Abstract
An impressive number of animal models to assess depression and anxiety are available today. However, the relationship between these models and the clinical syndromes of depression and anxiety is not always clear. Since human anxiety disorders represent a multifactorial phenomenon frequently comorbid with major depression andlor other psychiatric problems, the chance of creating animal models which consistently reflect the human situation is quite poor. When using experimental models to understand homologies between animal and human behavior, we have to consider the context in which an animal is investigated, and both the functional significance and relevance of the behavioral parameters that are quantified. Moreover, gender and interindividual and interspecies variabilities in behavioral responses to the test situation and in the sensitivity to pharmacological treatments are potential sources for confounding results. In the past, these aspects have been often neglected in preclinical approaches to behavioral pharmacology and psychopharmacology A pragmatic approach of combined preclinical and clinical efforts is necessary to imitate one or more aspects relevant to pathological anxiety disorders and depression. The resulting models may identify central nervous processes regulating defined behavioral output, the potential to develop more effective treatments.
Hoy en día existe un gran número de modelos animales para evaluar la depresión y la ansiedad. Sin embargo, la relación entre estos modelos y los síndromes clínicos de depresíon y ansiedad no siempre es clara. Ya que los trastornos de ansiedad en humanos representan un fenómeno multifactorial frecuentemente comórbido con la depresión mayor oly otros problemas psiquiátricos, la posibilidad de crear modelos animales que reflejen consistentemente la situación en humanos es baja. Cuando se utilizan modelos animales para intentar comprender las semejanzas entre la conducta animal y humana, se debe considerar el coniexto en el cual un animal está siendo investigado, y el significado funcional y la importancia de los parámetros conductuales que son cuantificados. Además, las variabilidades de género, interindividuales e interspecies en las respuestas conductuales a la situación de prueba y en la sensibilidad a los tratamientos farmacológicos son fuentes potenciales de resultados desconceriantes. En el pasado, estos aspectos han sido a menudo descuidados en las aproximaciones preclínicas a la farmacología conductual y a la psicofarmacologia. Para imitar uno o más aspectos relevantes de los trastornos de ansiedad patológica y de la depresión se requiere de una aproximación pragmática que combine esfuerzos preclínicos y clínicos. Los modelos resultantes pueden identificar procesos del sistema nervioso central que regulan la producción de una conducta definida, con la posibilidad de desarrollar tratamientos más efectivos.
Il existe aujourd'hui un grand nombre de modèles animaux pour évaluer la dépression et l'anxiété. Les relations entre ces modèles et les syndromes cliniques de dépression et d'anxiété ne sont cependant pas toujours claires. La possibilité de créer des modèles animaux imitant avec validité les troubles anxieux est assez faible, puisque ces troubles sont multifactoriels, se présentant fréquemment en comorbidité avec un état dépressif majeure et/ou d'autres problèmes psychiatriques. Il y a lieu de prendre en considération le contexte d'observation de l'animal, ainsi que le sens et la validité des comportements étudiés quantitativement, lors de l'utilisation de modèles expérimentaux afin de comprendre les points communs entre les comportements animaux et humains. De plus, le sexe et les différences interindividuelles et interespèces au niveau des réponses comportementales aux situations des tests ainsi qu'à la sensibilité aux traitements pharmacologiques sont une source potentielle d'erreur. Dans le passé ces aspects ont en pharmacologie du comportement et en psychopharmacologie. Une approche pragmatique combinant les thèmes précliniques et cliniques est nécessaire pour modéliser un ou plusieurs aspects pertinents de la dépression et de l'anxiété. Les modèles résultant de cette approche peuvent permettre l'identification de processus cérébraux gérant les conséquences comportementales, donc ayant un potentiel pour le développement de médicaments plus efficaces.
Animal models of psychiatric diseases attempt to capture various features of the human condition, from behavioral and physiological changes that are indicative of the emotional state to the etiology of the disease and the effects of therapeutic interventions. According to McKinney,Citation1 animal models are “experimental preparations developed in one species for the purpose of studying phenomena occurring in another species. In the case of animal models of human psychopathology one seeks to develop syndromes in animals which resemble those of humans in certain ways in order to study selected aspects of human psychopathology.” Later, other authors (eg,ref 2) proposed additional criteria that animal models need to fulfill. Suitable research models ought to display clear face validity (isomorphism), predictive validity (pharmacological correlation), and construct validity(homology and similarity in the underlying neurobiological mechanisms). Currently, the third criterion is regarded as having heuristic value because the central nervous processes that lead to anxiety/depression still have to be elucidated; therefore this criterion is regarded as desirable, but not essential.Citation3 Thus, in an ideal and perfect model one would like to have causative conditions, symptom profiles, and treatment responses identical to those seen in the human disease state.
Any animal model of depression, or of antidepressant activity, must account for the considerable symptom overlap between major depressive disorder (MDD) and anxiety disorders, eg, sleep disturbances, agitation, restlessness, irritability, difficulty concentrating, loss of control, fatigue, fear, distress and, of course, anxiety. Indeed, comorbidity of anxiety disorders and MDD is the rule rather than the exception (eg, refs 4-6)with more than 80% of adults with depression also having significant symptoms of anxiety.Citation7 Furthermore, most of the existing antidepressants successfully ameliorate anxiety as a component of depression (eg, ref 8).
