Abstract
Major depression is believed to be a multifactorial disorder involving predisposing temperament and personality traits, exposure to traumatic and stressful life events, and biological susceptibility. Depression, both unipolar and bipolar, is a “phasic” disease. Stressful life events are known to trigger depressive episodes, while their influence seems to decrease over the course of the illness. This suggests that depression is associated with progressive stress response abnormalities, possibly linked to impairments of structural plasticity and cellular resilience. It therefore appears crucial to adequately treat depression in the early stages of the illness, in order to prevent morphological and functional abnormalities. While evidence suggests that a severely depressed patient needs antidepressant drug therapy and that a non-severely depressed patient may benefit from other approaches (ie, “nonbiological”), little research has been done on the effectiveness of different treatments for depression. The assertion that the clinical efficacy of antidepressants is comparable between the classes and within the classes of those medications may be true from a statistical viewpoint, but is of limited value in practice. The antidepressant drugs may produce differences in therapeutic response and tolerability. Among the possible predictors of outcome in depression treatment, those derived from clinical assessment, neuroendocrine investigations, polysomnographic sleep parameters, genetic variables, and brain imaging techniques have been extensively studied. This article also reviews therapeutic strategies used when initial treatment fails, and describes briefly new concepts in antidepressant therapies such as the regulation of disturbances in circadian rhythms. The treatment of depressive illness does not stop with treatment of acute episodes, and has to be envisaged as a continuous therapeutic intervention, of which we are still not able to determine the optimal duration of treatment and the moment that it should be ceased.
Se cree que la depresión mayor es un trastorno multifactorial que involucra el temperamento y los rasgos de personalidad como predisponentes, la exposición a acontecimientos vitales estresante y a sucesos traumáticos, y la susceptibilidad biológica. Tanto la depresión unipolar como la bipolar son enfermedades “fásicas”. Los acontecimientos vitales estresantes pueden gatillar episodios depresivos, y su influencia parece decrecer a lo largo del curso de la enfermedad. Esto sugiere que la depresión se asocia con anormalidades progresivas en la respuesta de estrés, lo que posiblemente se relaciona con deterioros de la plasticidad estructural y de la resiliencia celular. Por lo tanto, parece crucial tratar adecuadamente la depresión en las etapas precoces de la enfermedad para prevenir las alteraciones morfológicas y funcionales. Mientras la evidencia sugiere que un paciente con una depresión grave necesita fármacos antidepresivos y que un paciente con una depresión de menor gravedad puede beneficiarse con otras estrategias (es decir. “no farmacológicas”), se ha realizado poca investigación sobre la eficacia de los diferentes tratamientos para la depresión. La afirmación que señala que la eficacia clínica de los antidepresivos es comparable entre las clases y dentro de las clases de estos medicamentos puede ser cierta desde un punto de vista estadístico, pero tiene un valor limitado en la práctica. Los antidepresivos pueden produtir diferencias en la respuesta terapéutica y en la tolerabilidad. Entre los posibles predictores de evolución en el tratamiento de la depresión se han estudiado extensamente aquéllos que derivan de la evaluación clínica, de las investigaciones neuroendocrinas, de los parámetros de polisomnografía del sueño, de las variables genéticas y de las técnicas de imágenes cerebrales. Este artículo también revisa las estrategias terapéuticas utilizadas cuando fracasa el tratamiento initial y describe brevemente los nuevos conceptos en las terapias antidepresivas como la regulación de las alteraciones en el ritmo circadiano. El tratamiento de la enfermedad depresiva no finaliza con la terapia de los episodios agudos, y debe ser visualizado como una intervención terapéutica continua, acerca de la cual aun no estamos en condiciones de determinar la duración óptima del tratamiento, ni el momento en que éste debe finalizar.
La dépression majeure est considérée comme un trouble multifactoriel associant un tempérament prédisposé et certains traits de personnalité, une exposition à des événements de vie traumatisants et stressants et une sensibilité biologique. La dépression, à la fois unipolaire et bipolaire, est une maladie «phasique». Les événements de vie stressants sont connus pour déclencher les épisodes dépressifs, alors que leur influence semble diminuer au cours de la maladie. Ceci suggère que la dépression est associée à des anomalies de réponse progressive au stress, probablement liées à des troubles de plasticité structurale et de résilience cellulaire. Il apparaît donc crucial de traiter la dépression de façon adéquate aux stades précoces de la maladie pour éviter les altérations morphologiques et fonctionnelles. Peu de recherches ont été faites sur l'efficacité des différents traitements pour la dépression, alors que des preuves suggèrent qu'un patient sévèrement déprimé a besoin d'un traitement antidépresseur tandis que celui dont la dépression est moindre peut bénéficier d'autres approches (par ex. «non biologiques»). L'affirmation que l'efficacité clinique des antidépresseurs est comparable entre les classes et dans les classes de ces médicaments peut être vraie d'un point de vue statistique, mais pas en pratique. Les réponses thérapeutiques et de tolérance aux antidépresseurs sont différentes. L'évaluation clinique, les investigations neuroendocriniennes, les paramètres polysomnographiques du sommeil, les variables génétiques et les techniques d'imagerie cérébrale ont été largement étudiés parmi les indices possibles d'évolution des antidépresseurs. Cet article passe aussi en revue les stratégies thérapeutiques utilisées en cas d'échec du traitement initial et décrit rapidement les nouveautés concernant les antidépresseurs telles que la régulation des troubles du rythme circadien. Le traitement de la dépression ne s'arrête pas avec le traitement des épisodes aigus et doit être envisage comme un traitement continu, pour lequel la durée optimale et le moment de l'arrêt ne peuvent pas encore être déterminés.
Depression is an incapacitating disorder with a lifetime prevalence of 16%,Citation1 with a female-to-male ratio of about 5:2. Research is beginning to allow us to fully grasp the complexity of factors- personal, genetic, biological, societal, and environmental - which are involved. Several efficient treatments and strategies exist, among which antidepressant drugs are a main choice. Although the criteria for choosing the best strategy remain empirical - there is some indication that the efficacy of antidepressants is comparable between and within classes - most patients are best treated with a combination of antidepressants and psychotherapy, modulated according to the course of their illness.Citation2
Schematically, one may categorize the treatment of depression into three phases: acute, continuation, and maintenance.Citation3,Citation4 As summarized in Table I, each phase is defined by specific aims and strategies. Some aspects remain under discussion, especially those concerning the appropriate duration of long-term treatment.
The first decision the clinician has to make is whether to hospitalize a depressed patient or to schedule outpatient treatment. Hospitalization is indicated when there is a risk of suicide or homicide associated or not with a severe depression- in particular with psychotic features- a notion of “treatment resistance” (supporting in fact the concept of therapeutic inefficacy or inadequacy, needing therefore an alternative therapeutic strategy), the absence of a patient support system, or the need for complementary diagnostic procedures.
Table I. Phases of treatment of depression (adapted from refs 3, 4).
Clinical and biological assessment
Depression is both clinically and biologically a heterogeneous entity. Typically the course of the disease is recurrent - 75% of patients experience more than one episode of major depression within 10 years. Although most patients recover from major depressive episode, about 50% have an inadequate response to an individual antidepressant trial.Citation5 Moreover, a substantial proportion of patients (about 10%Citation6) become chronic (ie, 2 years without clinical remission) which then leads to severe and cognitive functional impairment as well as psychosocial disability.Citation7 Therefore, the assertion that the clinical efficacy of antidepressants is comparable between the classes and within the classes of those medicationsCitation8 may be true from a statistical viewpoint but is of limited value in practice. For a given patient, antidepressant drugs may produce differences in therapeutic response and tolerability.
In order to predict outcome, it appears essential to determine parameters that would rationalize the therapeutic choice, taking into account not only the clinical features but also the “biological state” which is a major determinant in the antidepressant response.
