ABSTRACT
More than half of the world's population lives in cities, and their populations are rapidly increasing. Information on vertical and diurnal characterizations of volatile organic compounds (VOCs) in urban areas with heavy ambient air pollution can help further understand the impact of ambient VOCs on the local urban environment. This study characterized vertical and diurnal variations in VOCs at 2, 13, 32, 58, and 111 m during four daily time periods (7:00 to 9:00 a.m., 12:00 to 2:00 p.m., 5:00 to 7:00 p.m., and 11:00 p.m. to 1:00 a.m.) at the upwind of a high-rise building in downtown, Kaohsiung City, Taiwan. The study used gas chromatography–mass spectrometry to analyze air samples collected by silica-coated canisters. The vertical distributions of ambient VOC profiles showed that VOCs tended to decrease at greater heights. However, VOC levels were found to be higher at 13 m than at ground level at midnight from 11:00 p.m. to 1:00 a.m. and higher at 32 than 13 m between 7:00 and 9:00 a.m. These observations suggest that vertical dispersion and dilution of airborne pollutants could be jointly affected by local meteorological conditions and the proximity of pollution sources. The maximum concentration of VOCs was recorded during the morning rush hours from 7:00 to 9:00 a.m., followed by rush hours from 5:00 to 7:00 p.m., hours from 12:00 to 2:00 p.m., and hours from 11:00 p.m. to 1:00 a.m., indicating that the most VOC compounds in urban air originate from traffic and transportation emissions. The benzene-toluene-ethyl benzene-xylene (BTEX) source analysis shows that BTEX at all heights were mostly associated with vehicle transportation activities on the ground.
The vertical distributions of ambient VOC profiles tend to decrease with height. However, levels of VOCs that originate from upwind industrial sources can accumulate at higher altitudes because of thermal inversions. These observations suggest that vertical dispersion and dilution of airborne pollutants could be jointly affected by local meteorological conditions and the proximity of pollution sources. Whether such vertical variation in VOC profiles can affect the prediction of air quality model is of interest and needs to be considered in future air quality management.
INTRODUCTION
Deterioration of air quality in many of the world's cities is raising concerns over the health of their residents. Much research has been performed to monitor ambient air quality or the air near industrial parks,Citation1,Citation2 but until recently only a few have evaluated the vertical distribution of ambient volatile organic compounds (VOCs). One study investigated the vertical variation of motor vehicle emission in a street canyon formed by five-story buildings.Citation3 In Korea, another study was performed to evaluate the outdoor air quality for 112 homes in 56 high-rise apartment buildings with 10 or more stories.Citation4 Units on the lower levels had significantly higher methyl tertiary butyl ether, benzene, and toluene than those on the higher levels. In Greece, VOC data were collected using a specially instrumented Falcon 20-E5 research aircraft.Citation5 High concentrations of VOCs (150–350 parts per billion [ppb]) were observed near the ground and within the first 300–400 m ± relatively high (100–200 ppb) even at much higher altitudes (1400–1600 m). In Mexico, VOC levels were measured using tethered balloons floating from 0 to 200 m above ground.Citation6 The VOC concentration decreased with increasing height within the mixing layer before 6:00 a.m. and between 7:00 and 10:00 a.m. In Beijing in the autumn of 2005, atmospheric VOCs were measured at the heights of 8, 32, 140, and 280 m on the Beijing 325-m meteorological tower.Citation7 That study found concentrations of total volatile organic compounds (TVOCs) to range from 51.2 ± 39.7 to 83.6 ± 44.4 ppb on clear days but from 62.9 ± 19 to 105 ± 59.2 ppb on hazy days. Although the vertical distribution of VOCs is complex, most distribution profiles show that VOC levels decrease at greater heights on clear days. However, on hazy days, VOC concentrations gradually decrease at increasing heights from 8 to 140 m and then increase dramatically at heights greater than 140 m until 280 m is reached. These results may suggest that the vertical distributions of VOCs are affected jointly by several factors, including meteorological and transport factors. Additionally, principal component analysis and cluster analyses show that VOCs of different origins can be found at different heights.Citation7
More than half of the world's population lives in cities, and the populations of these urban areas are increasing rapidly. The vertical and diurnal characterization of VOCs in urban areas with heavy ambient air pollutionCitation8,Citation9 is important for the evaluation of exposure to airborne pollutants for indoor occupants of high-rise buildings in urban areas. This study measured the vertical distribution and concentrations of various VOC species near a high-rise building at four time periods during a day in downtown Kaohsiung, an industrial harbor city with heavy ambient air pollution in southern Taiwan.
