Abstract
Remarkable and continued growth in the field of brain aging research has been fueled by a confluence of factors. Developments in molecular biology, imaging, and genetics coupled with the imperative caused by the aging of the population has created fertile ground for improved understanding of the interaction between brain function and behavior. Aging changes in neurochemical systems may account for the spectrum of cognitive and behavioral states of successfully aged pen sons, but may also contribute to enhanced vulnerability to depressive or dementing illness. In particular, the refinement of in vivo imaging approaches to investigating the structure and function of the aging brain has provided the opportunity to strengthen our knowledge of the biological substrate of the aging brain and neuropsychiatrie disorders, and translate these into therapeutics.
El notable y continuo crecimiento en el campo de la investigación sobre el envejecimiento cerebral ha sido incentivado por una confluencia de factores. El desarrollo de la biología molecular, de las técnicas de imágenes y de la genética, en conjunto con la exigencia determinada por el envejecimiento de la población han creado un terreno fértil para mejorar la comprensión acerca de la interacción entre la función cerebral y la conducta. Los cambios que produce el envejecimiento en los sistemas neuroquímicos pueden explicar el espectro de estados cognitivos y conduciuales de personas de edad avanzada que han sido exitosas, pero también pueden contribuir a incrementar la vulnerabilidad a enfermedades depresivas o demencias. En particular, el refinamiento de las técnicas de imágenes in vivo que permiten investigar la estructura y función del cerebro que envejece ha dado la oportunidad de fortalecer nuestro conocimiento acerca del sustrato biológico del envejecimiento cerebral y de los trastornos neuropsiquiátricos, y traducir este conocimiento en terapias.
Un ensemble de facteurs convergents a contribué au développement remarquable et constant de la recherche sur le vieillissement cérébral. C'est ainsi que les avancées de la biologie moléculaire, de l'imagerie et de la génétique tout autant que les impératifs liés au vieillissement inéluctable de la population ont créé un terrain propice à la recherche d'une meilleure compréhension de l'interaction entre la fonction cérébrale et le comportement. Si les modifications des systèmes neurochimiques dues à l'âge peuvent expliquer l'éventail des états cognitifs et comportementaux de patients qui ont bien vieilli, elles peuvent aussi contribuer à une augmentation de la vulnérabilité aux maladies dépressives ou démentielles. Les perfectionnements des méthodes utilisant l'imagerie in vivo pour analyser la structure et la fonction du cerveau vieillissant ont permis de consolider nos connaissances sur le substrat biologique du cerveau vieillissant et les désordres neuropsychiatriques et de traduire celles-ci sous forme de thérapeutiques.
Man (and woman) has long been fascinated with the workings of the human mind. Yet, it is only recently that we have developed the tools to explore its biological underpinnings in the living state. The 1990 to 2000 interval was hailed as the Decade of the Brain. Advances in imaging, genetics, molecular biology, and pharmacology continue to advance our horizons in neuroscience research, but, the scientific yield from these highly productive past 10 years will surely both usher in the developments of the future and guide the research achievements to important clinical applications. The gap between bench and bedside is narrower than ever and, importantly, there is increasing focus on not only lengthening the life span, but also improving the quality of mental and physical health in aging.
Anatomical and neurochemical systems affected by brain aging
Imaging structural brain changes in aging
Structural brain changes accompanying normal aging and neurodegenerative and psychiatric disorders may parallel and provide insight, into the etiology of changes in cognition, mood, and motor function in the elderly. However, postmortem studies of brain morphology are plagued by artifacts caused by changes in hydration states just, prior to death and tissue fixation. These studies are biased toward end-stage disease states and permit, only retrospective correlations with measures of brain function and behavior. Magnetic resonance imaging (MRI) offers a means of assessing structural brain changes in vivo and provides the opportunity to evaluate the relationship of morphologic parameters to mood, neuropsychological dysfunction, and treatment response.
It is well known from both imaging and autopsy series that cerebrospinal fluid (CSF) increases and cerebral volume reductions accompany normal human aging.Citation1-Citation8
Several studies have suggested that age-related volume loss tends to affect some brain regions more than others. Jcrnigan et alCitation1 localized aging changes in brain volume to be most marked in the caudate nucleus, anterior diencephalic structures, association cortices, and mesial temporal structures, with no changes found in the thalamus and anterior cingulate cortex. Murphy et alCitation6 also found significantly larger MRI-determined volume losses in the caudate and lentiform nuclei than in the cerebral hemispheres in normal elderly men. These authors speculated that, this finding was in accord with motor abnormalities encountered in the elderly. Similarly, preferential reductions in the size of the hippocampal formation in normal aging have been shown to correlate with delayed memory performance.Citation9 It is important to bear in mind that age-related cerebral volume loss is highly variable among individuals and further accelerated by coincident medical illness. Conversely, DeCarli et alCitation10 showed that temporal lobe volumes did not change over a range of 19 to 92 years of age, when only successfully aged men were included.
Due to considerable intersubject variability of age-related structural brain changes, cross-sectional study designs provide limited information about, the rates and influences on such alterations. In a unique effort to determine the annual rate of brain volume changes in the healthy elderly, the Baltimore Longitudinal Study of Normal Aging has followed 94 elders with five annual MRI assessments. Preliminary findings support, substantial annual intrasubject whole -brain volume reductions estimated to average 5.5 mL, with 1.4 mL increases in total CSF volume.Citation11
Nonspecific foci of increased white matter signal may be observed by MRI in normal individuals of all ages, but, clearly increase in frequency with age, particularly after age 60.Citation7,Citation12,Citation13 Although of uncertain clinical significance, these white matter hyperintensities have been found to be especially prevalent in persons with prominent, risk factors for cerebrovascular disease, particularly hypertension.Citation13-Citation15 Pathologic correlates also point to an ischemic basis for these lesions,Citation16,Citation17 and blood flow reductions have been reported in association with white matter hyperintensities.Citation18-Citation21 Yet, whether white matter hyperintensities are associated with diminished cognitive function in aging is still unsettled.Citation14,Citation17,Citation22
Although substantial improvements in image processing and quantitative methods have recently been made, there are conflicting results among the numerous structural MRI studies of the aging brain due to a number of factors. These include methodological differences in imaging data acquisition and analysis, small sample size, and selection bias, such as failure to control for cerebrovascular risk factors. Still, morphologic changes in the brain that, accompany aging follow processes - often with substantial delay - that begin at the cellular level. For this reason, investigative techniques that reflect functional or physiologic brain changes are typically more sensitive approaches for identifying the earliest and potentially reversible changes of healthy or pathologic aging.
