ABSTRACT
Indoor air quality has become a critical issue because people spend most of their time in the indoor environment. The factors that influence indoor air quality are very important to environmental sanitation and air quality improvement. This study focuses on monitoring air quality, colony counts, and bacteria species of the indoor air of a nursing care institution. The regular colony counts in two different wards range from 55 to 600 cfu m−3. Regression analysis results indicate that the bacterial colony counts have close correlation with relative humidity or carbon dioxide (CO2) but not with carbon monoxide (CO) or ozone (O3). Real-time PCR was used to quantify the bacterial pathogens of nosocomial infection, including Acinetobacter baumannii, Citrobacter freundii, Escherichia coli, Klebsiella pneumoniae, and methicillin-sensitive Staphylococcus aureus. The most abundant bacteria species in the air of the nursing care institution is E. coli.
Indoor temperature, humidity, ventilation, accumulation of biological pollutants, and potential infection problems will seriously affect the indoor environments. Studying these factors is important to indoor environmental sanitation and air quality improvements. Results of using real-time PCR to evaluate the bacterial pathogens of nosocomial infection for a nursing care institution in Taiwan reveal that the main bacteria species existing in the indoor air is E. coli.
INTRODUCTION
Indoor air quality has become a critical issue because most people live, work, and enjoy their leisure activities in a highly dense and populated environment so that their exposure to many types of pathogens is greatly increased. Indoor air that contains various bacteria, viruses, fungi, and parasites may cause nosocomial infections. Patients in the medical center and the community hospitals experience a high rate of multidrug-resistant bacterial infections. Patients infected with resistant bacteria usually have a longer hospital stay with significantly higher costs of hospitalization than normal patients.Citation1–6 Poor indoor air quality has been reported to increase the risk of such infections,Citation7–9 and high airborne bacteria concentrations are positively correlated with adverse respiratory symptoms.Citation10 Temperature, humidity, ventilation, accumulation of biological pollutants, and infection potential seriously deteriorate indoor environments.Citation11 The indoor air quality of hospitals, nursing care institutions, schools, shopping malls, government administrations, and other public facilities causes more concern because these places, which are crowded with people, are at risk of spreading diseases caused by airborne bacteria. Quantification of specific bacteria associated with certain infections is essential to assess the air quality problem. Currently, real-time PCR is a promising tool that is capable of providing reproducible and accurate measurements of total micro-organism concentrations in environmental samples.Citation12 Real-time PCR has the benefit of performing rapid sample quantification and species-specific identification. In fact, real-time PCR has been used to quantify airborne myco-bacterium tuberculosis in healthcare settings.Citation13 This method is effective in detecting pathogens in wastewater to confirm the risk of exposure.Citation14 Oppliger et al.Citation15 applied real-time PCR to explore the quantification of airborne microorganisms and Staphylococcus in poultry houses. The results revealed that the bacteria colony densities were 53 × 107 cells m−3 for airborne microorganisms and 62 × 106 cells m−3 for Staphylococcus sp.
Monitoring and analyzing the factors that affect indoor air quality and types of airborne microorganisms are critical to maintaining proper indoor air quality. In this study, environmental quality parameters, including temperature, relative humidity (RH), carbon dioxide (CO2), carbon monoxide (CO), ozone (O3), and colony counts, were measured for monitoring the indoor air quality of a selected nursing care institution from June to September 2009. Moreover, the correlations between colony counts and the other monitoring items were evaluated using linear regression analyses to verify the factors that affect the indoor air quality. Specific types of microorganisms existing in the indoor air of a nursing care institution were identified by observing the microbial growth in the culture medium. Quantities of the bacteria identified were then analyzed using real-time PCR when the samples were collected so that the results will assist in evaluating the indoor air quality and early management of the indoor environment.
EXPERIMENTAL METHODS
Description of the Building
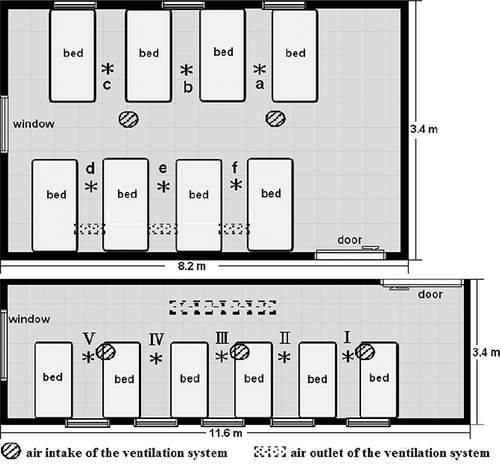
The nursing care institution selected for conducting this study is a two-story building located in Taichung, Taiwan, surrounded by three-story apartments. This institution was established for vegetative patients, terminally ill cancer patients, and dementia patients, along many others, and an acceptable indoor air quality and thermally comfortable indoor environment must be provided. The two wards to be studied were located on the first floor. Room A is 8.2 × 5.1 m with a clear ceiling of height 3 m and a usable space of 125.46 m3 shared by eight hospital beds. For Room B, the dimensions are 1.16 × 3.4 m with a clear ceiling of height 3 m and a usable space of 118.32 m3 occupied by six hospital beds. The indoor air sampling points are 0.9 m above ground between each bed in both rooms; there are six sampling points in room A (a, b, c, d, e, and f) and five sampling points in room B (I, II, III, IV, and V). presents the schematic diagram of the air sampling points in rooms A and B. The ventilation system has three air outlets and two air intakes in room A and one air outlet and three air intakes in room B. The doors in both rooms were always opened toward the hallway. There was no curtain between two adjacent beds.
