Abstract
For better management of mild cognitive impairment in elderly patients, clinicians should be provided with instruments to detect early changes and predict their progression. To define this cognitive status between optimal and pathological aging, many concepts have been proposed, which actually describe various conditions and provide more or less precise criteria, leaving room for variable implementation. As a consequence, application of these criteria gave highly variable prevalence rates, Neuropathological studies indicate that the different criteria have variable power in detecting incipient Alzheimer's disease (AD) and suggest that the transition between mild cognitive impairment and ÀD is not merely quantitative. Follow-up studies have produced, according to the criteria used, a 2.5% to 16,6% annual rate for progression toward dementia, and have also shown that the criteria differ in their stability and predictive power. Baseline cognitive performances have some predictive value, but are difficult to apply in first-line medicine. Investigational techniques (structural and functional imaging, magnetic resonance spectroscopy, magnetization transfer imaging, cerebrospinal fluid neuro-chemistry, and apolipoprotein E genotype) are promising tools in the early diagnosis of AD, which remains the most frequent type of dementia in elderly people and probably the most frequent type developed by patients with mild cognitive deficit. The final goal is to offer early treatment to those patients who will evolve towards dementia, once they can be identified, in the case of AD, recent findings question the adequacy of cholinergic replacement therapies. In its current state, the criteria for mild cognitive deficit are hardly transferable to first-line medicine. However, disseminating the concept could help increase the sensitivity of general practitioners to the importance of cognitive complaints and signs in their elderly patients.
Para un mejor manejo del deterioro cognitivo leve en los pacientes ancianos, los clínicos deben contar con instrumentas para detectar cambios precoces y predecir su progresión. Para definir este estado cognitivo entre el envejecimiento óptimo y el patológico se han propuesto muchos conceptos, los cuales incluyen diversas condiciones y proporcionan criterios más o menos precisos, dejando libertad para una implementación variable. Como consecuencia de esto, la aplicación de estos criterios determinó frecuencias de prevalencia altamente variables. Los estudios neuropatológicos indican que los diferentes criterios tienen un poder variable para detectar la Enfermedad de Alzheimer (EA) incipiente y sugieren que la transición entre el deterioro cognitivo leve y la EA no es meramente cuantitativa. Los estudios de seguimienio han determinado - de acuerdo con los criterios utilizados - una frecuencia anual de progresión hacia la demencia entre un 2,5% y un 16,6%, y también han mostrado que los criterios difieren en su estabilidad y poder predictor. Los resultados cognitivos basales tienen algún valor predictor, pero son difíciles de aplicar en la atenciôn primaria. Las têcnicas paradinicas (las imágenes estructurales y funcionales, la resonancia magnética por espectroscopía, las imágenes por transferencia de magnetización, la neuroquímica del líquido céfalo-raquídeo y el genotipo de la apolipoproteína E) constituyen herramientas promisorias en el diagnóstico precoz de la EA, la cual se mantiene como el tipo más frecuente de demencia en la población anciana y probablemente también el más frecuente que se desarrolla en pacientes con déficit cognitivo leve. El objetivo final es ofrecer un tratamiento precoz a aquellos pacientes que evolucionarán hacia la demencia, una vez que ellos se hayan podido identificar. En el caso de la EA los hallazgos recientes cuestionan la conveniencia de terapias colinérgicas de reemplazo. Actualmente los criterios para el déficit cognitivo leve son difíciles de aplicar a la atención primaria. Sin embargo, la divulgación del concepto podría ayudar a aumentar la sensibilización de los medicos generales respecto a la imporiancia de signos y quejas cognitivas en sus pacientes ancianos.
La prise en charge efficace des troubles cognitifs légers chez les patients âgés implique que les médecins puissent disposer d'instruments capables de détecter des modifications précoces et de prévoir leur évolution. Divers concepts, décrivant en fait des situations variées et utilisant des critères plus ou moins précis, ont été proposés pour définir cet état cognitif intermédiaire entre vieillissement optimal et pathologique. Par conséquent, l'application de ces critères a fourni des taux de prévalence eux-mêmes très variables. Les études neuropathologiques montrent que les différents critères ont un pouvoir variable pour détecter une maladie d'Alzheimer débutante (MA) et suggèrent que la transition entre trouble cognitif léger et MA avérée n'est pas seulement d'ordre quantitatif. Les études de suivi ont montré que les critères utilisés diffèrent quant à leur pouvoir prédictif et leur stabilité, aboutissant à des chiffres annuels de progression vers la démence variant entre 2,5 % et 16,6 %. Les performances cognitives basales ont une certaine valeur prédictive mais sont difficiles à appliquer en médecine de premier recours. Les examens paradiniques (imagerie fonctionnelle et structurelle, spectroscopie par résonance magnétique, imagerie par transfert de magnétisation, neurochimie du liquide céphalorachidien et génotype des apolipoprotéines E) sont des outils prometteurs dans le diagnostic précoce de la MA qui reste le type de démence le plus fréquent chez les sujets âgés et probablement la pathologie qui se développe le plus souvent chez les patients atteints de troubles cognitifs légers. Le but final est d'offrir un traitement précoce à ceux qui évolueront vers la démence dès qu'ils auront pu être identifiés. Pour ce qui est de la MA, des observations récentes remettent en cause les traitements substitutifs cholinergiques. Dans leur état actuel, les critères du déficit cognitif léger sont difficilement applicables en médecine de premier recours. Néanmoins, la diffusion de ce concept pourrait aider à sensibiliser les médecins généralistes à l'importance des manifestations tant subjectives qu'objectives indiquant une atteinte cognitive chez leurs patients âgés.
While promising therapeutic strategics are being explored, our capacity to diagnose dementias early in their evolution remains poor. Degenerative dementias are insidious and progressive in nature. It is therefore conceivable that a dementia picture is preceded by a “preclinical state” (ie, pathognomonic cerebral lesions coexisting with normal cognition) as described in Alzheimer's disease (AD),Citation1, Citation2 followed by mild deficits first experienced by patients themselves, then suspected by their family members, and eventually demonstrated through neuropsychological examination.
It is generally assumed that, normal aging involves cognitive changes, displaying large inter- and intraindividual variability.Citation3 Some studies challenged this common view, showing that the use of strict, criteria for the inclusion of cognitively normal subjects in longitudinal studies demonstrated long-term stability.Citation4-Citation7 It. was therefore argued that the elderly populations who were the basis of the “normal cognitive aging” concept were contaminated by individuals with very mild dementia.Citation1, Citation8, Citation9
As a result, there is currently no consensus on the definition or on the meaning of mild cognitive deficit, in an older individual, or on the attitude it should trigger in physicians. Periodic reassessment until the criteria for a dementia syndrome are fulfilled, as is currently practiced, avoids the risks of overdiagnosis, but conveys those of delaying the initiation of an effective treatment. This daily clinical dilemma would be resolved if physicians were provided with simple instruments allowing a clear differentiation between normal and prodromal cognitive status in a given elderly patient. The goal of this review is to assess to what, extent this need is currently met.
Main concepts and criteria
Since Kral's benign senescent forgetfulness,Citation10 several concepts have been proposed to understand this shadowy zone between optimal and pathological cognitive aging (Table I).Citation10-Citation23
Table I Age-related mild cognitive deficit: definitions and criteria.Citation10-Citation23 AACD, aging-associated cognitive decline; AAMI, age-associated memory impairment; ACMI, age-consistent memory impairment; BVRT, Benton Visual Retention Test; CAMDEX, Cambridge Examination for Mental Disorders in the Elderly; CDR, Clinical Dementia Rating; DSM-III-R, Diagnostic and Statistical Manual of Mental Health Disorders. 3rd ed, revised; GDS, Global Deterioration Scale; HIS, Hachinski Ischemia score; HRSD, Hamilton Rating Scale for Depression; ICD-10, International Statistical Classification of Diseases and Health-related Problems. 10th revision; LLF, Late-life forgetfulness; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; WAIS, Wechsler Adult Intelligence Scale; WMS, Wechsler Memory Scale.
