Abstract
This review summarizes the various conceptual paradigms for treating schizophrenia, and indicates how molecular biology and drug discovery technologies can accelerate the development of new medications. As yet, there is no convincing data that a crucial druggable molecular target exists which, if targeted, would yield medications with efficacies greater than any currently available. It is suggested, instead, that drugs which interact with a multiplicity of molecular targets are likely to show greater efficacy in treating the core symptoms of schizophrenia.
Esta revisión resume los diverses paradigmas conceptuales que existen para el tratamiento de la esquizofrenia e indica cómo la biología molecular y las tecnologías para el descubrimienio de fármacos pueden acelerar el desarrollo de nuevos medicamentos, Aun no se dispone de información convincente acerca de la exisiencia de una molécula específica, que pueda transformarse en un medicamento, y que de encontrarse pueda dar origen a fármacos más eficaces que cualquiera de los actualmente disponibles. Se sugiere, en cambio, que es probable que fármacos que interactúan con una multiplicidad de blancos moleculares muestren mayor eficacia en el tratamiento de los síntomas centrales de la esquizofrenia.
Cet article résume les différents modèles conceptuels de traitement de la schizophrénie et montre comment la biologie moléculaire et les technologies de découverte des médicaments peuvent accélérer le développement de nouveaux traitements. Nous ne disposons pas encore de données convaincantes sur l'existence d'une molécule décisive, transformable en médicament qui, si elle était choisie, déboucherait sur des traitements plus efficaces que ceux disponibles actuellement. Il est plutôt suggéré que les médicaments interagissant avec les nombreuses cibles moléculaires seraient plus efficaces dans le traitement des symptômes clés de la schizophrénie.
Psychiatric diseases represent a major cause of disability among individuals during their peak years of productivity (ages 15 to 44) and remain major causes of mortality in the developed world.Citation1 Because of this, governments and pharmaceutical companies have expended many billions of dollars on understanding the underlying causes of mental illnesses, and on discovering new and more effective treatments for them (Roth and Conn, unpublished report). The budget for the National Institute of Mental Health (NIMH) - the major funding agency for mental health-related research in the US - for the financial year 2006 stood at $1.4 billion, as stated on their Web site.Citation2 Despite this heavy investment, no psychiatric medications with greater efficacy than drugs discovered 50 years ago have yet appeared.Citation3,Citation4 Thus, for example, clozapine (which was synthesized nearly 50 years agoCitation4) continues to be the “gold standard” for treating schizophrenia.Citation5,Citation6
The recent sequencing and continued annotation of the human genomeCitation7 and the tentative identification of a large number of schizophrenia susceptibility genesCitation8 have raised the possibility that molecular biology and its associated technologies will lead to new and improved treatments for schizophrenia and related disorders.Citation9 The assumption underlying this hope is that “we should finally make rapid progress identifying some of the vulnerability genes and thus critical pathways for the pathophysiology of the major mental illnesses...”Citation1 The hypothesis is that if we can understand the pathophysiological basis of these diseases - based on their molecular neurobiological underpinning - we will be better able to develop curative therapeutics (or “cure therapeutics”Citation1) for schizophrenia and related disorders. Although this is a highly attractive hypothesis, it is founded on a number of assumptions, some of which are falsifiable, others of which are not (at least with the available technology). In this review, this hypothesis and its underlying assumptions will be examined, and suggestions will be put forward as to how molecular biology can (and cannot) provide tests of this hypothesis, as well as possibilities for novel medications for curative therapeutics of schizophrenia and related disorders.