Air Sampling and Culturing
Air samples were collected using the QuickTake impaction air sampler (SKC) placed between two adjacent beds and operated at 14.15-L min−1 airflow in both wards. The air sampler was sterilized with 70% ethanol between two sampling runs. BAP (Blood Agar Plate—a differential bacterial growth medium that contains 5% sheep blood) was used in this study to distinguish normal bacteria from pathogenic bacteria on the basis of the effect of bacterial hemolytic enzymes on erythrocytes.Citation16 The collected samples were cultured in BAP at 37 °C for 24 hr before the colonies (cfu m−3) were counted.
Air quality parameters monitored for evaluating the indoor air quality included temperature, including RH (RH%), CO2, CO, and O3, were monitored using the Air-Boxx IAQ monitor (KD AirBoxx).
Statistical Analyses
Data analyses were performed using the SAS program (version 9) executed on a personal computer. Relationships among colony counts and other measured items (CO2, CO, O3, temperature, and RH) were examined using simple linear regression and multiple regression analyses.
Dependent variables used in the statistical models were colony counts and other independent variables associated with colony counts in simple regression analyses.
Identification of Bacteria
Identifying the bacterial species that may have long existed in the wards is beneficial to prevent nosocomial infections. The colony morphology was observed first for selecting the strains that might cause nosocomial infections on the basis of whether the strain was Gram-negative or Gram-positive. The biselected bacteria species were then identified by performing clinical micro-organism examination.
Bacterial Sampling for Real-Time PCR
Airborne bacterial samples for performing real-time PCR were collected with an air sampler (Airport MD8, Sartorius) placed in each ward. The airborne bacteria were collected on gelatin membranes (3-μm pore size with 80-mm diameter; Sartorius) with the sampler operated at 50-L min−1 airflow for 5 min. For quantifying the specific bacteria in a room during each sampling period, one gelatin membrane was used to collect all air samples of all sampling points in either room A or room B. After samples were collected, the gelatin membranes were dissolved in a phosphate buffer (pH 7) for extracting bacterial genomic DNA using the BuccalAmp DNA extraction kit (Epicenter) following the manufacture's instructions. Subsequently, real-time PCR was used to assess the quantity of total airborne-specific species. The bacterial genomic DNA used for preparing standard concentration curves was extracted from pure cultures; a series of 10-fold diluted solutions containing pure culture genomic DNA was tested for real-time PCR amplification and cycle threshold (CT).
Real-time PCR analysis was performed in a 15-μL volume for each test using a StepOne Plus real-time PCR purchased from Applied Biosystems. Each of the final 15-μL volumes contained 0.2 μL of each primer (10 μM), 7.5 μL Power SYBR Green PCR Master Mix (Applied Bio-systems), 1 μL of template DNA, and 6.5 μL of sterilized superpure water. Using known microorganism concentrations as templates, standard curves were prepared for quantifying concentrations of the specific bacteria in an unknown sample. The real-time PCR amplification temperature was initially maintained at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 sec, and finally at 60 °C for 1 min. Fluorescence data were acquired at the end of each PCR cycle. Afterward, the melting curve analysis was performed immediately by increasing the temperature to 95 °C for 15 sec, followed by 60 °C for 1 min and 94 °C for 15 sec. Fluorescence was measured continually during melting curve analysis.
EXPERIMENTAL AND ANALYSIS RESULTS
Analyses of Colony Counts and Monitoring Data
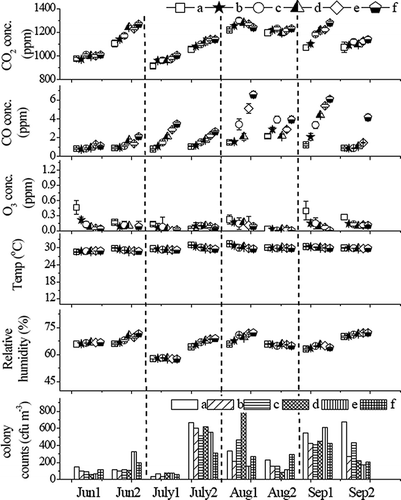
The sampling frequency was twice per month from June to September 2009, which covers the hottest summer month of July in Taiwan, with an average daily temperature of 28 °C (82.4 °F). shows the results of monitoring data in room A. The first sample in a month is represented by month 1 (e.g., Jun1, July1, Aug1, and Sep1), and the second sample in the same month is represented by month 2 (e.g., Jun2, July2, Aug2, and Sep2). The six sampling points between two adjacent beds are represented by a, b, c, d, e, and f, respectively. There are usually three or six patients lying in bed while two to four healthcare providers work in room A. The results reveal that the colony counts are higher in late July (308–671 cfu m−3) and early August (155–806 cfu m−3), whereas lower colony counts are observed in early July (35–78 cfu m−3) when the RH (57.5–58.1%) is also noted to be lower. The regular observation in room A shows that the concentrations of CO2, CO, and O3 are 900–1300, 0.8–6.5, and 0–0.46 parts per million (ppm), respectively; the room temperature is in the range from 28.5 to 31.4 °C; and humidity varies from 57.5 to 72.2%. The air conditioning system was always in the operating mode during the sampling period, but it was turned off on Jun1 and Aug1. All windows were closed when the air conditioning system was in operation. During the two days when the air conditioner was turned off, the windows were opened on Jun1 but were closed on Aug1. When the air conditioning system was not in operation while the windows were not opened in room A as on Aug1, the room ventilation might be inadequate in room 1 to cause poor indoor air quality problems such as higher CO2 and CO concentrations, higher RH, and higher colony counts. Hence, the air samples collected at all six sampling points in room A on Aug1 have higher CO2 and CO concentrations and higher RH than the samples collected at sampling point f (room B) while the air conditioning systems was continuously in operation. The observation may suggest that inadequate air circulation occurred in room B where sampling point f is located.