Cognitive impairment-no dementia (CIND) identifies cognitive impairment associated with various conditions, ranging from age-associated memory impairment (AAMI)Citation15 to cerebrovascular or general vascular diseases, to depression.Citation22, Citation24 Mild cognitive disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Citation20 and mild cognitive disorder in the International Statistical Classification of Diseases, 10th Revision (ICD-10) Citation18 refer to the cognitive consequences of somatic disorders. Limited dementiaCitation12 and minimal dementiaCitation14 clearly refer to a dementia state. AAMICitation15 and age-consistent memory impairment. (ACMI)Citation16 address normal cognitive aging. Zaudig's mild cognitive impairment,Citation17 mild cognitive decline,Citation13 and questionable dementiaCitation11 rely on global scores on cognitive -behavioral rating scales. The major drawback of this approach is that the same score can reflect different clinical profiles, making clinicopatho logical correlation and betwecn-study comparison difficult. Late life forgetfulness (LLF)Citation16 assesses cognitive deficits relative to what, is considered as normal for age, and aging-associated cognitive decline ( AACD)Citation19 compares in addition with an education- and gender-matched relatively healthy sample. Both provide explicit, inclusion and exclusion criteria and - in the case of LLF - examples of tests. LLF focuses on memory impairment, whereas AACD considers additional cognitive domains (attention and concentration, problem-solving and abstraction, language, and visuospatial function) and enforces a 6-month duration of decline. The Mayo Clinic criteria for mild cognitive impairment (MCI)Citation21 are less precise and their formulation has changed with time (Table II, page 66) .Citation21, Citation25-Citation33 As a consequence, the heading ”MCI“ covers highly variable diagnostic methodologies, hampering comparisons of studies from different research teams.
Table II. Definition and criteria for mild cognitive impairment (MCI).Citation21, Citation25-Citation26 ADL, activities of daily living; CDR, Clinical Dementia Rating; DSM-III-R, Diagnostic and Statistical Manual of Mental Health Disorders. 3rd ed, revised; MMSE, Mini-Mental State Examination. *Although CDR scoreCitation32, Citation33 was not a criterion, MCI subjects had CDR=0.5.
These different concepts and criteria have seldom been compared in the same population. In a recent, study,Citation34 111 subjects with informant, evidence of cognitive decline were classified as AAMI (n=37, 33.3%) after clinical assessment. When AACD criteria were also applied, they were fulfilled by 39 subjects (35.1 %), including 20 (54%)” of the AAMIs. Moreover, as illustrated in (seepage 66), the cognitive profiles of subjects with AACD or AAMI were different, with 35.9% of AACDs vs 27% of AAMIs impaired in the memory and learning domain according to AACD criteria (ie, at least 1 SD below age-appropriate norms), and 35.9% AACDs vs 18.9% AAMls impaired in more than one cognitive domain.Citation34
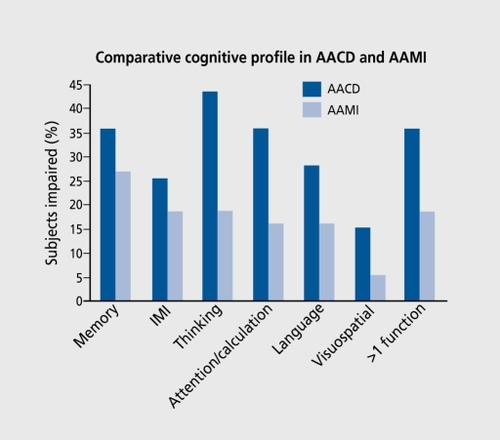
As expected according to their individual definitions and goals, the AAMI and AACD concepts only modestly overlap one another; the latter captures a more severe impairment.
In the Canadian Study of Health and Aging, specific criteria were applied in subjects classified as CIND.Citation22 Sixty-five percent did not meet any of them; none met the AAMI criteria of Bradford and LaRue.Citation16 When inclusion criteria were applied alone, 8.1% fitted the criteria for AAMI. 5.9% for ACMI, 7.4 % for LLF, and 34% AACD; after applying exclusion criteria, these figures dropped to 1.2 % (AAMI), 0.9 % (ACMI), 0 % (LLF), and 13 % (AACD). These data highlight the importance of exclusion criteria resulting from comprehensive clinical evaluation. Only 24% of those meeting one set of criteria also met one other or more (19.2 % met two, 3.8 % three, and 0.8 % four), suggesting that the different sets of criteria are mutually exclusive. In a sample of 60 - to 64 - year-old healthy people, Citation35 13.5% met criteria for AAMI, 6.5 % for ACMI, 1.5 % for LLF, and 23.5 % for AACD. Among subjects with AAMI, 22 % met the criteria for ACMI, 11 % for LLF, and 63 % for AACD. All the LLF subjects also fulfilled criteria for both AAMI and AACD.
Together these results are not very surprising. First, the different, sets of criteria refer to different concepts (AAMI and ACMI versus LLF and AACD, sec above). Second, they basically consist, of a priori constructs, differing in of clinical populations. Third, because of criteria's methodological vagueness (eg, no firm reference tests; no indication on whether one function should be assessed using one or several tests), they offer room for different, implementation across teams. The impact, of introducing changes in criteria is illustrated by the Eugeria Project.Citation36 Of 833 subjects recruited, 308 fulfilled the first two criteria for MCI (subjective memory complaint and normal general intellectual functioning, as assessed by performance on a vocabulary test); of these, 103 had a decrement of more than 1 SD on a memory task, relative to normal values for age and educational level (criterion 3); exclusion of subjects with difficulties in any other cognitive domain left only 27 subjects fulfilling the criteria; application of criterion 4 (normal activities of daily living) had no influence. Thus, modification of the third criterion reduced the prevalence of MCI from 12.4% to 3.2%. The AACD criteria applied to the same population identified 174 participants (20.8%), which included all the M.C1 subjects.
Neuropathological correlates
To the best of our knowledge, the only concept that has been compared with neuropathological examination is MCI as defined by the Mayo Clinic team.Citation21 In a followup study,Citation37 6 out. of 6 subjects with a Clinical Dementia Rating (CDR)Citation32 score 0.5 resulting from memory impairment alone were found to meet modifiedCitation38 KachaturianCitation39 neuropathological criteria for AD. This confirmed previous dataCitation40 showing that 10 out of 10 subjects with CDR=0.5 had histopathological AD, versus none of 4 with a score of 0. In another study,Citation41 subjects with a CDR>0.5 had large senile plaque densities in the neocortex and the degree of dementia seemed related to an increase in the ratio of neuritic to diffuse plaques. While cognitively healthy controls - and even individuals with preclinical AD - had no significant, decrease in neuronal count in the entorhinal cortex (ERC) as a whole, in ERC layer II or in the CA1 hippocampal field, the brains of subjects with CDR=0.5 were characterized by a significant neuronal loss in these areas.Citation42 These studies suggest. that “questionable dementia” or isolated memory impairment sufficient to yield CDR=0.5 actually represent very mild AD. It can be questioned whether CDR=0.5 equates to MCI. A series of studiesCitation43-Citation45 compared MCI subjects (defined as being impaired in one domain on neuropsychological testing, but. not being found to have dementia by the examining neurologist according to NINCDS/ ADRDA [National Institute of Neurological and Communicative Diseases and Stroke/ Alzheimer's Disease and Related Disorders Association] criteriaCitation46) with normal controls (NCs) and AD patients, all from a group of catholic clergy participating in the Religious Order Study (Table III). Both AD patients and MCI subjects had a lower ERC layer II neuronal count, remaining neuronal volume, and global volume. However, MCIs and AD patients significantly differed in layer II global volume only, despite a group effect in analysis of variance, while all the AD and NC values were significantly different. Global and neuronal atrophy were correlated with impairment of delayed and immediate recall.Citation44 Quantified ERC β-amyloid (βA) loadCitation43 in MCIs was intermediate, but not significantly different from that found in NCs and AD patients respectively (again, NC vs AD values were significantly different), although analysis of variance revealed a significant group effect with a trend to linear increase from NCs to MCIs to AD patients. It is noteworthy that, some NC or MCI subjects had PA load equal to or higher than that seen in many AD patients, and two MCIs had no detectable βA. The inverse correlation between Mini-Mental State Examination (MMSFs)Citation27 scores (mean scores: 27.3 in NCs and MCI patients, and 24 in AD patients) and βA load was not significant. In this study, 6 of 12 MCI subjects had a neuropathological diagnosis of possible AD according to the criteria of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD).Citation47 In a third study,Citation45 neurofibrillary tangles (NFTs) and neuropil threads (NTs) were present in perirhinal cortex and ERC in NCs, MCI subjects, and AD patients; the average number of NFTs increased with the diagnosis from NC to M'CI to AD. Retween-group differences analysis again found that MCI subjects were intermediate, but not significantly different from either NCs or AD patients. NFT density was inversely correlated with episodic memory score, but not with other, nonmemory, cognitive abilities across the three groups.