In this article we will discuss relevant animal models that have been developed and are used to enhance our understanding of the pathophysiology of the most common psychiatric disorders, depression and anxiety, and to guide the development of novel and more effective treatments.
Animal models of depression
The diagnosis of depressive illness and anxiety relies almost exclusively on observation of behavior and interpersonal relations, and on reported feelings and beliefs of the patient.Citation9 Therefore, several recent reviews claim that it is difficult to develop a true animal model of depressive disorders because mental illness may be a uniquely human condition. In particular, typical symptoms in depressed patients, such as recurring thoughts of suicide or death, or excessive thoughts of guilt, are impossible to model in animals. The creation of reasonably valid animal models of psychiatric diseases has been difficult, mainly due to both the verbal and personal nature of the symptoms to be modeled, eg, sadness or delusions, as well as the lack of clear etiological factors which can be used to design valid models. Moreover, unlike the situation with other neurological disorders such as Alzheimer's disease or Parkinson's disease, we still have only a vague idea about the pathophysiological processes that underlie depression.
The earliest models of depressive states in animals were based on maternal separation experiments in infant nonhuman primates.Citation10 In rodents, manipulation of early life environment such as, prenatal stress and maternal separation produces bio behavioral changes that persist well into adulthood, representing a risk factor for psychopathology.Citation11 Another behavioral approach to simulate a human depressive state in animals is the learned helplessness model. Originally described in dogs subjected to inescapable electric shock,Citation12 this model has received considerable attention in studies of “depression” in mice and rats (for review see refs 13, 14). limitations of the helplessness test as consequence of foot shock are that the test is difficult to replicate between laboratoriesCitation15 and that it cannot be routinely used in a number of countries because of ethical or regulator supervision.Citation14
The chronic mild stress model is based on exposure of animals (usually rats) to uncontrollable stressors. Animals arc subjected, in succession, to a range of mild stressors such as disrupted light-dark cycle, wet bedding, having an intruder rat placed in the home cage, or having the home cage tilted at an angle for 1 to 2 days.Citation16 The complex procedures of this model almost ensure that every laboratory will have at least slightly different experimental setups, and consequently, also different interpretations of the protocols.Citation14
Among the most potent factors known to trigger or induce depressive episodes are stressful life events.Citation17-Citation20
Stress is considered to perturb the homeostasis of an organism in a way that can lead to a long-lasting imbalance in neurotransmitter, neuroendocrine, and hormonal systems and thus finally to a psychiatric disease. The stress hypothesis of mood disorders has stimulated the development of a number of putative animal models of depression.Citation2-Citation21 Loss of rank and/or social status in humans is one example of loss experiences which are increasingly recognized as specific type of ”life event“ associated with a great risk of depression.Citation22 A number of behavioral models have sought to stimulate or model depression by manipulating social relationships in animals, and new powerful animal models using chronic psychosocial perturbations as stressors have been established (eg, ref 23). In recent years, our group has provided increasing evidence that chronic psychosocial stress in the male tree shrew (Tupaia belangeri) represents a natural and valid paradigm for studying the behavioral, endocrine, and neurobiological changes that may underlie stress-related disorders such as depression.Citation24 Recently, our group has described and validated a new model of chronic social stress in ratsCitation25 based on the resident-intruder paradigm originally described by MiczekCitation26 and Koolhaas et al.Citation27 This model, in which depressive -like behavior can be normalized by antidepressants, provides the opportunity to study gene expression in distinct brain areas.Citation28,Citation29 Although the relevant literature is constantly expanding, one can already summarize today that models of social stress greatly increased our understanding of processes that take place in the brain during depressive -like states of an animal. Also the understanding of antideprcssantinduced processes has greatly increased in the past years (eg, ref 30).
A summary of animal models of depression that are classified according to type and sensitivity to chronic drug treatment is presented in Table /.According to Willner and Mitchell,Citation31 the diathesis models summarize those paradigms that involve a genetically determined predisposition for the depressive illness, whereas in mere stress models external stimuli are the only factors triggering changes in behavior and physiology. Social dominance models are those that use natural (social) stressors and arc considered as a subset of the stress models.
Table I. Animal models of depression.
Many of the paradigms addressed above are more correctly described as models of stress rather than models of depression. Not all responses to stress are maladaptive, because the stress response may also fulfill adaptive or protective functions. Therefore, to truly model depression, other factors such as the genetic background that might cause a predisposition for the disease must also be taken into consideration. However, studies looking at stressful early life experiences and the type of stressresponsiveness later in life highlight a key area. They may help to understand the processes that in conjunction with environmental stress can lead to depression in some individuals but not in others.
With the emergence of specific genetic factors more defined models may be created in the near future. In the case of major depressive illness, we know that genetic factors can only account for about 30% of the variance, and environmental factors clearly play a major role in inducing the illness.Citation32 However, the development of models of depression based on the interaction between stress and genetic vulnerability appears plausible. Generation of specific strains or lines of rats or mice may be advantageous. Studies in knockout models with a mutation in a single gene may be of limited usefulness because of confounding factors such as developmental adaptational processes. Conditional knockouts may be considered as an improvement, but they also can inform us only about the role of a single gene. Therefore, the more complex models involving the interaction of genes and environment could supposedly yield more useful information.