Cell loss in normal aging
It, was widely accepted that substantial neuronal loss occurred during normal aging with values as high as 50% in some hippocampal subregions, and that this was likely to be responsible for age-related decline in memory. Most early neuropath ological studies used measures of neuronal density rather than cell number as the basis for measurement of cell loss. However, with the more recent, application of stereological techniques to this field, it has become clear that normal aging is not, accompanied by significant global decline in neuronal number.Citation23,Citation24 Within the hippocampus and associated cortical regions, there is no significant cell loss in entorhinal cortex, CA1, or temporal cortex of the undemented elderly.Citation25 Some agerelated cell loss does occur in the dentate gyrus and subiculum. It is, therefore, not possible to account for memory deficits in the elderly in terms of cell loss alone. The substrate for such change is more likely to be related to disruption of important hippocampal circuits short of cell loss. Such changes include the presence of neurofibrillary tangles within the entorhinal cortex,Citation25 synapse loss in the terminal zone of the perforant pathway of the dentate gyrus,Citation26 changes to dendrites, disruption of longterm potentiation,Citation27 and decreases in the expression of the iV-methyl-D-aspartate (NMDA) receptor in the molecular layer of the dentate gyrus.Citation24
Age-related alterations in brain metabolism and perfusion
Functional imaging tools, such as positron emission tomography (PET), single-photon emission tomography (SPECT), 133Xe- or xenon-enhanced computed tomography (CT), and optical imaging have permitted in vivo evaluation of brain perfusion and metabolic measurements. Yet, whether generalized physiologic measures such as resting cerebral blood flow (CBF) are altered in normal aging remains a point, of controversy. Using the 133Xe inhalation method, which suffers from particularly poor spatial resolution relative to other methods, several investigators have demonstrated a significant reduction in mean CBF throughout, the adult, life span.Citation28-Citation31 With PET, Leenders et alCitation32 similarly demonstrated a decline of 0.5%/year in CBF, cerebral blood volume (CBV), and cerebral metabolic rate of oxygen (CMRO2) in cortical brain regions. Several other investigators have also observed aging declines in CMRO2, with a milder influence of age on CBF and oxygen extraction.Citation33-Citation36 One important potential confound in many of these studies is the diluting influence of age-related cerebral volume loss on these measurements. Due to the limited spatial resolution of many functional imaging techniques, partial volume averaging of cortical signal with enlarged sulcal CSF spaces can result in underestimation of metabolic parameters in older subjects.Citation37 Applying MRI-based partial volume correction to [15O]water PET data, Meltzer et alCitation38 recently demonstrated no reduction in cortical CBF in healthy aging. This work challenges the interpretation of older studies, which did not account, for this source of artifact that may dilute metabolic measures in the elderly.Citation39 Although resting CBF may be normal in the successfully aged individual, age effects on small arterioles may reduce the autoregulatory capacity of the cerebrovascular system to respond to vasodilatory challenge;Citation40 thus diminishing the brain's ability to compensate for changes in systemic perfusion pressure and perhaps enhancing its susceptibility to ischemic damage. PET studies of brain glucose utilization have similarly demonstrated disagreement, among reports as to whether brain function declines with age. In 1982, Kuhl et alCitation41 reported a gradual aging decline in the mean cerebral metabolic rate of glucose utilization (CMRglc). Later studies supported a regional preference for agerelated reductions in brain glucose metabolism in the frontal lobes, which were most marked after age 60.Citation42,Citation43 Duara et al,Citation44 however, found no relationship between age and regional CMRglc in healthy men. Large intersubject variability in the neurobiologie effects of aging has been noted by several investigators.Citation44,Citation45 These reports, individually limited by small sample sizes, suggest, that aging effects on brain function are likely highly variable, affected by structural brain changes and systemic factors, and may differ between “successful aging” and individuals with substantial medical burden.
Alterations in neurotransmitter systems
The functional integrity of several neurotransmitter systems is altered by the aging process. Characterizing the profile of normal aging changes in neurotransmittcrmediated synaptic processes is the foundation upon which we will come to decipher the biological basis of behavioral and mood alterations accompanying aging. Further, the potential interaction between age effects and neurochemical disturbances associated with neuropsychiatrie disease states may influence the susceptibility of the elderly to certain neurobehavioral disorders. Our knowledge of the effect of age on neuroreceptor function is primarily inferred by postmortem studies, with limited and variable regional sampling of the brain, and by animal models, which may not appropriately represent, human brain aging. In contrast to studies of pathological changes in aging, there are many problems associated with the biochemical study of neurotransmission in humans. These include the effects of postmortem delay, hypoxia, and drug treatment, as well as the fundamental point that the material is removed most often removed following a terminal illness, which may itself influence neurotransmission regardless of the age at which the patient died. 'ITtic reader is referred to a comprehensive review of the subject, by DeKosky and Palmer.Citation46
With the development of highly selective radioligands for neuroreceptors, transporters, and other markers of neuronal function, it is possible to study the effects of aging and disease on brain neurotransmitter systems in vivo with PET. This approach permits whole-brain quantitative imaging in well-characterized subjects, with the potential for obtaining longitudinal measures. Such work has demonstrated specific aging reductions in dopamine and serotonin (5-hydroxytryptamine [5-HT]) receptor subtypes ().Citation47-Citation50 Interestingly, there is evidence that some neuroreceptors actually increase in density with age, a finding of note in the opiate system.Citation51 PET techniques are desirable relative to neuroendocrine challenge studies, which lack spatial localizing information and physiologic specificity. However, the combination of PET with neuropharmacologic probes is a powerful technique for localizing and quantifying neurotransmitter-mediated function in aging and disease.
![Figure 1. [18F]Altanserin positron emission tomography (PET) imaging of the 5-hydroxytryptamine (serotonin) type-2A receptor (5-HT2A). Left. [18F]Altanserin PET images (summed over 20 to 90 min postinjection and displayed with scale normalized to cerebellum) at three brain levels in healthy subjects, aged 20 (left) and 66 (right). Right. Scatter plot demonstrates a linear decline in the binding of [18F]altanserin to 5-HT2A receptors in the lateral orbitofrontal cortex over the adult life span.](/cms/asset/3059159b-d365-413a-805f-ad46fe777b34/tdcn_a_12130453_f0001_oc.jpg)
Cholinergic system
There is considerable evidence for a presynaptic cholinergic deficit during aging in many brain regions based on reductions in the enzyme responsible for the synthesis of acetylcholine, choline acetyltransferase (ChAT), in cortex and striatum (as reviewed Palmer and DeKoskyCitation52) and in acetylcholine synthesis in temporal cortex.Citation53 Furthermore, there arc decreases with age in both muscarinic and nicotinic cholinergic receptors.Citation54 Using proton magnetic spectroscopy, Cohen et alCitation55 demonstrated reductions in the uptake of circulating choline with advancing age. Selective imaging ligands for the cholinergic system have proved elusive. However, PET studies with the relatively nonselective cholinergic receptor ligands [11C]benztropine, [11C]tropanyl benzilatc, and [11C]-N-methylpiperidyl bcnzilate (NMPB) have supported in vivo losses in muscarinic receptor density with age, although they disagree on the magnitude of the reductions.Citation56-Citation58 Also, modest reductions in cholinergic terminal density with aging have been demonstrated by SPECT imaging of the vesicular acetylcholine transporter [123I]iodobenzovesamicol.Citation59
Monoaminergic systems
There is wide variation in the response of monoaminergic systems to aging. While postmortem studies show considerable loss of markers of the 5-HT system (5-HT, 5-HT1A, and 5-HT2A receptors), particularly in the neocortex, and of dopaminergic markers (dopamine, major metabolites, transporter, and receptors) in the striatum, there is little evidence of change outside those regions or in markers of the noradrenergic system.Citation52 The development, of selective imaging ligands for the 5-HT and dopamine binding sites has allowed these systems to be further studied in humans in vivo.