METHODS
Sampling Sites and Time
The vertical and diurnal distributions of VOCs in downtown Kaohsiung were explored by collecting atmospheric air samples at five heights during four time periods near a high-rise office building located at a heavy-traffic intersection. The five heights of 2, 13, 32, 58, and 111 m corresponded with the 1st, 4th, 9th, 16th, and 30th floors of the high-rise office building. The samples were collected continuously for 2 hr at a time four time periods in a day in August at 7:00 to 9:00 a.m., 12:00 to 2:00 p.m., 5:00 to 7:00 p.m., and 11:00 p.m. to 1:00 a.m. During each sampling, meteorological conditions including temperature, relative humidity, and pressure were recorded.
Analytical Methods
U.S. Environmental Protection Agency (EPA) Method TO-15Citation10 was used to quantitatively and qualitatively analyze the ambient VOCs. This method uses two sets of standards (i.e., the Urban Air Toxics and Photochemical Assessment Monitoring System) to analyze alkanes, alkenes, aromatics, and halogenated VOCs. Of the 101 VOCs measured, 40 are listed in the United States as hazardous air pollutants. Silica-coated canisters made of stainless steel were used to store the ambient VOC grab samples. Before sampling, all canisters were cleaned, moisturized, and checked for leaks to ensure that a vacuum could be created and maintained. A laboratory blank check was performed to guarantee that all canisters were properly cleaned. All team members who participated in the sampling activities were trained to follow standard sampling procedures. EPA Method TO-15Citation10 and Taiwan NIEA A715.13BCitation11 quality assurance/quality control protocols were followed during the course of sampling, preservation, transport, and analysis. Samples were collected using a 16-inlet-position autosampler and preconcentrated with an Entech 7000 before they were analyzed for polar and non-polar VOCs using a gas chromatography (GC) (Hewlett-Packard, HP6890) and mass spectrometry detector (HP5973) equipped with a DB-1 capillary column (Agilent, 60 m × 0.32 mm × 1.05 μm, 100% dimethylpolysiloxane, nonpolar, low-bleed column). The GC oven temperature was programmed at 45 °C for 5 min, increased at 8 °C min−1 to 180 °C, and held at 180 °C for 10 min.
RESULTS AND DISCUSSION
Concentrations and Species of VOCs
shows the concentrations and species of individual VOCs detected and not detected in the urban Kaohsiung area at five different heights during four time periods on August 3, 2008. The distribution of VOC species was similar in all samples collected at different heights and during various periods of the day. Ethene, acetylene, and ethane were the most abundant VOC species, followed by isopentane, toluene, pentane, methyl pentane, hexane, benzene-tolueneethyl benzene-xylene (BTEX), 1,2,4-trimethylbenzene, and butane. Moreover, the highest VOC concentrations were found during the morning traffic hours from 7:00 to 9:00 a.m. From morning to midnight, concentrations of TVOCs decreased from 272 to 42 ppb at the ground level, 165 to 55 ppb at 13 m, 183 to 30 ppb at 32 m, 125 to 12 ppb at 58 m, and 112 to 3 ppb at 111 m. The overall TVOC levels in this study were generally higher than those reported for Beijing in the autumn.Citation7 Concentrations and species of VOCs, which include trans-2-butene, isoprene, pentene, 2,3-dimethylbutane, heptane, methyl cyclohexane, and pentane, decreased dramatically above 32 m, or the ninth floor of the office building. In other words, the height of 32 m is the turning point above which the ambient air quality may not be a major concern in downtown Kaohsiung City during the sampling period.