Table III. Neuropathological characteristics of mild cognitive impairment in the Religious Order Study.Citation43-Citation45 CERAD, Consortium to Establish a Registry for Alzheimer's disease; MCI, mild cognitive impairment; NS, not significant; S, significant. Changes in MCI and AD group are given relative to normal controls.
These studies first, show that no more than 50% of MCIs were incipient. AD. This is less than in the studies by MorrisCitation37, Citation40 and Price,Citation41 and suggests that the populations described were not equivalent, although the use of different neuropathological diagnostic criteria makes the comparison difficult. Approximately the same proportion of NCs (45%) were also diagnosed as possible AD; this finding suggests that the clinical diagnostic tools were neither sensitive nor specific in the detection of incipient AD. This can be explained by the fact, that both the NC and MCI groups had high and similar MMSE scores, but the concept of MCI precisely intends to detect cases missed by more global testing. Nevertheless, MCI subjects globally were, for most. F,RC lesions, intermediate between NC and AD cases. This suggests that ERC lesions could be a better neuropathological marker of MCI than the presence of those required for a diagnosis of AD. However, the fact, that group-to-group comparisons failed to distinguish MCIs from ADs makes currently impossible to determine practically useful cutoff values. If this failure is due to sample size, larger studies should solve it. However, it. could also be due to heterogeneity; clinicopathological studies seeking pathological markers of both non- AD dementias and AD should confirm or rule out this possibility Awaiting further studies, the lack of significant difference between MCI and AD, which was also found for high (trkA)Citation48 and low (p75NTR) Citation49 expression of nerve growth factor receptors, suggests that the transition from MCI to AD is not merely quantitative.
Predictive value
Another way of understanding these concepts and criteria is through their ability to predict, the progression of patients. Follow-up studiesCitation21, Citation25, Citation36, Citation37, Citation50-Citation59 differ in their durations, making comparisons difficult; dividing the frequency of progression toward dementia by duration of follow-up gives an estimate of the annual rates of “conversion” (Table IV).
Thus, a significant proportion of subjects did not become demented. It could be argued that a longer follow-up would increase the “conversion” rate. However, data from some studies reporting multiple evaluationsCitation21, Citation58, Citation60-Citation62 suggest that the incidence of dementia could decrease over time. In a recent study with assessments at 3 and 6 years in subjects with CIND, aged 80 years or older,Citation61 it was found that, according to the severity of impairment at baseline, 84% to 89% of those who were demented at 6 years had already received the diagnosis at 3 years. In another study in oldest old (84 to 90 years old at baseline) over 6 years,Citation60 a decrease in the progression from MCI to dementia with time was also reported. 'Ms attenuation of the rate of progression with time could be an artifact, since in these two studies - and also in one in slightly younger subjectsCitation57 - MCI increased the risk of death by 1.7Citation58 to 7Citation60 during a 4-year period. In this case, there should be a correlation between the severity of cognitive impairment at baseline and the risk of death. Such a trend was found in one study,Citation58 but. not. in another,Citation61 and in a thirdCitation60 baseline performances in the deceased group were lower than those of survivors, but. higher than for those who progressed to dementia. Thus, the issue of the slope of the rate of progression deserves further attention, particularly in relation to age at. onset, of cognitive impairment.
Because the main criteria were set. to capture degenerative cognitive impairment (ie, without identifiable medical cause), an intriguing finding is that a substantial proportion of subjects were found to improve over time (4.8% after 3 years in subjects with CDR=0.5Citation63; 19.5% after 2.7 years in MCI as defined by ZaudigCitation54; 25% after 3 years and 12% to 17% after 6 years in CINDCitation61). In clinical practice, such an outcome would be ascribed to a diagnostic error (ie, impairment, was due to a unidentified medical condition). An alternative explanation is that the underlying process is different from AD. Indeed, a fluctuating course is classically described in vascular dementia (VaD)Citation61 and dementia with Lewy bodies (DLB).Citation65 In line with this hypothesis is the finding that, in a sample of MCI subjects, 20.5% developed VaD within 3.9 years; nothing in their baseline cognitive profile or their progression (based on MMSE) differentiated them from those who progressed to AD (47.9%).Citation62-Citation66 A third explanation is that the criteria do not. describe a stable state. The Eugeria Project compared MCI (with impairment, in memory, but not in any other domain) and AACD over 3 years,Citation36 and showed that. 7.5 % of MCI subjects retained the diagnosis from the first, to second assessment and 17.4 % from the second to third; the corresponding figures for AACD subjects were 56.3% and 59.4 %. Apart from those who became demented, subjects met criteria for the alternative diagnosis (from MCI to AACD and vice versa) or were found to be normal. In this study, the AACD diagnosis had a sensitivity of 94.7 % and a specificity of 54.1 %, whereas the MCI diagnosis had a sensitivity of 5.3 % and specificity of 91.3 % in the prediction of progression toward dementia after 2 years. In another community-based French study,Citation59 the MCT diagnosis was also found to be unstable.
According to the cited studies, there is no doubt that mild cognitive deficit in elderly subjects, whatever its definition and criteria, increases the risk of developing dementia. The available data provide a rather broad range of annual incidence of dementia and are not all in favor of a linear prevalence-time relationship in mildly impaired patients. The proposed sets of criteria have different, stability and predictive values. Also, they do not allow identification of individuals who will develop dementia or - more importantly - the type of dementia toward which they could evolve.
Beyond the criteria themselves, several studies found predictors of progression to dementia or even to AD in measures derived from the MMSE,Citation62 the CDR,Citation63 or impairment in memory, verbal fluency, and attention on more conventional neuropsychological tests.Citation52, Citation67-Citation69 As pointed out by Tuokko and Frcrichs,Citation70 a major shortcoming of these data is that, they are retrospective. No combination of cognitive tests has yet been assessed prospectively for its ability to predict, outcome in mildly impaired patients. If it were done using neuropsychological batteries that, were sufficiently refined for early identification of the characteristic signs of the major dementing diseases and determination of reliable cutoff scores, then this type of investigation would be reserved for specialized teams; however, the first person who people with cognitive complaints see is their general practitioner. It is expected that this dilemma will be partly solved in the near future by recourse to investigational techniques.