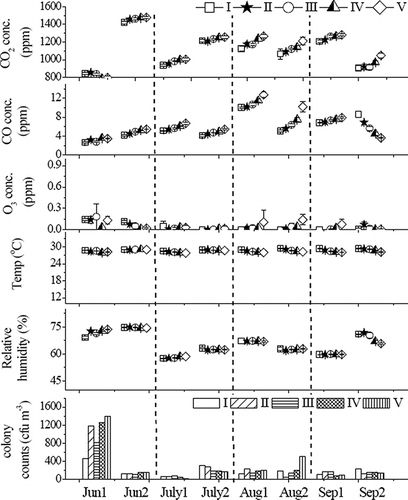
The results of monitoring data in room B from June to September 2009 are shown in . There are usually two or four patients lying in bed and two or three healthcare providers working in room B. The five sampling points between two adjacent beds are marked as I, II, III, IV, and V in room B. The results show that room B had higher colony counts (459–1392 cfu m−3) and RH (69.2–73.7%) but lower CO2 concentrations (739–857 ppm) on Jun1 than other days; the air contains lower colony counts (35–78 cfu m−3) and RH (57.5%–58.1%) in early July than other days. The air conditioner in room A was turned off on Jun1 (with windows opened) and on Aug1 (with windows closed). During other days, the air conditioner in room A was continuously in operation the same as in room B (with all windows closed). shows the monitoring results for all sampling points in rooms A and B. For room B, the colony counts of airborne bacteria are higher on Jun1 and Aug1. Additionally, the indoor CO2 concentration is higher if the air conditioning system is turned off with windows closed. The RH and colony counts are affected by other factors. When the conditioner is operated, samples collected at sampling point V have higher CO2 and CO concentrations and higher RH. This indicates that the air circulation is inadequate at sampling point V. In room B, the air contains 793–1478 ppm CO2, 2.6–12.7 ppm CO, and 0–0.18 ppm O3. The room temperature and RH range from 27.8 to 29.5 °C and 57.6 to 74.9%, respectively. Although rooms A and B have different areas and shapes, they have similar indoor air quality except for bacterial colony count, which varies from 35 to 806 cfu m−3 for room A and from 21 to 1392 cfu m−3 for room B. Although room B has higher colony counts than room A, the colony counts during the latter sampling period (Jul2 to Sep2) are usually higher in room A than room B. The observation might be because of the difference in the number of persons in the room, because during the latter sampling period, more people stayed in room A (7–10 persons) than in room B (2–3 persons).
Table 1. The results of monitoring data in rooms A and B on different sampling dates
Statistical Analyses
Linear regression analyses have been carried out to evaluate any correlation that may exist between colony counts and other monitoring items. and show the colony counts and other monitoring parameters for samples collected in rooms A and B during the sampling period. These data reveal that colony counts can be predicted based on the independent variables (). Results of simple regression suggest that colony counts are correlated with RH and CO2 concentrations. In the multiple linear regression model, CO2 concentrations (adjusted β: -0.819, P 0.0001) and RH (adjusted β: 33.04, P 0.0001) are shown to be significant for predicting colony counts. The model explained 39.2% (R2) variation from colony counts. Neither CO concentrations nor O3 concentrations showed independent associations with the colony counts.
Table 2. Predictors of colony counts among the environmental factors
Identification of Bacteria
Distinct colonies grown on each plate were selected for identification. The results show that the nursing care institution indoor air contains bacteria that may cause nosocomial infections. Among those bacteria identified in this study, Acinetobacter baumannii, Citrobacter freundii, and Klebsiella pneumonia are multidrug-resistant organisms that may cause urinary tract infections, wound infections, sepsis, meningitis, skin and soft-tissue infections, or eye infections. Aeromonas sobria, Leclercia adecarboxylata, and Escherichia coli were also identified. In addition, methicillin-sensitive Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) were found to exist in the air of rooms A and B.