Schizophrenia as a molecular disease
Currently, at least three overlapping paradigms drive the drug discovery effort for schizophrenia. These include, firstly, the molecular-genetic hypotheses which hypothesize strong effects of schizophrenia susceptibility genes.Citation8
A corollary of the molecular-genetic hypothesis is the proposal that targeting drugs at these genes might yield novel and more effective treatments for schizophrenia.Citation1,Citation10 Secondly, the neuronal network hypotheses propose strong effects of altered neuronal integration in schizophrenia. The corollary of this hypothesis predicts that drugs which fundamentally reset the tone of networks of neuronal interactions will prove efficacious in treating schizophrenia.Citation4,Citation11 Thirdly, the signal transduction hypothesis proposes that basic alterations in receptor-mediated signal transduction (cither at the receptor or post-receptor levels) induce schizophrenia-like pathology. It follows that ameliorating altered signaling via specific medications which target receptor/post-receptor molecules will prove efficacious in treating schizophrenia.Citation12-Citation16
These general hypotheses are highly interconnected and interdependent. Thus, one could suggest, for instance, that schizophrenia arises because of mutation in a specific susceptibility gene - oc7 nicotinic receptors for instance.Citation17 This mutation results in diminished oc7 expressionCitation18 which, in turn, leads to altered neuronal connectivity and signal transduction.Citation17 These alterations in neuronal signaling and connectivity lead to some of the symptoms of schizophrenia. The corollary is the proposal that a7 agonists will improve schizophrenia, symptomsCitation19 - a hypothesis that is now being tested.
The underlying assumption of these lines of reasoning is that if one can identify the critical node (Figure 1) in the pathogenesis of schizophrenia and alter its functioning, one will more effectively treat schizophrenia. The implicit assumption is that only one (or a small number) of molecular targets function as critical nodes in the pathogenesis of schizophrenia. The role of molecular biology in such an undertaking is relatively straightforward: (i) identify the “disease-inducing molecules” (genetic linkage studies, candidate gene approaches); (ii) express the molecule in a way suitable for high-throughput-screening of large chemical libraries to identify candidate ligands with appropriate pharmacology (agonist, antagonist, partial agonist, inverse agonist, allosteric modulatorCitation20); (iii) provide molecular-target based assays for profiling candidate ligands at a large variety of other druggable targets to verify that the final lead compounds arc suitably selective (or suitably nonselectiveCitation3,Citation21); and (iv) provide molecular-target based assays for profiling candidate ligands against various molecular targets which can lead to serious side effects. These can include prolongation of the QT interval via blockade of HERG K+-channels,Citation22 agonism of 5-HT 2B serotonin receptors which can lead to cardiovascular side effects,Citation23 carcinogenicity, genotoxicity, and alteration of cytochrome P450 isoforms leading to altered pharmacokinetics (see ref 24 for instance). In the case of antipsychotic medications, weight gain and adverse metabolic side effects (likely mediated in part via H1 -histamine and 5-HT2C-serotonin receptor blockade34) and extrapyramidal side effects (due to D2-dopamine receptor blockade) occur frequently. Indeed, much of preclinical drug discovery in both industry and academia is driven primarily via molecular target-based screening and profiling technologies. Despite our ability to screen millions of drug-like compounds at hundreds of druggable targets which comprise the “druggable genome,”Citation25,Citation26 no novel molecularly targeted treatments for schizophrenia have been approved. Indeed, as already mentioned, clozapine continues to be the “gold-standard treatment” for schizophrenia.