Contribution of investigational techniques
For decades, the scientific community has been seeking biomarkers of AD, using genetics, neurochemistry, and imaging techniques. It was rational to apply these techniques to mild cognitive deficit, in order to characterize these states and identify predictors of progression to AD. Neuropathological studies have shown the hippocampus to be one of the earliest, affected structures in AD,Citation71 and so it is a region of choice for neuroimaging studies. Although hippocampal atrophy, as measured by volumetric techniques is not entirely specific, it is now considered to be a hallmark of AD,Citation72 and its absence in addition to minor or unilateral atrophy is believed to be strong evidence against the diagnosis. In mild cognitive deficit, several studies have shown lesserCitation73-Citation76 or similarCitation77 hippocampal atrophy to that found in AD. Age transformation of combined hippocampal and amygdala volume increases the accuracy of classifying AD, MCI, and normal elderly subjects.Citation78 MCI subjects had hippocampal volume correlated with cognitive and performance measuresCitation79 and those who declined over time had also a greater annualized rate of hippocampal atrophy than nondeclincrs, close to that of AD patients.Citation80 Atrophy of various regions at baseline, including hippocampusCitation79, Citation81, Citation82 ERC,Citation83-Citation84 fusiform gyrus,Citation85 caudal cingulate cortex,Citation83 and medial temporal lobe,Citation76 was found predictive of progression to AD. White matter lesions have been found to be associated with subjective cognitive decline,Citation86 lowered attention and speed of mental processing,Citation87 and progression to dementia.Citation88
There is an agreement on the fact that established AD is characterized by altered cerebral blood flow (CBF) and metabolism in posterior parietal and temporal lobes as well as by, according to stage and neuropsychological profile, frontal cortex deficits, and hemispheric asymmetry.Citation89 That functional imaging is able to detect preclinical AD is suggested by positron emission tomography (PET) studies, which found regional cerebral glucose metabolism (rCMRGlu) alterations in nondemented subjects at risk of AD (ie, those carrying the apolipoprotein E type 4 allele [ ApoE ε4] and with familial history of AD)Citation90, Citation9190-91; those in the inferior parietal and posterior cingulate cortices correlated with later memory decline.Citation92
Studies comparing CBF and rCMRGlu in normal and mildly impaired subjects found deficits in the latter, in various regions including bilateral parietal cortex,Citation56 hippocampus,Citation77 and posterior cingulate gyrus.Citation93-Citation95 Prediction of outcome was found for defects in parietal or temporoparietal cortex,Citation56, Citation96 posterior cingulate gyrus,Citation94, Citation95 and for temporoparietal asymmetryCitation97 and lowered postcroanterior ratioCitation89; others were predictive when combined with performance on specific cognitive tasksCitation98, Citation99 and/or demographic characteristics.Citation99
Progress in functional imaging can come from activation studies. Comparisons of young to elderly healthy subjects have shown that poorer performances in tasks such as conflict resolution and episodic memory in the elderly corresponded to underactivation, whereas a performance similar to that of young controls in working memoir}' tasks was accompanied with recruitment, of additional brain regions.Citation100 Studies comparing activation during cognitive tasks in AD patients and controlsCitation101-Citation105 showed that, together with lower performances, AD patients had activation patterns characterized by absence of activation in some brain areas, activation with shifted peak foci, expansion of normally activated zones, and recruitment, of remote areas.Citation103 These differences were generally interpreted as due to compensation efforts; complementary interpretations are disconnection between regions normally involved in the task and predominant processing of accessory aspects of the stimuli (eg, emotional appearance in face recognition).Citation105 Passive pattern-flash stimulation elicited less activation in AD patients; this failure requires a less demanding stimulation to be disclosed in the modcrate-to-scvere group than in the mild group.Citation106
Cognitively normal subjects at risk for AD (defined as the presence of at least one ApoE ε4 allele, aloneCitation107 or combined with a history of AD in at. least one firstdegree relativeCitation108) were compared with low-risk controls for activation induced by cognitive tasks they performed with the same accuracy level. In the high-risk group, some regions were activated to a greater extent or magnitude (eg, nearly twice as much as in controls in hippocampal regionsCitation107); others displayed lower activation.Citation108 After a 2-year follow-up,Citation107 decline in verbal recall correlated with the number of regions activated in the left hemisphere at baseline.
Using a functional magnetic resonance imaging (fMRI) protocol specifically developed for hippocampal region analysis, one studyCitation109 compared cognitively NCs, subjects with isolated memory impairment (IMI), and AD patients during a simple task (gender discrimination of presented faces); all subjects performed the task with 100% accuracy. AD patients had lesser activation of the three regions studied, ie, ERC, subiculum, and the hippocampus proper. Among the IMI subjects, one third had an activation pattern similar to that of AD patients and the others displayed lesser activation in the subiculum only. Follow-up data would be necessary to determine whether the differences described in this study are predictive, but together these activation studies indicate that properly chosen activation paradigms could help identify AD in subjects with mild cognitive deficits.
Nuclear magnetic resonance affords additional approaches. Magnetic resonance spectroscopy (MRS) can assess the biochemical composition of living brain regions. To date, the most, consistent findings in ADCitation110 have been obtained with proton MRS showing a decrease in N-acetylaspartate (NAA) and an increase in myoinositol (MI). NAA and MI changes arc specific to neither AD nor brain disease, but the NAA/MI ratio can discriminate possible AD cases from NCs. In addition, NAA/MI, NAA, and the MI/creatine (Cr) ratio were shown to be correlated with MMSE score in controls and patients with probable ADCitation111; in a 12-month follow-up studyCitation112 NAA/Cr and NAA/MI at baseline were correlated with the progression of MMSE. scores. Few studies have used MRS in mild cognitive deficit. Ml/Cr was found to be higher in MCI subjectsCitation113, Citation114 and NAA lower in A AMI subjectsCitation115 and AD patients than in controls, whereas MI values were intermediate between AD patients and controls.Citation115 Follow-up studies are necessary to confirm the predictive value of such findings.
The magnetization transfer imaging (MTI)Citation116 signal arises from the magnetization exchange between waterand macromolecule -bound protons; this technique is useful in the study of membranes and membrane-linked diseases such as multiple sclerosis, in which decreased magnetic transfer ratio (MTR) is a marker of demyelinationCitation117 and axonal density loss.Citation118 MTI studies involving AD patientsCitation119-Citation125 agree on decreased values compared with NCs, expressing structural changes in the temporal lobe, and also the frontal lobe and the whole brain.Citation119-Citation120 Hippocampal MTR had a discrimination rate relative to controls of 85% in mild AD (CDR=0.5), 89% in mild AD (CDR=1), and 100% in moderate AD (CDR=2); the values for visually rated atrophy were of 73%, 80%, and 91% respectively.Citation124 MTR was also able to differentiate AD from non-AD dementia with a success rate of 77%.Citation123 Studies comparing MCI subjects with AD patients and healthy controlsCitation119-Citation122 identified structural changes in MCI in the absence of significant, atrophy; they were located in gray matter, whereas those found in AD patients involved white and gray matters.Citation122 These changes were found to be correlated with cognitive impairment.Citation119-Citation120 MTI thus seems able to identify structural changes before atrophy is manifest. Follow-up studies should confirm its predictive value and comparison with functional imaging should assess which technique detects the earlier changes.
The ApoE ε4 allele is acknowledged to be a risk factor for AD.Citation126, Citation127 Few studies have specifically addressed its influence on the evolution of MCI subjects. The ApoE ε4 carrier status was found the best predictor of conversion to AD (risk ratio=4.36),Citation21 a nonsignificant predictor (relative risk of 1 .49)Citation79 or to have no predictive value.Citation54, Citation128 In subjects with memory impairment and Global Deterioration Scale (GDS) score of 2 to 3,Citation129 ApoE ε4 alone predicted progression to dementia with a 73.8% accuracy; combining genotype and memory scores increased the accuracy to 92.5%. In subjects with MMSE, scores of 21 to 26, the ApoE, genotype was found to be associated with an odds ratio for progression to dementia of 3.31Citation130 and with memory decline.Citation131 Among the various substances that have been assayed in blood and CSF,Citation132 increased CSF-tau and decreased βA1-42 proteins are the best markers for AD to date.Citation132-Citation134 Their combination yielded a 94% and 88% sensitivity for probable and possible AD, respectively, and specificity of 100% versus psychiatric disorder, 89% versus no dementia, 67% versus DLB, and 48% versus VaD in clinical practice conditions.Citation135 CSF-tau levels were found to be higher in MCI than in healthy controls,Citation136-Citation140 lower thanCitation141 or similar toCitation139, Citation140 those found in AD patients. In follow-up studies, it identified MCI subjects who evolved to AD with a sensitivity of 65% to 68% and a specificity of 100% versus patients with memory complaintsCitation142 and 93% versus healthy controlsCitation139; baseline values in converters were higher than in nonconvcrtcrs.Citation137 In one study,Citation139 combining CSF-tau and βA, values did not. improve the predictability obtained with CSF-tau alone. In others, combined CSF-tau/βA1-42 values differentiated converters from healthy controls with 88% sensitivity and 80% specificityCitation138 and from nonconverters with a 90% sensitivity and specificity.Citation140
Medical diagnoses are rarely reached using a single marker; most often it results from the combination of different approaches, including thorough clinical evaluation. Once a consensus is obtained on cutoff values for the different techniques mentioned, it is likely that a combination of different markers for AD will allow early diagnosis with high sensitivity and specificity in individual cases.