Real-Time PCR
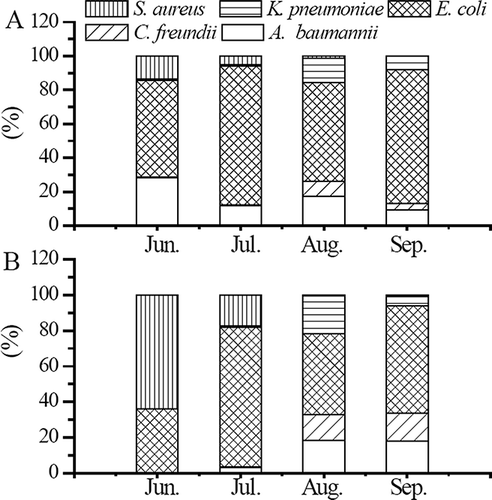
Because A. baumannii, C. freundii, E. coli, K. pneumonia, and S. aureus (MRSA), which may cause nosocomial infections, were found to exist in the indoor air of the institution, densities of these bacteria were monitored during each sampling period. The specific primers including forward primers and reverse primers are listed in . Results from gel electrophoresis confirm that the molecular weights of the amplicon are the correct size so that the primers are specific (). All real-time PCR reactions were followed by a melting curve analysis to verify that products of nonspecific amplification or primer dimers do not exist (data not shown). The results reveal no melting peaks for nonspecific amplicons, indicating that the quantity of the specific bacteria in the air samples can be estimated using standard curves on the basis of the relationships between CT values and bacterial concentrations. shows the percentage of bacteria detected to exist in the air of rooms A and B. The results of June (the average of Jun1 and Jun2 results) indicate the presence of a large percent of E. coli existing in rooms A and B from June to September. The average densities of E. coli are 2.02 × 108 to 1.33 × 1010 cells m−3 in room A and 7.37 × 107 to 1.94 × 1010 cells m−3 in room B. In the nursing care institution, E. coli was also detected in stool samples from most patients. The sampling time in this research was from 9:00 to 10:00 a.m. after healthcare providers changed diapers and sheets for patients from 7:30 to 8:30 a.m. This suggests that the routine work may have caused the observed higher concentrations of E. coli in the air because airborne microorganisms might be carried to great distances through airflow. The density of airborne S. aureus in room B is especially higher in June because two patients involved in conversation were sitting on their beds that are close to sampling point IV on Jun1, as seen in the sampling reports. One patient had a large, red, swollen sore that was oozing pus, indicating the sign of a skin infection caused by MRSA. This patient stayed in the nursing care institution from June to early August, which may contribute to the observed higher density of S. aureus in room B in June. In contrast, room A sometimes experienced a higher density of A. baumannii, whereas densities of A. baumannii, C. freundii, and K. pneumoniae in both rooms are relatively low.
Table 3. Specific primers for airborne-specific bacterial pathogens in real-time PCR assay
DISCUSSION
The density of airborne bacteria in the indoor air is affected by the activities of patients, care staffs, and visitors. Patients who have already been infected are the source of nosocomial bacteria transmission to the ambient environment (e.g., air, beds, and apparatus, among many others). Persons who have contacted these bacteria might further transmit the latter to other locations. Thus, indoor air quality and environment factors are mutually affected. Human bodies are usually considered to be the main sources of indoor air bacteriaCitation17; high bacteria counts are probably associated with high occupancy, the poor hygienic conditions of the occupants, and inadequate ventilation.Citation18 Chen et al.Citation13 found that there were 4-fold differences in the amounts of bacteria observed between rooms with and without occupants. Health authorities can reference the data as a basis for conducting disease risk assessment. Other forms of airborne contamination with infectious bioaerosols may occur when the occupant vomits in public or flushes toilets.Citation19 People generate particles of different sizes and air jets with different initial characteristics when they are coughing, sneezing, talking, or breathing, and particles with diameters of 10 μm or less are able to penetrate into the lungs of others.Citation20 Hence, the airborne route of transmission of pathogens to a healthy person may be through the generation of pathogen-laden droplets that originate in the respiratory tract of an infected individual. Some potent bacteria species such as A. baumannii, C. freundii, K. pneumonia, and S. aureus were observed in this nursing care institution. A. baumannii is a Gram-negative coccobacillus that causes outbreaks of nosocomial infections because of its multidrug-resistance patterns and its resistance to desiccation. A specific epidemic strain of A. baumannii causes infection or colonization of numerous patients.Citation21–24 reported that the bloodstream infections of the patients were caused by C. freundii. Citrobacter infections are often nosocomial, are commonly drug resistant, and have been seen and isolated in patients with significant underlying diseases.Citation26 Results of previous research show that the predominant infection site of C. freundii is the intra-abdominal region, with 22% mortality. Moreover, the resistance of C. freundii to most third-generation cephalosporins and broad-spectrum penicillins increases in nosocomial and community-acquired C. freundii bacteremia.Citation27 K. pneumoniae may cause severe morbidity and mortality, especially in newborn babies.Citation27–30 Compared with other Enterobacteriaceae, K. pneumonia is the most concerning pathogen for its severe morbidity and mortality.Citation31 Tsukadaira et al.Citation32 reported four cases of K. pneumonia infections, which were typical lobar pneumonia (Friedlander pneumonia), acute bronchopneumonia with subclinical aspiration, and chronic K. pneumonia with typical cavitary lung abscesses. E. coli, K. pneumoniae, and Enterococcus faecalis, which are often detected in the wastewater treatment process, can be used as indicators for pathogenic microorganisms in culture-based detection.Citation14 MRSA have also been identified as an important source of periprosthetic infection.Citation33