PET studies confirm substantial aging reductions in specific binding to dopamine D1 and D2 receptors.Citation47,Citation48,Citation60 Further, alterations in cognition and coordination of motor activity that frequently accompany aging have been shown to correlate with PET measures of dopamine receptor function.Citation61 Aging losses of presynaptic dopamine transporter sites have also been demonstrated with PET and SPECT, suggesting that age affects the integrity of dopaminergic neuronal pathways.Citation62-Citation65
Recently developed PET ligands for several 5-HT receptor subtypes and the 5-HT transporter have facilitated in vivo imaging of this important neurotransmitter system, which is central to mood and sleep regulation.Citation66,Citation67 Marked widespread aging reductions in binding of the pharmacologically well-characterized 5-HT2A receptor using [18F]altanserin and [18F]setoperone have been shown by several investigators.Citation49,Citation50,Citation68 The magnitude of the inverse relationship between age and 5-HT2A receptor binding supports the hypothesis that loss of serotonergic function in aging may contribute to the susceptibility of the elderly to alterations in mood and 5-HT-mcdiated behaviors. Intriguing preliminary evidence suggests gender differences in the effect of age on the 5-HT1A receptor.Citation69
Amino acid systems
Glutamic acid decarboxylase, responsible for the synthesis of γ-vinyl γ-aminobutyric acid (GABA), declines with age in cortex, hippocampus, and striatum, while there is limited evidence for decreases in markers of the glutamatergic system (transporter and NMDA receptor).Citation46,Citation70 It is, however, difficult to assess the status of the presynaptic glutamatergic system since the neurotransmitter is a ubiquitous component, of all cells.Citation71
While no changes have been reported in [3H]MK801 binding (to the ion channel) from middle age to old age, age-related changes in the ability of glutamate and glycine binding sites to influence binding within the channel have been observed.Citation72,Citation73 For example, the ability of glutamate and glycine to enhance [3H]MK801 binding in the frontal cortex is reduced from a 44% increase in young adults to a 35% increase in 80- to 100-year-old humans.Citation74 Furthermore, spermine stimulation of [3H]MK801 binding via the polyamine site disappears by 80 years of age and zinc inhibition also declines with increased age.Citation74 Reduction in binding to one or more sites on the NMDA receptor complex with age may reflect, losses of the entire receptor complex, a selective loss of certain subunits, or both. There is some evidence from studies in mice that changes in receptor subunit composition occur with age and may form the basis for changes in the affinity of certain binding sites.Citation75
Influence of gender on brain aging
The profound impact of sex steroids on brain structure and function is evidenced by sexual dimorphisms in brain organization and development,Citation76 which have been associated with gender-based differences in behavior and learning.Citation77 Recent evidence of male-female differences in brain aging supports an ongoing dynamic relationship between sex steroids and neural structure and function. This includes work by Honeycutt et al,Citation78 which demonstrates differential aging patterns for the morphology of mesial temporal structures, particularly the amygdala, in men and women. In vivo evidence of male-female differences in neuroreceptor distribution has been shown for 5-HT2A receptors, and a specific age-gender interaction on 5-HT1A receptors has recently been reported.Citation69 Gender preferences for psychiatric disorders, particularly depressive illness, also support, a biological underpinning for functional brain differences in men and women. Women clearly exhibit higher rates of depression in early and middle adulthood, with enhanced risk associated with surgical menopause and antiestrogen treatment for breast, cancer.Citation79,Citation80 However, there is evidence for a narrowing of the gender gap in mood disorders in older middle adulthood, for which a neuroendocrine basis is speculated.Citation81,Citation82
Animal models demonstrating a positive effect of estrogen on memory task performance, particularly working memory,Citation83,Citation84 and the observation that normal hormonal cycling affects cognitive performance in womenCitation85-Citation88 suggest a role for ovarian steroids in mediating cognitive function. However, variable outcomes have resulted from clinical investigations of hormone replacement therapy (HRT) and cognition in aging women. In a community-based study of over 700 postmenopausal women, Jacobs and colleaguesCitation89 noted higher cognitive measures in HRT users relative to nonusers. They also found slight, improvements in verbal memory performance over the follow-up interval. However, these findings were not, independent of age and education level. Other investigators have reported no clear beneficial effect of estrogen replacement therapy on cognitive function,Citation90,Citation91 and no relationship between endogenous estrogen levels on cognitive test performance.Citation92,Citation93 (Interestingly, an association between higher endogenous testosterone levels and cognitive performance has been noted in women.Citation92 ) It has been further suggested that a lack of epidemiological evidence of gender differences in cognitive decline with aging argues against a link between estrogen deficiency and cognitive dysfunction.Citation94 Research to date on male aging has been limited and the clinical relevance of the aging decline of testosterone levels in men is debated.Citation95 Although androgens clearly play a role in brain development, and sexual brain dimorphisms, central mechanisms for modulating human behavior are less well characterized (for a review, see reference 96). Androgen receptors are found in many brain regions with particular localization to the hippocampus,Citation97 where, similar to estrogen, they modulate hyperpolarization of pyramidal cells in the CA1 region.Citation98 In healthy young men, testosterone levels have been shown to correlate positively with spatial cognitive function and negatively with verbal performance.Citation99 Beneficial effects on spatial cognitive function in men have been associated with an optimal level of testosterone, with deterioration of performance observed at, both high and low levels.Citation100 Although the concept of testosterone supplementation remains controversial, randomized, controlled trials of androgen replacement therapy in healthy older men have demonstrated enhanced spatial cognitive ability.Citation101 Overall, the potential benefits of androgen replacement in elderly men appear to weigh favorably against minor potential added risks to cardiovascular and prostate health.Citation102
Late-life neuropsychiatrie disorders
Depression
The association of evidence of disruption of structural brain integrity (eg, white matter lesions) and late-life, particularly late-onset, depression further underscores the potential multiplicity of biological factors relevant to depressive illness occurring in the elderly.Citation103-Citation105 The correlation between white matter hyperintensities and hypertension and/or atherosclerotic disease may parallel and provide insight into the role of cerebral ischemia in the etiology of late-life depression, since an increased prevalence of both cerebrovascular risk factorsCitation103 and white matter hyperintensities has been observed in elderly depressed patients,Citation106 particularly those with a late onset, of their disease.Citation22,Citation104,Citation107,Citation108 These observations have led some investigators to postulate an MRI-defined vascular or atherosclerotic form of depression,Citation107,Citation109 which supports a strong link between aging and biological factors in depression occurring in the elderly. Also, evidence for serotonergic control of the regulation of CBFCitation110,Citation111 and the potential influence of age on this regulatory mechanismCitation112 suggest an interaction - although as yet, illdefined - between disturbances in serotonergic function and risk of cerebral ischemic injury.
Depression has been widely attributed to deficient 5-HT neurotransmission. In the unique setting of geriatric depression, age-related alterations in the 5-HT system may perturb its functional integrity and thus potentially contribute to the high prevalence and distinct, character of depression in late life. Postmortem studies have reported conflicting findings of 5-HT receptor status in suicide victims.Citation113-Citation119 In a small group of elderly nonsuicide depressed patients, reductions in binding to 5HT2A, but not to 5-HT1A, sites in temporal, frontal, and parietal cortex were reported using membranes.Citation120 Using autoradiographic techniques, the density (Bmax) for the 5-HT1A receptor was reduced by approximately 40% in the superficial layers of frontal cortex (Brodmann area 9) from patients undergoing surgery for intractable depression.Citation71 It is also worth noting that there was a 30% to 40% reduction in the concentration of the 5-HT metabolite, 5-hydroxyindoleacetic acid (5-HIAA), in the ventricular CSF of these depressed patients, underlining the possible relationship between disturbances of serotonergic neurotransmission and depressive symptoms.
Evaluation of serotonergic function through imaging techniques has offered a unique approach to evaluating the heterogeneity of depression in the elderly. In an attempt, to assign neurochemical specificity to the blood flow and metabolic disturbances reported in depression,Citation121-Citation123 fluorodeoxyglucose (FDG) PET coupled with the 5-HT-releasing agent, dl-fenfluramine provided indirect yet in vivo evidence of serotonergic dysfunction in the prefrontal cortex in depressed patients, who exhibited a blunted response relative to healthy controls.Citation124,Citation125 With the subsequent development, of selective imaging agents for serotonergic receptor sites, it became possible to quantify central 5-HT binding in depressed patients. Of particular interest are the 5-HT2A and 5-HT1A receptors and the 5-HT transporter, which are all heavily implicated in the antidepressant, response (). There are few published studies to date of serotonergic PET imaging in mood disorders and fewer conducted in elderly patients. Biver et alCitation126 demonstrated a significant, reduction in the binding of [18F]altanserin to right, orbitofrontai 5-HT2A receptors in a group of mid-life depressed subjects. However, this agent failed to show a change in 5-HT2A receptor binding in late-life depression.Citation127 Drevets et alCitation128 recently reported reductions in 5-HT1A binding of [11C]WAY100635 to mcsiotemporal and brainstem raphe areas in familial mood disorders including bipolar dépressives. Whether this finding is generalizable to nonfamilial forms of mood disorders and late-life depression is yet uncertain. The capability to selectively evaluate neurotransmitter binding sites in vivo will likely continue to be a valuable tool for determining the biological underpinnings of late-life depression and sources of treatment response variability among patients.
-McN5652 (summed over 40 to 90 min postinjection). High-resolution T1-weighted magnetic resonance imaging (MRI) (middle) provides detailed anatomic information. However, the coregistration of the PET and MRI depicted in the overlay image (right) offers a unique combination of structural and functional data. The localization of the raphe nuclei, which are not visible by MRI alone, are functionally and anatomically defined by the coregistered images.](/cms/asset/23a55132-0afe-429a-954f-bb795e0f077e/tdcn_a_12130453_f0002_oc.jpg)
Alzheimer's disease: breaking the disease barrier
Alzheimer's disease (AD), the most common form of dementia, has enormous and growing public health significance. A disease of aging, the financial and social burdens of AD are compounded by recent and continued increases in the average life span.Citation129,Citation130 It has been estimated that the prevalence of AD will continue to climb at, a rapid rate, with an expected quadrupling of cases in the United States over the next, 50 years.Citation130 Thus, the need for developing early diagnostic markers to complement new therapeutic approaches is more acute than ever before. Indeed, a modest goal of instituting treatment that could delay disease onset by just 2 years would profoundly impact these projections, resulting in 2 million fewer cases by 2050.