Clinical predictors
Typical symptoms of depression include depressed mood, diminished interest or pleasure (anhedonia), feelings of worthlessness or inappropriate guilt, decrease in appetite and libido, insomnia, and recurrent thoughts of death or suicide (in about half of patients). Up to 15% of patients with severe depression die from suicide.Citation9 Suicidal risk should be assessed not only at the initiation of the treatment, but repeatedly throughout treatment (typically this risk is increased during the first 2 weeks of treatment). In fact, it appears that the risk of suicide attempt does not differ among antidepressants, but the rate of death from overdose is higher with tricyclics (owing to their cardiotoxicity) than with nontricyclics.Citation10 This may have implications for the choice of an antidepressant for a depressed patient at risk for suicidal behavior. On the other hand, about half of suicide victims with major depression had received inadequate treatment.Citation11
It has been argued that all subtypes of depressive disorders may be an indication for antidepressants,Citation12 but the main “intuitive” criteria for prescribing antidepressants remains the severity of the depressive symptoms (eg, Hamilton Depression Rating Scale [HDRS] score >18). Indeed, some investigators have concluded that there is no evidence for greater effectiveness of a standard reference treatment (ie, imipramine plus clinical management) versus brief psychotherapies (interpersonal therapy, cognitive behavioral therapy) or placebo plus clinical management for the less severely depressed and functionally impaired outpatients.Citation13 On the other hand, greater depression severity at baseline generally predicts a poorer response to pharmacotherapy or psychotherapy.Citation14,Citation15
Many prospective studies have been published - with conflicting results - on the predictive value of clinical variables such as temporal aspects (age at onset, duration of the disorder, number of recurrences), treatment-related variables (out- or inpatients, number of prior treatments, nature of treatment, dosage, duration, and compliance), demographic characteristics (age, gender), social and family variables (marital status, social support), and comorbidity (eg, Axis II personality disorders; Axis I comorbidity such as anxiety, especially panic disorder and/or substance and alcohol abuse; Axis III such as hypothyroidism, diabetes, stroke, coronary artery disease, Parkinson's disease, cancer, immunodeficiency syndromes, and chronic pain syndrome [for review see ref 4]). Comorbidity is generally considered to be a factor contributing to poor treatment response.
However, some clinical features may lead to specific strategies. For example, psychotic depression, representing over 15% of severely depressed patients, generally does not respond favorably to antidepressant monotherapy. Initial studies have shown that tricyclic antidepressants (TCAs) combined with typical antipsychotics have greater efficacy than TCAs alone.Citation16 More recently, selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs) combined with typical or atypical antipsychotics, have demonstrated efficacy in psychotic depression.Citation17 The antipsychotic medication can be tapered off and stopped when psychotic symptoms have subsided (generally after 1 to 3 months). Electroconvulsive therapy (ECT), despite many drawbacks, remains indicated in some “refractory” cases.Citation18
Bipolar depression also requires specific strategies, since response to antidepressant treatment is often partial. Indeed, both TCAs and SSRIs are moderately efficacious in this population.Citation19 Moreover, manic switch may occur (substantially more often with TCAs [approximately 11%] than SSRIs [both approximately 4 %]Citation20,Citation21). Therefore, it is suggested to use a mood stabilizer (carbamazepine/oxcarbazepine, valproate, lithium carbonate, lamotrigine) as the first-line therapy at an optimal dose (and drug plasma concentration when available) and to add an antidepressant in case of partial/nonresponse. The recommended period of mood stabilizer monotherapy is 1 monthCitation4; it is also expected that the mood stabilizer treatment would prevent a switch into mania when an antidepressant is added. Recent trials suggest that SSRIs and SNRIs combined with atypical antipsychotics are also efficacious (for review see ref 22). Olanzapine and risperidone, for example, are now approved for maintenance treatment in bipolar disorder.
Biological predictors
During the past years there has been increased interest in the identification of biological predictors of outcome in depression. Among the possible predictors, those derived from neuroendocrine investigations, polysomnographic sleep parameters, genetic variables, and brain imaging techniques have been extensively studied.
Neuroendocrine investigations
These predictors can be measured at baseline (ie, after a sufficient drug-withdrawal period) and/or during the course of treatment.
The hypothalamic-pituitary-adrenal (HPA) axis in the psychobiology of depression has been widely documented (for review see ref 23). Increased Cortisol secretion, failure to suppress Cortisol in response to dexamethasone- the dexamethasone suppression test (DST) and increased corticotropin (ACTH)/cortisol response to the combined dexamethasone/corticotropin-releasing hormone (DEX/CRH) test have been consistently associated with severe depression.Citation24-Citation27 It has been hypothesized that this stress axis overdrive is primarily a reflection of abnormal limbic-hypothalamic activation, with chronic increased secretion of corticotropin releasing hormone (CRH) and consequent excessive adrenal Cortisol secretion linked to impaired negative feedback at the level of the pituitary corticotroph (ie, decreased type II glucocorticoid receptor function). The presence of an abnormal DST or DEX/CRH test indicates the need for a biological treatment, while the initial status of these tests has no predictive value in the choice of given antidepressants.Citation28 Serial DST or combined DEX/CRH test monitoring of depressed patients undergoing drug treatment showed that normalization typically precedes or coincides with (rather than follows) clinical recovery, and failure to normalize portends poorly for clinical outcome.Citation29-Citation31 These results suggest that lowering HPA activity - via the restoration of type II glucocorticoid receptor function with a subsequent re-establishment of HPA axis negative feedback-and clinical response are related.
The hypothalamic-pituitary-thyroid (HPT) axis is often abnormal in depression. The main abnormality is a chronobiological dysfunction of this axis as reflected by the decreased mean and amplitude of the 24-hour thyroid-stimulating hormone (TSH) secretionCitation32,Citation33 and the blunted TSH test (ie, difference in ∆∆TSH response between 11 PM and 8 AM protirelin [TRH] tests) in about 80% of depressed inpatients.Citation34 When HPT dysregulation is more pronounced, the 11 PM TRH-TSH test is also abnormal (in about 40% of patients), while the 8 AM TRH-TSH test is blunted in only a minority of inpatients (<20%). It has been suggestedCitation34 that blunted TRH-induced TSH stimulation might be a reflection of downregulation of the thyrotropin-releasing hormone (TRH) receptors in the pituitary gland secondary to a prolonged increase in hypothalamic TRH stimulation. Furthermore, the shift to higher iodothyronine levels in euthyroid depressed inpatients, both in the morningCitation35 and in the eveningCitation36 may contribute to the blunting of TSH response to TRH.Citation37,Citation38 The presence of a blunted TRH test or an abnormal ∆∆TSH indicates the need for a biological treatment, while the initial status of these tests has no predictive value in the choice of given antidepressants. However, patients with the lowest pretreatment evening TSH secretion (basal and after 11 PM TRH stimulation) have been found to have the lowest rate of antidepressant response.Citation39 The reduced ∆∆TSH values is a “state” marker of depression since its normalization is associated with remission.Citation39 Conversely, persistence of blunted responses (ie, 8 AM-∆TSH and/or 11 PM-∆TSH and/or ∆∆TSH) during remission could represent a “vulnerability” marker of depression.
Investigation of the noradrenergic (NA) system. One of the most consistently reported abnormal findings in depression is a blunted growth hormone (GH) response to acute administration of clonidine, a partial α2-adrenoreceptor agonist, in drug-free patients. This abnormality suggests subsensitive postsynaptic α2-adrenoreceptors at the hypothalamic level, via growth hormone-releasing hormone release, linked to an erratic release of norepinephrine.Citation40 This finding has led to a reformulation of the original NA depletion hypothesis of depression into the “noradrenergic dysregulation hypothesis,”Citation40 which emphasizes a primary subsensitivity or downregulation in nerve terminal α2-adrenoreceptors, leading to impaired negative feedback on the presynaptic neuron, which in turn may induce a disinhibition of NA output and exaggerated NA release in response to any activation of the catecholaminergic system. However, blunted GH response to clonidine does not appear specific to depression but rather to the “anxiety spectrum,” since this blunting has also been observed in generalized anxiety disorder,Citation41 panic disorder,Citation42,Citation43 and social phobia.Citation44 It has also been argued that deficiencies in noradrenergic function could lead to differential response to noradrenaline and serotonin reuptake inhibitors.Citation45 In a study by Coote et alCitation46 the decreased GH response, before treatment, was correlated with subsequent good clinical response to desipramine (a “noradrenergic” antidepressant). In a recent study, Correa et alCitation47 reported that amitriptyline, which primarily increases noradrenergic function, was more efficacious than fluoxetine in depressed patients showing blunted GH to clonidine at baseline (amitriptyline is at least 100 times most potent than fluoxetine in the inhibition of the noradrenaline transporterCitation48). Taken together, these results suggest that the noradrenergic function might influence response to antidepressant treatment.
Polysomnographic sleep parameters
Depression is often associated with difficulties in initiation and maintenance of sleep, as well as poor subjective quality of sleep. Although a low level of correspondence between subjective complaints of sleep and objective measurements, alterations can be observed polysomnographically in approximately 90% of depressed patients (for review see ref 49). Many of the sleep abnormalities in depression also occur in other psychiatric disorders. The most characteristic alterations in the sleep electroencephalogram (EEG) during major depression are a shortened latency to rapid eye movement (REM) sleep and an increase in REM density. These changes might represent vulnerability markers. Recently it has been reported that the increased REM density was observed not only in depressed patients, but also in their healthy relatives who subsequently developed an affective disorder.Citation50 Furthermore, increased REM density has been found to be predictive for the occurrence of recurrences in follow-up and has been related to excessive stress hormone response in the DEX/CRH-test (owing to HPA axis overdrive).Citation51 This suggests that EEG and HPA disturbances may reflect important mechanisms responsible for causing and maintaining the disease process of depression.