Table 1. Diurnal variation of individual VOC concentrations (ppb) detected at five heights during four time periods on August 3, 2008
Vertical Variations of TVOCs and VOCs
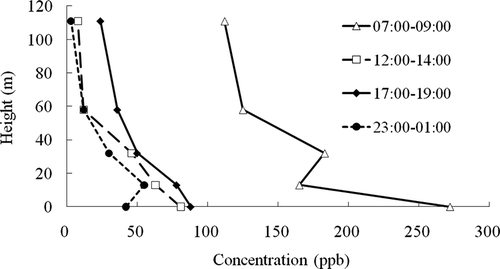
In general, the concentrations of TVOCs exhibit a well-defined vertical pattern during all sampling periods except for two measurements (7:00 to 9:00 a.m. and 11:00 p.m. to 1:00 a.m.). TVOCs have the highest concentrations near the ground level and the smallest concentrations at 111 m (). This is mostly because of the shorter distance between the ground level and transportation pollution sources, consistent with the study conducted in Beijing.Citation7 However, at midnight from 11:00 p.m. to 1:00 a.m., TVOC concentrations are higher at 13 m than near ground level, and the concentration of TVOCs suddenly becomes higher at 32 m than at 13 m from 7:00 to 9:00 a.m., a result of increased levels of styrene, ethyl benzene, and toluene at that time (). A large quantity of toluene and ethyl benzene is detected in all VOC samples collected between 11:00 p.m. and 1:00 a.m. at 13 m and between 7:00 and 9:00 a.m. at 32 m. Moreover, styrene was only detected in large amounts at these two measurement times. Styrene is produced industrially from ethyl benzeneCitation12 and from toluene and methanol.Citation13 Thus, ambient VOCs near the height levels of 13 and 32 m may have nearby industrial origins that are located upwind of the sampling site.
Figure 1. Vertical profiles of TVOC concentrations at heights of 2, 13, 32, 58, and 111 m at four periods of time during a day (7:00–9:00 a.m., 12:00–2:00 p.m., 5:00–7:00 p.m., and 11:00 p.m.–1:00 a.m.).
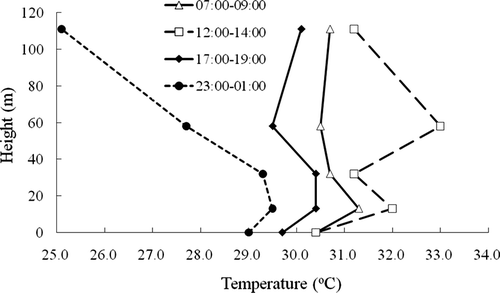
The temperature lapse rates or environmental lapse rate (ELR), calculated as the difference of temperatures measured at different heights, can be used to characterize the stability of the atmospheric layer between two different altitudes.Citation14 Most ELR values are found between the saturated adiabatic lapse rate of 5.4 °C/km and the dry adiabatic lapse rate (DALR) of 9.8 °C/km or more than DALR, which indicates conditional instability.Citation14 Thus, generally speaking, the atmospheric layers are not stable and facilitate the dispersal and dilution of airborne pollutants. However, the vertical temperature data presented here indicated that there was a temperature-reversing zone located somewhere between 13 and 32 m () that significantly affected the vertical profiles of VOCs. This impact was especially obvious between 11:00 p.m. and 1:00 a.m. when the temperature difference was the largest (between 2 and 111 m) (). Temperature reversion basically hinders pollutants from diffusing, making it easy for some VOCs that originate from the place near the reversing zone to accumulate, as was found for styrene in this study.