Biological basis of Alzheimer's disease
Cell death and histopathological changes affecting a number of neuronal systems are considered to result in the development of the typical symptomatology of AD characterized by gross and progressive impairments of cognitive function. The histopathological features are intracellular neurofibrillary tangles formed from a hyperphosphorylated form of the microtubule-associatcd protein, tau, and extracellular deposits of a 40/42 amino acid peptide, Aβ (derived from amyloid precursor protein [APP]), often in the form of senile or neuritic plaques. Plaques, tangles, and cell loss have a characteristic regional and temporal distribution in the AD brain, affecting entorhinal, hippocampal, and temporal cortical structures first and frontal and parietal cortices later in the disease process, while sparing primary sensory and primary motor areas.Citation131 Indeed, this pathology is reflected in the characteristic regional pattern of blood flow and metabolic disturbances demonstrated by PET or SPECT imaging in early AD. Evidence from biochemical studies also indicates that certain subcortical stuctures, including the nucleus basalis of Meynert and the dorsal raphe are also affected early in the disease.Citation132
Most, cases of AD are considered to be sporadic and therefore of unknown cause; however, there are a number of autosomal dominant mutations (Table I) that cause AD and several genetic risk factors, the best known of which is apolipoprotein E (apo E) ε4 polymorphism, the presence of which carries an approximately sixfold greater risk of developing AD.Citation133 While the disease-causing mechanism behind apo E remains controversial, most studies indicate that mutations in the genes APP, prcscnilin 1 (PS1), and presenilin 2 (PS2) alter the metabolism of APP so as to favor production of a long form of Aβ (Aβ 1-42) (see, for example, reference 134).
Table I. Genes causing Alzheimer's disease (AD).
Neurotransmitter changes in Alzheimer's disease
The majority of biochemical studies of AD have relied on information derived from postmortem brain, which typically represents the late stage of the disease (8-10 years after onset, of symptoms). In these studies, there is considerable evidence for multiple neurotransmitter abnormalities affecting many brain regions. However, investigations of biopsy tissue taken from AD patients 3 to 5 years (on average) after the onset, of symptoms indicate that a selective neurotransmitter pathology occurs early in the course of the disease.Citation132
Acetylcholine. Changes affecting many aspects of the cholinergic system in patients with AD have been reported since the initial discovery of deficits in ChAT activity in postmortem brains.Citation135-Citation137 In biopsy samples from AD patients, presynaptic markers of the cholinergic system were also uniformly reduced.Citation132 Thus, ChAT activity, choline uptake, and acetylcholine synthesis are all reduced to between 30% and 60% of control values. The clinical correlate of this cholinergic deficit, in AD was until recently considered to be cognitive dysfunction. Such a conclusion was supported by clinicopathological studies in AD and parallel experiments in nonhuman primates or rodents, which demonstrated disruptive effects of basal forebrain cholinergic lesions on cognitive functions. Furthermore, cholinergic deficits in AD occur to the greatest extent in cortical areas primarily concerned with memory and cognition: the hippocampus, adjacent temporal lobe regions, and selected frontal areas. Such studies led to the “cholinergic hypothesis of geriatric memory dysfunction.”Citation138
On the basis of the above evidence, neocortical cholinergic innervation appears to be lost at an early stage of the disease and this is supported by a recent study where the cholinergic deficit (reduced ChAT activity) has been related to Braak staging.Citation131 Braak stages I and II are considered to represent the earliest, presentation of AD with neurofibrillary tangles in entorhinal cortex, and a 20% to 30% loss in ChAT activity was reported in brains from patients at, these stages of AD.Citation139 However, another study using the Clinical Dementia Rating (CDR) scale suggests that the greatest reduction in markers of the cholinergic system occurs between moderate (CDR 2.0) and severe (CDR 5.0) disease with little change between nondemented and the mild stage (CDR 0-2).Citation140
There has been a recent shift of emphasis regarding the clinical significance of cholinergic deficits. Noncognitive or neuropsychiatrie, in addition to cognitive, symptoms also appear to have a cholinergic component.Citation141 For example, visual hallucinations relate to neocortical cholinergic deficits,Citation142 such deficits (eg, loss of ChAT) being greater in dementia with Lewy bodies (DLB), where hallucinations are common, than in AD, where they are less common.Citation143 Reductions in cortical ChAT activity in patients with dementia, in addition to correlating with cognitive decline, are also related to overactivity and aggressive behavior.Citation144
Glutamate. Although neurochemical studies of glutamate neurotransmission have failed to demonstrate extensive alterations, this may be related to the difficulty in distinguishing the transmitter pool of glutamate from the metabolic pool. Nevertheless, glutamate concentration was reduced by 14% in temporal lobe biopsy samples and by 86% in the terminal zone of the perforant pathway at autopsy of AD patients.Citation145 Uptake of D-aspartate, a putative marker of glutamatergic nerve endings, is also reduced in many cortical areas in the AD brain.Citation146 In addition, loss of synapses and pyramidal cell perikarya (both considered to be markers of glutamatergic neurones) from the neocortex of AD patients correlate with measures of cognitive decline.Citation71 Thus, additional factors other than impaired cholinergic function are likely to contribute to cognitive impairment in AD. However, it, is important to remember that glutamatergic neurons of the neocortex and hippocampus are influenced by acetylcholine through nicotinic and muscarinic receptors.Citation147-Citation148 Thus, treatment of patients with cholinomimetics is likely to increase glutamatergic function.
Other neurotransmitters. In biopsy samples from AD patients, some noradrenergic markers are affected, whereas markers for dopamine, GABA or somatostatin are not altered. When postmortem studies of AD brain are considered many neurotransmitter systems, including GABA and somatostatin, are involved or are affected to a greater extent.Citation71 Changes in serotonergic neurotransmission seen at biopsy, postmortem, and recently in vivoCitation68,Citation149 may be linked to the behavioral disturbances of AD, such as depression, rather than cognitive dysfunction. For example, patients with AD who were also depressed had lower numbers of serotonin reuptake sites in the neocortex than AD patients without this symptom.Citation150 Furthermore, both reduced serotonergicCitation151,Citation152 and increased noradrenergic activities and sensitivityCitation153,Citation154 have been linked to aggressive behavior.
Neurotransmitter receptors. The majority of neurotransmitter receptors appear to be unaffected in AD; however, studies have demonstrated a reduced numbers of nicotinic and muscarinic (M2) acetylcholine receptors, some of which are considered to be located on presynaptic cholinergic terminals. The Mj subtype is unchanged, but the coupling of the receptor to its Gprotein may be impaired.Citation155,Citation156
A highly consistent receptor abnormality in AD is the loss of the nicotinic receptor,Citation157-Citation159 which appears to primarily reflect loss of the oc4-containing subtype (generally associated with α2), as opposed to α3 or α7 subtypes.Citation160 Immunohistochemically, loss of α4 and α2 reactive fibers has been observed in temporal cortex, associated with reactive neuropil threads, tangles, and plaques.Citation161
Links between neurotransmission and neuropathology
There is increasing evidence that various neurotransmitter systems are capable of influencing the metabolism of APP, favoring nonamyloidogenic processing.Citation162 In particular, stimulation of muscarinic M1 receptors increases APP secretion, while decreasing β-amyloid production.Citation163 These results suggest that compounds developed for symptomatic treatment may have a serendipitous effect on the continuing emergence of pathology by reducing the production of Aβ.