Antidepressants have class- and compound- specific effects on polysomnographic profiles. Most antidepressants (eg, TCAs, SSRIs) induce a decrease or suppression of REM sleep and increase REM sleep onset latency.Citation52 The decrease in amount of REM sleep appears to be greatest early in treatment, and gradually diminishes during long-term treatment. On the other hand, some antidepressants such as bupropion may increase or intensify REM sleep. Sleep initiation and maintenance are also affected by antidepressants. Some antidepressants such as the SSRIs (particularly fluoxetine) and the SNRIs (particularly venlafaxineCitation53) may be sleep disturbing early in treatment, and some others such as amitriptyline, mianserine, and the newer serotonin (5-HT)2-receptor antagonists (eg, nefazodone, mirtazapine), are sleep-promoting. This may be an important clinical goal in some patients. Generally the sleep of depressed patients (ie, objective measures, and subjective impression) improves over 3 to 4 weeks of effective antidepressant treatment with most agents. The new antidepressant agomelatine, a melatonergic MT1/MT2 receptor agonist with 5-HT2c antagonist properties,Citation54,Citation55 has shown beneficial effects on sleep in depressed patients, with reorganization of sleep architecture and without sedative or hangover effects from the first week of treatment.Citation56-Citation58 Patients with other sleep disorders such as restless legs syndrome should be identified before choosing a treatment, as some antidepressants (such as TCAs, SSRIs) may worsen this syndrome.
Genetic variables
Pharmacogenetics (ie, the variability in drug response and metabolism due to genetic variants) may explain in part the heterogeneity in response to antidepressant drugs.Citation59 Antidepressant targets are primarily the monoaminergic neurotransmitter systems (eg, 5-HT, NA, and to a lesser extent dopamine [DA]) by inhibiting (more or less specifically) neurotransmitter transporters or receptors, leading to an increase in synaptic levels of monoamines.
For example, the serotonin transporter (5-HTT) is a target of SSRIs, SNRIs, and most TCAs. It has been found that the short (S) allele reduces the transcriptional activity of the 5-HTT gene promoter, leading to reduced 5-HTT expression and 5-HT uptake.Citation60 Patients carrying the S allele are more vulnerable to stress and depression.Citation61,Citation62 In a Caucasian population, the 5-HTT promoter polymorphism seems to play a role in the response to SSRIs: the S/S genotype has been associated with poor response to citalopram and fluvoxamine, while the individuals carrying at least one L allele were good responders to fluvoxamine and paroxetine.Citation63,Citation64 However, in an Asian population, the S/S genotype was associated with good response to antidepressant treatment, suggesting complex interactions between 5-HTT variants and treatment response according to the ethnicity of the population studied. Discrepant results have also been reported concerning other functional gene variants coding for the NA and DA systems (for review see ref 59).
Concerning the drug-metabolizing enzymes, those of the cytochrome P-450 (CYP) family are largely involved in the phamacokinetic/pharmacodynamic variability of the antidepressants. Inter- and intraindividual differences in activity of the CYPs are due to genetic variants, but the CYP activity may be induced or inhibited by some drugs or environmental factors (for review see ref s 65,66). All the interactions have significant effect on the bioavailability of the antidepressant drugs when such drugs and/or environmental factors are combined. In some specific cases (treatment inefficacy, severe adverse effects [eg, confusion]) CYP genotyping (which is not influenced by environmental factors and represents a “trait marker”) and/or phenotyping (which represents a “state marker”) may be indicated in association with plasma drug concentration.
Brain imaging techniques
Structural brain imaging studies have revealed abnormalities in major depression. Among the most consistent abnormalities are enlarged lateral ventricles, decreased size of certain brain structures involved in the modulation of emotional behavior (eg, hippocampus, frontal lobe volume, basal ganglia,)Citation67 and increased subcortical white matter hyperintensity (SCH).Citation68,Citation69 SCH has been related to poor treatment response and thus might have some value in clinical decision-making.Citation70
Functional brain imaging studies have shown decreased blood flow and metabolism in the the frontal cortex, temporal cortex, cingulate gyrus, basal ganglia, amygdala, hippocampus, and thalamus. Older studies had found that increased activity in the cingulate gyrus at rest was predictive of a good response to sleep deprivationCitation71-Citation73 or treatment with fluoxetine.Citation74 Abnormalities in regional cerebral blood flow are at least partly reversed by antidepressant treatment including SSRIs,Citation75 SNRIs (eg, venlafaxineCitation76), and vagus nerve stimulation.Citation77
Toward an integrative model of depression: therapeutic implications
Major depression is believed to be a multifactorial disorder including predisposing temperament and personality traits, exposure to traumatic and stressful life events, and biological susceptibility. Depression, both unipolar and bipolar, is characterized by recurrence of mood episodes, and consequently may be seen as a “phasic” disease. Stressful life events that involve loss, threat, humiliation, or defeat are known to trigger depressive episodes, while their influence seem to decrease over the course of the illness.Citation78,Citation79
This has suggested that depression is associated with progressive stress response abnormalities possibly linked to impairments of structural plasticity and cellular resilience.Citation80 We will review the kindling hypothesis and the HPA axis hypothesis of depression, and will describe a recent theory of the pathogenesis of depressive illness, namely neuro plasticity. Furthermore, another promising area of research in depression, resynchronization of circadian rhythms and its therapeutic implications will be briefly commented on.
The kindling hypothesis
The primary conceptual framework to some of the phenomena of illness initiation and progression is the “kindling“ hypothesis,Citation81,Citation82 inspired by temporal and developmental similarities between the clinical course of affective disorders and that of seizure disorders. Kindling is a form of sensitization of the brain tissue (eg, limbic and other subcortical areas) leading to functional and structural alteration, including the induction of gene transcription factors such as c-fos. Induction of c-fos leads to neurochemical changes at neurotransmitter and receptor levels.
The kindling model proposes that certain types of stressors, repetitively experienced in a predisposed individual, will lead to mood symptoms of increasing intensity and duration until a full-blown depressive (or manic episode) occurs. This model may explain some of the key aspects of depression: () (i) the first lifetime episodes are more strongly associated with major life stress than are successive recurrences; (ii) the severity and duration of the nontreated episodes increase with clinical course (the corollary of this is a possible treatment resistance); and (iii) the interval between episodes decreases with the duration of the illness. This hypothesis has nevertheless its limitations, although has recently regained some interest.Citation83
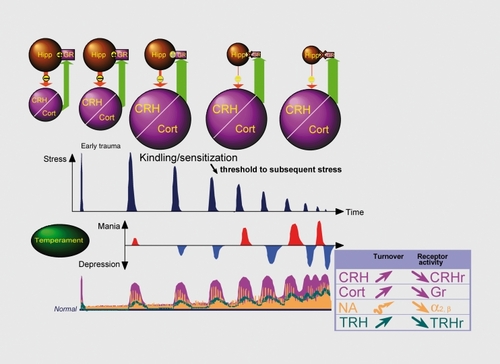
The hypothalamic-pituitary-adrenal (HPA) axis, stress, and depression
Classically, stress is defined as a threat to homeostasis, to which the organism, in order to survive, responds with a large number of adaptive responses implicating the activation of the sympathetic nervous system and the HPA axis. Several forebrain structures, including the prefrontal cortex, hippocampus, amygdala, and septum have been shown to influence stress responsivity. Synaptic inputs from several brain regions converge on the paraventricular nucleus in the hypothalamus, which is the final integrator of the stress response. Neurons of this nucleus produce CRH leading to behavioral activation and to the secretion of adrenocorticotropin (ACTH) from the anterior pituitary gland. ACTH elicits release of Cortisol from the adrenal cortex. Cortisol inhibits its own release by inhibiting the secretion and synthesis of ACTH at the level of the pituitary and of CRH at hypothalamic and upstream sites. Thus, the HPA system is the key effector of the stress response, and it has been demonstrated that chronic exposure to heightened glucocorticoid levels can lead to permanent changes in the HPA axis. Damage to the hippocampus, as a result of the reduction in cellular density and glucocorticoid receptors, impairs the negative feedback system that dampens HPA activation.Citation84 Moreover, clinical and experimental data suggest that glucocorticoids affect the activity of catecholamineCitation85,Citation86 and thyroidCitation87 systems, which have consistently been found to be dysregulated in depression.Citation88-Citation90 A recent neuroendocrine study, conducted in a selected sample of unipolar depressed inpatients with melancholic and psychotic features,Citation91 supports a pathophysiological link between hypercortisolemia and dysregulation of the NA, dopamine (DA), and HPT systems.