The results of statistical analyses obtained in this research are similar to those of the previous studies confirming that airborne microorganisms are influenced by environmental factors such as relative CO2 and humidity; the concentrations of these microorganisms are in turn determined by ventilation conditions and human activities.Citation34–36 Excess moisture may also lead to excessive growth of various microbes (e.g., molds, fungi, and bacteria) that subsequently emit spores, cells, fragments, and volatile organic compounds into the indoor air. The correlation between RH and airborne microorganisms has been shown to be statistically significant,Citation35 and mid range humidity conditions (40–60%) are more lethal to nonpathogenic bacteria.Citation34 Higher levels of humidity in the vicinity may favor the growth of S. aureus.Citation37 Environmental factors (e.g., CO2) have positive effects on the vitality of airborne microorganisms: Higher CO2 concentrations are associated with increasing concentrations of airborne bacteria.Citation38
Results of previous studies indicate that some specific bacteria isolated from patients caused nosocomial infections.Citation21–31 Thus, the activity of occupants may influence the presence of infectious bioaerosols in the environment. The fluctuation of bacterial flora in the air is also noted when patients and medical devices are transferred into and out of the room; it then remains steady but adaptable thereafter.Citation39 Previous research results show that E. coli is the dominant microbe within the airborne bacterial consortium.Citation40 E. coli originating from fecal materials was found in aerosols that spread to outdoor air via air exchange.Citation41 Therefore, spread of E. coli via aerosols in the nursing care institution is suggested. The results of real-time PCR obtained in this study confirm that the predominant bacterial species in these two rooms of the nursing care institution is E. coli. Hence, more aggressive enforcement of hand washing may be the best strategy to curb the spread of E. coli throughout the institution. Further, changing disposable diapers and bed sheets in a more sanitary manner may be also helpful to reduce the risk of E. coli infection.
The density of airborne S. aureus is noted to be higher in June than other months. Many airborne pathogenic microorganisms, including viruses and bacteria, can be transmitted to great distances through airflow.Citation42–46 Staph ylococci, including MRSA, are known to survive in dry conditions and persist in clinical areas that are inadequately cleaned.Citation47 Hence, airborne transmission may contribute to infection by S. aureus and be responsible for contaminating the environment.Citation37 Strains resistant to methicillin (MRSA) are now the major cause of nosocomial infection in burn units in Europe.Citation48 Colonization may also occur indirectly by contact with a contaminated object. Fabrics can also be responsible for the transmission of bacteria.Citation49,Citation50 A previous study shows that the number of S. aureus-containing airborne particles is significantly higher 15 min after bed-making than during the rest period.Citation51 S. aureus has been reported to survive on the white coatsCitation52 and pantsCitation53,Citation54 worn by healthcare staff. Although gloves will prevent direct contact, they may also spread staphylococci from one patient to another if not changed between patients.Citation55
The amounts of these bacteria need to be monitored because they may cause nosocomial infection. In addition, the information will assist in developing prompt methods to improve indoor air quality and decrease infections. Using real-time PCR to quantify the specific bacteria in this research is valuable for evaluating the indoor air quality and the predominant bacterial species existing in nursing care institutions. Future studies should include more detailed records of individual patient characteristics and the activities of healthcare staff and visitors so that the influence of human factors that influence the density and species of airborne bacteria can be factored in the evaluation.
CONCLUSIONS
This study presented here emphasizes the significance of using real-time PCR for quantifying airborne-specific bacterial pathogens in a nursing care institution; the dominant bacterial species was E. coli. Real-time PCR is a practical method for observing variations in the indoor airborne-specific bacterial species. The results will assist in monitoring indoor air quality and evaluating the efficiency of disinfection methods. Moreover, the RH and CO2 concentrations are the important factors related to colony counts. The results also suggest that the room should be properly ventilated so that the indoor air RH and CO2 concentrations will meet the air quality standards for protecting the health of inhabitants.
ACKNOWLEDGMENTS
This research was supported by the National Science Council, Taiwan, Republic of China (NSC-97-3114-E-167-001). The authors are grateful to Ms. Ju-Ling Hsiao for her valuable suggestions. The views and opinions expressed in this article are those of the authors and should not be construed as the opinions of the U.S. Environmental Protection Agency.
REFERENCES
- Abramson , M.A. and Sexton , D.J. 1999 . Nosocomial Methicillin-Resistant and Methicillin-Susceptible Staphylococcus Aureus Primary Bacteremia—at What Costs? . Infect. Control Hosp. Epidemiol. , 20 : 408 – 411 .
- Carmeli , Y. , Troillet , N. , Karchmer , A.W. and Samore , M.H. 1999 . Health and Economic Outcomes of Antibiotic Resistance in Pseudomonas aeruginosa . Arch. Intern Med. , 159 : 1127 – 1132 .
