Sleep and depression
Two qualitatively different brain states characterize normal human sleep: non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. NREM sleep is further subdivided into four stages: stage 1 is the lightest and stage 4 the deepest. Stages 3 and 4 are often defined as δ sleep or slow-wave sleep (SWS) due to the occurrence of slow (0.5-3.5 Hz) “delta” waves. REM sleep (also called paradoxical sleep) alternates with NREM throughout the night in recurrent NREM-REM cycles of about 90 min. Sleep-wake regulation is classically viewed as resulting from the interaction of two regulating processes (homeostatic [S] and circadian [C]).Citation1 In this model, the propensity to sleep or be awake at any given time is a consequence of a sleep debt (Process S) and its interaction with signals coming from the circadian clock located in the suprachiasmatic nucleus (Process C).
In 1982, Borbely and Wirz-JusticeCitation2 suggested that the characteristic sleep disturbances of major depressive patients reflect a homeostatic Process S deficiency, ie, a failure to accumulate SWS pressure during the daytime, leading to sleep initiation and maintenance difficulties, and early emergence of REM sleep. Indeed, characteristic sleep EEG changes such lengthening of sleep latency, sleep disruption, and disturbances in REM sleep organization have been consistently identified in depressive illness.Citation3 Spectral analysis of NREM sleep in major depressed patients has shown lower δ activity (power spectra in the δ wave) in NREM sleepCitation4-Citation6 and decreased δ incidence particularly in the first non-REM period,Citation7 supporting the “Process S” deficiency hypothesis. Using spectral analysis of the sleep-onset period, we have brought support to this hypothesis: we found that homeostatic sleep regulation processes are partially maintained in primary insomniacs, but not in major depressed patients with insomnia.Citation8
Sleep EEG and antidepressant response
Some studies have shown that the clinical response to various antidepressant therapies could be predicted by sleep electroencephalography (EEG) parameters. For instance, the amount of REM sleep suppression observed after the first dose of antidepressant may predict ultimate clinical response.Citation9,Citation10 REM rebound following antidepressant withdrawal was also found predictive of antidepressant response. Kupfer et alCitation11 demonstrated that the antidepressant response to two consecutive days of pulse loading of clomipramine followed by placebo was positively correlated with the amount of REM rebound. Similarly, Gillin et alCitation9 noted that patients who improved during treatment with amitriptyline exhibit a clear REM sleep rebound during withdrawal, whereas patients with no improvement show no such REM sleep rebound. Induction of cytokine synthesis and fever has been shown to suppress REM sleep and improve mood in patients with major depression.Citation12 Finally, some studies showed that increased REM activity (ie, more rapid eye movements occurring during REM sleep) identify depressive patients who do not respond to psychotherapy and may warrant somatic treatment.Citation13,Citation14
The results of some studies cast doubt on the value of REM suppression as a predictor of antidepressant response. For instance, data suggest that effective long-term pharmacotherapy of recurrent major depression with imipramineCitation15 or nortriptylineCitation16 is associated with higher REM activity than that observed in patients relapsing while receiving these drugs. Other studies were unable to demonstrate a consistent relationship between REM decreases and the alleviation of depression during treatment with antidepressants.Citation17-Citation19 The REM suppressant effect may play an important role in the mechanism underlying treatment response, but is insufficient for use in prediction.
It is also not clear whether changes in NREM sleep, including SWS, are related to improvement in depression. Quantification of NREM sleep changes by visual scoring of sleep EEG in terms of changes in duration or proportion may be insufficient for detection of such a relationship. A more accurate method may be to investigate whether clinical response is related to drug-induced modification of sleep microstructure. For instance, the number of transient polysomnographic activations suggestive of an awake state (ie, microarousal) occurring during stage 2 observed after the first doses of doxepine was found to be positively associated with antidepressant response.Citation20 Other studies have shown that methods involving spectral analysis of NREM sleep are useful for prediction of clinical responsiveness to antidepressants.
Power spectral analysis and antidepressant response
A classical way to describe an EEG signal is in terms of frequency of the common EEG bands. One of the most useful methods to decompose EEG signals into frequency components is Fourrier analysis, and the fast Fourrier transform (FFT) algorithm has been extensively used in EEG analysis. In FFT spectral analysis, signal intensity is calculated per bandwidth and a power spectral density can be obtained for each frequency band of interest.