Diurnal Variations of TVOCs and VOCs
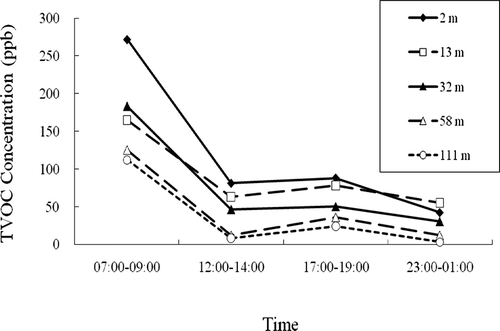
and indicate that the highest concentrations of TVOCs and the largest number of VOC species were measured in the morning (7:00 to 9:00 a.m.) at all heights, corresponding more or less to the heavy traffic during morning rush hours in downtown Kaohsiung. However, the pollution may also have come from photochemical reactions to form VOCs, although solar radiation is usually not very strong during early-morning hours. Seibert et al.Citation15 suggest that another important reason for this observed morning high concentration is that the low atmospheric mixing height in the early morning may lead to insufficient vertical mixing, making VOCs' vertical dilution less possible than at other times of the day. It is also clear in and that the levels of TVOCs decreased at noon (12:00 to 2:00 p.m.) and increased again during the evening (5:00 to 7:00 p.m.), then decreased to the lowest levels around midnight (11:00 p.m. to 1:00 a.m.). This observation is consistent with still another study that implied that a strong effect of vertical dilution corresponded with an increasing mixing layer at noon.Citation6 The increased TVOCs in the evening can be explained by the elevated traffic volume during the evening rush hours. Furthermore, the lowest TVOCs are expected because of the diminishing traffic volume at midnight in downtown Kaohsiung. Moreover, it is worth mentioning that the concentrations of TVOCs during the evening rush hours (5:00 to 7:00 p.m.) are not as high as in the morning (7:00 to 9:00 a.m.), although both periods have a high volume of traffic. This may be explained by two observations. First, the wind speed and direction changes during this time frame. Second, the traffic flow is large but not dense enough in the evening rush hours because people tend to get off work at different times whereas they almost all go to work at the same time.
Figure 3. Diurnal profiles of TVOCs at heights of 2, 13, 32, 58, and 111 m at four periods of time during a day (7:00–9:00 a.m., 12:00–2:00 p.m., 5:00–7:00 p.m., and 11:00 p.m.–1:00 a.m.).
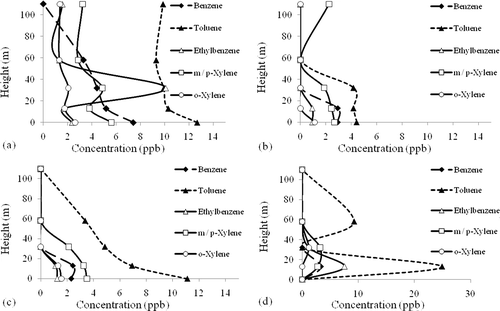
Regarding individual VOC species (shown in ), concentrations were the highest for most cases during the morning traffic hours from 7:00 to 9:00 a.m. at all heights. This is consistent with the above-stated diurnal variation of TVOCs. However, some variations of individual VOCs were different from the pattern of TVOCs for the rest of the day, especially at the 13-m level, where the highest concentrations occurred around midnight (11:00 p.m. to 1:00 a.m.) for the species of toluene, ethyl benzene, and styrene. Although the reasons for this observation are not clear, these individual VOCs are likely to be coming from nearby industrial sources rather than traffic and automotive emissions.
Vertical and Diurnal Variations of BTEX
Aromatic hydrocarbons such as BTEX, which are widely used in industry, cause serious adverse effects on environmental air quality. Benzene is a carcinogenic compound capable of causing leukemia. The World Health Organization has estimated that a lifetime exposure of 1 μg m−3 of benzene leads to approximately six cases of leukemia per 1 million inhabitants.Citation16
In general, the vertical profiles of BTEX over 24 hr remained consistent (); for example, at noon and in the evening, concentrations of BTEX were the highest at the ground level and decreased with height until 111 m. However, a few exceptions have been observed. The ethyl benzene concentration was greater at 32 m than at ground level in the morning (). Additionally, concentrations of some BTEX species were highest at 13 or 32 m than at ground level around midnight (11:00 p.m. to 1:00 a.m., ). This was especially obvious for toluene, which had the highest concentration at 13 m of the measurements during the midnight hours. These observations may be caused by the combined effect of the temperature reversion between 13 and 32 m and proximity of the sampling site to pollution sources.