Therapeutic aspects
The final goal of constructing criteria for age-associated mild cognitive deficit, is to treat, it, and many therapeutic approaches are available.Citation143-Citation144
Some benefits have been reported, in terms of global stability and improved memory with the acetylcholinesterase inhibitors (AChEI) donepezilCitation145 and rivastigmineCitation146 and the dopamine receptor agonist/α2 antagonist, piribedilCitation147; trials are underway using donepezil and vitamin E, rivastigmine, the cyclooxygenase-2 (COX-2) inhibitors celccoxib and rofecoxib with the goal of delaying patients' progression to dementiaCitation144 and the Ampakine® CX516 with the aim of short-term symptomatic improvement.Citation148 Treating MCI using approaches initially intended for AD premises either that MCI equates to early AD in all cases, which is unlikely, or that the underlying mechanism is the same in both cases, the difference being merely a matter of intensity, which is not. confirmed by neuropathological data. Even if improved criteria or techniques were able to predict, the progression to AD in a given patient, this strategy deserves discussion. As regards the cholinergic and glutamatergic systems in AD, it has been proposed that, the final deficiency state is preceded, in the early stages, by a hyperactive stateCitation149, Citation150 originating in βA-induced N-methyl-D-aspartate (NMDA) receptor hypersensitivity. It was recently found that the number of choline acetyltransferase (ChAT)-positive neurons in the nucleus basalis of Meynert was no lower in MCI than in NCs.Citation151 Further, ChAT activity was shown to be increased in hippocampus and in the superior frontal cortex compared with NCs, and similar in anterior cingulate, superior temporal, and inferior parietal cortices.Citation152 Although such data are lacking for the glutamatergic system, these findings suggest that, among the currently available treatments, those aiming at downmodulating the NMDA receptor responsiveness could be more appropriate than replacement therapies in degenerative, AD-type MCI. Mild cognitive deficit will be the condition of choice for administration of future treatments addressing basic mechanisms of degenerative dementias, provided they can be reliably identified in these patients.
Conclusion
Introducing criteria for mild cognitive deficit should:
Help its detection, mostly in first-line medicine.
Improve the accuracy of early dementia diagnosis.
Through harmonization of practice in research settings, permit progress in pathophysiological and therapeutic research.
All the sets of criteria, whatever their formulation, require the input, of a neuropsychologist and a thorough, comprehensive examination. This approach is available in specialized centers only, and not transferable to first-line medicine. The criteria by themselves do not predict which individual will progress to dementia and still less the nature of this potential disease. On the other hand, early, reliable diagnosis of AD through a proper combination of investigational procedures can be expected in the near future. Epidemiological data show that AD remains the most, frequent dementia type in elderly people.Citation153 Followup studies suggest that it. is also the most, frequent dementia type developed by subjects with mild cognitive deficit. Therefore, early identification or rejection of AD would solve the majority of cases. It can thus be thought that the priority is to optimize our battery of investigational tools by defining appropriate cutoff values, comparing them in the same patient samples, and defining their individual powers.
From a practical and clinical point of view, refining the sets of criteria to improve their specificity, which implies skilled professional intervention, is probably useless. Efforts should rather be made to define simple, sensitive tools, usable by general practitioners.
From a research point of view, it seems mandatory to reach a consensus on several points. Should the reference population be matched for age only, or for gender and education as well? Using age-related references implies admitting that cognitive decline occurs in healthy aging; using education-related ones implies that, low education is an independent risk factor for cognitive declineCitation154, Citation155; using gender requires taking into account the hormonal status of women. Should impairment, in a cognitive domain be established on the basis of a single test, or of several ones addressing the same function? Should studies include subjects with memory impairment, only, or with decline in other domains as well? Stratifying the participants according to their cognitive profile would allow assessment, of the predictivity of this item, relative to the underlying disease, as proposed by Petersen et al.Citation156 It would also permit comparison of the effect of treatments in these subsets.
These issues cannot, be solved by any single research team. Collaborative or, at least, comparable studies require the strict definition of common basic inclusion (eg, the tests to be used with standard cutoff scores) and exclusion criteria. Before being applicable in daily practice, the available sets of criteria need to be further defined and standardized. The current lack of treatment is a hurdle to its acceptance. However, disseminating the concept could help increase the sensitivity of general practitioners to the importance of cognitive complaints and signs in their elderly patients.
Selected abbreviations and acronyms
| AACD | = | aging-associated cognitive decline |
| AAMI | = | age-associated memory impairment |
| ACMI | = | age-consistent memory impairment |
| AD | = | Alzheimer's disease |
| CDR | = | Clinical Dementia Rating |
| CERAD | = | Consortium to Establish a Registry for Alzheimer's Disease |
| CIND | = | cognitive impairment-no dementia |
| DLB | = | dementia with Lewy bodies |
| ERC | = | entorhinal cortex |
| IMI | = | isolated memory impairment |
| LLF | = | late-life forgetfulness |
| MCI | = | mild cognitive impairment |
| MMSE | = | Mini-Mental State Examination |
| MTI | = | magnetization transfer imaging |
| NC | = | normal control |
| NFT | = | neurofibrillary tangle |
| NT | = | neuropil thread |
| VaD | = | vascular dementia |
| βA | = | β-amyloid |
REFERENCES
- HuletteCM.Welsh-BohmerKA.MurrayMG.SaundersAM.MashDC.MclntyreLM.Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals.J Neuropathol Exp Neurol.199857116811749862640
- PriceJL.MorrisJC.Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease.Ann Neurol.19994535836810072051
- ChristensenH.What cognitive changes can be expected with normal aging?Aust NZ J Psychiatry.200135768775
- JohanssonB.ZaritS.BergS.Changes in cognitive functioning of the oldest old.J Gerontol.199247P75P801538071
- HowiesonDB.DameA.CamicioliR.SextonG.PayamiH.KayeJA.Cognitive markers preceding Alzheimer's dementia in the healthy oldest old.J Am Geriatr Soc.1997455845899158579
- RubinEH.StorandtM.MillerJP.et al.A prospective study of cognitive function and onset of dementia in cognitively healthy elders.Arch Neurol.1998553954019520014
- WilsonRS.BeckettLA.BennettDA.AlbertS.EvansDA.Change in cognitive function in older persons from a community population.Arch Neurol.1999561274127910520945
- SilwinskiM.LiptonRB.BuschkeH.StewartW.The effects of preclinical dementia on estimates of normal cognitive functioning in aging.J Gerontol.199651BP217P225
- MorrisJC.PriceJL.Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease,J Mol Neurosci.20011710111811816784
- KraiVA.Senescent forgetfulness: benign and malignant.Can Med Assoc J.19628625726014459267