Interestingly, there is accumulating evidence (for review see ref 92) that TRH is a key central nervous system (CNS) homeostatic modulator. TRH not only regulates thyroid axis activity, but owing to its large distribution in the CNS (especially in limbic-cortical regions) TRH is also involved in regulation of many neurotransmitters (eg, NA, DA, 5-HT, acethylcholine). In depression TRH hypersecretion (as reflected by TRH-TSH abnormalities) may be regarded as a compensatory mechanism in order to correct neurotransmitter alterations (particularly those involving 5-HT and NA systemsCitation91,Citation93). TRH also modulates a variety of vegetative and chronobiological functions and has a role in the adaptative response to stress. The homeostatic properties are further suggested by the fact that TRH is an anticonvulsifiant (TRH is stimulated by kindling and seizures and TRH inhibits seizure), analeptic (only when the organism is sedated), promnesic (TRH increases learning and memory) and antiapoptotic. Finally, previous studies have shown that TRH has antidepressant effectsCitation94,Citation95 but owing to its short half-life (about 3 minutes) and the uncertain ability to the peptide to gain access to the CNS after peripheral administration inconsistent findings have been reported with native TRH.
HPA axis and neural plasticity: implications for antidepressant treatment
Recent studies have shown that chronic or cumulated stress induce neuronal atrophy in some vulnerable brain structures such as the hippocampus (atrophy of CA3 pyramidal neurons, decreased number and length of apical dendrites) and decreased neurogenesis (for reviews see refs 84,96). Reduced volume has been associated with duration of depression and cognitive difficulties such as impairment list learning and a specific recollection task.Citation97 The relationship between hippocampus volume and illness duration further suggests that it is possible to stop or delay progression of the morphological changes associated with depression. Indeed, it has been shown that antidepressant treatment (eg, tianeptine, TCAs, SSRIs, ECT) increases neural plasticity at the level of neurogenesis (by increasing the number of newborn neurons contributing to reverse hippocampal atrophy), signal transduction and gene expression (for review see refs 84,96). Since decreased hippocampal volume has been correlated with duration of depressive illnessCitation97,Citation98 and CRH has a critical role in long-term effects of early-life stress on hippocampal integrity and functionCitation99 it is suggested that chronic hypercortisolemia associated with alterations in neuroplasticity and neurogenesis may underlie the vulnerability to subsequent depressive episodes.
It appears therefore crucial to adequately treat depression in the early stages of illness in order to prevent morphological and functional abnormalities.
Circadian rhythms and depression
Depressive disorder is characterized by a profound disturbance of circadian rhythms, mainly characterized by a reduction in the amplitude.Citation100 This flattened amplitude has been reported for temperature, TSH, plasma melatonin, Cortisol, and motor activity. Most importantly, in depression, the deregulation of circadian rhythms is reflected in disturbed sleep-wake cycles.Citation49 It has been shown that circadian rhythms are normalized during remission and that unavoidable disturbances in circadian rhythms can trigger depressive episodes in humans.Citation101 This could suggest that circadian abnormalities may play a role in the pathogenesis of depression. Agomelatine, a melatonergic receptor agonist and 5HT2c receptor antagonist, resynchronizes human circadian rhythms in healthy volunteersCitation102,Citation103 and depressed patients,Citation57 and has shown a powerful antidepressant efficacy in major depressive disorder.Citation104-Citation106 The wide prevalence of circadian dysfunction in depression and the improvement of depression after treatment with this new antidepressant add to the suggestion that the circadian abnormalities may be part of the core of depression, rather than a consequence of the illness.
Acute treatment
The choice of the antidepressant treatment (Table II) needs to be tailored to the particular patient's medical condition and personal preferences. Evidence suggests that a severely depressed patient needs antidepressant drug therapy, while a while a nonsevere patient with major depression may benefit from other approaches (ie, “nonbiological”). However, little research has been done on the effectiveness of different treatments for depression, and the fact that clinicians can individually predict the evolution of patients has been rarely studied.Citation107 In some cases specific treatment may be recommended. For example, bright light (BL) treatment is indicated in seasonal affective disorder and depression during pregnancy.Citation108 The probable mechanisms of action of BL treatment are synchronization of biological rhythms and increase in serotonin transmission in the human brain. In general this treatment is safe and well tolerated.Citation109
Table II. Specific depression subscales derived from the HAM-D by the microanalytic approach. SRI, Serotonin reuptake inhibitor; NRI, Noradrenaline reuptake inhibitor; DRI, Dopamine reuptake inhibitor; MAOI, monoamine oxidase inhibitor
How and when should antidepressants be prescribed?
Optimal treatment starts with appropriate patient education about the nature of the illness and the nature of the proposed treatment. Specific psychological treatments are effective for major depression, with greatest evidence for mild-to-moderate depression, while no specific psychotherapy emerges as being superior to others. In moderate depression, the decision to prescribe an antidepressant can be taken over the course of a few weeks” In severely or recurrently depressed patients, the use of antidepressants is recommended, since the neurobiological substrate is too severely disturbed to be responsive to psychotherapy alone.Citation110
Given the supposed equivalence of therapeutic effect, the choice of antidepressant drug is based on the type of symptomatology as well as severity of the symptoms, avoidance of side effects (eg, sedation, weight gain, sexual dysfunction), presence of comorbid psychiatric and/or somatic disorders, prior positive and/or negative response (and tolerability/adverse effects) to a given antidepressant. Other considerations are the contraindications and potential toxicity of the drug and, to a lesser degree, its cost. Moreover, patient preference- after being informed about the benefit-risk ratio - may be expected to enhance compliance.
It has been suggested that SSRIs are more effective than primarily noradrenergic antidepressants (eg, maprotiline) in reducing irritability/aggression and anxious symptoms.Citation111-Citation114 On the other hand, severely depressed patients with psychomotor retardation respond more favorably to treatment with noradrenergic antidepressants than with SSRIs.Citation115 Some studiesCitation116 suggest that monoamine oxidase inhibitors (MAOIs) are highly effective in out-patients with “atypical depression” (characterized by fatigue, excessive need for sleep, increased appetite/weight gain, and rejection sensitivity). However, given the dietary restriction needed and the numerous interactions with other drugs, MAOIs remain a second-line treatment in this group of patients.
Drug selection
Evidence-based guidelinesCitation117,Citation118 suggest that newer antidepressants, mainly SSRIs, should be prescribed in mildly and moderately depressed patients, and TCAs or venlafaxine in severely depressed patients. Controversy exists about whether the newer antidepressants are as effective as the TCAs. Some meta-analyses,Citation119-Citation122 but not all,Citation123 indicate that TCAs (eg, amitriptyline, clomipramine) and venlafaxine (at a dose of 150 mg or greater) are more effective than SSRIs (citalopram, paroxetine) or moclobemide in severely depressed inpatients. Although this is not unanimously admitted, the efficacy of TCAs is related to the administered dose (a dose of 150 mg/day is more effective than 75 mg/day in severe depression). However, owing to their anticholinergic adverse effects (such as dry mouth, constipation, blurred vision, impaired concentration, and confusion) TCAs are usually prescribed below recommended doses (for review see ref 4). Thus, in order to minimize adverse effects, TCAs have to be started at a low dose and increased gradually (every 3 to 7 days); the delayed clinical response also makes it difficult to establish the optimal dose quickly (TCAs take 2 to 4 weeks before an antidepressant effect is evident).
On the other hand, SSRIs and newer antidepressants are better tolerated than TCAs and are safer in overdose. Moreover, their dose formulation tends to ensure adequate dosing, and they can be administered at the recommended dose after a few days of treatment at a lower dose. The adverse reactions associated with the SSRIs (eg, nausea, diarrhea, anxiety, agitation, sexual dysfunction, insomnia, and anorexia) may also occur with SNRIs. Some drugs have specific adverse effects, such as hypertension with venlafaxine and agranulocytosis with mianserin. It is noteworthy that mirtazapine, induce weight gain.
New treatment options such as agomelatine will have to be taken into consideration, once available.