Validity of animal models
The importance of chronic drug treatment
Pharmacological tests and models sensitive to acute drug treatment are not included in this overview. These models, perhaps more appropriately called “screens,” Citation33 have been designed to detect most existing antidepressants. The mechanism(s) of action by which test compounds produce positive results in such screens may not be identical, or even not similar to the mechanisms underlying their clinical effects. One might have concerns about how drugs are applied to animals in preclinical experiments when comparing the routes of administration with those generally used in clinical settings. Many screens try to detect antideprcssant-like activity quite quickly, within minutes or hours, and the drugs are given prior to the testing, thus producing a behavioral alteration rather than preventing a disease-induced type of behavior. It is obvious that such an approach bears no similarity to the clinical situation where drugs are administered only after disease symptoms have already appeared, and where a delayed onset of therapeutic effects for at least 2 to 3 weeks has to be expected. In light of such data, we would suggest that one important characteristic factor for animal models with predictive validity is the reproduction of a lime course of the “therapeutic effects.”
Furthermore, in most studies drugs arc given intraperitoneally (IP) instead of orally, although the oral administration provides several advantages: (i) it mimics the clinical situation, where most patients take the drug orally; (ii) drugs taken orally produce metabolite concentrations that differ from those obtained after IP or intravenous (IV) administration; and (iii) it minimizes the uncontrollable stress effects of injections.
Also, little attention has so far been paid to potential species-specific differences in the metabolism of the applied drugs and their dosages. To exclude the effects of sub- or supraeffective doses there is an urgent need for monitoring the concentrations of circulating antidepressants and their pharmacologically active metabolites in the animals to be tested in the studies. Equally important is the observation that the drug effects are seen at clinically relevant doses that do not produce other, potentially confounding effects on physiology and behavior.Citation34
The clinical requirement for chronic treatment regimes has produced extensive literature describing the effects of chronic antidepressant treatment in normal animals without paying attention to the basal state of targeted neural systems. Administration of antidepressant medication, or electroconvulsive stimulation, to nondepressed humans almost certainly does not elicit the same neural changes as when applied to a depressed patient. Therefore, we should make sure that the basal state of laboratory animals undergoing trials of (putative) antidepressants closely mirrors what is known about the neural changes that occur in depressed humans.
As outlined above, the ideal model should respond to chronic, but not acute, treatment with conventional antidepressants. The importance of this feature should not be underestimated, since only when a model shows a gradual response reflecting a drug's gradual onset of action is it possible to detect the actual time point of the therapeutic onset. Two models for which the clearest evidence for gradual onset of action has been obtained are the chronic mild stress model and the social stress/resident in trader paradigm.
It is well known that the environment plays an important role in determining behavior and, eg, lighting conditions and familiarity of the experimental settings have a profound impact on the behavior that an experimental animal displays. Therefore, it is a major problem that in the laboratory, the “daytime” when an animal's behavior is observed is determined purely by the experimenter. It is quite frequently neglected that laboratory rodents are nocturnal, and thus generally quiescent during the light phase of the day. Therefore, in rodents determination of the effect of psychotropic drugs on natural action patterns of behavior should be performed during the dark phase of the light-dark cycle. This means that animals must be housed under a reversed light-dark schedule.Citation34
Glucocorticoids and depression
Major depressive disorder is a complex, multifactorial and heterogeneous mental disorderCitation9 and its phenotypic heterogeneity requires the development of “multi-phenomenon” animal models. As an example of problematic clinical heterogeneity and its impact upon the utility of animal modeling, we will briefly discuss the hypothesis of hypcrcortisolism that has been widely considered as one of the fundamental neurobiological abnormalities of depression, and thus has dominated the relevant literature for many years.
If we are developing or using a valid animal model based upon perturbed corticosteroid function as a core aspect of depression, we must be confident that such perturbation is a reliable feature of the clinical presentation of depression. However, the clinical situation reveals that depressed subjects show a remarkable heterogeneity of neuroendocrine functions and that patients with hypothalamo-pituitary-adrenal (HPA) axis hyperactivity during acute depression may be in the range of only 35 %.Citation35 Interestingly, hypcrcortisolism has also been described in patients with quite different diagnoses such as Alzheimer's diseaseCitation36 or substance abuse.Citation37
A recent study by Strickland et alCitation38 in women revealed that, although well-defined adverse life events were associated with increased Cortisol concentrations in saliva, depression was not. In light of these and other findings in patients, Matthews et alCitation35 posed the question of the validity and relevance of studies modeling depression in animals with the focus predominantly on corticosteroid function and regulation. However, although these data are not incompatible with the theory that stress predisposes to depression through its effects on the HPA axis, one cannot exclude that pre-existing HPA-axis abnormalities represent a contributory factor in the genesis of some forms of depression.
Animal models of anxiety
Anxiety enables the individual to recognize danger and to deal with an unknown or vague internal or external threat. Fear is a similar alerting signal, but differs from anxiety in that it is regarded as response to a known, definite, nonconflictual threat. Clinicians assessing anxiety distinguish between “normal” and “pathological” anxiety. Normal anxiety is an advantageous response to a threatening situation that accompanies many aspects of daily life. By contrast, pathological anxiety is an inappropriate response to an external or internal stimulus. In light of the high complexity of anxiety disorders and the comorbidity with MDD, the chance of succeeding in developing comprehensive animal models that accurately reflect the relative influences of contributing factors in humans is probably quite poor.Citation39 However, as outlined below, ample opportunity exists to better define and extend existing models and to develop new experimental setups that consider the impact of combined factors in determining anxious behavior. The examples summarized in this part of the article have been selected because they model cardinal symptoms of anxiety but not depressive disorders.