- Chow , J.W. , Fine , M.J. , Shlaes , D.M. , Quinn , J.P. , Hooper , D.C. , Johnson , M.P. , Ramphal , R. , Wagener , M.M. , Miyashiro , D.K. and Yu , V.L. 1991 . Enterobacter Bacteremia—Clinical Features and Emergence of Antibiotic Resistance during Therapy . Ann. Intern. Med. , 115 : 585 – 590 .
- Kim , T. , Oh , P.I. and Simor , A.E. 2001 . The Economic Impact of Methicillin-resistant Staphylococcus aureus in Canadian Hospitals . Infect. Control Hosp. Epidemiol. , 22 : 99 – 104 .
- Kreméry , V. Jr. , Spanik , S. , Krupova , I. , Trupl , J. , Kunova , A. , Smid , M. and Pichnova , E . 1998 . Bacteremia Due to Multi-Resistant Gram-Negative Bacilli in Neutropenic Cancer Patients—a Case Controlled Study . J. Che-mother. , 10 : 320 – 325 .
- Sheng , W.H. , Wang , J.T. , Lu , D.C.T. , Chie , W.C. , Chen , Y.C. and Chang , S.C. 2005 . Comparative Impact of Hospital-Acquired Infections on Medical Costs, Length of Hospital Stay and Outcome between Community Hospitals and Medical Centres . J. Hosp Infect. , 59 : 205 – 214 .
- Emmenlin , A. and Wall , S. 2007 . Indoor Air Pollution: a Poverty-Related Cause of Mortality among the Children of the World . Chest , 132 : 1615 – 1623 .
- Kovesi , T. , Gibert , N.L. , Stocco , C. , Fugler , D. , Dales , R.E. , Guay , M. and Miller , J.D. 2007 . Indoor Air Quality and the Risk of Lower Respiratory Tract Infections in Young Canadian Inuit Children . CMAJ , 177 : 155 – 160 .
- Ishihama , K. , Koizumi , H. , Wada , T. , Tanaka , I.S. , Yamanishi , T. and Enomoto , A. 2009 . Evidence of Aerosolized Floating Blood Mist during Oral Surgery . J. Hosp. Infect. , 71 : 359 – 364 .
- Bjornsson , E. , Norback , D. , Janson , C. , Widstrom , J. , Palmgren , U. , Strom , G. and Boman , G. 1995 . Asthmatic Symptoms and Indoor Levels of Micro-Organisms and House Dust Mites . Clin. Exp. Allergy , 25 : 423 – 431 .
- Graudenz , G.S. , Oliveira , C.H. , Tribess , A. , Mendes , C. Jr. , Latorre , M.R. and Kalil , J. 2005 . Association of Air-Conditioning with Respiratory Symptoms in Office Workers in Tropical Climate . Indoor Air , 15 : 62 – 66 .
- An , H.R. , Mainelis , G. and White , L. 2006 . Development and Calibration of Real-Time PCR for Quantification of Airborne Microorganisms in Air Samples . Atmos. Environ. , 40 : 7924 – 7939 .
- Chen , Y.S. and Li , C.S. 2005 . Quantification of Airborne Mycobacterium tuberculosis in Health Care Setting Using Real-Time QPCR Coupled to an Air-Sampling Filter Method . Aerosol Sci. Technol. , 39 : 371 – 376 .
- Shannon , K.E. , Lee , D.Y. , Trevors , J.T. and Beaudette , L.A. 2007 . Application of Real-Time Quantitative PCR for the Detection of Selection Bacterial Pathogens during Municipal Wastewater Treatment . Sci. Total Environ. , 382 : 121 – 129 .
- Oppliger , A. , Charrière , N. , Droz , P.O. and Rinsoz , T. 2008 . Exposure to Bioaerosols in Poultry Houses at Different Stages of Fattening: Use of Real-Time PCR for Airborne Bacterial Quantification . Ann. Occup Hyg. , 52 : 405 – 412 .
- 1998 . “ Laboratory Methods for the Diagnosis of Meningitis Caused by ” . In Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenza , 1 – 95 . Atlanta , GA : Centers for Disease Control and Prevention .
- An , H.R. , Mainelis , G. and White , L. 2006 . Development and Calibration of Real-Time PCR for Quantification of Airborne Microorganisms in Air Samples . Atmos. Environ. , 40 : 7924 – 7939 .
- Lee , S.C. , Guo , H. , Li , W.M. and Chan , L.Y. 2002 . Inter-Comparison of Air Pollutant Concentrations in Different Indoor Environments in Hong Kong . Atmos Environ. , 36 : 1929 – 1940 .
- Bolashikov , Z.D. and Melikov , A.K. 2009 . Methods for Air Cleaning and Protection of Building Occupants from Airborne Pathogens . Building Environ. , 44 : 1378 – 1385 .
- Hind , W.C. 1999 . Aerosol Technology. Properties, Behavior, and Measurement of Airborne Particles. 11 Respiratory Depositions , 2nd , 233 – 257 . New York : John Wiley & Sons .