- HughesCP.BergL.DanzigerWL.CobenLA.MartinRL.A new clinical scale for the staging of dementia.Br J Psychiatry.19821405665727104545
- GurlandBJ.DeanL.CopelandJ.GurlandR.GoldenR.Criteria for diagnosis of dementia in the community elderly.Gerontologist.1982221801867084739
- ReisbergB.FerrisSH.de LeonMJ.CrookT.The Global Deterioration Scale for assessment of primary degenerative dementia. AmJ Psychiatry.198213911361139
- RothM.TymE.MountjoyCQ.et al.CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia.Br J Psychiatry.19861496987093790869
- CrookT.BartusRT.FerrisSH.WhitehouseP.CohenGD.GershonSG.Age-associated memory impairment: proposed diagnostic criteria and measures of clinical changes. Report of a National Institute of Mental Health Work Group.Dev Neuropsychol.19862261276
- BradfordRC.La RueA.Criterion for diagnosing age-associated memory impairment: proposed improvement from the field.Dev Neuropsychol.19895298306
- ZaudigM.A new systematic method of measurement and diagnosis of “mild cognitive impairment” and dementia according to ICD- 10 and DSM-lll-R criteria.Int Psychogeriatr.19924(suppl 2)2032191288663
- World Health Organization.The ICD-10 Classification of Mental and Behavioural Disorders. Diagnostic Criteria for Research. Geneva, Switzerland: World Health Organization1993
- LevyR.Aging-associated cognitive decline. Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization.Int Psychogeriatr.1994663688054494
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association;1994
- PetersenRC.SmithGE.IvnikRL.et al.Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory-impaired individuals.JAMA.1995273127412787646655
- EblyEM.HoganDB.ParhadIM.Cognitive impairment in the nondemented elderly. Results from the Canadian Study of Health and Aging.Arch Neurol.1995526126197763211
- TengEL.The modified Mini-Mental State (3MS) examination.J Clin Psychiatry.1987483143183611032
- GrahamJE.RockwoodK.BeattieBL.et al.Prevalence and severity of cognitive impairment with and without dementia in an elderly population.Lancet.1997349179317969269213
- PetersenRC.SmithGE.WaringSC.IvnikRJ.TangalosEG.KomkenE.Mild cognitve impairment. Clinical characterization and outcome.Arch Neurol.19995630330810190820
- PetersenRC.StevensJC.GanguliM.TangalosEG.CummingsJL.DeKoskyST.Practice parameters: early detection of dementia. Mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology.Neurology.2001561133114211342677
- FolsteinMF.FolsteinSE.McHughPR.“Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975121891981202204
- KokmenE.NaessensJM.OffordKP.A short test of mental status: description and preliminary results.Mayo Clin Proc.1987622812883561043
- BlessedG.TomlinsonBE.RothM.The association between quantitative measures of dementia and of senile change in the cerebral gray matter of elderly subjects.Br J Psychiatry.196811479781 1 5662937
- WeintraubS.BaratzR.MesulamMM.Daily living activities in the assessment of dementia. In: Corkin S, Davis KL, Growdon JH, Usdin E, Wurtman RJ, eds.Alzheimer's Disease: A Report of Progress in Research. New York, NY: Raven Press;1982189192
- American Psychiatric Association:Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised. Washington, DC: American Psychiatric Association;1987
- BergL.Clinical Dementia Rating (CDR).Psychopharmacol Bull.1988246376393249765
- MorrisJC.The clinical dementia rating: current version and scoring rules.Neurology.199343241224148232972
- RichardsM.TouchonJ.LedesertB.RitchieK.Cognitive decline in ageing: are AAMI and AACD distinct entities?Int J Geriat Psychiatry.199914534540
- SchroderJ.KratzB.PantelJ.MinnemannE.LehrU.SauerH.Prevalence of mild cognitive impairment in an elderly community sample.J Neural Transm.1998(suppl54)5159
- RitchieK.ArteroS.TouchonJ.Classification criteria for mild cognitive impairment. A population-based validation study.Neurology.200156374211148233
- MorrisJC.StorandtM.MillerJP.et al.Mild cognitive impairment represents early-stage Alzheimer disease.Arch Neurol.20015839740511255443
- BergL.McKeelDW.MillerJP.et al.Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype.Arch Neurol.1998553263359520006
- KachaturianZS.Diagnosis of Alzheimer's disease.Arch Neurol.198542109711052864910
- MorrisJC.McKeelDW.StorandtM.et al.Very mild Alzheimer's disease: informant-based clinical, psychometric, and pathological distinction from normal aging.Neurology.1991414694782011242
- PriceJL.DavisPB.MorrisJC.WhiteDL.The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease.Neurobiol Aging .1991122953121961359
- PriceJL.KoAI.WadeMJ.TsouSK.McKeelDW.MorrisJC.Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease.Arch Neurol.2001581395140211559310
- MufsonEJ.ChenEY.CochranEJ.BeckettLA.BennettDA.KordowerJH.Entorhinal cortex [3-amyloid load in individuals with mild cognitive impairment.Exp Neurol.199915846949010415154
- KordowerJH.ChuY.StebbinsGT.et al.Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment.Ann Neurol.20014920221311220740
- MitchellTW.MufsonEJ.SchneiderJA.et al.Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer's disease.Ann Neurol.20025118218911835374
- McKhannG.DrachmanD.FolsteinM.KatzmanR.PriceD.StadlanEM.Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease.Neurology.1984349399446610841
- MirraSS.HeymanD.McKeelSM.et al.The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathological assessment of Alzheimer's disease.Neurology.1991414794862011243
- ChuY.CochranEJ.BennettDA.MufsonEJ.KordowerJH.Down-regulation of trkA mRNA within nucleus basalis of Meynert neurons in individuals with mild cognitive impairment and Alzheimer's disease.J Comp Neurol.200143729630711494257
- MufsonEJ.MaSY.DillsJ.et al.Loss of basal forebrain P75NTR immunoreactivity in subjects with mild cognitive impairment and Alzheimer's disease.J Comp Neurol.200244313615311793352
- NielsenH.LolkA.Kragh-SorensenP.Age-associated memory impairment - pathological memory decline or normal aging?Scand J Psychol.19983933379619130
- HänninenT.HallikainenM.KoivistoK.et al.A follow-up study of ageassociated memory impairment: neuropsychological predictors of dementia.J Am Geriatr Soc.199543100710157657916
- DevanandDP.FolzM.GorlynM.MoellerJR.SternY.Questionable dementia: clinical course and predictors of outcome.J Am Geriatr Soc.1997453213289063278
- SmallGW.La RueA.KomoS.KaplanA.Mnemonics usage and cognitive decline in age-associated memory impairment.Int Psychogeriatr.1997947569195278
- WolfH.GrunwaldM.EckeGM.et al.The prognosis of mild cognitive impairment in the elderly.J Neural Transm.199854(suppl)3150
- McKelveyR.BergmanH.SternJ.RushC.ZahirneyG.ChertkowH.Lack of prognostic significance of SPECT abnormalities in non-demented elderly subjects with memory loss.Can J Neurol Sci.199926232810068803
- BerentS.GiordaniB.FosterML.et al.Neuropsychological function and cerebral glucose utilization in isolated memory impairment and Alzheimer's disease.J Psychiatr Res.19993371610094234
- BozokiA.GiordaniB.HeidenbrinkJL.BerentS.FosterNL.Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss.Arch Neurol.20015841141611255444
- BennettDA.WilsonRS.SchneiderJA.et al.Natural history of mild cognitive impairment in older persons.Neurology.20025919820512136057
- LarrieuS.LetenneurL.OrgogozoJM.et al.Incidence and outcome of mild cognitive impairment in a population-based prospective cohort.Neurology.2002591594159912451203
- JohanssonB.ZaritSH.Early cognitive markers of the incidence of dementia and mortality: a longitudinal study of the oldest old.Int J Geriatr Psychiatry.19971253599050424
- PalmerK.WangHX.BäckmanL.WinbladB.FratiglioniL.Differential evolution of cognitive impairment in nodemented older persons: results from the Kungsholmen project.Am J Psychiatry.200215943644211870008