Cholinergic neurotransmission may be a specific target for Aβ, since it has been shown to reduce both choline uptake and acetylcholine release in vitro.Citation164 Furthermore, Aβ is reported to bind with high affinity to the β7 subtype of the nicotinic receptor, suggesting that cholinergic function through this receptor may be compromised because of high levels of (soluble) peptide in AD brains.Citation165
Translation of discoveries into therapeutics
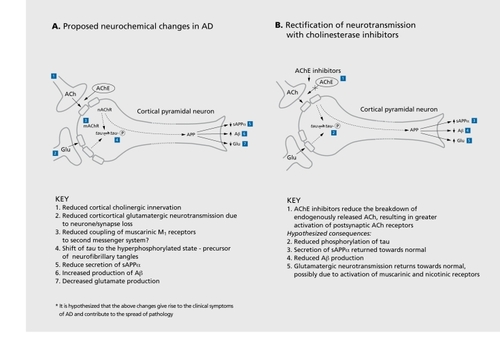
Biochemical studies in AD have generated a large number of therapeutic strategies for AD, many of which have been tested in same-scale, inconclusive studies. Only a few strategies have gone on to full-scale clinical trials. The best known of these is related to the cholinergic deficit. Moreover, while there are a number of rational approaches, including precursor loading and the use of muscarinic or nicotinic agonists, the use of acetylcholinesterase inhibitors (AChE-Is) is the most welldeveloped approach to the treatment, of AD to date ().Citation166 Tacrine underwent large-scale clinical studies and clearly established the benefits of AChE-I treatment in patients with a diagnosis of probable AD. Statistically significant, dose-related improvements on objective performance-based tests of cognition, clinician- and caregivcr-rated global evaluations of patient well-being, and also quality of life measures have been reported.Citation167 Tacrine was subsequently approved for use in some, but, not, all, countries. Adverse side effects, including raised liver enzymes, have limited the use of this compound. Further AChE-Is have been developed including donepezil, rivastigmine, metrifonate, and galantamine.Citation166 Such compounds demonstrate a clinical effect and magnitude of benefit, of at least that, reported for tacrine, but with a more favorable clinical profile including fewer and less serious side effects. Furthermore, evidence is emerging from clinical trials of cholinomimetics that such drugs may improve the abnormal noncognitive, behavioral symptoms of AD. The cholinesterase inhibitors physostigmine, tacrine, rivastigmine, and metrifonate have variously been reported in controlled trials to decrease psychoses (hallucinations and delusions), agitation, apathy, anxiety, disinhibition, pacing and aberrant motor behavior, and lack of cooperation in AD.Citation141,Citation168
Reproduced from reference 166: Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137-147. Copyright © 1999, Journal of Neurology Neurosurgery, and Psychiatry.
Future directions: merqinq technologies
Investigational ncuropharmacologic techniques comprise a powerful and complementary collection of research tools for studying the effects of aging and disease on regional and specific measures of brain function. These have allowed us to characterize both the normal neurochemical changes that accompany successful aging and the accelerated or aberrant, alterations seen in neuropsychiatrie and behavioral dysfunction. Future work will carry the findings of the past decade into the realm of intervention. Advancements in structural and functional imaging naturally complement those in molecular neurobiology and genetics, but, we are just beginning to realize their potential combined power. For example, the recent, availability of animal PET scanners presents the opportunity for the in vivo study of genetic models of disease, such as AD. Further, neuropharmacologic approaches to cognitive enhancement and slowing of dementia progression may be evaluated and monitored by imaging strategies. Indeed, the challenges posed by an increasingly aged population in industrialized nations are formidable, but, may best, be met, by the combined application of developing technologies.
Selected abbreviations and acronyms
| AChE-I | = | acetylcholinesterase inhibitor |
| AD | = | Alzheimer's disease |
| APP | = | amyloid precursor protein |
| CBF | = | cerebral blood flow |
| CBV | = | cerebral blood volume |
| ChAT | = | choline acetyltransferase |
| CMRglc | = | cerebral metabolic rate of glucose utilization |
| CMRO2 | = | cerebral metabolic rate of oxygen |
| CSF | = | cerebrospinal fluid |
| GABA | = | γ-aminobutyric acid |
| HRT | = | hormone replacement therapy |
| 5-HT | = | 5-hydroxytryptamine |
| MRI | = | magnetic resonance imaging |
| NMDA | = | N-methyl-D-aspartate |
| PET | = | positron emission tomography |
| SPECT | = | single-photon emission computed tomography |
REFERENCES
- JerniganT.ArchibaldS.BerhowM.SowellE.FosterD.HesselinkJ.Cerebral structure on MRI, part I: localization of age-related changesBiol Psychiatry.19912955672001446
- BlatterD.BiglerE.GaleS.et al.Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of lifeAm J Neuroradiol.1995162412517726068
- MillerAKH.AlstonRL.CorsellisJAN.Variation with age in the volumes of grey and white matter in the cerebral hemispheres of man: measurements with an image analyserNeuropathol Appl Neurobiol.198061191327374914
- JerniganTL.PressGA.HesselinkJR.Methods for measuring brain morphologic features on magnetic resonance imagesArch Neurol.19904727322294890
- ItoM.HatazawaJ.YamauraH.MatsuzawaT.Age-related brain atrophy and mental deterioration - a study with computed tomographyBr J Radiol.1981543843907237008
- MurphyD.DeCarliC.SchapiroM.RapoportS.HorwitzB.Age-related differences in volumes of subcortical nuclei, brain matter, and cerebrospinal fluid in healthy men as measured with magnetic resonance imagingArch Neurol.1992498398451343082
- ChristiansenP.LarssonH.ThomsenC.WieslanderS.HenriksenO.Age dependent white matter lesions and brain volume changes in healthy volunteersActa Radiol.1994351171228172734
- WahlundLO.AgartzI.AlmqvistO.et al.The brain in healthy aged individuals: MR imagingRadiology.19901746756792305048
- GolombJ.KlugerA.de LeonMJ.et al.Hippocampal formation size in normal human aging: a correlate of delayed secondary memory performanceLearn Mem.19941455410467585
- DeCarliC.MurphyD.GilletteJ.et al.Lack of age-related differences in temporal lobe volume of very healthy adultsAm J Neutoradiol.19941 5689696
- ResnickS.DavatzikosC.KrautM.ZondermanA.Longitudinal changes in MRI volumes in older adults. Presented at First International Meeting of the Alzheimer's Imaging Consortium 2000. Washington DC 2000
- AuttiT.RaininkoR.VanhanenSL.KallioM.SantavuoriP.MRI of the normal brain from early childhood to middle age. I. Appearances on T2- and proton density-weighted images and occurrence of incidental high-signal fociNeuroradiology.1994366446487862287
- HorikoshiT.YagiS.FukamachiA.Incidental high-intensity foci in white matter on T2-weighted magnetic resonance imaging. Frequency and clinical significance in symptom-free adultsNeuroradiology.1993351511558433794
- LongstrethW.ManolioT.ArnoldA.et al.Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health StudyStroke.199627127412828711786
- YetkinF.FischerM.PapkeR.HaughtonV.Focal hyperintensities in cerebral white matter on MR images of asymptomatic volunteers: correlation with social and medical historiesAm J Roentgenol.19931618558587980728
- KalariaR.Cerebrovascular degeneration is related to amyloid-β protein deposition in Alzheimer's diseaseAnn NY Acad Sci.19978262632719329698
- PantoniL.GarciaJ.Cognitive impairment and cellular/vascular changes in cerebral white matterAnn NY Acad Sci.1997826921019329683
- HatazawaJ.ShimosegawaE.SatohT.ToyoshimaH.OkuderaT.Subcortical hypoperfusion associated with asymptomatic white matter lesions on magnetic resonance imagingStroke.199728194419479341700
- KuwabaraY.IchiyaY.SasakiM.et al.Cerebral blood flow and vascular response to hypercapnia in hypertensive patients with leukoaraiosisAnn Nucl Med.1996102932988883704