BTEX Ratios and Source Determination
The BTEX concentration ratios were compared to assess the VOC emission sources near the sampling site. The ground-level BTEX ratios of (3:5:1:3) in the morning, (3:5:1:4) at noon, and (2:9:1:4) in the evening are similar to the BTEX ratios of (3:5:1:3), (3:14:1:4), (4:11:1:3), and (7:11:1:3) reported for the Lincoln Tunnel at Newark, NJ.Citation17 These findings demonstrate a similarity between the sources of BTEX measured in the two studies, suggesting that both had similar emission sources, mostly associated with vehicle transportation activities. However, different ground-level BTEX ratios were reported for the same time periods in other studies on vehicle exhaust; for example, (1.3:11.5:1:2.8), (1:10.6:2.1:3.1), (1.4:6.1:1:2.6), (1:6.9:1.3:2.1), and (3.3:6.3:1.3:1) in Hong KongCitation18 and (3.6:4.7:1:7.4), (1:1.7:1.3:2.9), (1:2.7:1:2.7), (1:8.6:1.7:4.9), (3.1:2.8:1:3.3), and (1.2:3.6:1:3.8) in southern Taiwan.Citation19 These discrepancies may be caused by a difference in pollution sources for the various sampling locations. Other factors such as climate, topography, characteristics of vehicle fuel used, orientation and alignment of buildings, etc., may also contribute to the observed differences in BTEX ratios.
Differentiating pollution sources is difficult for air samples, and forecasting the true influence of different emission sources is not an easy task. However, ratios of toluene/benzene (T/B) and xylene/ethyl benzene (X/E) ratios can be used to clarify the characteristics of BTEX emission. Nelson and QuigleyCitation20,Citation21 demonstrated that the ratio between m,p-xylene and ethyl benzene (X/E) indicates the extent of atmospheric photochemical reactivity. Subsequently, the X/E ratio is useful for the estimation of the photochemical age of air mass,Citation22 as was suggested by Nelson and Quigley.Citation20 Recently, the species ratios (T/B and X/B) could be used to estimate the photochemical age of air mass,Citation9 as suggested by Nelson and QuigleyCitation20 and Monod et al.Citation22 Xylenes are considered a highly reactive species, whereas ethyl benzene is considered a lower activity species. A low X/E ratio suggests an aged air parcel.Citation20
All T/B ratios obtained in this research are shown in . Relatively higher T/B ratios (7.53 and 4.81) were found at 13 m at midnight and at ground level in the evening. As mentioned above, the high T/B ratio suggests that there were additional pollution sources of toluene from upwind industrial sites that contributed significantly to the receptor during the sampling period. On the basis of the wind patterns of Kaohsiung, toluene may be blown from upwind industrial sources. It might have then been trapped in the atmosphere approximately 13 m above the ground because of inversion layer and low mixing height during the two sampling periods.
Table 2. T/B, m,p-X/B, o-X/B, and X/E concentration ratios in this study
The X/E ratios measured in downtown Kaohsiung agree with the observed X/E ratios of 2.2–3.8 in a nearby industrial areaCitation19 except for two low ratios observed at 13 m at midnight and at 32 m in the morning. As mentioned previously, these two low ratios may have been caused by the extraordinarily large amount of ethyl benzene observed at 13 m at midnight and at 32 m in the morning. The rest of the X/E ratios measured at 13 m or above were comparable with those observed at ground level, suggesting a quick and fresh mixture of automotive exhausts at this height. Finally, it is worth noting that the X/E ratios observed in downtown Kaohsiung were generally a little higher than those observed in Hong Kong (1.5–2.2).Citation26 This is not surprising because the profiles of Kaohsiung are somehow complicated by various surrounding industries whereas Hong Kong is not.