- MeyerJS.XuG.ThornbyJ.ChowdhuryM.QuachM.Longitudinal analysis of abnormal domains comprising mild cognitive impairment (MCI) during aging.J Neurol Sci.2002201192512163189
- DalyE.ZaitchikD.CopelandM.SchmahmannJ.GuntherJ.AlbertM.Predicting conversion to Alzheimer disease using standardized clinical information.Arch Neurol.20005767568010815133
- RomanGC.TatemichiTK.ErkinjunttiT.et al.Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop.Neurology.1993432502608094895
- McKeithIG.GalaskoD.KosakaK.et al.Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop.Neurology.199647111311248909416
- MeyerJS.XuG.ThornbyJ.ChowdhuryMH.QuachM.Is mild cognitive impairment prodromal for vascular dementia like Alzheimer's disease?Stroke.2002331981198512154249
- FlickerC.FerrisSH.ReisbergB.Mild cognitive impairment in the elderly: predictors of dementia.Neurology.199141100610092067629
- TierneyMC.SzalaiJP.SnowWG.et al.Prediction of probable Alzheimer's disease in memory-impaired patients: a prospective longitudinal study.Neurology.1996466616658618663
- TierneyMC.SzalaiJP.SnowWG.FisherRH.The prediction of Alzheimer's disease: the role of patients and informant perceptions of cognitive deficits.Arch Neurol.1996534234278624217
- TuokkoH.FrerichsRJ.Cognitive impairment with no dementia (CIND): longitudinal studies, the findings, and the issues.Clin Neuropsychol.20001450452511262720
- BraakH.BraakE.Neuropathologic staging of Alzheimer-related changes.Acta Neuropathologies.199182239259
- LaaksoMP.Structural imaging in cognitive impairment and the dementias: an update.Curr Opin Neurol.20021541542112151837
- DuA.SchuffN.AmendD.et al.MRI of entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease.J Neurol Neurosurg Psychiatry.20017144144711561025
- WolfH.GrunwaldM.KruggelF.et al.Hippocampal volume discriminates between normal cognition; questionable and mild dementia in the elderly.Neurobiol Aging.20012217718611182467
- BottinoCM.CastroCC.GomesRL.BuchpiguelCA.MarchettiRL.NetoMR.Volumetric MRI measurements can differentiate Alzheimer's disease, mild cognitive impairment, and normal aging.Int Psychogeriatr.200214597212094908
- VisserPJ.ScheltensP.VerheyFRJ.et al.Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment.J Neurol.1999214647748510431775
- De SantiS.de LeonMJ.RusinekH.et al.Hippocampal formation glucose metabolism and volume losses in MCI and AD.Neurobiol Aging.20012252953911445252
- HampelH.TeipelSJ.BayerW.Age transformation of combined hippocampus and amygdala volume improves diagnostic accuracy in Alzheimer's disease.J Neurol Sci.2002194151911809161
- GrundmanM.SencakovaD.JackCR.et al.Brain MRI hippocampal volume and prediction of clinical status in a mild cognitive impairment trial.J Mol Neurosci.200219232712212787
- JackCR.PetersenRC.XuYC.et al.Rates of hippocampal atrophy correlates with change in clinical status in aging and AD.Neurology.20005548448910953178
- de LeonM.GeorgeA.ConvitA.et al.The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation.AJNR Am J Neuroradiol.1993148979068352162
- JackCR.PetersenRC.XuYC.et al.Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment.Neurology.1999521397140310227624
- KillianyRJ.Gomez-islaT.MossM.et al.Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease.Ann Neurol.20004743043910762153
- DickersonBC.GoncharovaI.SullivanMP.et al.MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease.Neurobiol Aging.2001 2274745411705634
- ConvitA.de AsisJ.de LeonMJ.et al.Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in non-demented elderly predict decline to Alzheimer's disease.Neurobiol Aging.200021192610794844
- De GrootJC.de LeeuwFE.OudkerkM.HofmanA.JoliesJ.BretelerMMB.Cerebral white matter lesions and subjective cognitive dysfunction. The Rotterdam Scan study.Neurology.2001561539154511402112
- YlikoskiR.YlikoskiA.ErkinjunttiT.SulkavaR.RaininkoR.TilvisR.White matter changes in healthy elderly persons correlate with attention and speed of mental processing.Arch Neurol.1993508188248352667
- WolfH.EckeGM.BettinS.DietrichJ.GertzHJ.Do white matter changes contribute to the subsequent development of dementia in patients with mild cognitive impairment? A longitudinal study.Int J Geriat Psychiatry.200015803812
- JelicV.NordbergA.Early diagnosis of Alzheimer disease with positron emission tomography.Alzheimer Dis Assoc Disord.200014(suppl1)S109S11310850738
- SmallGW.MazziottaJC.CollinsMT.et al.Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease.JAMA.19952739429477884953
- ReimanEM.CaselliRJ.YunLS.et al.Preclinical evidence of Alzheimer's disease in persons homozygous for the e4 allele for apolipoprotein E.N Engl J Med.19963347527588592548
- SmallGW.ErcoliLM.SilvermanDHS.et al.Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease.Proc Natl AcadSciUSA.20009760376042
- MinoshimaS.GiordaniB.FreyKA.FosterNL.KuhlDE.Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease.Ann Neurol.19974285949225689
- KogureD.MatsudaH.OhnishiT.et al.Longitudinal evaluation of early Alzheimer's disease using brain perfusion SPECT.J Nucl Med.2000411155116210914904
- HuangC.WahlundLO.SvenssonL.WinbladB.JulinP.Cingulate cortex hypoperfusion predicts Alzheimer's disease in mild cognitive impairment.BMC Neurology.2002291412227833
- JohnsonKA.JonesK.HolmanBL.et al.Preclinical prediction of Alzheimer's disease using SPECT.Neurology.199850156315719633695
- CelsisP.AgnielA.CardebatD.DémonetJF.OussetPJ.PuelM.Agerelated cognitive decline: a clinical entity? A longitudinal study of cerebral blood flow and memory performance.J Neurol Neurosurg Psychiatry.1997626016089219746
- ArnaizE.JelicV.AlmkvistO.et al.Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment.Neuroreport.20011285185511277595
- SmallGW.LaRueA.KomoS.KaplanA.MandelkernMA.Predictors of cognitive changes in middle-aged and older adults with memory loss.Am J Psychiatry.1995152175717648526242
- Reuter-LorenzP.New visions of the aging mind and brain.Trends Cogn Sci.2002639440012200182
- BeckerJT.MintunMA.AlevaK.WisemanMB.NicholsT.DeKoskyST.Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer's disease.Neurology.1996466927008618669
- WoodardJL.GraftonST.VotawJR.GreenRC.DobraskiME.HoffmanJM.Compensatory recruitment of neural resources during overt rehearsal of word lists in Alzheimer's disease.Neuropsychology.1998124915049805319
- SaykinAJ.FlashmanLA.FrutigerSA.et al.Neuroanatomy substrates of semantic memory impairment in Alzheimer's disease: patterns of functional MRI activation.J int Neuropsychol Soc.1999537739210439584
- BackmanL.AnderssonJLR.NybergL.WinbladB.NordbergA.AlmkvistO.Brain regions associated with episodic retrieval in normal aging and Alzheimer's disease.Neurology.1999521861187010371535
- GradyCL.FureyML.PietriniP.HorwitzB.RapoportSI.Altered brain functional connectivity and impaired short-term memory in Alzheimer's disease.Brain.200112473975611287374
- MentisMJ.AlexanderGE.KrasukiJ.et al.Increasing required neural response to expose abnormal brain function in mild versus moderate or severe Alzheimer's disease: PET study using parametric visual stimulation.Am J Psychiatry.19981557857949619151
- BookheimerSY.StrojwasMH.CohenMS.et al.Patterns of brain activation in people at risk for Alzheimer's disease.N Engl J Med.200034345045610944562
- SmithCD.AndersenAH.KryscioRJ.et al.Women at risk for AD show increased parietal activation during a fluency task.Neurology.2002581197120211971086
- SmallSA.PereraGM.DeLaPazR.MayeuxR.SternY.Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease.Ann Neurol.19994546647210211471
- ValenzuelaMJ.SachdevP.Magnetic resonance spectroscopy in AD.Neurology.20015659259811261442