The critical node assumption has not (yet) yielded better drugs for schizophrenia
Based on the “critical node” assumption, a large number of potential nodes have been identified for therapeutic drug discovery. These have been identified via the three general strategies outlined above (eg, molecular genetic, neuronal network, or signal transduction) and a large number of these candidate nodes have been a theme of research over the past decade. As we have recently summarized as part of a larger study of psychiatric drug discovery, nearly 150 investigational compounds directed against many individual molecular targets (“nodes”) have been subjected to at least early-phase clinical trials (Roth and Conn, unpublished report). Representative compounds for each node are listed in Table I. In this table, antipsychotic drugs have been classified based on molecular target (eg, “node”)/targets (“nodes”) and whether the compounds were validated with preclinical and clinical studies. Lastly, it is indicated whether the compounds were found, based on clinical trials, to be superior to a standard comparator medication (typically haloperidol). Based on the currently available data, we were unable to find any evidence to support the hypothesis that targeting any single molecular target (“node”) other than D2 dopamine receptors will yield a drug which effectively treats the core symptoms of schizophrenia. Additionally, we were unable to find any support for the hypothesis that drugs targeting a single node are more effective at treating schizophrenia than drugs targeting a large number of nodes. Indeed, clozapine, which targets at least 50 nodes, remains superior to all other medications.Citation3,Citation5 The results obtained arc consistent with the proposal that “D2 dopamine receptors represent the critical node in schizophrenia pathogenesis.”Citation13 It is unknown whether any single molecular target of greater promise will ever be found.
There are many ways in which these findings can be interpreted, although each interpretation relies mainly on untested assertions. A typical criticism one can make of these findings is that “we have not yet found the critical node” and that once this key node is discovered, the pathway towards drugs with greater efficacy and fewer side effects will be clarified. The untested assumptions are (i) that such a special node associated with efficacy exists; (ii) that it can be discovered; and (iii) that, once discovered, using techniques of molecular biology, a drug can be designed to target it. An implicit assumption underlying this argument relates to the need for an enhanced understanding of the molecular pathogenesis of schizophrenia in order to discover and validate suitable molecular targets.Citation1,Citation9
Based upon our current understanding of the molecular pathogenesis of schizophrenia, no critical, node other than the D2 dopamine receptor has yet been convincingly and reproducibly elucidated, although a large number of candidate genes and susceptibility factors have been described. These include neuregulin-1 ,Citation27 dysbindin,Citation28 disrupted in schizophrenia-1 (DISC-1)Citation29 and many others (eg, rcelin, regulator of G protein signaling-4, catccholO-methyltransferase, mGluR3 glutamate receptor, and so on; see ref 8 for recent review). As weCitation3 and othersCitation30 have pointed out (.) these susceptibility gene products are found in a variety of cell types (both neuronal and glial) and show differential subcellular localizations. As Figure I shows, the molecular targets identified are frequently found in circuits which are targeted by drugs with a “promiscuous” pharmacology (eg, clozapine). No single node is an obvious target for therapeutic drug discovery efforts, although nearly all of the identified nodes have been reported to be targets of therapeutic drug discovery (Roth and Conn, unpublished report).
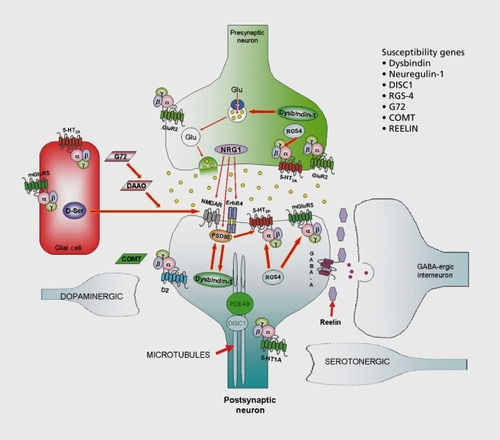
Another possibility is that schizophrenia, can be most effectively treated by influencing several nodes simultaneously.Citation3 Indeed, based on the demonstrated superiority of clozapine for treatment-resistant schizophreniaCitation5 and the relative inferiority of all other medications,Citation6 there is strong support for this hypothesis. A great deal of effort has been expended to discover an optimal clozapinemimetic devoid of the side effects of clozapine which include agranulocytosis, seizures, sialorrhea, weight gain, sedation, and hypotension. We, and others, have suggested that the massively parallel screening of large numbers of molecular targets allows one to efficiently discover “toxic” vs “therapeutic” targets.Citation32-Citation34 Antipsychotic drug-induced weight gain might be due to H1 -histamine and 5-HT7C-reccptor blockade,Citation35,Citation36 agranulocytosis to H4 histamine agonism,Citation2 sedation to H1 histamine antagonism,Citation4 and so on. Thus far, these molecular targets implicated in clozapine's side effects (H1 -histamine, -histamine, 5-HT2C serotonin) are not identical with those targets thought to be involved in its superiority as an antipsychotic drug (5-HT2A serotonin, D4-dopamine, 5HT6 and 5-HT7 serotonin). A problem with the approach of designing selectively nonselective drugs is that it is very difficult to rationally design in new pharmacological properties during the drug discovery process.Citation24 This is an emerging paradigm, however, and some successful strategics have recently been elucidated.Citation37
Table I. Multiple candidate nodes have been subjected to testing as targets for treating schizophrenia. This shows an abstracted analysis from a recent study' examining the evidence for and against various molecular-target based approaches for treating schizophrenia *, various animal models which have been tested and for which the drug has efficacy; **, clinical trials are ongoing and information is not available; ***, dropped from development with no further data available; EPS, extrapyramidal syndrome.
