Abstract
In order to stress the importance of P300 responses in drug development, we describe the spatiotemporal characteristics of this objective, evoked event-related potential. These brain activations reflect mnemonic function, in which limbic structures play a role. It is demonstrated that a pharmacological challenge concerning, for example, the cholinergic system in young healthy volunteers induces modifications in P300 reminiscent of the aging brain. We use this type of observation to build a model in which it can be verified whether the deterioration can be counteracted by treatment with “cognition-enhancing” drugs. If we accept the extrapolation of the pharmacological effects to symptomatology, scalp potential analysis offers an appropriate tool for the study of drug interactions in early proof-of-concept models.
Para enfatizar la importancia de las respuestas de la onda P300 en el desarrollo de fármacos, nosotros describimos las características témporo-espaciales de este potencial evocado objetivo que describe una respuesta a un estimulo. Estas activaciones cerebrales reflejan la función mnémica en la cual juegan un papel las estructuras Iímbicas. Está demostrado que un desafío farmacológico, que se relacione por ejemplo con el sistema colinérgico en voluntarios sanos jóvenes, induce modificaciones en la onda P300 que hacen pensar en un cerebro envejecido. Nosotros utilizamos este tipo de observación para construir un modelo en el cual se pueda verificar si el deterioro se puede neutralizar mediante el tratamiento con fármacos “aumentadores de la cognición.” De aceptar la extrapolación de los efectos farmacológicos a la sintomatología, el análisis de la actividad eléctrica del cuero cabelludo ofrece una herramienta apropiada para el estudio de las interacciones de fármacos en los modelos iniciales de “proof of concept.”
Afin de souligner l'importance des réponses cognitives appelées P300 dans le développement des molécules, nous décrivons les caractéristiques tem porospatiales de ces potentiels évoqués objectifs en réponse à une stimulation cible. Ces activations cérébrales reflètent la fonction mnésique dans laquelle les structures limbiques jouent un rôle. Il a été démontré qu'un test de provocation pharmacologique utilisant, par exemple, le système cholinergique chez déjeunes volontaires sains, entraînait des modifications des réponses P300 rappelant celles retrouvées dans le cerveau vieillissant. Nous avons utilisé ce type d'observations pour construire un modèle qui permet de vérifier si cette détérioration peut être contrebalancée par des molécules « procognitives ». Si l'on accepte l'extrapolation des effets pharmacologiques à la sympiomaiologie, l'analyse des potentiels au niveau du cuir chevelu constitue un outil approprié pour l'étude des interactions médicamenteuses dans des modèles utilisés en phase précoce de démonstration de concept.
A model of cognitive enhancement would be of benefit, as a screening tool in the search for new therapies for cognitive disorders such as Alzheimer's disease. This article provides arguments in favor of neurophysi ological assessments during performance in psychometric tests to fulfil such aims. The first, part, concerns the basic characterization of event-related potentials (ERPs) and, in particular, the generators of the cognitive response called P300, in terms of temporal and spatial properties. Next, we investigate the effects of both noncholinergic and cholinergic drugs and their interaction in healthy young male and elderly subjects using the extracted ERP parameter as readout.
Temporal and spatial characterization of cognitive responses
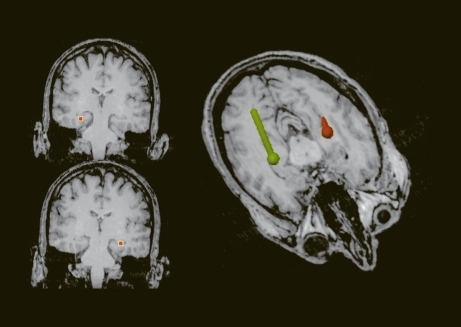
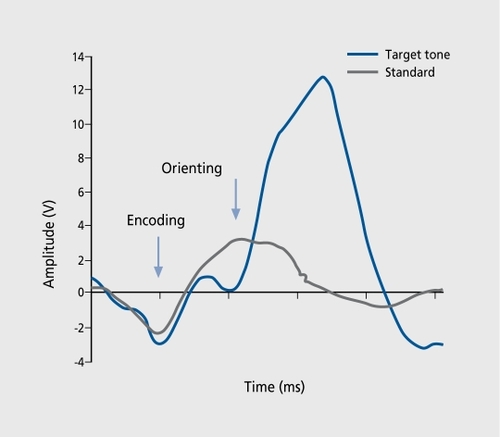