Short-term outcome
It is important to review patients regularly (usually weekly for the first 4 to 6 weeks) to monitor response, compliance, side effects with treatment, and suicidal ideation. Adverse effects are often dose-dependent, and a dose reduction may alleviate the problem. Moreover, tolerance occurs for many of the acute adverse effects of the antidepressants, probably as a result of receptor downregulation. For this reason, the dose can be gradually re-escalated, if needed for optimal efficacy, without the adverse effect necessarily recurring. If there is no response (< 25% improvement) after 3 weeks it is possible to increase the dose, while in case of a partial response, one could wait another 2 weeks before increasing the dose.Citation124
What are the options in ease of nonresponse?
When a patient does not respond to the first-choice antidepressant at an adequate dose, three strategies exist: increasing the dose of the antidepressant, switching to another antidepressant, or combining several drugs.
Increasing the dose of the initial antidepressant,Citation118 although not recommended by all authors, seems a logical step, since there is wide interindividual variability in plasma concentration of antidepressants. Checking plasma concentrations of the antidepressant (target ranges are available for most drugs) and the parent compound/metabolite ratio may be helpful to evaluate the metabolite state and compliance of the patient.Citation125 For example, norfluoxetine is a more selective and more potent 5-HT reuptake inhibitor than fluoxetine (the parent compound) and has an extremely long half-life (7 to 15 compared with 1 to 3 days). Thus, the metabolite plays an important role for the therapeutic effect of fluoxetine. CYP 2D6 and CYP 2C9 polymorphisms contribute to the interindividual variability in fluoxetine and norfluoxetine pharmacokinetics at steady-state.Citation126 While some studies found no advantage of increasing the dose of fluoxetine (for review see ref 127), Fava and alCitation128 have reported a better outcome with 60 mg/day of fluoxetine in poor responders to 20 mg/day for 8 weeks.
Venlafaxine has a dual profile, predominantly serotonin reuptake inhibitor (SRI) at low doses (≤ 75 mg/day) and noradrenaline reuptake inhibitor (NRI) at higher doses (the maximum recommended dose is 375 mg/day). Interindividual variability has been reported with venlafaxine and its main active metabolite, O-desmethyl-venlafaxine - which also inhibits 5-HT and NA, and has comparable therapeutic activity to that of the parent drug.Citation129 It has been found, but not by all investigators, that there is a superior effect on depression of higher dose compared with a lower dose (but with more frequent adverse effects).Citation130 Two studies to date have used venlafaxine above the maximum recommended dose (450 to 600 mg/day) in treatment-resistant depression and both have shown clinical improvement.Citation131,Citation132 The tolerability was good (1 transient elevated blood pressure on 14 patients studied).
If it is decided to switch treatment, a drug with a different or broader mechanism of action should preferably be chosen. It may be necessary to have a drug-free interval before starting the new treatment to avoid drug interactions. Irreversible and nonselective monoamine oxidase inhibitors should be used only in special cases because of their potentially severe adverse effects.
Combining several drugs
The drugs most often added to antidepressant therapy are lithium, tri-iodothyronine, or, for patients receiving SSRIs, a compound acting on the NA and/or DA system. However, adding another antidepressant to the existing regimen may increase the risk of drug interactions (venlafaxine, SSRIs, or TCAs should not be combined with IMAOs, and fluoxetine should not been combined with TCAs). For nonresponders to SSRIs, buspirone/gepirone (both are 5-HT1A receptor agonists) or pindolol (a 5-HT1A receptor antagonist) have been used as adjunctive medication. The presumed mechanism of pindolol involves interruption in the short-loop negative feedback system, allowing for an increase in synaptic concentrations of 5-HT. The use of mood stabilizers is well documented in unipolar and bipolar patients (especially lithium in TCAs nonresponders), and two modalities of response have been described: one group responds during the first week, while the second responds after a delay of 4 to 6 weeks (for review see ref 133). The dose of lithium used in this strategy (ie, 450 to 600 mg/day) is generally lower than that used for the treatment of acute mania or prophylaxis of bipolar disorder. Similarly, plasma lithium levels are lower in the range of 0.4 to 0.8 mEq/L. However the risks associated with lithium augmentation compared with that of switching antidepressant drugs needs to be weighted. Concerning the antiepileptic drugs (such as carbamazepine/oxcarbazepine, valproate, lamotrigine) their efficacy as adjuvant therapy has been demonstrated in bipolar patients (especially in rapid-cyclers).
Thyroid hormones are useful in euthyroid patients for converting nonresponders into responders. It has been assumed that tri-iodothyronine (T3) would be preferentially indicated in unipolar patients (a 25-μg to 37.5-μg daily dose accelerates the time of response to antidepressants), while thyroxine (T4) combined with lithium would be useful in the prevention of mood episodes in bipolar patients (however the daily dose is generally high, about 200 to 400 μg, and this may lead to possible adverse effects [thyrotoxicosis]).
Dopamine agonists such as bromocriptine, pergolide, pramipexole, and ropinirole have been used with promising results as adjuvant to antidepressants especially in bipolar patients.Citation134 These agonists are also useful in depressed patients with Parkinson's disease and in patients with restless legs syndrome.
Atypical antipsychotics such as risperidone,Citation135 olanzapine,Citation136 and aripiprazoleCitation137 may also be useful as adjunctive medication in nonpsychotic treatment-resistant patients. Psychostimulants such as d-amphetamine, methylphenidate, and modafinil added to antidepressants have also been found to be effective in resistant depression.Citation138,Citation139
Electroconvulsive therapy (ECT) remains an option for resistant depression, although there is only a weak possibility that a given patient will respond to ECT if he or she has previously failed to respond to pharmacotherapyCitation140
Transcranial magnetic stimulation (which involves the depolarization of neurons in a localized area of the brain by applying a powerful magnetic field in rapid flux), vagus nerve stimulation, and deep brain stimulation have been proposed as alternatives to ECT.Citation141 The efficacy of these approaches is promising, but needs further confirmation.
Chronotherapeutics such as wake therapy- single or repeated sleep deprivation, total (all night) or partial (second half of the night) - and light therapy have been proposed as adjuvant to conventional antidepressants in unipolar patients, or lithium in bipolar patients (for review see ref 142).
“Antiglucocorticoid” treatments have been proposed, assuming that cortisol-lowering treatments may be of clinical benefit in patients refractory to traditional antidepressants (for review see refs 143,144). Ketoconazole, which inhibits Cortisol biosynthesis, and acts at the receptor level as a glucocorticoid antagonist, has led to mixed results: some authors have found antidepressant properties, while others, despite the inhibition of Cortisol, found only a weak impact on depression. Moreover, the numerous side effects of ketoconazole (including hepatotoxicity) mandate frequent laboratory monitoring.
Mifepristone (RU-486), a potent glucocorticoid and progesterone receptor antagonist, may be effective in the treatment of psychotic and bipolar depression and may re-regulate the HPA axis.Citation145 CRH1 receptor antagonists have therapeutic potential in disorders that involve excessive CRH activityCitation146 and some are currently under investigation as antidepressants (eg, antalarmin; CP-154,526; CP-36,311; GW876008; SSR125543; DMP 696; ONO-2333Ms; JNJ-19567470; R121919).Citation147
Conclusion
The treatments of depressive states are based on rational approaches involving the understanding of the pathophysiogenetic mechanisms and the mechanisms of action of the therapeutics. The noninversion of the mood has to be considered as therapeutic failure: the rule is to obtain the cessation of depressive symptoms and then the recovery from the episode.
Of course, the symptoms are cured but not necessary the illness; and the problem of eventual recurrence is still present.
The measures to prevent relapses require:
On the one hand, the perfect understanding of pathophysiogenesis of depressive illness, which is something we are not always able to do,
On the other hand, the use of chronic treatments for depression, which can be envisaged only if therapeutics having few or no side effects are available, and these need to be specifie. They can be normothymic drugs, but their side affects are not negligible. They can be antidepressant drugs; most of these have significant side effects. The use of agomelatine, a melatoninergic agonist with 5HT2c antagonist properties, can be emphasized, since this new antidepressant has been shown in long-term therapy to have antidepressant efficacy accompanied by good tolerance. In the more or less near future, products still in development (CRH1 receptor antagonists, TRH analogs) may be available, if they prove to be efficacious in clinical trials in depressed patients.
The treatment of depressive illness does not stop with treatment of acute episodes, and has to be envisaged as a continuous treatment; of which, for the moment, we are still not able to determine the appropriate duration and the time of treatment cessation.