A systems level approach
The neuronal systems approach similarly proposes that there might be crucial nodes in the network that are amenable to target-based discovery efforts.Citation4 Spedding and colleagues have cogently argued that a systems-level approach using animal models will lead to more effective treatment for psychiatric diseases.Citation4 Based on a model which involves specific alterations in hippocampal-cortical circuitry, they propose testing compounds in animals in which these circuits are disrupted by phenycyclidinc (PCP). In support of this systems-level approach, nearly every approved antipsychotic drug will ameliorate PCPinduced alterations in neuronal functioning.Citation37 However, it is also true that drug classes with demonstrated ability to ameliorate PCP-induced deficits (eg, 5-HT2A antagonistsCitation38) are only marginally effective in treating schizophrenia.Citation39-Citation40
Thus, in vivo systems-level screens can be highly effective tools to verify in vivo actions of putative atypical antipsychotic drugs. It does not appear that any of the available in vivo screening models are able to predict relative efficacy at treating schizophrenia, however. In addition, none of the available models appears to adequately recapitulate the entirety of the human phenotype.Citation37
One can easily provide the counterargument that a “suitable animal model will eventually be found which recapitulates the schizophrenia phenotype,” although it is also plausible that “no suitable preclinical model will ever be found which adequately recapitulates schizophrenia, pathology.” Clearly, despite decades of research we have not yet discovered an adequate preclinical model, and it is within the realm of possibility that “schizophrenia is a uniquely human disease which cannot be adequately modeled in rodents.” In large measure, this is likely to be due to the fact that a number of genetic “hits” as well as nongenomic factors converge to produce the final phenotype in humans.Citation41 At present, we have no way to predict either way, and continued research in this arena will be based more on untested assumptions than on data.
Is schizophrenia similar to hypertension in being complex, polygenic, and epigenetic?
Another possibility is that schizophrenia represents a complex disease with genetic and epigenetic factors and which is both chronic and progressive, resulting in irreversible end-organ damage - similar to hypertension. Indeed, there is accumulating evidence for epigenetic factors involved in the etiology of schizophrenia - particularly relating to reelin.Citation42-Citation45 There has also been abundant evidence accumulated over the past several decades that schizophrenia is associated with subtle but reproducibly documented neurodegeneration (reviewed in refs 46,47).