- Jang , T.N. , Lee , S.H. , Huang , C.H. , Lee , C.L. and Chen , W.Y. 2009 . Risk Factors and Impact of Nosocomial Acinetobacter baumannii Bloodstream Infections in the Adult Intensive Care Unit: a Case-Control Study . J. Hosp. Infect. , 73 : 143 – 150 .
- Monterrubio-Villar , J. , Conzález-Velasco , C. , Valdezate-Ramos , S. , Córdoba-López , A. , Villalón-Panzano , P. and Saéz-Nieto , J.A. 2009 . Outbreak of Multiresistant Acinetobacter baumannii in a Polyvalent Intensive Care Unit: Clinical, Epidemiological Analysis and PFGE-Printing Evolution . Eur. J. Clin. Microbiol. Infect. , 28 : 1281 – 1284 .
- Park , Y.K. , Choi , J.Y. , Jung , S.I. , Park , K.H. , Lee , H. and Jung , D.S. 2009 . Two Distinct Clones of Carbapenem-Resistant Acinetobacter baumannii Isolates from Korean Hospitals . Diagn. Microbiol. Infect. Dis. , 64 : 389 – 395 .
- Towner , K.J. 2009 . Acinetobacter: an Old Friend, but a New Enemy . J. Hosp. Infect. , 73 : 355 – 363 .
- Liu , C.P. , Wang , L.C. , Tseng , H.K. , Wang , N.Y. and Lee , C.M. 2007 . Cefotaxime-Resistant Citrobacter freundii in Isolates from Blood in a Tertiary Teaching Hospital in Northern Taiwan . J. Infect. , 55 : 363 – 368 .
- Mohanty , S. , Singhal , R. , Sood , S. , Dhawan , B. , Kapil , A. and Das , B.K. 2007 . Citrobacter Infections in a Tertiary Care Hospital in Northern India . J. Infect. , 54 : 58 – 64 .
- Cheng , Y.S. , Wong , W.W. , Fung , C.P. , Yu , K.W. and Liu , C.Y. 2002 . Clinical Features and Antimicrobial Susceptibility Trends in Citrobacter freundii Bacteremia . J. Microbiol. Immunol. Infect. , 35 : 109 – 114 .
- Bingen , E. 1993 . Molecular Markers and Epidemiology of Nosocomial Infections in Pediatric Hospital Units . Pathol. Biol. , 41 : 716 – 723 .
- Casolari , C. , Pecorari , M. , Fabio , G. , Cattani , S. , Venturelli , C. and Piccinini , L.A. 2005 . Simultaneous Outbreak of Serratia marcescens and Klebsiella pneumoniae in a Neonatal Intensive Care Unit . J. Hosp. Infect , 61 : 312 – 320 .
- Coovadia , Y.M. , Johnson , A.P. , Bhana , R.H. , Hutchinson , G.R. , George , R.C. and Hafferjee , I.E. 1992 . Multiresistant Klebsiella pneumoniae in a Neonatal Nursery: the Importance of Maintenance of Infection Control Policies and Procedures in the Prevention of Outbreaks . J. Hosp. Infect. , 22 : 197 – 205 .
- Vernet , V. , Philippon , A. , Madoulet , C. , Vistelle , R. , Jaussaud , R. and Chippaux , C. 1995 . Virulence Factors (Aerobactin and Mucoid Phenotype) in Klebsiella pneumoniae and Escherichia coli Blood Culture Isolates . FEMS Microbiol. Lett. , 130 : 51 – 57 .
- Tsukadaira , A. , Okubo , Y. , Kobayashi , T. , Wakamatsu , T.T. , Sasabayashi , M. and Hotta , J. 2004 . Four Cases of Klebsiella pneumonia . J. Jpn. Respir Soc. , 40 : 530 – 535 .
- Mohantv , S.S. and Kay , P.R. 2004 . Infection in Total Joint Replacements. Why We Screen MRSA When MRSE Is the Problem . J. Bone Joint Surg. Br. , 86 : 266 – 268 .
- Hatch , M.Y. and Wolochow , H. 1969 . Bacterial Survival: Consequences of Airborne State: an Introduction to Experimental Aerobiology , 267 – 295 . New York : John Wiley and Sons .
- Kim , K.Y. , Kim , H.T. , Kim , D. , Nakajima , J. and Higuchi , T. 2009 . Distribution Characteristics of Airborne Bacteria and Fungi in the Feedstuff-Manufacturing Factories . J. Hazard. Mater. , 169 : 1054 – 1060 .
- Walter , M.V. , Marthi , B. , Fieland , V.P. and Ganio , L.M. 1990 . Effect of Aerosolization on Subsequent Bacterial Survival . Appl. Environ. Microbiol. , 56 : 3468 – 3472 .
- Khojasten , V.J. , Edwards-Jones , V. , Childs , C. and Foster , H.A. 2007 . Prevalence of Toxin Producing Strains of Staphylococcus aureus in a Pediatric Burns Unit . Burns , 33 : 334 – 340 .
- Otten , J.A. and Burge , H.A. Bioaerosols: Assessment and Control; Macher, J . American Conference of Governmental Industrial Hygienists . Cincinnati , OH . Chapter 18
- Narui , K. , Noguchia , N. , Matsunaga , N. , Namiki , Y. , Yamanaka , Y. and Kumaki , Y. 2009 . Change in Environmental Bacterial Flora in a New Hospital Building . J. Hosp. Infect. , 73 : 24 – 33 .