Selected abbreviations and acronyms
| 5-HT | = | serotonin |
| ACTH | = | adrenocorticotropin |
| CRH | = | corticotropin-releasing hormone |
| DA | = | dopamine |
| HPA | = | axis hypothalamic-pituitary-adrenal axis |
| NA | = | noradrenaline |
| SNRI | = | serotonin and noradrenaline reuptake inhibitor |
| SSRI | = | selective serotonin reuptake inhibitor |
| TCA | = | tricyclic antidepressant |
REFERENCES
- KesslerRC.BerglundP.DemlerO.et al.National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R).JAMA.20032893095310512813115
- FrankE.NovikD.KupferDJ.Antidepressants and psychotherapy: a clinical research review.Dialogues Clin Neurosci.2005726327216156384
- PrienRF.KocsisJH.Long-term treatment of mood disorders. In: Bloom FE; Kupfer DJ, eds.Psychopharmacology: the Fourth Generation Of Progress. New York, NY: Raven Press;199510671079
- KupferDJ.The pharmacological management of depression.Dialogues Clin Neurosci.2005719120516156378
- TrivediMH.RushAJ.WisniewskiSR.et al.STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice.Am J Psychiatry.2006163284016390886
- KelllerMB.HirschfeldRM.HanksD.Double depression: a distintive subtype of unipolar depression.J Affect Disord.19974565739268776
- ZarateCA.TohenM.LandM.CavanaghS.Functional impairment and cognition in bipolar disorder.Psychiatry Q.200071309329
- American Psychiatric Association.Practice Guidelines for Patients With Major Depressive Disorder. 2nd ed. Washington, DC: American Psychiatric Association;2000
- DilsaverSC.ChenYW.SwannAC.ShoaibAM.KrajewskiKJ.Suicidality in patients with pure and depressive mania.Am J Psychiatry.1994151131213158067486
- KapurS.MieczkowskiT.MannJJ.Antidepressant medications and the relative risk of suicide attempt and suicide.JAMA.199226344134451460734
- IsometsaET.HenrikssonMM.AroHM.HeikkinenME.KuoppasalmiKl.LonnqvistJK.Suicide in major depression.Am J Psychiatry.19941515305368147450
- SchulzP.MacherJP.The clinical pharmacology of depressive states.Dialogues Clin Neurosci.20024475622034133
- ElkinI.SheaMT.WatkinsJT.et al.National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments.Arch Gen Psychiatry.1989469719822684085
- MollerHJ.FugerJ.KasperS.Efficacy of new generation antidepressants: meta-analysis of imipramine-controlled studies.Pharmacopsychiatry.1994272152237870742
- TedlowJ.FavaM.UebelackerL.NierenbergAA.AlpertJE.RosenbaumJ.Outcome definitions and predictors in depression.Psychother Psychosom.1998672662709693355
- SpikerDG.WeissJC.DealyRS.et al.The pharmacological treatment of delusional depression.Am J Psychiatry.19851424304363883815
- WijkstraJ.LijmerJ.BalkFJ.GeddesJR.NolenWA.Pharmacological treatment for unipolar psychotic depression: systematic review and meta-analysis.Br J Psychiatry.200618841041516648526
- DannonPN.LowengrubK.GonopolskiY.KotlerM.Current and emerging somatic treatment strategies in psychotic major depression.Expert Rev Neurother.20066738016466314
- ThaseME.SachsGS.Bipolar depression: pharmacotherapy and related therapeutic strategies.Biol Psychiatry.20004855857211018227
- PeetM.Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants.Br J Psychiatry.19941645495508038948
- VisserHM.Van Der MastRC.Bipolar disorder, antidepressants and induction of hypomania or mania. A systematic review.World J Biol Psychiatry.2005623124116272078
- YathamLN.GoldsteinJM.VietaE.et al.Atypical antipsychotics in bipolar depression: potential mechanisms of action.J Clin Psychiatry.200566(suppl 5)404816038601
- HatzingerM.Neuropeptides and the hypothalamic-pituitary-adrenocortical (HPA) system: review of recent research strategies in depression.World J Biol Psychiatry.2000110511112607206
- CarrollBJ.FeinbergM.GredenJF.et al.A specific laboratory test for the diagnosis of melancholia: standardisation, validation, and clinical utility.Arch Gen Psychiatry.19813815227458567
- APA Task Force On Laboratory Tests. The dexamethasone suppression test: an overview of its current status in psychiatry.Am J Psychiatry.19871412531262
- NelsonJC.DavisJM.DST studies in psychotic depression: a meta-analysis.Am J Psychiatry.1997154149715039356556
- HeuserI.YassouridisA.HolsboerF.The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders.J Psychiatr Res.1994283413567877114
- AnsseauM.PapartP.PitchotW.Timsit-BerthierM.LegrosJJ.von FrenckellR.Dexamethasone suppression test and the prediction of treatment response to selective antidepressants.Eur Psychiatry.19927191194
- GredenJF.GardnerR.KingD.GrunhausL.CarrollBJ.KronfolZ.Dexamethasone suppression tests in antidepressant treatment of melancholia.Arch Gen Psychiatry.1983404935006340634
- PeselowED.StanleyM.FilippiAM.BaroucheF.GoodnickP.FieveR.The predictive value of the dexamethasone suppression test: a placebo-controlled study.Br J Psychiatry.19891556676722532946
- Holsboer-TrachslerE.StohlerR.HatzingerM.Repeated administration of the combined dexamethasone-human corticotropin releasing hormone stimulation test during treatment of depression.Psychiatry Res.1991381631711661430
- DuvalF.MacherJP.MokraniMC.Difference between evening and morning thyrotropin response to protirelin in major depressive episode.Arch Gen Psychiatry.1990474434482109971
- MokraniMC.DuvalF.MonrealJ.ChampevalC.MauriceD.MacherJP.Chronobiological HPT axis dysfunction in depression. In:New Research Abstracts. 159th Meeting, American Psychiatric Association, Toronto, Canada: APA;2006
- LoosenPT.PrangeAJ.Serum thyrotropin response to thyrotropin-releasing hormone in psychiatric patients: a review.Am J Psychiatry.19821394054166802002
- WhybrowPC.BauerMS.Effects of peripheral thyroid hormones on the central nervous system: relevance to disorders of mood.Current Topics Neuroendocrinology.19888309327
- DuvalF.MokraniMC.CrocqMA.BaileyP.MacherJR.Influence of thyroid hormones on morning and evening TSH response to TRH in major depression.Biol Psychiatry.1994359269348080892
- CallowaySP.DolanRJ.FonagyP.De SouzaVF.WakelingA.Endocrine changes and clinical profiles in depression: II. The thyrotropin-releasing hormone test.Psychol Med.1984147597656443618
- KirkegaardC.FaberJ.Influence of free thyroid hormone levels on the TSH response to TRH in endogenous depression.Psychoneuroendocrinology.1986114914973104947
- DuvalF.MokraniMC.CrocqMA.et al.Effect of antidepressant medication on morning and evening thyroid function tests during a major depressive episode.Arch Gen Psychiatry.1996538338408792760
- SieverLJ.DavisKL.Overview: toward a dysregulation hypothesis of depression.Am J Psychiatry.1985142101710312862799
- AbeIsonJL.GlitzD.CameronOG.et al.Blunted growth hormone response to clonidine in patients with generalized anxiety disorder.Arch Gen Psychiatry.1991481571621989571
- UhdeTW.SteinMB.VittoneBJ.et al.Behavioral and physiolologic effect of short-term and long-term administration of clonidine in panic disorder.Arch Gen Psychiatry.1989461701772643934
- BrambillaF.PernaG.Garberiet al.A2-adrenergic receptor sensitivity in panic disorder: I. GH response to GHRH and clonidine stimulation in panic disorder.Psychoneuroendocrinology.199520197838898