Accordingly, optimal treatment of schizophrenia would be similar to that for other progressive and complex diseases such as hypertension, where individuals at risk would be identified and then treated to avoid end-organ damage. Such an approach has already been attempted, with a mixed degree of success.Citation48 In this study, individuals at risk were identified and then prophylactlcally treated with placebo or olanzapine. Although the results were not statistically significant, there was a trend toward protection of conversion to overt psychosis among individuals treated with olanzapine.Citation48
Conclusion
As is clear from the foregoing, the tools of molecular biology can, at least theoretically, accelerate drug discovery in schizophrenia. In the main, molecular biological approaches have been more useful in providing reagents for high-throughput screening campaigns than for providing better animal models - at least to date. With the continued discovery of schizophrenia susceptibility genes, it is at least conceivable that better preclinical models will be produced. To a great degree, lack of progress in developing more effective antipsychotic drugs has stemmed mainly from the failure both to fully appreciate the pharmacological robustness of clozapine and to discover medications which reproduce the essential features without producing serious side effects. It is not clear whether any of the paradigms outlined will lead to more effective medications, although it is likely that continued molecular target-based screening will eventually yield medications with fewer side effects.
The work from the author's lab was supported entirely by grants from the National Institute of Health (MH57635, MH61887, DA017237) and the NIMH Psychoactive Drug Screening Program.
- InselTRScolnickEMCure therapeutics and strategic prevention: raising the bar for mental health research.Mol Psychiatry.200611111716355250
- National Institutes of Mental Health. Facts about NIMH. Available at: http://www.nimh. nih.gov/about/nimh.cfm. Accessed August 8. 2006
- RothBLShefflerDJKroezeWKMagic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. WatRev Drug Discov.20043353359
- SpeddingMJayTCosta eSilva JPerretLA pathophysiological paradigm for the therapy of psychiatric disease.Nat Rev Drug Discov.2005446747615931256
- KaneJHonigfieldGSingerJMeltzerHYGroup atCCSClozapine for the treatment-resistant schizophrenic.Arch Gen Psychiatry.1988457897963046553
- McEvoyJPLiebermanJAStroupTSDavisSMMeltzerHYRosenheckRAet al.Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment.Am J Psychiatry.200616360061016585434
- VenterJCAdamsMDMyersEWLiPWMuralRJSuttonGGet al.The sequence of the human genome.Science.20012911304135111181995
- HarrisonPJWeinbergerDRSchizophrenia genes, gene expression, and neuropathology: on the matter of their convergence.Mol Psychiatry.200510406815263907
- InselTRCollinsFSPsychiatry in the genomics era.Am J Psychiatry.200316061662012668345
- SawaASnyderSHSchizophrenia: neural mechanisms for novel therapies.Mol Med.200393912765334
- HymanSENestlerEJInitiation and adaptation: a paradigm for understanding psychotropic drug action.Am J Psychiatry.19961531511628561194
- CarlssonMCarlssonASystems within the basal ganglia: implications for schizophrenia and Parkinson's disease.Trends Neurosci.1990132722761695402
- CarlssonAThe current status of the dopamine hypothesis of schizophrenia.Neuropsychopharmacology.198811791863075131
- MeltzerHYClinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia.Psychopharmacology.198999S18S272682729
- JavittDCZukinSRRecent advances in the phencyclidine model of schizophrenia.Am J Psychiatry.1991148130113081654746
- FreedmanRAdlerLEBickfordPet al.Schizophrenia and nicotinic receptors.Harv Rev Psychiatry.199421791929384901
- FreedmanRCoonHMyles-WorsleyMet al.Linkage of a neurophysiological deficit in schizophrenia to a chromosome 1 5 locus.Proc Natl Acad SciUSA.199794587592
- FreedmanRHallMAdlerLELeonardSEvidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia.Biol Psychiatry.19953822337548469
- MartinLFKemWRFreedmanRAlpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia.Psychopharmacology (Bert).20041745464
- NeubigRRSpeddingMKenakinTChristopoulosAInternational Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology.Pharmacol Rev.20035559760614657418
- MorphyRKayCRankovicZFrom magic bullets to designed multiple ligands.Drug DiscovToday.20049641651
- RecanatiniMPoluzziEMasettiMCavalliADe PontiFQT prolongation through hERG K(+) channel blockade: current knowledge and strategies for the early prediction during drug development.Med Res Rev.20042513316615389727
- RothmanRBBaumannMHSavageJERauserLMcBrideAHufeisenSJet al.Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications.Circulation.20001022836284111104741
- LessardEYessineMAHamelinBAO'HaraGLeBlancJTurgeonJInfluence of CYP2D6 activity on the disposition and cardiovascular toxicity of the antidepressant agent venlafaxine in humans.Pharmacogenetics.1999943544310780263
- HopkinsALGroomCRThe druggable genome.Nat Rev Drug Discov.2002172773012209152
- RussAPLampelSThe druggable genome: an update.Drug Discov Today.2005101607161016376820
- StefanssonHSigurdssonESteinthorsdottirVet al.Neuregulin 1 and susceptibility to schizophrenia.Am J Hum Genet.20027187789212145742
- StraubREJiangYMacLeanCJet al.Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia.Ami J Hum Genet.200271337348
- St ClairDBlackwoodDMuirWet al.Association within a family of a balanced autosomal translocation with major mental illness.Lancet.199033613161973210
- HarrisonPJOwenMJGenes for schizophrenia? Recent findings and their pathophysiological implications.Lancet.200336141741912573388
- VorthermsTARothBLReceptorome screening for CNS drug discovery.Drugs.20058491496
- ArmbrusterBNRothBLMining the receptorome.J Biol Chem.20052805129513215590622
- FliriAFLogingWTThadeioPFVolkmannRABiological spectra analysis: Linking biological activity profiles to molecular structure.Proc Natl Acad SciUSA.2005102261266
- KroezeWKHufeisenSJPopadakBAet al.H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs.Neuropsychopharmacology.20032851952612629531
- WirshingDAWirshingWCKysarLet al.Novel antipsychotics: comparison of weight gain liabilities.J Clin Psychiatry.1999603586310401912
- MorphyRRankovicZDesigned multiple ligands. An emerging drug discovery paradigm.J Med Chem.2005486523654316220969
- GeyerMAEllenbroekBAnimal behavior models of the mechanisms underlying antipsychotic atypicality.Prog Neuropsychopharmacol Biol Psychiatry.2003271071107914642967
- VartyGBBakshiVPGeyerMAM100907, a serotonin 5-HT2A receptor antagonist and putative antipsychotic, blocks dizocilpine-induced prepulse inhibition deficits in Sprague-Dawley and Wistar rats.Neuropsychopharmacology.19992031132110088132
- PotkinSGShipleyJBeraRet al.Clinical and PET Effects of M100907, a selective 5HT-2A receptor antagonist.Schizophr Res.200149242
- MeltzerHYArvanitisLBauerDReinWPlacebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder.Am J Psychiatry.200416197598415169685
- EllenbroekBAAnimal models in the genomic era: possibilities and limitations with special emphasis on schizophrenia.Behav Pharmacol.20031440941714501254
- CostaEChenYDavisJet al.REELIN and schizophrenia: a disease at the interface of the genome and the epigenome.Moi Interv.20022475714993361
- TremolizzoLCarboniGRuzickaWBet al.An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability.Proc Natl Acad Sci U S A.200299170951710012481028
- AbdolmalekyHMChengKHRussoAet al.Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report.Am J Med Genet B Neuropsychiatr Genet.2005134606615717292
- GraysonDRJiaXChenYet al.Reelin promoter hypermethylation in schizophrenia.Proc Natl Acad Sci U S A.20051029341934615961543
- BergerGEWoodSMcGorryPDIncipient neurovulnerability and neuroprotection in early psychosis.Psychopharmacol Bull.2003377910114566217
- de HaanLBakkerJMOverview of neuropathological theories of schizophrenia: from degeneration to progressive developmental disorder.Psychopathology.2004371714988644
- McGlashanTHZipurskyRBPerkinsDet al.Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis.Am J Psychiatry.200616379079916648318