- MiyazawaN.SatohT.HashizumeK.FukamachiA.Xenon contrast CT-CBF measurements in high-intensity foci on T2-weighted MR images in centrum semiovale of asymptomatic individualsStroke.1997289849879158638
- YamajiS.IshiiK.Sasaki M, et al. Changes in cerebral blood flow and oxygen metabolism related to magnetic resonance imaging white matter hyperintensities in Alzheimer's diseaseJ Nucl Med.199738147114749293811
- LesserI.BooneK.MehringerC.WohlM.MillerB.BermanN.Cognition and white matter hyperintensities in older depressed patientsAm J Psychiatry1996153128012878831435
- ColemanP.FloodD.Neuron numbers and dendritic extent in normal aging and Alzheimer's diseaseNeurobiol Aging.198785215453323927
- MorrisonJH.HofPR.Life and death of neurons in the aging brainScience.19972784124199334292
- Gomez-lslaT.HollisterR.WestH.et al.Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's diseaseAnn Neurol.19974117249005861
- GeinismanY.Detoledo-MorrellL.MorrellF.HellerR.Hippocampal markers of age-related memory dysfunction: behavioral, electrophysiological and morphological perspectivesProg Neurobiol.1995452232527777673
- BarnesC.Normal aging: regionally specific changes in hippocampal synaptic transmissionTrends Neurosci.19941713187511843
- DavisS.AckermanR.CorreiaJ.et al.Cerebral blood flow and cerebrovascular CO2 reactivity in stroke-age normal controlsNeurology.1983333913996403889
- ShawT.MortelK.MeyerJ.RogersR.HardenbergJ.CutaiaM.Cerebral blood flow changes in benign aging and cerebrovascular diseaseNeurology.1984348558626539861
- MelamedE.LavyS.BentinS.CooperG.RinotY.Reduction in regional cerebral blood flow during normal aging in manStroke.19801131357355426
- GurR.GurR.ObristW.SkolnickB.ReivichM.Age and regional cerebral blood flow at rest and during cognitive activityArch Gen Psychiatry.1987446176213606327
- LeendersK.PeraniD.LammertsmaA.et al.Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of ageBrain.199011327472302536
- PantanoP.BaronJC.Lebrun-GrandieP.DuquesnoyN.BousserMG.ComarD.Regional cerebral blood flow and oxygen consumption in human agingStroke.1984156356416611613
- MarchalG.RiouxP.Petit-TaboueMC.et al.Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human agingArch Neurol.199249101310201417508
- YamaguchiT.KannoI.UemuraK.et al.Reduction in regional cerebral metabolic rate of oxygen during human agingStroke.198617122012283492786
- TakadaH.NagataK.HirataY.et al.Age-related decline of cerebral oxygen metabolism in normal population detected with positron emission tomographyNeurol Res.199214(suppl)1281311355868
- KesslerRM.EllisJR.EdenM.Analysis of emission tomographic scan data: limitations imposed by resolution and backgroundJ Comput Assist Tomogr.198485145226609942
- MeltzerC.CantwellM.GreerP.et al.Does cerebral blood flow decline in healthy aging? A PET study with partial volume correctionJ Nucl Med.2000411842184811079492
- FazioF.PeraniD.Importance of partial-volume correction in brain PET studiesJ Nucl Med.2000411849185011079493
- ToyodaK.FujiiK.TakataY.IbayashiS.FujikawaM.FujishimaM.Effect of aging on regulation of brain stem circulation during hypotensionJ Cereb Blood Flow Metab.1997176806859236724
- KuhlD.MetterE.RiegeW.PhelpsM.Effects of human aging on patterns of local cerebral glucose utilization determined by the [18F] fluorodeoxyglucose methodJ Cereb Blood Flow Metab.198221631716978885
- LoessnerA.AlaviA.LewandrowskiKU.MozleyD.SouderE.GurR.Regional cerebral function determined by FDG-PET in healthy volunteers: normal patterns and changes with ageJ Nucl Med.199536114111497790936
- SalmonE.MaquetP.SadzotB.DegueldreC.ternaireC.FranckG.Decrease of frontal metabolism demonstrated by positron emission tomography in a population of healthy elderly volunteersActa Neurol Belg.1991912882951781265
- DuaraR.GradyC.HaxbyJ.et al.Human brain glucose utilization and cognitive function in relation to ageAnn Neurol.198416702713
- RappP.AmaralD.Individual differences in the cognitive and neurobiological consequences of normal agingTrends Neurosci.1992153403451382333
- DeKoskyS.PalmerA.Neurochemistry of aging. In: Albert M, Knoefel J, edsClinical Neurology of Aging. 2nded. New York, NY: Oxford Press;199479101
- WongWF.PearlsonGD.TuneLE.et al.Quantification of neuroreceptors in the living human brain: IV. Effect of aging and elevations of D2-like receptors in schizophrenia and bipolar illnessJ Cereb Blood Flow Metab.1997173313429119906
- WongD.WagnerHJ.DannalsR.et al.Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brainScience.1984226139313966334363
- MeltzerC.SmithG.ReynoldsC.et al.Reduced binding of [18F]altanserin to serotonin type 2A receptors in aging: persistence of effect after partial volume correctionBrain Res.19988131671719824691
- RosierA.DupontP.PeuskensJ.et al.Visualisation of loss of 5-HT2A receptors with age in healthy volunteers using [18F] and positron emission tomographic imagingPsychiatr Res.1996681122
- ZubietaJK.DannalsRF.FrostJJ.Gender and age influences on human brain mu-opioid receptor binding measured by PETAm J Psychiatry.199915684284810360121
- PalmerAM.DeKoskyST.Monoamine neurons in aging and Alzheimer's diseaseJ Neural Transm.199391135159
- AllenS.BentonJ.GoodhardtM.et al.Biochemical evidence of selective nerve cell changes in the normal ageing human and rat brainJ Neurochem.1983412562656134787
- NordbergA.AlafuzoffI.WinbladB.Nicotinic and muscarinic subtypes in the human brain: changes with aging and dementiaJ Neurosci Res.1992311031111613816
- CohenB.RenshawP.StollA.WurtmanR.Yurgelun-ToddD.BabbS.Decreased brain choline uptake in older adults. An in vivo proton magnetic spectroscopy studyJAMA.19952749029077674505
- DeweyS.VolkowN.LoganJ.et al.Age-related decreases in muscarinic cholinergic receptor binding in the human brain measured with positron emission tomography (PET)J Neurosci Res.1990275695752079718
- LeeKS.FreyKA.KoeppeRA.BuckA.MulhollandGK.KuhlDE.In vivo quantification of cerebral muscarinic receptors in normal human aging using positron emission tomography and [11C]tropanyl benzilateJ Cereb Blood Flow Metab.1996163033108594063
- SuharaT.InoueO.KobayashiK.SuzukiK.TatenoY.Age-related changes in human muscarinic acetylcholine receptors measured by positron emission tomographyNeurosci Lett.19931492252288474698
- KuhlDE.MinoshimaS.FessierJA.et al.In vivo mapping of cholinergic terminals in normal aging, Alzheimer's disease, and Parkinson's diseaseAnn Neurol.1996403994108797529
- WangY.ChanGL.HoldenJE.et al.Age-dependent decline of dopamine D1 receptors in human brain: a PET studySynapse.19983056619704881
- VolkowND.GurRC.WangGJ.et al.Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individualsAm J Psychiatry.19981553443499501743
- VolkowND.WangGJ.FowlerJS.et al.Parallel loss of presynaptic and postsynaptic dopamine markers in normal agingAnn Neurol.1998441431479667606
- VolkowND.DingYS.FowlerJS.et al.Dopamine transporters decrease with ageJ Nucl Med.1996375545598691238
- RinneJO.SahlbergN.RuottinenH.NagrenK.LehikoinenP.Striatal uptake of the dopamine reuptake ligand [11C]beta-CFT is reduced in Alzheimer's disease assessed by positron emission tomographyNeurology1998501521569443472
- KazumataK.DhawanV.ChalyT.et al.Dopamine transporter imaging with fluorine-18 FPCIT and PETJ Nucl Med.199839152115309744335
- LuckiI.The spectrum of behaviors influenced by serotoninBiol Psychiatry.1998441511629693387
- CrouzelC.GuillaumeM.BarreL.LemaireC.PikeVW.Ligands and tracers for PET studies of the 5-HT system - current statusInt J Rad Appl Instrum B.1992198578701428914
- BlinJ.BaronJC.DuboisB.et al.Loss of brain 5HT2 receptors in Alzheimer's diseaseBrain.19931164975108513389