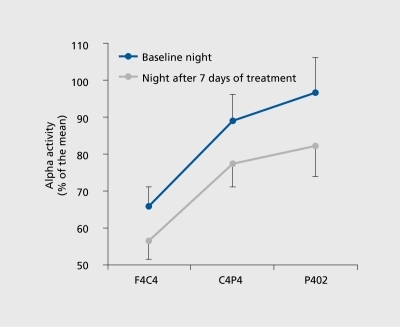
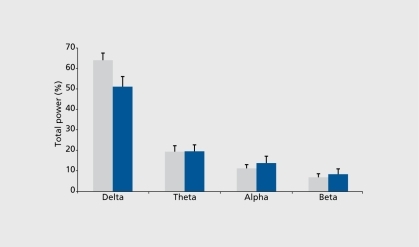
Predicting clinical response through the sleep EEG spectral analysis of depressed patients has received little interest to date. The few studies on the topic showed that high pretreatment δ activityCitation5,Citation21 and redistribution of δ activity to the earlier part of the night may predict ultimate clinical response. Citation22 For instance, we have shown a clear relationship between baseline sleep EEG spectral density values and clinical response (). Another study has shown that different forms of δ activity can distinguish between acute depression and the risk of recurrence in previously recovered patients. On the basis of fundamental studiesCitation23 showing that the δ bandwidth (thought to be generated in thalamic nuclei) also contains slow oscillations (0.5-1 Hz) originating in the cortex, Buysse et alCitation24 demonstrated that high δ activity (2-3 Hz) was more related to the acute depressive state, while the lower frequencies (0.5-1 Hz) were linked to risk of recurrence. Other research efforts focused on the significance of a frequency during REM sleep. In a topographic study, we found that REM α power spectra are reduced after antidepressant administration in healthy volunteers (),Citation25 a finding that needs to be extended to depressed patients in order to assess its potential predictive value.
Another promising direction for future research is the study of change in dynamic relationships, or coherence, between frequency ranges.Citation26 Coherence evaluates the strength of covariation between two frequency rhythms: if two frequencies have high coherence, they are likely to be controlled by the same or similar timing mechanism. In this context, Armitage et alCitation27 reported that β and δ rhythms were less coherent in depressive patients than in healthy controls, and Röschke et alCitation28 suggested that 4 weeks' administration of paroxetine in healthy volunteers significantly increased coherence between β and δ frequencies.
Conclusion
Clinical research on the influence of antidepressant drugs on sleep microarchitecture will become increasingly important for interpreting the effects of antidepressants on sleep physiology and for the development of new antidepressant therapies. In this regard, spectral EEG sleep profiles represent a promising tool for the prediction of clinical response to antidepressant treatment.