CONCLUSIONS
This study characterized vertical and diurnal variations of VOCs at five different heights during four daily time periods near a high-rise building in downtown Kaohsiung, Taiwan. The maximum concentration of VOCs was recorded during the morning rush hours, followed by evening rush hours, afternoon hours, and the midnight hours, indicating that most VOC compounds originated from traffic emissions. This implication was further supported by the source analysis showing that BTEX in most of the samples was associated with vehicle exhausts at ground level. However, T/B and X/E ratios suggest that some of the samples were obviously affected by the upwind industrial sources. The vertical distributions of VOC profiles showed that VOC levels were in general lower at higher altitudes. However, probably because of additional industrial sources upwind and a thermal inversion somewhere between 13 and 32 m, higher VOC levels were observed at 13 m than at ground level during the midnight hours and at 32 m than 13 m during the morning rush hours. These results suggest that vertical dispersion and dilution of airborne pollutants could be jointly affected by local meteorological conditions and the proximity of pollution sources. Whether such vertical variation in VOC profiles can affect the prediction of air quality model is of interest and needs to be considered in future air quality management. Finally, it is worth mentioning that concentrations and species of VOCs decreased dramatically above 32 m. In other words, the height of 32 m is the turning point above which the ambient air quality may not be a major concern in downtown Kaohsiung City during the sampling period.
ACKNOWLEDGMENTS
This work was financed by the National Science Council (NSC) of Taiwan under contract no. NSC-97-2815-C-022-002-E. The authors are grateful to NSC for the financial support provided for the pursuit of this project.
REFERENCES
- Blanchard , C.L. , Tanenbaum , S. and Lawson , D.R. 2008 . Differences between Weekday and Weekend Air Pollutant Levels in Atlanta, Baltimore, Chicago, Dallas-Fort Worth, Denver, Houston, New York, Phoenix, Washington, DC, and Surrounding Areas . Journal of the Air & Waste Management Association , 58 : 1598 – 1615 . doi: 10.3155/1047-3289.58.12.1598
- Chang , T.Y. , Lin , S.J. , Shie , R.H. , Tsai , S.W. , Hsu , H.T. , Tsai , C.T. , Kuo , H.W. , Chiang , C.F. and Lai , J.S. 2010 . Characterization of Volatile Organic Compounds in the Vicinity of an Optoelectronics Industrial Park in Taiwan . Journal of the Air & Waste Management Association , 60 : 55 – 62 . doi: 10.3155/1047–3289.60.1.55
- Ilgen , E. , Karfich , N. , Levsen , K. , Angerer , J. , Schneider , P. , Heinrich , J. , Wichmann , H. , Dunemann , L. and Begerow , J. 2001 . Aromatic Hydrocarbons in the Atmospheric Environment: Part I. Indoor versus Outdoor Sources the Influence of Traffic . Atmos. Environ. , 35 : 1235 – 1252 .
- Jo , W.K. , Kim , K.Y. , Park , K.H. , Kim , Y.K. , Lee , H.W. and Park , J.K. 2003 . Comparison of Outdoor and Indoor Mobile Source-Related Volatile Organic Compounds between Low-and High-Floor Apartments . Environ. Res. , 92 : 166 – 171 .
- Flocas , H.A. , Assimakopoulos , V.D. and Helmis , C.G. 2003 . VOC and O3 Distribution over the Densely Populated Area of Greater Athens, Greece . J. Appl. Meteorol. , 42 : 1799 – 1810 .
- Wöhrnschimmel , H. , Márquez , C. , Mugica , V. , Stahel , W.A. , Staehelin , J. and Cárdenas , B. 2006 . Vertical Profiles and Receptor Modeling of Volatile Organic Compounds over Southeastern Mexico City . Atmos. Environ. , 40 : 5125 – 5136 .
- Ting , M. , Yue-Si , W. , Jie , J. , Fang-Kun , W. and Mingxing , W. 2008 . The Vertical Distributions of VOCs in the Atmosphere of Beijing in Autumn . Sci. Total Environ. , 390 : 97 – 108 .
- Chen , C.L. , Fang , H.Y. and Shu , S.M. 2006 . Mapping and Profile of Emission Sources for Airborne Volatile Organic Compounds from Process Regions at a Petrochemical Plant in Kaohsiung, Taiwan . Journal of the Air & Waste Management Association , 56 : 824 – 833 .
- Hsieh , C.C. and Tsai , J.H. 2003 . VOC Concentration Characteristics in Southern Taiwan . Chemosphere , 50 : 545 – 556 .
- 1997 . Compendium Method TO-15. Determination of Volatile Organic Compounds (VOCs) in Air Collected in Specially Prepared Canister and Analyzed by Gas Chromatography/ Mass Spectrometry (GC/MS) , Cincinnati , OH : Center for Environmental Research Information, Office of Research and Development; U.S. Environmental Protection Agency .