Validity criteria for animal models of anxiety disorders
Numerous procedures with experimental animals have been developed to facilitate preclinical research on the behavioral pharmacology of anxiety and, as a result of this aim, are often referred to as “animal models of anxiety.” This is an unfortunate error in terminology, not only because it implies that anxiety is a unitary emotional state, but also because of the apparent inability of many tests to consistently detect the anxiolytic effects of novel drugs.Citation40 The discovery of benzodiazepines (BZs) about 50 years ago, and their therapeutic and commercial success in the treatment of anxiety, has stimulated the development of a number of experimental test procedures. Because BZs were the only effective anxiolytic drugs at that time, the predictive validity of the animal models has been mainly based on their ability to detect the pharmacological action of BZs and related compounds. Later, clinicians discovered that patients can become addicted to BZ, and consequently paid more attention to non-benzodiazepine anxiolytics. However, it turned out that these new drugs were a challenge to the validity of the existing screening models. The best known example is buspirone, a clinically effective serotonin (5-HT)1A receptor partial agonist whose anxiolytic potential was missed by conventional screening procedures in animals, in particular conflict tests in rats, and was only recognized during clinical assessments for possible antipsychotic efficacy.Citation41 This was the time when unconditioned conflict tests such as the elevated plus-maze were developed.Citation42
A further complication appeared when it became evident that anxiety is not a unitary phenomenon, but could be divided into various forms including “normal” or “state” anxiety on the one hand and “pathological” or “trait” anxiety on the other hand. According to today's terminology, pathological anxiety should not be considered just as an excess of normal anxiety, but it rather appears that the pathological forms have a different neurobiological basis. Furthermore, the various forms of human anxiety disorders have been shown to be differentially sensitive to pharmacological treatment.
Most of the experimental paradigms involve exposure of animals to external stimuli (eg, cues paired with foot shock, bright light for rodents, or exposure to a predator) or internal stimuli (eg, drugs) that are assumed to induce anxiety. Because none of these models involves pathological anxiety, that is an anxiety-like state independent of an obvious (external) stimulus, ListerCitation43 described them as animal models of state anxiety. In these experimental set-ups, subjects experience normal anxiety at a particular moment in time and their emotional state is just potentiated by an external anxiogenic stimulus.
Despite these problems in the use of animals to study anxiety, these models have been, and are still, indispensable for neurobiological/neuropharmacological research. Much of our understanding of the neural substrates of anxiety has emerged from studies employing animal models that emulate aspects of the presumed etiology, physiology, and behavioral expression of fear and anxiety. There are several excellent book chapters and review articles describing and discussing extensively these models.Citation2,Citation39,Citation40,Citation43-Citation46 However, a survey of current literature reveals a confusing diversity of experimental procedures with more than 30 behavioral paradigms claiming face, construct, and/or predictive validity as animal models of anxiety disorders (for review see refs 47-49).
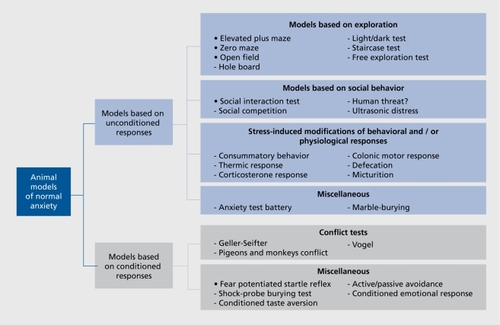
Models for normal anxiety
An overview of the existing models for normal anxiety is given in . As proposed by GriebelCitation47 these models are distinguished according to the following categories: (i) Models based on unconditioned responses; and (ii) models based on conditioned responses. The first category is further divided into four subgroups: models based on exploratory behavior in rodents (eg, elevated plusmaze and the light-dark test), models based on social behavior in rodents (social interaction test) or in nonhuman primates (human threat), and models based on somatic stress reactions (eg, stress-induced hyperthermia). In the fourth group, other paradigms are summarized which do not fit easily into the other subgroups such as the anxiety/fear test battery.
Elevated plus-maze
Today, the majority of studies using animal models of normal or state anxiety employ unconditioned-based procedures that rely on the natural behavior of the animals. Among these, the elevated plus-maze has become one of the most popular behavioral tests.Citation42,Citation48 Its popularity is mainly due to practical reasons, because the elevated plus-maze permits a quick screening of potential anxiety-modulating drugs or of genetically modified laboratory rodents without training the animals or involvement of complex schedules.Citation48 The elevated maze consists of two opposite open and two closed alleys. When the animal is taken straight from its home cage it explores the different alleys and the total number of entries is counted. Anxiolytics help to overcome the fear-induced inhibition of open-alley exploration, while anxiogenic agents suppress open-alley exploration. Unfortunately, the plus-maze behavior patterns may be influenced by variations in test parameters that are not always obvious, eg, the species or strain investigated, housing conditions, day time of the testing, intensity of the light, and scoring method.Citation50 As a result, a vast number of studies employing the elevated plus-maze have yielded inconsistent findings. To overcome these problems, Rodgers and JohnsonCitation51 have developed an “etiological” version of the mouse plus-maze that incorporates species-specific behavioral postures (eg, risk assessment, head-dipping) together with the conventional spatiotemporal measures of open-arm avoidance.