REFERENCES
- DijckDJ.CzeislerCA.Paradoxal timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans.Neurosci Lett.199416663688190360
- BorbelyAA.Wirz-JusticeA.Sleep, sleep deprivation and depression. A hypothesis derived from a model of sleep regulation.Hum Neurobiol.198212052107185793
- BencaRM.ObermeyerWH.ThistedRA.GillinJC.Sleep and psychiatric disorders. A meta-analysis.Arch Gen Psychiatry.1992496516681386215
- BorbelyAA.ToblerI.LoepfeM.et al.All-night spectral analysis of the sleep EEG in untreated depressives and normal controls.Psychiatry Res.19841227336589657
- KupferDJ.FrankE.EhlersCL.EEG sleep in young depressives: first and second night effect.Biol Psychiatry.19892587972912511
- ArmitageR.EmslieGJ.HoffmannRF.RintelmannJ.RushJA.Delta sleep EEG in depressed adolescent females and healthy controls.J Affect Disord.20016313914811246090
- KupferDJ.UlrichRF.CoblePA.et al.Applications of an automated REM and slow-wave sleep analysis. II. Testing the assumptions of the two-process model of sleep regulation in normal and depressed subjects.Psychiatry Res.1984133353436596589
- StanerL.CornetteF.MauriceD.et al.Sleep microstructure at sleep onset differentiates major depressive insomnia from primary insomnia.J Sleep Res.20031231933014633244
- GillinJC.WyattRJ.FramD.et al.The relationship between changes in REM sleep and clinical improvement in depressed patients treated with amitriptyline.Psychopharmacology.197859267272216045
- HochliD.RiemannD.ZulleyJ.et al.Initial REM sleep suppression by clomipramine: a prognostic tool for treatment response in patients with major depressive disorder.Biol Psychiatry.198621121712203756267
- KupferDJ.ReynoldsCF III.EhlersCL.Comparison of EEG sleep measures among depressive subtypes and controls in older individuals.Psychiatry Res.19892713212922441
- BauerJ.HohagenF.GimmelE.et al.Induction of cytokine synthesis and fever suppresses REM sleep and improves mood in patients with major depression.Biol Psychiatry.1995386116218573663
- ThaseME.SimonsAD.ReynoldsCF III.Abnormal electroencephalographic sleep profiles in major depression. Association with response to cognitive behavior therapy.Arch Gen Psychiatry.199653991088629894
- BuysseDJ.TuXM.CherryCR.et al.Pretreatment REM sleep and subjective sleep quality distinguish depressed psychotherapy remitters and non-remitters.Biol Psychiatry.1999452052139951568
- KupferDJ.PollockBG.PerelJM.et al.Effect of pulse loading with clomipramine on EEG sleep.Psychiatry Res.1994541611757761550
- ReynoldsCF III.BuysseDJ.BrunnerD.et al.Maintenance nortriptyline effects on electroencephalographic sleep in elderly patients with recurrent major depression: double-blind, placebo- and plasma-level-controlled evaluation.Biol Psychiatry.1997425605679376452
- WareJC.BrownFW.MooradPJ Jr.et al.Effects on sleep: a double-blind study comparing trimipramine to imipramine in depressed insomniac patients.Sleep.1989125375492688037
- StanerL.KerkhofsM.DetrouxD.et al.Acute, subchronic, and withdrawal sleep EEG changes during treatment with paroxetine and amitriptyline: a double-blind randomized trial in major depression.Sleep.1995184704777481419
- Van BemmelAL.BeersmaDGM.Van Den HoofdakkerRH.Changes in EEG power density of non-REM sleep in depressed patients during treatment with trazodone.J Affect Disord.19953511198557883
- StaedtJ.HünerjägerH.RütherE.et al.Sleep cluster arousal analysis and treatment response to heterocyclic antidepressants in patients with major depression.J Affect Disord.1998492212279629952
- LuthringerR.MinotR.ToussaintM.et al.All-night spectral analysis as a tool for the prediction of clinical response to antidepressant treatment.Biol Psychiatry.199538981047578656
- EhlersCL.HavstadJW.KupferDJ.Estimation of time course of slow-wave sleep over the night in depressed patients: effects of clomipramine and clinical response.Br J Psychiatry.199639171181
- SteriadeM.Mc CormickDA.SejnowskiTJ.Thalamocortical oscillations in the sleeping and aroused brain.Science.19932626796858235588
- BuysseDJ.FrankE.LoweKK.et al.Electroencephalographic sleep correlates of episode and vulnerability to recurrence in depression.Biol Psychiatry.1997414064189034535
- ToussaintM.BrandenbergerG.StanerL.LuthringerR.MacherJP.Topography of EEG alpha activity during sleep before and after 1 week of fluvoxamine treatment.J Sleep Res.20009(suppl 1)190
- ArmitageR.Microarchitectural findings in sleep EEG in depression: diagnostic implications.Biol Psychiatry.19953772847718683
- ArmitageR.RoffwargH.RushA.et al.Digital period analysis of EEG in depression: periodicity, coherence, and interhemispheric relationships during sleep.Prog Neuropsychopharmacol Biol Psychiatry.1993173633728475318
- RöschkeJ.KögelP.SchlössserR.et al.Analysis of sleep EEG microstructure in subchronic paroxetine treatment of healthy subjects.Psychopharmacology.199713244479272758