Abstract
Drug transporters are membrane proteins present in various tissues such as the lymphocytes, intestine, liver, kidney, testis, placenta, and central nervous system. These transporters play a significant role in drug absorption and distribution to organic systems, particularly if the organs are protected by blood-organ barriers, such as the blood-brain barrier or the maternal-fetal barrier. In contrast to neurotransmitters and receptor-coupled transporters or other modes of interneuronal transmission, drug transporters are not directly involved in specific neuronal functions, but provide global protection to the central nervous system. The lack of capillary fenestration, the low pinocytic activity, and the tight junctions between brain capillary and choroid plexus endothelial cells represent further gatekeepers limiting the entrance of endogenous and exogenous compounds into the central nervous system. Drug transport is a result of the concerted action of efflux and influx pumps (transporters) located both in the basolateral and apical membranes of brain capillary and choroid plexus endothelial cells. By regulating efflux and influx of endogenous or exogenous substances, the blood-brain barrier and, to a lesser extent, the blood-cerebrospinal barrier in the ventricles, represents the main interface between the central nervous system and the blood, ie, the rest of the body. As drug distribution to organs is dependent on the affinity of a substrate for a specific transport system, membrane transporter proteins are increasingly recognized as a key determinant of drug disposition. Many drug transporters are members of the adenosine triphosphate (ATP)-binding cassette (ABC) transporter superfamily or the solute-linked carrier (SLC) class. The multidrug resistance protein MDR1 (ABCB1), also called P-glycoprotein, the multidrug resistance-associated proteins MRP1 (ABCC1) and MRP2 (ABCC2), and the breast cancer-resistance protein BCRP (ABCG2) are ATP-dependent efflux transporters expressed in the blood-brain barrier. They belong to the superfamily of ABC transporters, which export drugs from the intracellular to the extracellular milieu. Members of the SLC class of solute carriers include, for example, organic ion transporting peptides, organic cation transporters, and organic ion transporters. They are ATP-independent polypeptides principally expressed at the basolateral membrane of brain capillary and choroid plexus endothelial cells that also mediate drug transport through central nervous system barriers.
Los transportadores de fármacos son proteínas de membrana presentes en varios tejidos como linfocitos, intestino, hígado, riñón, testículos, placenta y el sistema nervioso central. Estos transportadores juegan un papel significativo en la absorción de fármacos y en la distribución a los sistemas del organismo, especialmente si los órganos están protegidos por barreras sangre-órgano, como la barrera hémato-encefálica o la barrera materno-fetal. En contraste con los neurotransmisores y los transportadores acoplados a receptores u otros modos de transmisión interneuronal, los transportadores de fármacos no están directamente involucrados en funciones neuronales específicas, pero proveen una protección global al sistema nervioso central. La falta de capilarización, la baja actividad de los pinocitos, y las uniones estrechas entre los capilares cerebrales y las células endoteliales de los plexos coroídeos representan más barreras que limitan la entrada de compuestos endógenos y exógenos al sistema nervioso central. El transporte de fármacos es el resultado de una acción concertada de bombas de entrada y salida (transportadores) ubicadas en las membranas basolaterales y apicales de los capilares cerebrales y de las células endoteliales de los plexos coroídeos. En la regulación de la entrada y salida de las sustancias endógenas o exógenas, la barrera hémato-encefálica, y en menor medida la barrera hémato-cerebroespinal en los ventrículos, representan el principal punto de contacto entre el sistema nervioso central y la sangre, es decir, el resto del organismo. Como la distribución de los fármacos a los órganos depende de la afinidad de un sustrato por un sistema de transporte específico, las proteínas transportadoras de membrana están siendo cada vez más reconocidas como un factor déterminante en la disponibilidad de los fármacos. Muchos transportadores de fármacos son miembros de la superfamilia del transportador (ABC) unido a adenosín trifosfato (ATP) o de la clase de transportadores unidos a soluto (SLC). La proteína de resistencia a multifármacos MDR1 (ABCB1), también llamada P-glicoproieína, las proteínas asociadas a resistencia a multifármacos MRP1 (ABCC1) y MRP2 (ABCC2), y la proteína de resistencia al cáncer de mama BCRP (ABCG2) son transportadores de salida dependientas de ATP que se expresan en la barrera hémato-encefálica. Ellos pertenecen a la superfamilia de transportadores ABC, los cuales llevan fármacos desde el medio intracelular al extracelular. Los miembros de la clase SLC de los transportadores de soluto incluyen, por ejemplo, péptidos transportadores de iones orgánicos, transportadores de cationes orgánicos y transportadores de iones orgánicos. Ellos son polipéptidos independientes de ATP que se expresan principalmente en la membrana basolateral de los capilares cerebrales y las células endoteliales de los plexos coroídeos que también median el transporte de fármacos a través de las barreras del sistema nervioso central.