- RoseSE.de ZubicarayGl.WangD.et al.A 'H MRS study of probable Alzheimer's disease and normal aging: implications for longitudinal monitoring of dementia progression.Magn Reson imaging.19991729129910215485
- DoraiswamyPM.CharlesHC.Rama KrishnanKR.Prediction of cognitive decline in early Alzheimer's disease.Lancet.199835216789853445
- KantarciK.JackCR.XuYC.et al.Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: a:H MRS study.Neurology.20005521021710908893
- CataniM.CherubiniA.HowardR.et al.(1)H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging.Neuroreport.2001122315231711496102
- ParnettiL.LowenthalDT.PresciuttiO.1H-MRS, MRI-based volumetry, and 99mTc-HMPAO-SPECT in normal aging, age-associated memory impairment, and probable Alzheimer's disease.J Am Geriatr Soc.1996441331388576501
- GrossmanRl.GomoriJM.RamerKN.LexaFJ.SchnallMD.Magnetization transfer: theory and clinical application in neuroradiology.Radiographics.1994142792908190954
- DoussetV.GrossmanRl.RamerKN.et al.Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging.Radiology.19921824834911732968
- Van WaesbergheJHTM.KamphorstW.De GrootJA.et al.Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability.Ann Neurol.19994674775410553992
- Van der WM.Van den HeuvelDM.Weverling-RinjsburgerAW.et al.Cognitive decline in AD and mild cognitive impairment is associated with global brain damage.Neurology.20025987487912297570
- Van der FlierWM.Van den HeuvelDMJ.Weverling-RinjsburgerAWE.et al.Magnetization transfer imaging in normal aging, mild cognitive impairment, and Alzheimer's disease.Ann Neurol.200252626712112048
- KabaniNJ.SledJG.ChertkowH.Magnetization transfer ratio in mild cognitive impairment and dementia of Alzheimer's type.Neuroimage.20021560461011848703
- KabaniNJ.SledJG.ShuperA.ChertkowH.Regional magnetization transfer ratio changes in mild cognitive impairment.Magn Reson Med.20024714314811754453
- HanyuH.AsanoT.IwamotoT.TakasakiM.ShindoH.AbeK.Magnetization transfer measurements of the hippocampus in patients with Alzheimer's disease, vascular dementia, and other types of dementia.AJNR Am J Neuroradiol.2000211235124210954274
- HanyuH.AsanoT.SakuraiH.TakasakiM.ShindoH.AbeK.Magnetization transfer measurements of the hippocampus in the early diagnosis of Alzheimer's disease.J Neurol Sci.2001188798411489289
- BozzaliM.FranceschiM.FaliniA.et al.Quantification of tissue damage in AD using diffusion tensor and magnetization transfer MRI.Neurology.2001571135113711571355
- StrittmatterWJ.SaundersAM.ScmechelD.et al.Apolipoprotein E: high avidity binding to β amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer's disease. ProcNatl Acad Sci U S A.19939019771981
- SlooterAJ.CrutsM.KalmijnS.et al.Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam study.Arch Neurol.1998559649689678314
- ChertkowH.Mild cognitive impairment.Curr Opin Neurol.20021540140712151835
- TierneyMC.SzalaiJP.SnowWG.et al.A prospective study of the clinical utility of ApoE genotype in the prediction of outcome in patients with memory impairment.Neurology.1996461491548559365
- JonkerC.SchmandB.LindenboomJ.HavekesLM.LaunerLJ.Association between apolipoprotein E ε4 and the rate of cognitive decline in community-dwelling elderly individuals with and without dementia.Arch Neurol.1998106510699708956
- DikMG.JonkerC.BouterLM.GeerlingsMl.van KampGJ.DeegDJ.APOE-ε4 is associated with memory decline in cognitively impaired elderly.Neurology.2000541492149710751265
- TeunissenCE.de VenteJ.SteinbuchHWM.De BruijnC.Biocemical markers related to Alzheimer's dementia in serum and cerebrospinal fluid.Neurobiol Aging.20022348550812009495
- The Ronald and Nancy Reagan Research Institute of the Alzheimer's Association and the National Institute on Aging Working Group. Consensus report of the working group on: “Molecular and biochemical markers of Alzheimer's disease.”Neurobiol Aging.1998191091169558143
- RosierN.WichartI.JellingerKA.Clinical significance of neurobiochemical profiles in the lumbar cerebrospinal fluid of Alzheimer's disease patients.J Neural Transm.200110823124611314776
- AndreasenN.MinthonL.DavidssonP.et al.Evaluation of CSF-tau and CSF-A(342 as diagnostic markers for Alzheimer's disease in clinical practice.Arch Neurol.20015837337911255440
- de LeonMJ.SegalS.TarshishCY.et al.Longitudinal cerebrospinal fluid tau load increases in mild cognitive impairment.Neurosci Lett.200233318318612429378
- BuergerK.TeipelSJ.ZinkowskiR.et al.CSF-tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects.Neurology.20025962762912196665
- AndreasenN.MinthonL.VanmechelenE.et al.Cerebrospinal fluid tau and A(342 as predictors of development of Alzheimer's disease in patients with mild cognitive impairment.Neurosci Lett.19992735810505638
- MaruyamaM.AraiH.SugitaM.et al.Cerebrospinal fluid amyloid β1-42 levels in the mild cognitive impairment stage of Alzheimer's disease.Exp Neurol.200117243343611716567
- RiemenschneiderM.LautenschlagerN.WagenpfeilS.DiehlJ.DrzezgaA.KurzA.Cerebrospinal fluid tau and β-amyloid 42 proteins identify Alzheimer disease in subjects with mild cognitive impairment.Arch Neurol.2002591729173412433260
- BlombergM.JensenM.BasunH.LannfeltL.WahlundLO.Increasing cerebrospinal fluid tau levels in a subgroup of Alzheimer patients with apolipoprotein E allele ε4 during 14-month follow-up.Neurosci Lett.19962141631668878109
- AraiH.IshiguroK.OhnoH.et al.CSF phosphorylated tau protein and mild cognitive impairment: a prospective study.Exp Neurol.200016620120311031097
- BuccafuscoJJ.Terry AVJr.Multiple central nervous system targets for eliciting beneficial effects on memory and cognition.J Pharmacol Exp Ther.200029543844611046074
- SramekJJ.VeroffAE.CutlerNR.The status of ongoing trials for mild cognitive impairment.Exp Opin Invest Drugs.200110741752
- MeyerJS.LiY.XuG.ThornbyJ.ChowdhuryMH.QuachM.Feasability of treating mild cognitive impairment with cholinesterase inhibitors.Int J Geriatr Psychiatry.20021758658812112184
- FreoU.PizzolatoG.DamM.OriC.BattistinL.A short review of cognitive and functional neuroimaging studies of cholinergic drugs: implications for therapeutic potentials.J Neural Transm.200210985787012111473
- NagarajaD.JayashreeS.Randomized study of the dopamine receptor agonist piribedil in the treatment of mild cognitive impairment.Am J Psychiatry.20011581517151911532743
- JohnsonSA.SimmonVF.Randomized, double-blind, placebo-controlled international clinical trial of the Ampakine CX516 in elderly participants with mild cognitive impairment.J Mol Neurosci.20021919720012212780
- OlneyJW.WozniakDF.FarberNB.Excitotoxic neurodegeneration in Alzheimer disease. New hypothesis and new therapeutic strategies.Arch Neurol.199754123412409341569
- NewcomerJW.FarberNB.OlneyJW.NMDA receptor function, memory, and brain aging.Dialogues Clin Neurosci.2000221923222034391
- GilmorML.EricksonJF.VaroquiH.et al.Preservation of nucleus basalis neurons containing choline acetyltransferase and the vesicular acetylcholine transporter in the elderly with mild cognitive impairment and early Alzheimer's disease.J Comp Neurol.199941169370410421878
- DeKoskyST.IkonomovicMD.StyrenSD.et al.Upregulation of choline acetyl transferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment.Ann Neurol.20025114515511835370
- LoboA.LaunerLJ.FratiglioniL.et al.Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts.Neurology.200054(suppl5)S4S910854354
- AnsteyK.ChristensenH.Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: a review.Gerontology.20004616317710754375
- LetenneurL.LaunerLJ.AndersenK.et al.Education and the risk for Alzheimer's disease: sex makes a difference.Am J Epidemiol.20001511064107110873130
- PetersenRC.DoodyR.KurzA.et al.Current concepts in mild cognitive impairment.Arch Neurol.2001581985199211735772