- MeltzerCC.DrevetsWC.PriceJC.et al.Gender-specific aging effects on the serotonin 1A receptorBrain Res.200189591711259754
- PalmerA.DeKoskyS.Neurochemistry. In: Pathy M, edPrinciples and Practice of Geriatric Medicine. Chichester, UK: John Wiley & Sons;19986576
- FrancisP.SimsN.ProcterA.BowenD.Cortical pyramidal neurone loss may cause glutamatergic hypoactivity and cognitive impairment in Alzheimer's disease: investigative and therapeutic perspectivesJ Neurochem.199360158916048473885
- MouradianM.ContrerasP.MonahanJ.ChaseT.[3H]MK-801 binding in Alzheimer's diseaseNeurosci Lett.1988932252302853845
- ProcterA.StirlingJ.StratmannG.CrossA.BowenD.Loss of glycine-dependent radioligand binding to the N-methyl-D-aspartate-phencyclidine receptor complex in patients with Alzheimer's diseaseNeurosci Lett.198910162662570384
- PiggottM.PerryE.PerryR.CourtJ.[3H]MK-801 binding to the NMDA receptor complex, and its modulation in human frontal cortex during development and agingBrain Res.19925882772861393579
- MagnussonKR.Declines in mRNA expression of different subunits may account for differential effects of aging on agonist and antagonist binding to the NMDA receptorJ Neurosci.2000201666167410684868
- HutchisonJB.WozniakA.BeyerC.KarolczakM.HutchisonRE.Steroid metabolising enzymes in the determination of brain genderJ Steroid Biochem Mol Biol.199969859610418982
- SchmidtPJ.RubinowDR.Neuroregulatory role of gonadal steroids in humansPsychopharmacol Bull.1997332192209230633
- HoneycuttN.LiQ.YatesK.et al.Gender differences in hippocampal, amygdaloid and frontal volumes across the lifespan: a cross-sectional studyBiol Psychiatry.20004798S
- CathcartC.JonesSE.PumroyC.PetersG.KnoxSM.CheekJH.Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifenBreast Cancer Res Treat.1993272772818312586
- LebowitzBD.PearsonJL.SchneiderLS.et al.Diagnosis and treatment of depression in late life. Consensus statement updateJAMA.1997278118611909326481
- BebbingtonP.DunnG.JenkinsR.et al.The influence of age and sex on the prevalence of depressive conditions: report from the National Survey of Psychiatric MorbidityPsychol Med.1998289199483679
- EatonWW.AnthonyJC.GalloJ.et al.Natural history of Diagnostic Interview Schedule/DSM-IV major depression. The Baltimore Epidemiologic Catchment Area follow-upArch Gen Psychiatry.1997549939999366655
- LuineVN.RichardsST.WuVY.BeckKD.Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. WormBehav.199834149162
- O'NealMF.MeansLW.PooleMC.HammRJ.Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory taskPsychoneuroendocrinology19962151658778904
- HampsonE.KimuraD.Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skillsBehav Neurosci.19881024564593395456
- HampsonE.Variations in sex-related cognitive abilities across the menstrual cycleBrain Cogn.19901426432223043
- HampsonE.Estrogen-related variations in human spatial and articulatory-motor skillsPsychoneuroendocrinology199015971112359813
- PhillipsSM.SherwinBB.Variations in memory function and sex steroid hormones across the menstrual cyclePsychoneuroendocrinology1992174975061484916
- JacobsDM.TangMX.SternY.et al.Cognitive function in nondemented older women who took estrogen after menopauseNeurology.1998503683739484355
- Barrett-ConnorE.Kritz-SilversteinD.Estrogen replacement therapy and cognitive function in older womenJAMA.1993269263726418487446
- YaffeK.BlackwellT.GoreR.SandsL.ReusV.BrownerWS.Depressive symptoms and cognitive decline in nondemented elderly women: a prospective studyArch Gen Psychiatry.19995642543010232297
- Barrett-ConnorE.Goodman-GruenD.Cognitive function and endogenous sex hormones in older womenJ Am Geriatr Soc.1999471289129310573435
- YaffeK.GradyD.PressmanA.CummingsS.Serum estrogen levels, cognitive performance, and risk of cognitive decline in older community womenJ Am Geriatr Soc.199846816219670866
- Barrett-ConnorE.Kritz-SilversteinD.Gender differences in cognitive function with age: the Rancho Bernardo studyJ Am Geriatr Soc.1999471591649988286
- GoorenLI.The age-related decline of androgen levels in men: clinically significant?Br J Urol.1996787637688976775
- RubinowDR.SchmidtPJ.Androgens, brain, and behaviorAm J Psychiatry.19961539749848678193
- KerrJE.AlloreRJ.BeckSG.HandaRJ.Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampusEndocrinology1995136321332217628354
- PouliotWA.HandaRJ.BeckSG.Androgen modulates N -methyl-D-aspartate-mediated depolarization in CA1 hippocampal pyramidal cells.Synapse.19962310198723131
- ChristiansenK.KnussmannR.Sex hormones and cognitive functioning in menNeuropsychobiology19871827363444523
- MoffatS.HampsonE.A curvilinear relationship between testosterone and spatial cognition in humans: possible influence of hand preferencePsychoneuroendocrinology.1996213233378817730
- JanoskyJ.OviattS.OrwollE.Testosterone influences spatial cognition in older menBehav Neurosci.19941083253328037876
- MaasD.JochenA.LalandeB.Age-related changes in male gonadal function. Implications for therapyDrugs Aging.19971145609237040
- BaldwinRC.TomensonB.Depression in later life. A comparison of symptoms and risk factors in early and late onset casesBr J Psychiatry.19951676496528564322
- FigielG.KrishnanK.DoraiswamyP.RaoV.NemeroffC.BoykoO.Subcortical hyperintensities on brain magnetic resonance imaging: a comparison between late age onset and early onset elderly depressed subjectsNeurobiol Aging.1991122452471876230
- AlexopoulosG.YoungR.ShindledeckerR.Brain computed tomography findings in geriatric depression and primary degenerative dementiaBiol Psychiatry.1992315915991581438
- IidakaT.NakajimaT.KawamotoK.et al.Signal hyperintensities on brain magnetic resonance imaging in elderly depressed patientsEur Neurol.1996362932998864711
- KrishnanK.HaysJ.BlazerD.MRI-defined vascular depression. Am J Psychiatry.19971544975019090336
- LesserI.Hill-GutierrezE.MillerB.BooneK.Late-onset depression with white matter lesionsPsychosomatics.1993343643678351313
- AlexopoulosGS.MeyersBS.YoungRC.CampbellS.SilberswiegD.CharlsonM.“Vascular depression” hypothesisArch Gen Psychiatry.1997549159229337771
- McBeanDE.SharkeyJ.RitchieIM.KellyPA.Evidence for a possible role for serotonergic systems in the control of cerebral blood flowBrain Res.19905373073102128199
- McBeanDE.SharkeyJ.RitchieIM.KellyPA.Cerebrovascular and functional consequences of 5-HT1A receptor activationBrain Res.19915551591631834308
- HajduMA.McElmurryRT.HeistadDD.BaumbachGL.Effects of aging on cerebral vascular responses to serotonin in ratsAm J Physiol.1993264(6 Pt2)H2136H21408322944
- ArangoV.ErnsbergerP.MarzukP.et al.Autoradiographic demonstration of increased serotonin 5-HT2 and β-adrenergic receptor binding sites in the brain of suicide victimsArch Gen Psychiatry.199047103810472173513
- StanleyM.MannJ.Increased serotonin-2 binding sites in the frontal cortex of suicide victimsLancet.1983i2142166130248
- ArangoV.UnderwoodM.GubbiA.MannJ.Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victimsBrain Res.19956881211338542298
- StockmeierC.DilleyG.ShapiroL.OverholserJ.ThompsonP.MeltzerH.Serotonin receptors in suicide victims with major depressionNeuropsychopharmacology1996161621739015799
- StockmeierC.DilleyG.ShapiroL.KolliT.ThompsonP.RajkowskaG.Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression - postmortem evidence for decreased serotonin activity?Soc Neurosci Abstr.1997231676
- AustinM.WhiteheadR.EdgarC.LewisD.Decreased serotonin transporter-immunoreactive axons in the prefrontal cortex of suicide victims with major depressionSoc Neurosci Abstr.1999252098
- ArangoV.UnderwoodM.BakalianM.et al.Reduction in serotonin transporter sites in prefrontal cortex is localized in suicide and widespread in major depressionSoc Neurosci Abstr1999251798