Elevated zero maze
This is a recent modification of the plus-maze designed for investigations in mice. It is an elevated annular platform with two opposite open and two closed quadrants. Animals are placed in one of the closed quadrants designated as the starting quadrant and anxiety related behaviors are recorded by both the observer and through a video system.
Open field test
Rodents arc night-active animals that prefer darkness and avoid bright areas. This has to be taken into account when using the open field test, a very common observational method.Citation52 For the open field test, the animal is taken from its home cage and placed in a novel and relatively lit arena that is large enough for the animal to move around in. The area is divided into peripheral and central units, and locomotion and rearing can be recorded in these units. Because of its photophobicity, the animal avoids the brightly lit open spaces and prefers to stay close to the walls. Exploratory or locomotor behavior is therefore measured while determining the distance from the wall, and autonomic activity such as urination and defecation is evaluated. By using infrared beam array systems, locomotion, rearing and time spent in certain predefined areas of the open field are measured automatically One also has to consider that the behavior displayed in the open field - similar to that in the elevated plus maze - is remarkably sensitive to a variety of internal and external factors.
Social interaction test
The social interaction test that was originally introduced by File,Citation53 and that quantifies the level of social behavior between animals, is a valuable behavioral paradigm for testing anxiolytic drugs. Experimental animals unfamiliar to each other are placed in pairs into an open arena. When the arena is brightly illuminated the situation is aversive for the animals, so that they reduce their social interactions. Anxiolytics usually increase the time spent in social interactions.
Fear-potentiated startle test
Davis and colleaguesCitation54 have utilized the fear-potentiated startle lest to study the fear circuitry in the brain. TMs test includes a classical fear conditioning in that a stimulus (eg, light) is paired with a mild electric foot shock. During the fear-conditioning phase a light stimulus signals the occurrence of a shock. The startle response is elicited by a loud noise, and its amplitude is augmented when the light and the noise are presented together. BZs have anxiolytic effects in this paradigm in that they inhibit the enhancement of the startle response but do not block the startle response per se. Briefly, the paradigm involves placing the animal in a cage equipped to measure the amplitude of the startle response elicited by the noise, either in the presence or absence of a light previously paired with an electric shock. Animals that have already been exposed to the shock-paired light show a greater startle response to the noise in the presence of the light than in its absence. Using this kind of potentiated startle response as an operational measure, it was found that the central nucleus of the amygdala and a variety of hypothalamic and brain stem areas are involved in physiological (eg, activation of the sympathetic and the parasympathetic system, release of “stress hormones”) and behavioral responses (eg, changes in locomotor activity, freezing) that reflect fear and anxiety.Citation54,Citation55
Defense tests
Defensive behaviors in mammals are thought to constitute a significant parameter that can be studied to understand human emotional disorders, including anxiety.Citation56 These behaviors occur in response to a number of threatening stimuli including predators, attacks by conspecifics, or presence of dangerous objects. The mouse defense test battery (MDTB) consists of an oval runway that allows the investigation of state anxiety by extensive etiological analyses to generate comprehensive behavioral profiles following drug treatment.Citation57-Citation58 Specific situational and behavioral components of the anxiety defense test battery, including reactivity to stimuli associated with potential threat such as presentation of an anesthetized predator (a rat), are incorporated into the M.DTB. Drug experiments have demonstrated that anxiolytic compounds generally tend to decrease defensive behaviors. It is noteworthy that some responses are specifically or mainly modulated by certain classes of drugs, and it has been suggested that risk assessment, flight, defensive threat/attack and escape attempts probably reflect different aspects of anxiety-related reactions.Citation59 These tests may thus represent a considerable methodological improvement because a major concern with traditional animal models of state anxiety that are based on single measures is that they are often unable to discriminate between effects of different classes of anxiolytics (benzodiazepines, 5-HT1A agonists, 5-HT reuptake inhibitors), whereas clinical findings strongly indicate differential therapeutic efficacy of these agents. Based on present observations in mice, the etiological plus-maze and the MDTB provide new tools to differentiate anxiolytic drugs of various classes that induce specific behavioral profiles.
Animal models for pathological anxiety
Pathological anxiety in humans is often an enduring feature of the individual, at least in part due to a genetic predisposition. To model genetically based anxiety, mice with target mutations in distinct genes were created that exhibit phenotypic changes indicative of increased anxiety. In addition rat or mouse lines were bred to select for high or low emotional reactivity.
The neurotransmitter 5-HT is centrally involved in the neuropathology of many neuropsychiatrie disorders. More than a dozen pharmacologically distinct serotonin receptor subtypes regulate a wide range of functions in different brain areas and in the periphery of the body (for review, see ref 60). There is pharmacological and neuroanatomical evidence that at least one 5-HT receptor, 5-HT]A, is involved in the regulation of anxiety-like behaviors.Citation49,Citation61 Results of recent studies employing mutant mice with targeted deletions of the 5-HT1A receptor gene further support a role of this receptor in anxiety.Citation62 5-HT, 1A receptor null mutant mouse lines have been independently generated in three laboratories from mice with different genetic backgrounds, C57BL/6,Citation63 129/Sz,Citation62 or through outbreeding from Swiss-Webster.Citation64 Given the interlaboratory variability that occurred in other cases of behavioral studies on genetically modified mice,Citation65 it is notable that concordant findings on 5-HT1A receptor null mutants were reported in all three laboratories and across the three mouse strains.
