilibrium Adsorption of Phenol-, Tire-, and Coal-Derived Activated Carbons for Organic Vapors . J. Environ. Eng. , 130 : 231 – 241 .
- Ramirez , D. , Qi , S. , Rood , M.J. and Hay , J.K. 2005 . Equilibrium and Heat of Adsorption for Organic Vapors and Activated Carbons . Environ. Sci. Technol. , 39 : 5864 – 5871 .
- Lillo-Ródenas , M.A. , Cazorla-Amorós , D. and Linares-Solano , A. 2005 . Behaviour of Activated Carbons with Different Pore Size Distributions and Surface Oxygen Groups for Benzene and Toluene Adsorption at Low Concentrations . Carbon , 43 : 1758 – 1767 .
- Tsai , J.H. , Chiang , H.M. , Huang , G.Y. and Chiang , H.L. 2008 . Adsorption Characteristics of Acetone, Chloroform and Acetonitrile on Sludge-Derived Adsorbent, Commercial Granular Activated Carbon and Activated Carbon Fibers . J. Hazard. Mater. , 154 : 1183 – 1191 .
- Lippens , B.C. and De Boer , J.H. 1965 . Studies on Pore System in Catalyst, V; the T Method . J. Catal. , 4 : 319 – 323 .
- Sun , J. , Chen , S. , Rood , M.J. and Rostam-Abadi , M. 1998 . Correlating N2 and CH4 Adsorption on Microporous Carbon Using a New Analytical Model . Energy Fuels , 12 : 1071 – 1078 .
- Gregg , S.J. and Sing , K.S.W. 1982 . Adsorption, Surface Area and Porosity , 2nd , London , , United Kingdom : Academic Press .
- Ruthven , D.M. 1984 . Principles of Adsorption & Adsorption Process , New York : John Wiley and Sons .
- Dubinin , M.M. 1989 . Fundamentals of the Theory of Adsorption in Micro-pores of Carbon Adsorbents: Characteristics of Their Adsorption Properties and Microporous Structures . Carbon , 27 : 457 – 467 .
- Ponec , V. , Knor , Z. and Cerny , S. 1974 . Adsorption on Solid , Cleveland , OH : Chemical Rubber Company .
- Gauden , P.A. , Terzyk , A.P. , Rychlicki , G. , Kowalczyk , P. , Cwiertnia , M.S. and Garbacz , J.K. 2004 . Estimating the Pore Size Distribution of Activated Carbons from Adsorption Data of Different Adsorbates by Various Methods . J. Colloid Interface Sci. , 273 : 39 – 63 .