- TancerME.SteinMB.UhdeTW.Growth hormone response to intravenous clonidine in social phobia: comparison to patients with panic disorder and healthy volunteers.Biol Psychiatry.1993345915958292687
- AnsseauM.Von FrenckellR.MaassenD.et al.Prediction of treatment response to selective antidepressants from clonidine and apomorphine neuroendocrine challenges. In, Briley M, Fillion G, eds.New Concepts in Depression. London, UK: McMillan Press;1988269276
- CooteM.WilkinsA.WerstiukES.SteinerM.Effects of electroconvulsive therapy and desipramine on neuroendocrine responses to the clonidine challenge test.J Psychiatry Neurosci.1998231721789595891
- CorreaH.DuvalF.MokraniMC.et al.Noradrenergic dysfunction and antidepressant treatment response.Eur Neuropsychopharmacol.20011116316811313162
- NemeroffCB.Psychopharmacology of affective disorders in the 21st century.Biol Psychiatry.1998445175259787875
- ArgyropouIosSV.WilsonSJ.Sleep disturbances in depression and the effects of antidepressants.Int Rev Psychiatry.20051723724516194795
- ModellS.IsingM.HolsboerF.LauerCJ.The Munich vulnerability study on affective disorders: premorbid polysomnographic profile of affected high-risk probands.Biol Psychiatry.20055869469916018976
- HatzingerM.HemmeterUM.BrandS.IsingM.Holsboer-TrachslerE.Electroencephalographic sleep profiles in treatment course and long-term outcome of major depression: association with DEX/CRH-test response.J Psychiatr Res.20043845346515380395
- WilsonS.ArgyropoulosS.Antidepressants and sleep: a qualitative review of the literature.Drugs.20056592794715892588
- Salin-PascuaIRJ.Galicia-PoloL.Drucker-ColinR.Sleep changes after 4 consecutive days of venlafaxine administration in normal volunteers.J Clin Psychiatry.1997583483509515972
- AudinotV.MaillietF.Lahaye-BrasseurC.et al.New selective ligands of human cloned melatonin MT1, and MT2 receptors.Naunyn-Shmiedeberg's Arch Pharmacol.2003367553561
- MillanMJ.GobertA.LejeuneF.et al.The novel melatonin agonist Valdoxan (S20098) is an antagonist at 5-hydroxytryptamine2C Receptors, blokade of which enhances the activity of frontocrotical dopaminergic and adrenergic pathways.JPET.2003306954964
- GuilleminaultC.Efficacy of agomelatine versus venlafaxine on subjective sleep of patients with Major Depressive Disorder.Eur Neuropsychopharmacol.200515(suppl 3)S419
- Quera SalvaMA.VanierB.ChapototF.et al.Effect of agomelatine on the sleep EEG in patients with major depressive disorder (MMD).Eur Neuropsychopharmacol.200515(suppl 3)S435
- LamRW.Sleep disturbances and depression: a challenge for antidepressants.Int Clin Psychopharmacol.200621(suppl 1)S25S2916436937
- BondyB.Pharmacogenomics in depression and antidepressants.Dialogues Clin Neurosci.2005722323016156381
- HeilsA.TeufelA.PetriS.et al.Allelic variation of human serotonin transporter gene expression.J Neurochem.199666262126248632190
- CaspiA.SugdenK.MoffittTE.et al.Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene.Science.200330138638912869766
- WilhelmK.MitchellPB.NivenH.et al.Life events, first depression onset and the serotonin transporter gene.Br J Psychiatry.200618821021516507960
- SerrettiA.LilliR.SmeraldiE.Pharmacogenetics in affective disorders.Eur J Pharmacol.200243811712811909602
- MancamaD.KerwinRW.Role of pharmacogenomics in individualising treatment with SSRIs.CNS Drugs.20031714315112617694
- MrazekDA.SmollerJW.de LeonJ.Incorporating pharmacogenetics into clinical practice: Reality of a new tool in psychiatry.CNS Spectr.200611(3, suppl 3)113
- BinderEB.HolsboerF.Pharmacogenomics and antidepressant drugs.Ann Med.200638829416581694
- HastingsRS.ParseyRV.OquendoMA.ArangoV.MannJJ.Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression.Neuropsychopharmacology.20042995295914997169
- ManjiHK.QuirozJA.SpornJ.et al.Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression.Biol Psychiatry.20035370774212706957
- CampbellS.McQueenG.An update on regional brain volume differences associated with mood disorders.Curr Opin Psychiatry.200619253316612175
- IosifescuDV.RenshawPF.LyooIK.et al.Brain white-matter hyperintensities and treatment outcome in major depressive disorder.Br J Psychiatry.200618818018516449707
- WuJC.GillinJC.BuchsbaumMS.HersheyT.JohnsonJC.BunneyWE.Effect of sleep deprivation on brain metabolism of depressed patients.Am J Psychiatry.19921495385431554042
- EbertD.FeistelH.BarockaA.KaschkaW.Increased limbic flow and total sleep deprivation in major depression with melancholia. 199455101109
- EbertD.EbmeierKP.The role of the cingulate gyrus in depression - from functional anatomy to neurochemistry.Biol Psychiatry.199639104410508780840
- MaybergHS.BrannanSK.MahurinRK.et al.Cingulate function in depression - a potential predictor of treatment response.NeuroReport.19978105710619141092
- VlassenkoA.ShelineYI.FischerK.MintunMA.Cerebral perfusion response to successful treatment of depression with different serotoninergic agents.J Neuropsychiatry Clin Neurosci.20041636036315377745
- MartinSD.MartinE.RaiSS.RichardsonMA.RoyallR.Brain blood flow changes in depressed patients treated with interpersonal psychotherapy or venlafaxine hydrochloride: preliminary findings.Arch Gen Psychiatry.20015864164811448369
- ZobelA.JoeA.FreymannN.ClusmannH.SchrammJ.ReinhardtM.BiersackHJ.MaierW.BroichK.Changes in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: an exploratory approach.Psychiatry Res.200513916517916043331
- EzquiagaE.Ayuso GutierrezJL.Garcia LopezA.Psychosocial factors and episode number in depression.J Affect Disord.1987121351382955004
- MitchellPB.ParkerGB.GladstoneGL.WilhelmK.AustinMP.Severity of stressful life events in first and subsequent episodes of depression: the relevance of depressive subtype.J Affect Disord.20037324525212547293
- ManjiHK.DrevetsWC.CharneyDS.The cellular neurobiology of depression.Nat Med.2001754154711329053
- PostRM.Transduction of psychosocial stress into the neurobiology of recurrent affective disorder.Am J Psychiatry.199214999910101353322
- WeissSR.PostRM.Kindling: separate vs. shared mechanisms in affective disorders and epilepsy.Neuropsychobiology.1998381671809778605
- MonroeSM.HarknessKL.Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective.Psychol Rev.200511241744515783292
- McEwenBS.Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders.Ann N Y Acad Sci.200410321715677391
- O'ConnorTM.O'HalloranDJ.ShanahanF.The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia.Q J Med.200093323333
- CarrascoGA.Van de KarLD.Neuroendocrine pharmacology of stress.Eur J Pharmacol.200346323527212600714
- TsigosC.ChrousosGP.Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress.J Psychosom Res.20025386587112377295
- BrownAS.GershonS.Dopamine and depression.J Neural Transm Gen Sect.199391751098099801
- JacksonIM.The thyroid axis and depression.Thyroid.199889519569827665
- AnandA.CharneyDS.Norepinephrine dysfunction in depression.J Clin Psychiatry.200061162410910013
- DuvalF.MokraniMC.Monreal-OrtizJA.et al.Cortisol hypersecretion in unipolar major depression with melancholic and psychotic features: dopaminergic, noradrenergic and thyroid correlates. In press.Psychoneuroendocrinology.