Further examples of models for pathological anxiety are mice that were gene targeted for the corticotropin-releasing factor (CRF)Citation66 or for the γ2 subunit of the GABAA receptor. This receptor subunit is known to be essential in mediating the anxiolytic actions of benzodiazepines.Citation67 An “anxious” phenotype was also observed in mutant mice lacking the gene for the neuroactive peptide NPYCitation68; (see also the review on genetic models of anxiety).Citation69
At first glance, these lines of mutant mice seem to provide a unique opportunity to model pathological or trait anxiety. Moreover, compared with the state anxiety models in which the baseline level of anxiety of a subject is increased artificially by exposure to external (aversive) stimuli, the new models seem to be advantageous in that they may represent a kind of “general anxiety” due to a certain genetic modification. This sounds reasonable since genetic studies in humans have indicated that there are genetic components contributing to the development of anxiety disorders. However, one has to consider that in humans, the modulation of anxiety processes involves multiple genes. In the future, the use of mice strains that display elevated emotionality due to a distinct “genetic background,” or mice selected for their high levels of anxiety using gene targeting experiments may lead to greater progress in our understanding of the neurobiological substrates of anxiety. Such animals would exhibit increased anxiety not because of a defect in a single gene, but because of a complex set of genes that result in an enduring feature of the strain/individual, thus determining its phenotype in combination with environmental factors.Citation46
Inbred strains which show constantly high levels of anxiety/fcarfulness have already been created. In mice, the BALB/c strain has been considered to be a realistic model of trait anxiety, which is probably not related to only one particular target gene but to abnormalities in various neurotransmitter circuits such as the G ABA ergic, dopaminergic and the opioid system.Citation46 Also in rats, several strains of trait anxiety have been described, eg, the Maudsley rat,Citation70 the Wistar-Kyoto,Citation71 the Roman,Citation72 or the Sardinian alcohol-preferring line.Citation73
Recently, two breeding lines were generated from the same strain of Wistar rats showing a maximum difference in anxiety-related behavior and a minimum difference in other behaviors as well as in physiological parameters not directly related to anxiety. These two rat lines are now called high anxiety-related behavior (HAB) and low anxiety-related behavior (LAB).Citation74 Their overall performance in various behavioral tests suggests that selective breeding has resulted in lines not only differing markedly in their innate anxiety -related behavior but also in stressrelated behavioral performances, indicating a close link between the emotional evaluation of a novel and stressful situation and a subject's capability to cope with such situations.
Developing novel models relevant to depression and anxiety disorders
One striking aspect of most anxiety disorders and MDD is the higher incidence in females compared with males.Citation9 Furthermore, gender differences in psychotropic drug metabolism and clearance can have direct effects on the efficacy of pharmacological treatments of mental disorders in women.Citation75 Thus, biological, hormonal, and cultural factors may contribute to gender differences in some disorders and to gender-specific efficacy of pharmacological interventions. Basic research in animals may help to determine the degree to which these features arc caused by differences in brain physiology.Citation76 Given the preponderance of sex differences in many aspects of anxiety disorders and MDD, it is surprising to find how few basic animal studies have considered gender as a determining factor for depression and anxiety disorders. A recent survey revealed that approximately 90% of the animal studies on serotonergic drugs and anxiety-like behaviors utilized males exclusively.Citation77 Clearly, this major deficiency has delayed progress towards an understanding of the processes contributing to anxiety disorders and MDD, and most likely hindered the development of gender-specific treatments.
Conclusion
In conclusion, animal models are indispensable tools for research on the neurobiological mechanisms underlying MDD and/or anxiety disorders and for the development of new antidepressant/anxiolytic drugs. However, besides the obvious progress in research that could only be achieved because of the existence of these models, one also has to bear in mind that each animal model has its pros and cons. Currently, it appears that the use of several models, either successively or in parallel, provides the greatest chance to elucidate the neurobiological processes of psychiatric diseases and to identify new, effective antidepressant and anxiolytic compounds.