ERPs are transient, modifications in electromagnetic brain signals, which are time-locked to cognitive, motor, or sensory processing. They represent activity directly at the level of neuronal networks and hence form a good method for studying the working brain and obtaining neurophysiological indices of attentional mechanisms and cognitive function. In so-called “oddball” paradigms, in which a subject is instructed to count the number of target, stimuli, a positive scalp potential with a maximum amplitude of around 300 ms is recorded and is referred to as P300 (). Before the emergence of this type of activation, the brain signals display a sequence of components related to consecutive steps of information processing in the central nervous system (CNS), like encoding of stimulus, orienting reaction, etc. These occur in certain time-windows during normal functioning, and the term chronometry is often used. Hence, such electrical responses from the scalp are relevant because they are the result of a coordinated synchronization in distributed neuronal populations. Topographic analysis reveals patterns in agreement, with such a hypothesis (, top). The reversal in polarity in the posterior part (oriented toward the right) suggests the presence of parietotemporal sources. Several lines of evidence indicate that the sources must, be located in deep structures, such as the hippocampal formation. Multiple generators located in limbic frontal and inferotemporal brain regions are described as neuronal substratesCitation2,Citation3 as has been confirmed by positron emission tomography (PET) imagingCitation4 and, more recently, by hemodynamic responses in functional magnetic resonance imaging (fMRI).Citation5-Citation9 Speculations are made that the underlying generators are located in medial temporal lobe regions.
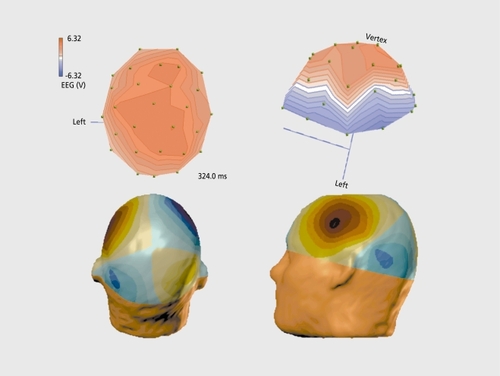
A method that is promising to better characterize these phenomena is the recording of the magnetic fields. Regarding auditor P300, contradictory results have been reported, ranging from no responseCitation10 to an effect in the left hemisphere,Citation11 or more global activations during a lexical testCitation12 or equivalent, oddball tasks in the visual modality.Citation13 These studies have been carried out. either with gradiomcters or with a limited number of sensors, covering only a small portion of the cortex. We used a more sensitive method, namely a whole-head magnetometer array (148 channels, Magnes 2500 WH, BTI, San Diego, Calif) to study the equivalent P300 generators. Figure 2 displays a top view (bottom left) and lateral view (bottom right.) of the average activation pattern obtained in the P300 paradigm for the same views as for the electrical responses (Figure 2, top). As can be seen, a highly structured pattern with a positive and a negative pole, called the magnetic dipole, is present. A similar but mirror-imaged pattern is present over the right hemisphere. The rotation by about 90° with respect to the orientation of the electrical pattern should be noted. Many researchers have developed three-dimensional reconstruction or source localization techniques. With my colleague L. Soufflet, we have also implemented such noninvasive imaging procedures. We recently demonstrated that analysis using magnetoencephalography (MEG) can model this type of cognitive response, yielding, as expected, a relatively simple solution of the complex neuronal interactions, by a preliminary process of localizing the sources using a spherical model of the head, topologically adapted for the anatomical substrate, as shown in . The locations of current vectors, which explain more than 90% of the observed brain signals, have their origin in the hippocampal formation. Hence, limbic structures contribute to information processing during cognitive discrimination in the auditory paradigm.