- Zucker , B.A. , Trojan , S. and Müller , W. 2000 . Airborne Gram-Negative Bacterial Flora in Animal Houses . J. Vet. Med. B , 40 : 37 – 46 .
- Duan , H. , Chai , T. , Liu , J. , Zhang , X. , Qi , C. , Gao , J. , Wang , Y. , Cai , Y. , Miao , Z. , Yao , M. and Schlenker , G. 2009 . Source Identification of Airborne Escherichia coli of Swine House Surroundings Using ERIC-PCR and REP-PCR . Environ. Res. , 109 : 511 – 517 .
- Brown , J.K.M. and Hovmoller , M.S. 2002 . Aerial Dispersal of Pathogens on the Global and Continental Scales and Its Impact on Plant Disease . Science , 297 : 537 – 541 .
- Donaldson , A. 1999 . Airborne Spread of Foot-and-Mouth Disease . Microbiol. Today , 26 : 118 – 119 .
- Lee , A.K.Y. , Lau , A.P.S. , Cheng , J.Y.W. , Fang , M. and Chan , C.K. 2007 . Source Identification Analysis for the Airborne Bacteria and Fungi Using a Biomarker Approach . Atmos. Environ. , 41 : 2831 – 2843 .
- Mims , S.A. and Mims , F.M. III. 2004 . Fungal Spores Are Transported Long Distances in Smoke from Biomass Fires . Atmos. Environ. , 38 : 651 – 655 .
- Shinn , E.A. , Smith , G.W. , Prospero , J.M. , Betzer , P. , Hayes , M.L. , Garrison , V. and Barber , R.T. 2000 . African Dust and the Demise of Caribbean Coral Reefs . Geophys. Res. Lett. , 27 : 3029 – 3032 .
- Kramer , A. , Schwebke , I. and Kampf , G. 2006 . How Long Do Nosocomial Pathogens Persist on Inanimate Surfaces? A Systematic Review . BMC Infect. Dis. , 6 : 130 – 137 .
- Vincent , J.L. , Bihari , D.J. , Suter , P.M. , Bruining , H.A. , White , J. , Nicolas-Chanoin , M.H. , Wolff , M. , Spencer , R.C. and Hemmer , M. 1995 . The Prevalence of Nosocomial Infection in Intensive Care Units in Europe . JAMA , 274 : 639 – 644 .
- Bischoff , W.E. , Tucker , B.K. , Wallis , M.L. , Reboussin , B.A. , Pfaller , M.A. , Hayden , F.G. and Sherertz , R.J. 2007 . Preventing the Airborne Spread of Staphylococcus aureus by Persons with the Common Cold: Effect of Surgical Scrubs, Gowns, and Masks . Infect. Control Hosp. Epidemiol. , 28 : 1148 – 1154 .
- Neely , A.N. 2000 . A Survey of Gram-Negative Bacteria Survival on Hospital Fabrics and Plastics . J. Burn Care Rehab. , 21 : 523 – 527 .
- Shiomori , T. , Miyamoto , H. , Makishima , K. , Yoshida , M. , Fujiyoshi , T. , Udaka , T. , Inaba , T. and Hiraki , N. 2002 . Evaluation of Bed Making-Related Airborne and Surface Methicillin-Resistant Staphylococcus aureus Contamination . J. Hosp. Infect. , 50 : 30 – 35 .
- Wong , D. , Nye , K. and Hollis , P. 1991 . Microbial Flora on Doctor's White Coats . BMJ , 303 : 1602 – 1604 .
- Datz , C. , Jungwirth , A. , Dusch , H. , Galcan , G. and Weigert , T. 1997 . What's on Doctor's Ball-Point Pens? . Lancet , 350 : 1284
- French , G. , Rayner , D. , Branson , M. and Walsh , M. 1998 . Contamination of Doctors’ and Nurses’ Pens with Nosocomial Pathogens . Lancet , 351 : 513
- Bolyard , E.A. , Tablan , O.C. and Williams , W.W. 1998 . Guidelines for Infection Control in Health Care Personnel . Am. J. Infect. Control , 26 : 289 – 354 .
- Hogg , G.M. , Mckenna , J.P. and Ong , G. 2008 . Rapid Detection of Methicillin-Susceptible and Methicillin-Resistant Staphylococcus Aureus Directly from Positive BacT/Alert® Blood Culture Bottles Using Real-Time Polymerase Chain Reaction: Evaluation and Comparison of 4 DNA Extraction Methods . Diag. Micro. Infect. Dis. , 61 : 446 – 452 .
![Figure 4. Gel electrophoresis. M = DNA ladder (100 base pairs [bp]), A = A. baumannii (101 bp), C = C. freundii (221 bp), E = E. coli (446 bp), K = K. pneumoniae (119 bp), S = S. aureus (188 bp).](/cms/asset/6612d464-0e0c-4d6f-be93-40f57585d90f/uawm_a_10412137_o_f0004g.gif)