REFERENCES
- McKinneyWT.Animal models of depression: an overview.Psychiatr Dev.1984277966483850
- WillnerP.Behavioural Models in Psychopharmacology: Theoretical, industrial and Clinical Perspectives. Cambridge, UK: Cambridge University Press;1991
- CryanJF.MarkouA.LuckiI.Assessing antidepressant activity in rodents: recent developments and future needs.Trends Pharmacol Sci20022323824512008002
- MinekaS.WatsonD.ClarkLA.Comorbidity of anxiety and unipolar mood disorders.Annu Rev Psychol.1998493774129496627
- KaufmanJ.CharneyD.Comorbidity of mood and anxiety disorders.Depress Anxiety.200012 (suppl I) 697611098417
- NemeroffC.Comorbidity of mood and anxiety disorders: the rule, not the exception.Am J Psychiatry.20021593411772680
- GormanJM.Comorbid depression and anxiety spectrum disorders.Depress Anxiety.199741601689166648
- NelsonJC.A review of the efficacy of serotonergic and noradrenergic reuptake inhibitors for treatment of major depression.Biol Psychiatry.1999461301130810560035
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association;1994
- JesbergerJA.RichardsonJS.Animal models depression: parallels and correlates to serve depression in humans.Biol Psychiatry.1985207647842860930
- SanchezMM.LaddCO.PlotskyPM.Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models.Dev Psychopathol.20011341944911523842
- eligmanM.MaierS.Failure to escape traumatic shock.J Exp Psychol.196774196032570
- NestlerEJ.GouldE.ManjiH.et al.Preclinical models: status of basic research in depression.Biol Psychiatry.20025250352812361666
- O'NeilMFO.MooreNA.Animal models of depression: Are there any?Human Psychopharmacol.200318239254
- VollmayrB.HennFA.Learned helplessness in the rat: improvements in validity and reliability.Brain Res Protocols.2001817
- WillnerP.MuscatR.PappM.Chronic mild stress-induced anhedonia: A realistic animal model of depression.Neurosci Biobehav Rev.1992165255341480349
- nismanH.ZacharkoRM.Depression the predisposing influence of stress.Behav Brain Sci.1982589137
- KesslerRC.The effects of stressful life events on depression.Annu Rev Psychol.1997481912149046559
- KendlerKS.KarkowskiLM.PrescottCA.Causal relationship between stressful life events and the onset of major depression.Am J Psychiatry.199915683784110360120
- PaykelES.Stress and affective disorders in humans.Sernin Clin Neuropsychiatry.20016411
- adidG.NakashR.DeriI.et al.Elucidation of the neurobiology of depression: insights from a novel genetic animal model.Prog Neurobiol.20006235337810856609
- BrownG.Life events and illness. In: Stanford SC, Salamon P, eds.Stress: from Synapse to Syndrome.London, UK: Academic Press 1993;xx2040
- BlanchardDC.BlanchardRJ.Behavior correlates of chronic dominancesubordination relationships of adult male rats in a seminatural situation.Neurosci Biobehav Rev.1990144554622287482
- FuchsE.FlùggeG.Social stress in tree shrews: effects on physiology, brain function and behavior of subordinate individuals.Pharmacol Biochem Behav.20027324725812076743
- RygulaR.AbumariaN.FluggeG.et al.Anhedonia and motivational deficits in rats: impact of chronic social stress.Behav Brain Res.200516212713415922073
- MiczekKA.Tolerance to the analgesic, but not discriminative stimulus effects of morphine after brief social defeat in rats.Psychopharmacology (Bed).1991104181186
- KoolhaasJM.De BoerSF.De RutterAJ.et al.Social stress in rats and mice.Acta Physiol Scand Suppl.199764069729401610
- RygulaR.AbumariaN.FluggeG.et al.Citalopram counteracts depressivelike symptoms evoked by chronic social stress in rats.Behav Pharmacol.200617192916377960
- AbumariaN.RygulaR.Havemann-ReineckeU.RutherE.BodemerW.RoosC.FluggeG.Identification of genes regulated by chronic social stress in the rat dorsal raphe nucleus.Cell Mol Neurobiol.2006 Apr 22 [Epub ahead of print]
- DumanRS.Neuroplasticity: consequences of stressand actions of antidepressant treatment.Dialogues Clin Neurosci.2004615716922034207
- WillnerP.MitchellPJ.Animal models of depression: a diathesis/stress approach. In: D'haenen HAH, Den Boer JA, Willner P, eds.Biological Psychiatry.Chichester, UK: Wiley 2002703726
- SullivanPF.NealeMC.KendlerKS.Genetic epidemiology of major depression: review and meta-analysis.Am J Psychiatry.20001571552156211007705
- FrazerA.MorilakDA.What should animal models of depression model?Neurosci Biobehav Rev.20052951552315893377
- MitchellPJ.RedfernPH.Animal models of depressive illness: the importance of chronic drug treatment.Curr Pharmaceutical Design.200511171203
- MatthewsK.ChristmasD.SwanJ.et al.Animal models of depression: navigating through the clinical fog.Neurosci Biobehav Rev.20052950351315925695
- MartignoniE.PetragliaF.CostaA.et al.Dementia of the Alzheimer type and hypothalamus-pituitary-adrenocortical axis: changes in cerebrospinal fluid corticotropin releasing factor and plasma Cortisol levels.Acta Neurol Scand.1990814524562375247
- ContoreggiC.HerningRI.MaP.et al.Stress hormone responses to corticotropin-releasing hormone in substance abusers without severe comorbid psychiatric disease.Biol Psychiatry.200354 87387814573313
- StricklandPL.DeakinJFW.PercivalC.et al.Bio-social origins of depression in the community: interactions between social adversity, Cortisol and serotonin neurotransmission.Br J Psychiatry.200218016817311823330
- hekharA.McCannUD.MeanyMJ.et al.Summary of a National Institute of Mental Health workshop: developing animal models of anxiety disorders.Psychopharmacology.200115732733911605091