Functional aspects and neuropharmacology
P300 characteristics such as amplitude and latency are altered during aging.Citation14,Citation15 The pathophysiological state is reflected in the brain activity.Citation16-Citation18 Moreover, the deterioration effect has been shown to coincide with the clinical severity of mental illness in demented patients.Citation19-Citation21
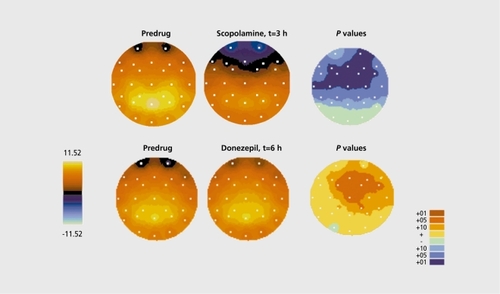
The activation pattern has been shown to be under cholinergic control: scopolamine is able to attenuate or even abolish the P300 response in young healthy volunteers.Citation22-Citation24 shows the postdosing evolution and statistical comparison of the effect, of the cholinergic antagonist scopolamine (0.5 mg, subcutaneous [SC], top). As can be seen, a significant frontocentral attenuation is present in healthy volunteers (upper right panel). Such effects parallel the deterioration in mnemonic capacities induced by the drug (see below). Acetylcholinesterase (AchE) inhibitors, on the contrary, induce increases in these topographic regions after oral administration. Indeed, oral administration of donepezil, a representative of the class of nootropic compounds, induces the opposite effects (Figure 4, bottom). This type of effect, is in full agreement with the existing literature.Citation26,Citation27
The message so far is that the relationship between neuropharmacology of the cholinergic system, which is fairly straightforward, on the one hand, and evoked (cognitive) responses reflecting conscious attention, which fits with the functional brain anatomy of limbic circuitry, on the other, form an ideal basis to study drug interaction in research in the field of psychiatric disorders.
Proof of concept
The “pseudo-state/trait marker” concept
As mentioned previously, the aging brain provides a natural decline in the properties of the cognitive response, such as the P300 waves (, top). Comparison of healthy male subjects aged 55 years and older with young subjects aged between 18 and 35 years yields significant attenuation in large parietal and temporal scalp regions. On basis of this change in CNS state, a proof of concept, can be proposed by administration of equivalent drugs presumed to be beneficial and by verifying the presence of improvement in healthy elderly subjects. The idea is that of a selective marker because in the elderly particular nootropic drugs are able to significantly restore P300.Citation28
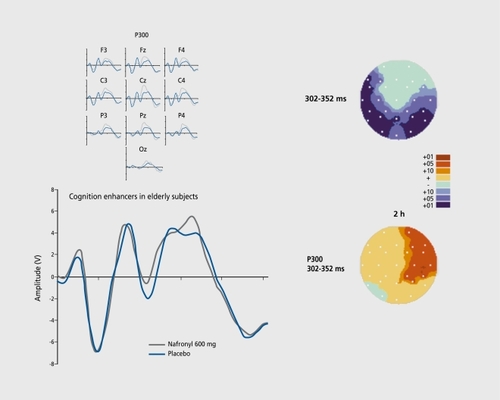
Due to the lack of clinical efficacy of AchE inhibitors,Citation29 more and more alternative mechanisms of action on central receptors or enzymes are being explored. An example of the effect of a noncholinergic drug is given in Figure 5 (bottom).Citation30 Indeed, a clear-cut indication of recovery can be observed, even though the increase in absolute terms is modest.
The concept of a pharmacological model in young volunteers
The established approach in behavioral neuropsychopharmacology is the use of a pharmacological challenge to reversibly provoke symptoms. As an example, we refer to the model that makes use of the comparison of performance in a battery of psychometric tests (eg, digit vigilance speed) and recording of continuous electrical cerebral activity.Citation31 Both types of examination undergo changes with scopolamine and some of these effects have been shown to be reversed by AchE inhibitors.
Hence P300 responses constitute a useful tool in neuropsychopharmacology in exactly the same way as continuous electrical cerebral activity, for the reasons shown in Figure 4. Interestingly, manipulation with benzodiazepines in order to provoke - like scopolamine - symptoms of cognitive impairment at the clinical level in, for example, free word recall,Citation32 induces similar collapses in P300 in auditoryCitation33-Citation36 (eg, lorazepam, ) and visuallyCitation37 evoked cognitive responses.
In our experience, the effects on neurophysiological parameters are often much more sensitive than the effects seen in performance changes. Schematically, the procedure can be summarized as follows:
Drug 1 induces a simulation of the acute state of “nontreated” patient (symptom provocation).
Drug 2 is used to verify its potency to (partially) reverse the deterioration (validation for pharmacotherapy).
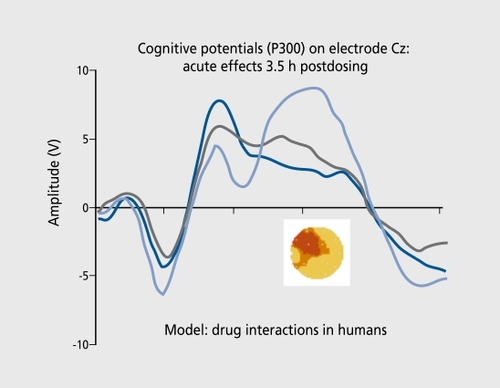
An example of the reversal of the challenge-induced deterioration (drug 1) with an AchE inhibitor (drug 2) is shown in Figure 6 (for study design see reference 38). The interesting aspect of such a model is the possibility of preventing the induction of symptoms by compounds with out, direct, cholinergic effectsCitation39 and using a neurophysiological readout, as surrogate marker at the same time.