- Determination of Volatile Organic Compounds (VOCs) in Air Collected by Canister and Analyzed by Gas Chromatography/Mass Spectrometry . NIEA A715.12B . 2005 . Taiwan Environmental Protection Administration: Taipei, Taiwan
- Denis , H. , William , J. and Castor , M. 2005 . Ullmann's Encyclopedia of Industrial Chemistry , Weinheim , , Germany : Wiley-VCH .
- Yashima , T. , Sato , K. , Hayasaka , T. and Hara , N. 1972 . Alkylation on Synthetic Zeolites: III. Alkylation of Toluene with Methanol and Formaldehyde on Alkali Cation Exchanged Zeolites . J. Catal. , 6 : 303 – 313 .
- Chan , C.Y. , Xu , X.D. , Li , Y.S. , Wong , K.H. , Ding , G.A. and Chan , L.Y. 2005 . Characteristics of Vertical Profiles and Sources of PM2.5, PM10 and Carbonaceous Species in Beijing . Atmos. Environ. , 39 : 59 – 71 .
- Seibert , P. , Beyrich , F. , Gryning , E. , Joffre , S. , Rasmussen , A. and Tercier , P. 2000 . Review and Intercomparison of Operational Methods for Determination of the Mixing Height . Atmos. Environ. , 34 : 1001 – 1027 .
- 1999 . Air Quality Guidelines for Europe , Copenhagen , Denmark : World Health Organization (WHO) Regional Publication; European Series; World Health Organization; Regional Office for Europe .
- Harkov , R. , Kebbekus , B. , Bozzelli , J.W. and Lioy , P.J. 1983 . Measurement of Selected Volatile Organic Compounds at Three Locations in New Jersey during the Summer Season . J. Air Pollut. Control Assoc. , 33 : 1177 – 1183 .
- Lee , S.C. , Chiu , K.F. , Ho , S.C. , Zou , X.M. and Wang , X. 2002 . Volatile Organic Compounds (VOCs) in Urban Atmosphere of Hong Kong . Atmos. Environ. , 48 : 375 – 382 .
- Hsieh , L.T. , Hsien , Y.H. and Wen , C.H. 2006 . Ambient BTEX and MTBE in the Neighborhoods of Different Industrial Parks in Southern Taiwan . J. Hazard. Mater. , A128 : 106 – 115 .
- Nelson , P.F. and Quigley , S.M. 1983 . The m,p-Xylenes: Ethylbenzene Ratio, a Technique for Estimating Hydrocarbon Age in Ambient Atmospheres . Atmos. Environ. , 17 : 659 – 662 .
- Nelson , P.F. and Quigley , S.M. 1984 . The Hydrocarbon Composition of Exhaust Emitted from Gasoline Fueled Vehicles . Atmos. Environ. , 18 : 79 – 87 .
- Monod , A. , Sive , C.S. , Avino , P. , Chen , T. , Blake , D.R. and Rowland , S. 2001 . Monoaromatic Compounds in Ambient Air of Various Sites: a Focus on Correlations between the Xylenes and Ethylbenzene . Atmos. Environ. , 35 : 135 – 149 .
- Brocco , D. , Fratarcangeli , R. , Lepore , L. , Petricca , M. and Ventrone , I. 1997 . Determination of Aromatic Hydrocarbons in Urban Air of Rome . Chemosphere , 31 : 557 – 566 .
- Stevenson , K.J. , Stacey , B. and Willis , P.G. Air Quality at Heathrow Airport: Annual Report for 1996 . London , United Kingdom. AEA Technology .
- Guicherit , R. 1997 . Traffic as a Source of Volatile Hydrocarbons in Ambient Air . Sci. Total Environ. , 205 : 201 – 213 .
- Ho , K.F. , Lee , S.C. , Guo , H. and Tsai , W.Y. 2004 . Seasonal and Diurnal Variations of Volatile Organic Compounds (VOCs) in the Atmosphere of Hong Kong . Sci. Total Environ. , 322 : 155 – 166 .