- GaryKA.SevarinoKA.YarbroughGG.Prange JrAJ.WinokurA.The thyrotropin-releasing hormone (TRH) hypothesis of homeostatic regulation: implications for TRH-based therapeutics.J Pharmacol Exp Ther.200330541041612606661
- DuvalF.MokraniMC.BaileyP.CorreaH.DiepTS.CrocqMA.MacherJP.Thyroid axis activity and serotonin function in major depressive episode.Psychoneuroendocrinology.19992469571210451906
- ItilM.PattersonCD.PolvanN.MehtaD.BergeyB.Clinical and CNS effects of oral and i.v. thyrotropin releasing hormone (TRH). Psychopharmacol Bull. 197511214222
- MarangellLB.GeorgeMS.CallahanAM.et al.Effects of intrathecal thyrotropin-releasing hormone (protirelin) in refractory depressed patients.Arch Gen Psychiatry.1997542142229075462
- DumanRS.Neural plasticity: consequences of stress and actions of antidepressant treatment.Dialogues Clin Neurosci.2004615716922034207
- MacQueenGM.CampbellS.McEwenBS.et al.Course of illness, hippocampal function, and hippocampal volume in major depression.Proc Natl Acad Sci U S A.20031001387139212552118
- ShelineYI.SanghaviM.MintunMA.GadoMH.Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression.J Neurosci.1999195034504310366636
- NemeroffCB.ValeWW.The neurobiology of depression: inroads to treatment and new drug discovery.J Clin Psychiatry.200566(suppl 7)51316124836
- SouêtreE.SalvatiE.WehrTA.SackDA.KrebsB.DarcourtG.Twenty-four hour profile of body temperature and plasma TSH in bipolar patients during depression and during remission and in normal controls subjects.Am J Psychiatry.1988139282286
- Wirz-JusticeA.Biological rhythms in mood disorders. In: Bloom FE, Kupfer DJ, eds.Psychopharmacology: the Fourth Generation of Progress. New York, NY: Raven Press;19959991017
- KrauchiK.CajochenC.MoriD.GrawP.Wirz-JusticeA.Early evening melatonin and S-20098 advance circadian phase and nocturnal regulation of core body temperature.Am J Physiol.1997272R1178R889140018
- LeproultR.Van OnderbergenA.L'hermite-BaleriauxM.et al.Phase-shifts of 24-h rhythms of hormonal release and body temperature following early evening administration of the melatonin agonist agomelatine in healthy older men.Clin Endocrinol.200563298304
- LooH.HaleA.D'haenenH.Determination of the dose of agomelatine, a melatoninergic agonist and selective 5-HT2c antagonist, in the treatment of major depressive disorder: a placebo-controlled dose range study.Int Clin Psychopharmacol.20021723924712177586
- KennedySH.EmsleyR.Placebo-controlled trial of agomelatine in the treatment of major depressive disorder.Eur Neuropsychopharmacol.2006169310016249073
- Den BoerJA.BoskerFJ.MeestersY.Clinical efficacy of agomelatine in depression: the evidence.Int Clin Psychopharmacol.200621(suppl 1)S21S2416436936
- SchulzP.BerneyP.Clinician's predictors of patient response to psychotropic medication.Dialogues Clin Neurosci.2004610511122033598
- Wirz-JusticeA.Biological rhythm disturbances in mood disorders. IntClin Psychopharmacol.200621S11S15
- KrzystanekM.Krupka-MatuszczykI.Bargiel-MatusiewiczK.Observations of toleranc of bright light treatment in psychiatry.Psychiatr Pol.20053944945816149755
- FrankE.NovickD.KupferDJ.Antidepressants and psychotherapy: a clinical research review.Dialogues Clin Neurosci.2005726327216156384
- Aberg-WistedtA.A double-blind study of zimelidine, a serotonin uptake inhibitor, and desipramine, a noradrenaline uptake inhibitor, in endogenous depression. 1: Clinical findings.Acta Psychiatr Scand.1982661291386215831
- HumbleM.Noradrenaline and serotonin reuptake inhibition as clinical principles: a review of antidepressant efficacy.Acta Psychiatr Scand.2000402(suppl)2836
- ErikssonE.Antidepressant drugs: does it matter if they inhibit the reuptake of noradrenaline or serotonin?Acta Psychiatr Scand.2000402(suppl)1217
- ErikssonE.HedbergMA.AnderschB.et al.The serotonin reuptake inhibitor paroxetine is superior to the noradrenaline reuptake inhibitor maprotiline in the treatment of premenstrual syndrome.Neuropsychopharmacology.1995121671767779245
- Aberg-WistedtA.Comparison between zimelidine and desipramine inendogenous depression: a cross-over study.Acta Psychiatr Scand.1982661292136215831
- ThaseME.RushAJ.Treatment-resistant depression. In: Bloom FE, Kupfer DJ, eds.Psychopharmacology: the Fourth Generation of Progress. New York, NY: Raven Press;199510811097
- SpigsetO.MartenssonB.Fortnightly review: drug treatment of depression.BMJ.19993181188119110221948
- AndersonIM.NuttDJ.DeakinJF.Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 1993 British Association for Psychopharmacology guidelines. British Association for Psychopharmacology.J Psychopharmacol.20001432010757248
- AndersonIM.SSRIs versus tricyclic antidepressants in depressed inpatients: a meta analysis of efficacy and tolerability.Depression Anxiety.19987(suppl 1)1117
- Danish University Antidepressant Group (DUAG). Citalopram: clinical effect profile in comparison with clomipramine. A controlled multicenter study.Psychopharmacology.1986901311382876451
- Danish University Antidepressant Group (DUAG). Paroxetine: a selective serotonin reuptake inhibitor showing a better tolerance, but weaker antidepressant effect than clomipramine in a controlled multicenter study.J Affective Disord.199018289299
- Danish University Antidepressant Group (DUAG). Moclobemide: a reversible MAO-A inhibitor showing weaker antidepressant effect than clomipramine in a controlled multicenter study.J Affective Disord.199328105116
- TrindadeE.MenonD.Selective Serotonin Reuptake Inhibitors (SSRIs) for Major Depression. Part 1: Evaluation of the Clinical Literature. Ottawa, Canada: Canadian Coordinating Office for Health Technology Assessment; 1997 (Report 3E.)
- KasperS.BechP.de JongheF.et al.Treatment of unipolar major depression: algorithms for pharmacotherapy.Int J Psychatry Clin Pract.199715557
- BaumannP.UlrichS.EckermannG.et al.Arbeitsgemeinschaft fur Neuropsychopharmakologie und Pharmakopsyhiatrie - Therapeutic Drug Monitoring Group. The AGNP-TDM Expert Group Consensus Guidelines: focus on therapeutic monitoring of antidepressants.Dialogues Clin Neurosci.2005723124716156382
- ScordoMG.SpinaE.DahlML.GattiG.PeruccaE.Influence of CYP2C9, 2C19 and 2D6 genetic polymorphisms on the steady-state plasma concentrations of the enantiomers of fluoxetine and norfluoxetine.Basic Clin Pharmacol Toxicol.20059729630116236141
- BerneyP.Dose-response relationship of recent antidepressants in the short-term treatment of depression.Dialogues Clin Neurosci.2005724926216156383
- FavaM.RosenbaumJF.McGrathPJ.StewartJW.AmsterdamJD.QuitkinFM.Lithium and tricyclic augmentation of fluoxetine treatment for resistant major depression: a double-blind, controlled study.Am J Psychiatry.1994151137213748067495
- MuthFA.MoyerJA.HaskinsJT.AndreeTH.HusbandsGEM.Biochemical, neurophysiological and behavioral effects of WY 45,233, its enantiomers, and other identified metabolites of the antidepressant venlafaxine.Drug Dev Res.199123191199
- RudolphRL.FabreLF.FeighnerJP.RickelsK.EntsuahR.DerivanAT.A randomized, placebo-controlled, dose-response trial of venlafaxine hydrochloride in the treatment of major depression.J Clin Psychiatry.1998591161229541154
- MbayaP.Safety and efficacy of high dose of venlafaxine XL in treatment resistant major depression.Hum Psychopharmacol Clin Exp.200217335339
- AbuzzahabFS.KayongoMN.High doses of venlafaxine in treatment-resistant depression.Bipolar Disord. (Abstract).20046(suppl 1)35
- RouillonF.GorwoodP.The use of lithium to augment antidepressant medication.J Clin Psychiatry.199859(suppl 5)32399635546
- DeBattistaC.LembkeA.Update on augmentation of antidepressant response in resistant depression.Curr Psychiatry Rep.2005743544016318821
- BlierP.SzaboST.Potential mechanisms of action of atypical antipsychotic medications in treatment-resistant depression and anxiety.J Clin Psychiatry.200566(suppl 8)304016336034
- NemeroffCB.Use of atypical antipsychotics in refractory depression and anxiety.J Clin Psychiatry.200566(suppl 8)132116336032
- SimonJS.NemeroffCB.Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder.J Clin Psychiatry.2005661216122016259533
- HolmesVF.Related Medical use of psychostimulants: an overview. IntJ Psychiatr Med.199525119
- MenzaMA.KaufmanKR.CastellanosA.Modafinil augmentation of antidepressant treatment in depression.J Clin Psychiatry.20006137838110847314
- O'ConnorMK.KnappR.HusainM.et al.The influence of age on the response of major depression to electroconvulsive therapy: a C.O.R.E. Report.Am J Geriatr Psychiatry.2001938239011739064
- CarpenterLL.Neurostimulation in resistant depression.J Psychopharmacol.200620354016644770
- Wirz-JusticeA.BenedettiF.BergerM.et al.Chronotherapeutics (light and wake therapy) in affective disorders.Psychol Med.20053593994416045060
- ReusVI.WolkowitzOM.Antiglucocorticoid drugs in the treatment of depression.Expert Opin Investig Drugs.20011017891796
- RothschildAJ.Challenges in the treatment of depression with psychotic features.Biol Psychiatry.20035368069012706954
- FloresBH.KennaH.KellerJ.SolvasonHB.SchatzbergAF.Clinical and biological effects of mifepristone treatment for psychotic depression.Neuropsychopharmacology.20063162863616160710
- HolsboerF.The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety.J Psychiatr Res.19993318121410367986
- HeldK.KunzelH.IsingM.et al.Treatment with the CRH1-receptor-antagonist R121919 improves sleep-EEG in patients with depression.J Psychiatr Res.20043812913614757326