Perspectives and conclusions
Using the P300 as a marker of relevant, brain function, a pharmacological challenge in young healthy volunteers to provoke symptoms like in aging can be counteracted by treatment with cognition-enhancing drugs. A longer pretreatment with nootropics (such as AchE inhibitors), which simulates more closely the clinical, setting, may have more persistent effects on challenge-induced deterioration in P300, but this hypothesis remains to be investigated. A lower dose of symptom-provoking agents associated with P300 changes may also increase the probability of detecting an antagonism in this model. The first steps toward a validation of a surrogate marker can now be considered as accomplished.
The author wishes to express his gratitude to Drs R. Luthringer and L-A. Granier for their support of this work, and A. Poignard and I. Jantzi for their help in the documentation and secretarial assistance.
REFERENCES
- Liogier d'ArdhuyX.BoeijingaPH.RenaultB.et al.Effects of serotoninselective and classical antidepressants on the auditory P300 cognitive potential.Neuropsychobiology.19994020721310559704
- HalgrenE.MarinkovicK.ChauvelP.Generators of the late potentials in auditory and visual oddball tasks.Electroencephalogr Clin Neurophysiol.19981061561649741777
- NishitaniN.NagamineT.FujiwaraN.YazawaS.ShibasakiH.Cortical-hippocampal auditory processing identified by magnetoencephalography.J Cogn Neurosci.1998102312479555109
- EbmeierKP.SteeleJD.MacKenzieDM.et al.Cognitive brain potentials and regional cerebral blood flow equivalents during two- and three-sound auditory “oddball tasks.”Electroencephalogr Clin Neurophysiol.1995954344438536572
- KirinoE.BelgerA.Goldman-RakicP.McCarthyG.Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: an event-related functional magnetic resonance imaging study.J Neurosci.2000206612661810964966
- LindenDEJ.PrlovicD.FormisanoE.et al.The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks.Cereb Cortex.1999981582310601000
- McCarthyG.LubyM.GoreJ.Goldman-RakicP.Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI.J Neurophysiol.199777163016349084626
- MenonV.FordJM.LimKO.GloverGH.PfefferbaumA.Combined eventrelated fMRI and EEG evidence for temporal-parietal cortex activation during target detection.Neuroreport.19978302930379331910
- OpitzB.MecklingerA.FriedericiAD.von CramonDY.The functional neuroanatomy of novelty processing: integrating ERP and fMRI results.Cereb Cortex.1999937939110426417
- SiedenbergR.GoodinDS.AminoffMJ.RowleyHA.RobertsTPL.Comparison of late components in simultaneously recorded event-related electrical potentials and event-related magnetic fields.Electroencephalogr Clin Neurophysiol.1996991911978761055
- YoshidaH.IwahashiM.YamaguchiM.TonoikeM.Neuromagnetic activity in the human left cerebral hemisphere concerning logical processing during auditory oddball stimulation.Neuroreport.19989126112659631409
- EulitzC.EulitzH.ElbertT.Differential outcome from magneto and electroencephalography for the analysis of human cognition.Neurosci Lett.19972271851889185681
- MecklingerA.MaessB.OpitzB.PfeiferE.CheyneD.WeinbergH.An MEG analysis of the P300 in visual discrimination tasks.Electroencephalogr Clin Neurophysiol.199810845569474061
- PolichJ.EEG and ERP assessment of normal aging.Electroencephalogr Clin Neurophysiol.19971042442569186239
- FriedmanD.KazmerskiV.FabianiM.An overview of age-related changes in the scalp distribution of P3b.Electroencephalogr Clin Neurophysiol.19971044985139402892
- HoltLE.RaineA.PaG.SchneiderLS.HendersonVW.PollockVE.P300 topography in Alzheimer's disease.Psychophysiology.199532(suppl 3)2572657784534
- KraiuhinC.GordonE.MearesR.Psychometrics and event-related potentials in the diagnosis of dementia.J Gerontol.1986411541623950340
- PolichJ.LadishC.BloomFE.P300 assessment of early Alzheimer's disease.Electroencephalogr Clin Neurophysiol.1990771791891691970