- odgersRJ.Animal models of 'anxiety': where next?Behav Pharmacol.199784774969832964
- oaKL.WardA.Buspirone: a preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic.Drugs.1986321141292874976
- ListerRG.The use of a plus-maze to measure anxiety in the mouse.Psychopharmacology.1987921801853110839
- ListerRG.Ethologically-based animal models of anxiety disorders.Pharmacol Ther.1990463213402188266
- TreitD.Animal models for the study of anti-anxiety agents: a review.Neurosci Biobehav Rev.198592032222861589
- SangerDJ.Animal models of anxiety and the screening and development of novel anxiolytic drugs. In: Boulton AA, Baker GB, Martin-lverson MT, eds.Animal Models in Psychiatry II, Neuromethods. Vol 19. Clifton, NJ: Humana Press;1991147198
- BelzungC.GriebelG.Measuring normal and pathological anxiety-like behaviour in mice: a review.Behav Brain Res.200112514114011682105
- GriebelG.5-Hydroxytryptamine-interacting drugs in animal models of anxiety disorders: more than 30 years of research.Pharmacol Ther.1995653193957644567
- RodgersRJ.CaoBJ.DalviA.et al.A. Animal models of anxiety: an ethological perspective.Brazj Med Biol Res.199730289304
- eissSM.LightowlerS.StanhopeKJ.KennettGA.DourishCT.Measurement of anxiety in transgenic mice.Rev Neurosci.200011597410716656
- HoggS.A review of the validity and variability of the elevated plus-maze as an animal model of anxiety.Pharmacol Biochem Behav.19965421308728535
- RodgersRJ.JohnsonNJT.Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety.Pharmacol Biochem Behav.1995522973038577794
- KelleyAE.Locomotor activity and exploration. In: Sahgal A, ed.Behavioural Neuroscience. A Practical Approach. Vol II. Oxford, UK: IRL Press;1993121
- FileSE.Animal models for predicting clinical efficacy of anxiolytics: social behaviour.Neuropsychobiology.19851355622863779
- DavisM.The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety.Trends Pharmacol Sci.19921335411542936
- DavisM.FallsWA.CampeauS.KimM.Fear-potentiated startle: a neural and pharmacological analysis.Behav Brain Res.1993581751988136044
- BlanchardRJ.BlanchardDC.Affect and aggression: an animal model applied to human behavior. In: Blanchard RJ, Blanchard DC, eds.Advances in the Study of Aggression. Orlando, Fla: Academic Press Inc;1984162
- GriebelG.BlanchardDC.JungA.BlanchardRJ.A model of 'antipredator' defense in Swiss- Webster mice: effects of benzodiazepine receptor ligands with different intrinsic activities.Behav Pharmacol.1995673274511224376
- BlanchardDC.GriebelG.BlanchardRJ.Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic.Neurosci BiobehavRev. 200125205281
- GriebelG.SangerDJ.The mouse defense test battery: an experimental model of different emotional states. In: Haug M, Whalen RE, eds.Animal Models of Human Emotion and Cognition. Washington, DC: American Psychological Association;19997585
- BarnesMM.SharpT.A review of central 5-HT receptors and their function.Neuropharmacology.1999381083115210462127
- FileSE.GonzalezLE.AndrewsN.Comparative study of pre- and postsynaptic 5HT1 A receptor modulation of anxiety in two ethological animal tests.J Neurosci.199616481048158764667
- RambozS.CostingR.AmaraDA.et al.Serotonin receptor 1A knockout: an animal model of anxiety-related disorder.Proc Natl Acad Sci U S A.19989514476144819826725
- HeislerLK.ChuHM.BrennanTJ.et al.Elevated anxiety and antidepressant-like responses in serotonin 5-HT 1 A receptor mutant mice.Proc Natl Acad Sci US A.1998951504915054
- ParksCL.RobinsonPS.SibilleE.et al.Increased anxiety of mice lacking the serotonin 1A receptor.Proc Natl Acad Sci U S A.19989510734107399724773
- CrabbeJC.WahlstenD.DudekBC.Genetics of mouse behavior: interactions with laboratory environment.Science.19992841670167210356397
- Stenzel-PooreMP.DuncanJE.RittenbergMB.et al.CRH overproduction in transgenic mice: behavioral and immune system modulation.Ann NY Acad Sci.199678036488602738
- CrestaniF.LorezM.BaerK.et al.Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues.Nat Neurosci.1999283383910461223
- BannonAW.SedaJ.CarmoucheM.et al.Behavioral characterization of neuropeptide Y knockout mice.Brain Res.2000868798710841890
- FinnDA.Rutledge-GormannMT.CrabbeJC.Genetic animal models of anxiety.Neurogenetics.2003410913512687420
- BroadhurstPL.The Maudsley reactive and non-reactive strains of rats: a survey.Behav Genet.10755 2993191191155
- GotoSH.ConceicaoIM.RibeiroRA.FrussaFilhoR.Comparison of anxiety measured in the elevated plus-maze, open-field and social interaction tests between spontaneously hypertensive rats and Wistar EPM-1 rats.BrazJ Med Biol Res.1993269659698298531
- ChaouloffF.CastanonN.MormedeP.Paradoxical differences in animal models of anxiety among the Roman rat lines.Neurosci Lett.19941822172217715814
- ColomboG.AgabioR.LobinaC.et al.Sardinian alcohol-preferring rats: a genetic animal model of anxiety.Physiol Behav.199557118111857652041
- LiebschG.MontkowskiA.HolsboerF.et al.Behavioural profiles of two Wistar lines selectively bred for high or low anxiety-related behaviour.Behav Brain Res.1998943013109722280
- PigottTA.Gender differences in the epidemiology and treatment of anxiety disorders.J Clin Psychiatry.19996041510487250
- PalanzaP.Animal models of anxiety and depression: how are females different?Neurosci Biobehav Rev.20012521923311378178
- BlanchardDC.GriebelG.BlanchardRJ.Gender bias in preclinical psychopharmacology: male models for (predominantly) female disorders.J Psychopharmacol.199597982