- BowenDM.NajlerahimA.ProcterA.FrancisP.MurphyE.Circumscribed changes of the cerebral cortex in neuropsychiatrie disorders of late lifeProc Natl Acad Sci U S A.198986950495082574463
- MaybergHS.BrannanSK.MahurinRK.et al.Cingulate function in depression: a potential predictor of treatment responseNeuroreport.19978105710619141092
- DrevetsW.PriceJ.SimpsonJ.et al.Subgenual prefrontal cortex abnormalities in mood disordersNature.19973868247709126739
- BenchC.FristonK.BrownR.ScottL.FrackowiakR.DolanR.The anatomy of melancholia - focal abnormalities of cerebral blood flow in major depressionPsychol Med.1992226076151410086
- MannJ.McBrideP.MaloneK.DeMeoM.KeilpJ.Blunted serotonergic responsivity in depressed patientsNeuropsychopharmacology19951353648526971
- MannJ.MaloneK.DiehlD.PerelJ.CooperT.MintunM.Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patientsAm J Psychiatry.19961531741828561196
- BiverF.WiklerD.LotstraF.DamhautP.GoldmanS.MendlewiczJ.Serotonin 5-HT2 receptor imaging in major depression: focal changes in orbitoinsular cortexBr J Psychiatry.19971714444489463603
- MeltzerC.PriceJ.MathisC.et al.PET imaging of serotonin type 2A receptors in late-life neuropsychiatric disordersAm J Psychiatry.19991561871187810588399
- DrevetsW.FrankE.PriceJ.et al.PET imaging of serotonin 1A receptor binding in depressionBiol Psychiatry.1999461375138710578452
- ErnstRL.HayJW.The US economic and social costs of Alzheimer's disease revisitedAm J Publ Health.19948412611264
- BrookmeyerR.GrayS.KawasC.Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onsetAm J Publ Health.19988813371342
- BraakH.BraakE.Neuropathological staging of Alzheimer-related changesActa Neuropathol (Berl).1991822392591759558
- FrancisPT.PangalosMN.StephensPH.et al.Antemortem measurements of neurotransmission: possible implications for pharmacotherapy of Alzheimer's disease and depressionJ Neurol Neurosurg Psychiatry.19935680847679142
- Pericak-VanceMA.BeboutJL.GaskellPC Jr.et al.Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkageAm J Hum Genet.199148103410502035524
- CitronM.WestawayD.XiaW.et al.Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic miceNat Med.1997367728986743
- BowenD.SmithC.WhiteP.DavisonA.Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophiesBrain.19769945949611871
- DaviesP.MaloneyA.Selective loss of central cholinergic neurons in Alzheimer's diseaseLancet.19762140363862
- PerryE.GibsonP.BlessedG.PerryR.TomlinsonB.Neurotransmitter abnormalities in senile dementiaJ Neurol Sci.197734247265144789
- BartusR.DeanR.BeerB.LippaA.The cholinergic hypothesis of geriatric memory dysfunctionScience.19822174084177046051
- BeachT.KuoY.SpiegelK.et al.The cholinergic deficit coincides with Abeta deposition at the earliest histopathologic stages of Alzheimer diseaseJ Neuropathol Exp Neurol.20005930831310759186
- DavisP.MohsR.MarinD.et al.Cholinergic markers in elderly patients with early signs of Alzheimer diseaseJAMA.19992811401140610217056
- CummingsJ.KauferD.Neuropsychiatrie aspects of Alzheimer's disease: the cholinergic hypothesis revisitedNeurology1996478768838857712
- PerryE.MarshallE.ThompsonP.et al.Monoaminergic activities in Lewy-body-dementia - relation to hallucinosis and extrapyramidal featuresJ Neural Transm.19936167177
- PerryE.HaroutunianV.DavisK.et al.Neocortical cholinergic activities differentiate Lewy body dementia from classical Alzheimer's diseaseNeuroreport199457477498018843
- MingerS.EsiriM.McDonaldB.et al.Cholinergic deficits contribute to behavioural disturbance in patients with dementiaNeurology.2000551460146711094098
- HymanB.Van HoesenG.DamasioA.Alzheimer's disease: glutamate depletion in the hippocampal perforant pathway zoneAnn Neurol.19872237402443073
- ProcterA.PalmerA.FrancisP.et al.Evidence of glutamatergic denervation and possible abnormal metabolism in Alzheimer's diseaseJ Neurochem.1988507908023339353
- DijkS.FrancisP.StratmannG.BowenD.Cholinomimetics increase glutamate outflow by an action on the corticostriatal pathway: implications for Alzheimer's diseaseJ Neurochem.199565216521697595503
- ChessellI.HumphreyP.Nicotinic and muscarinic receptor-evoked depolarisations recorded from a novel cortical brain slice preparationNeuropharmacology.199534128912968570026
- MeltzerC.SmithG.DeKoskyS.et al.Serotonin in aging, late life depression, and Alzheimer's disease: the emerging role of functional imagingNeuropsychopharmacology1998184074309571651
- ChenCH.AlderJ.BowenD.et al.Presynaptic serotonergic markers in community-acquired cases of Alzheimer's disease: correlations with depression and neuroleptic medicationJ Neurochem.199666159215988627315
- CoccaroE.Central serotonin and impulsive aggressionBr J Psychiatry.1989155(suppl8)5262
- ProctorAW.FrancisPT.StratmannGC.BowenDM.Serotonergic pathology is not widespread in Alzheimer patients without prominent aggressive symptomsNeurochem Res.1992179179221357564
- PeskindE.WingersonD.MurrayS.et al.Effects of Alzheimer's disease and normal aging on cerebrospinal fluid norepinephrine responses to yohimbine and clonidineArch Gen Psychiatry.1995527747827654129
- RaskindM.Evaluation and management of aggressive behavior in the elderly demented patientJ Clin Psychiatry.199960454910418815
- FlynnD.WeinsteinD.MashD.Loss of high-affinity agonist binding to M1 muscarinic receptors in Alzheimer's disease: implications for the failure of cholinergic replacement therapiesAnn Neurol.1991292562622042942
- JopeR.SongL.PowersR.Agonist-induced, GTP-dependent phosphoinositide hydrolysis in postmortem human brain membranesJ Neurochem.199462178263508
- AubertI.AraujoD.CecyreD.RobitailleY.GauthierS.QuirionR.Comparative alterations of nicotinic and muscarinic binding sites in Alzheimer's and Parkinson's diseasesJ Neurochem.1992585295411729398
- CourtJ.PerryE.CNS nicotinic receptors - possible therapeutic targets in neurodegenerative disordersCNS Drugs.19942216233
- PerryE.MorrisC.CourtJ.et al.Alteration in nicotine binding sites in Parkinson's disease, Lewy body dementia and Alzheimer's: possible index of early neuropathologyNeuroscience.1995643853957700528
- MartinRuizC.CourtJ.MolnarE.et al.Alpha 4 but not alpha 3 and alpha 7 nicotinic acetylcholine receptor subunits are lost from the temporal cortex in Alzheimer“ s diseaseJ Neurochem.1999731635164010501210
- SparksD.BeachT.LukasR.Immunohistochemical localization of nicotinic-2 and -4 receptor subunits in normal human brain and individuals with Lewy body and Alzheimer's disease: preliminary observationsNeurosci Lett.19982561511549855362
- NitschR.From acetylcholine to amyloid: neurotransmitters and the pathology of Alzheimer's diseaseNeurodegeneration.199654774829117566
- NitschR.SlackB.WurtmanR.GrowdonJ.Release of Alzheimer amyloid precursor stimulated by activation of muscarinic acetylcholine receptorsScience.19922583043071411529
- AuldD.KarS.QuirionR.Beta-amyloid peptides as direct cholinergic neuromodulators: a missing link?Trends Neurosci.19982143499464686
- WangH.LeeD.DandreaM.PetersonP.ShankR.ReitzA.Beta-amyloid(1-42) binds to alpha 7 nicotinic acetylcholine receptor with high affinity - implications for Alzheimer's disease pathologyJ Biol Chem.20002755626563210681545
- FrancisP.PalmerA.SnapeM.WilcockG.The cholinergic hypothesis of Alzheimer's disease: a review of progressJ Neurol Neurosurg Psychiatry.19996613714710071091
- DavisK.ThalL.GamzuE.et al.A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer's diseaseN Engl J Med.1992327125312591406817
- PerryE.WalkerM.GraceJ.PerryR.Acetylcholine in mind: a neurotransmitter correlate of consciousness?Trends Neurosci.19992227328010354606