- BallSS.MarshJT.SchubarthG.BrownWS.StrandburgR.Longitudinal P300 latency changes in Alzheimer's disease.J Gerontol.198944(suppl M)M195M2002809106
- GoodinDS.AminoffMJ.Electrophysiological differences between subtypes of dementia.Brain.1986109110311132947660
- GoodinDS.AminoffMJ.The distinction between different types of dementia using evoked potentials.Electroencephalogr Clin Neurophysiol Suppl.1987836956982961553
- Frodl-BauchT.BottlenderR.HegerlU.Neurochemical substrates and neuroanatomical generators of the event-related P300.Neuropsychobiology.199940869410474063
- MeadorKJ.LoringDW.AdamsRJ.PatelBR.DavisHC.HammondEJ.Central cholinergic systems and the P3 evoked potential.Int J Neurosci.198733(suppl3-4)1992053596949
- MeadorKJ.LoringDW.HendrixN.NicholsME.OberzanR.MooreEE.Synergistic anticholinergic and antiserotoninergic effects in humans.J Clin Exp Neuropsychol.1995176116217593479
- DagoKT.LuthringerR.LengelléR.RinaudoG.MacherJP.Statistical decision tree: a tool for studying pharmaco-EEG effects of CNS-active drugs.Neuropsychobiology.19942991968170530
- DierksT.FrölichL.IhlR.MaurerK.Event-related potentials and psychopharmacology. Cholinergic modulation of P300.Pharmacopsychiatry.19942772747913237
- MünteTF.HeinzeHJ.ScholzMB.BartuschSM.DietrichDE.Eventrelated potentials and visual spatial attention: influence of a cholinergic drug.Neuropsychobiology.19892194992615925
- SemlitschHV.AndererP.SaletuB.BinderGA.DeckerKA.Cognitive psychophysiology in nootropic drug research: effects of Ginkgo biloba on event-related potentials (P300) in age-associated memory impairment.Pharmacopsychiatry.1995281341427491367
- Van GoolWA.WaardenburgJ.PosthumusFE.WiensteinHC.De WildeA.The effect of tetrahydroaminoacride (THA) on P300 in Alzheimer's disease.Biol Psychiatry.1991309539571747439
- BoeijingaPH.MuzetM.GamandS.d'AnielloF.LuthringerR.MacherJP.Double-blind, randomized, placebo-controlled study of the effects on wake-EEG of 2 doses of Praxilene® using a cross-over design in elderly healthy male volunteers.Eur Neuropsychopharmacology.200010(suppl 3)S363
- EbertU.OertelR.WesnesKA.KirchW.Effects of physostigmine in quantitative electroencephalogram and cognitive performance.Hum Psychopharmacol.199813199210
- CurranHV.PooviboonsukP.DaltonJA.LaderMH.Differentiating the effects of centrally acting drugs on arousal and memory: an event-related potential study of scopolamine, lorazepam and diphenhydramine.Psychopharmacology.199813527369489931
- DominoEF.FrenchJ.PohoreckiR.GalusCF.PanditSK.Further observations on the effects of subhypnotic doses of midazolam in normal volunteers.Psychopharmacol Bull.198925(suppl 3)4604652626519
- RayPG.MeadorKJ.LoringDW.Diazepam effects on the P3 eventrelated potential.J Clin Psychopharmacol.199212(suppl 6)4154191335460
- SamraSK.BradshawEG.PanditSK.PapanicolaouAC.MooreBD.The relation between lorazepam-induced auditory amnesia and auditory evoked potentials.Anesth Analg.1988675265333377206
- SemlitschHV.AndererP.SaletuB.Acute effects of the anxiolytics suriclone and alprazolam on cognitive information processing utilizing topographic mapping of event-related brain potentials (P300) in healthy subjects.Eur J Clin Pharmacol.199549(suppl 3)1831918665994
- EngelhardtW.FriessK.HartungE.SoldM.DierksT.EEG and auditory evoked potential P300 compared with psychometric tests in assessing vigilance after benzodiazepine sedation and antagonism.Br J Anaesth.19926975801637608
- BoeijingaPH.ChalonS.GranierLA.et al.Effects of rivastigmine on lorazepam-induced changes in EEG: a double-blind placebo-controlled study. Methods and findings.Exp Clin Pharmacol.200224(suppl D)155. O-12
- DukaT.OttH.RohloffA.VoetB.The effects of a benzodiazepine receptor antagonist beta-carboline ZK-93426 on scopolamine-induced impairment on attention, memory and psychomotor skills.Psychopharmacology.19961233613738867876