Les transporteurs de médicaments (drug transporters) sont des protéines situés dans les membranes cellulaires de différents tissus comme les lymphocytes, l'intestin, le foie, les reins, les testicules, le placenta ou le SNC (système nerveux central). Ces transporteurs jouent un rôle primordial dans l'absorption et la distribution des médicaments dans les organes cibles, surtout s'ils sont protégés par des structures comme la barrière hématoencéphalique (BHE) ou la barrière maternofœtale. Contrairement aux transporteurs liés à des récepteurs ou à la transmission interneuronale, ces transporteurs membranaires ne sont pas directement impliqués dans une fonction neuronale spécifique mais assurent une protection globale du système nerveux central. Le transport de médicaments est la résultante des mécanismes d'efflux (sortie) et d'influx (entrée) situés au niveau de la membrane apicale et basolatérale des cellules endothélials des capillaires cérébraux et du plexus choroïdien. En régulant l'efflux et l'influx des substances endogènes ou exogènes, la BHE et la barrière hémato-cérébro-spinale représentent les principales interfaces entre le système nerveux central et le sang. La distribution d'un médicament vers les organes cibles est dépendante de son affinité pour un système de transport spécifique ; les transporteurs membranaires sont ainsi reconnus comme un élément clé dans la disposition des médicaments au niveau du système nerveux central. Plusieurs transporteurs membranaires font partie de la superfamille ABC (ATP-Binding Cassette) ou SLC (Solute Carriers). La Multi-Drug Resistance protein MDR1 (ABCB1) nommée aussi P-glycoprotéine, les Multidrug Resistance-associated Proteins MRP1 (ABCC1) et MRP2 (ABCC2), ainsi que la Breast Cancer Resistance Protein BCRP (ABCG2) sont des transporteurs d'efflux ATP-dépendants présents au niveau de la BHE et qui appartiennent à la superfamille des transporteurs ABC, Les OATPs (Organic Anion Transporter Proteins), OCTs (Organic Cation Transporters) et OATs (Organic Anion Transporters) font partie des transporteurs ATP-indépendants de type SLC constitués de polypeptides (solute carriers). Les conséquences cliniques des transporteurs membranaires semblent jouer un rôle de plus en plus important dans le développement de nouveaux médicaments, particulièrement en neurologie et en psychiatrie. Les différents mécanismes qui régulent le transport de molécules à travers les membranes cellulaires et les barrières organiques sont des éléments clés pour expliquer les mécanismes d'échec ou de résistance au traitement, ainsi que certains effets indésirables et variations individuelles à la réponse thérapeutique attendue.
The consequences of transporters on central nervous system (CNS) drug development are becoming increasingly important, due to their influence on clinical outcome. Membrane transporters provide insight into the mechanisms of treatment failure, adverse drug reactions, and individual differences in the management of neurological and psychiatric disorders.
The presence of uptake and efflux transporters in capillary endothelial cells mediates drug transport from the bloodstream to the organs. For example, these membrane transporters play an essential role in the digestive tract, the kidney, and the liver, as well as other organic systems such as the blood cells, the placenta, and the central nervous system.Citation1
All cells selectively transport endogenous and exogenous compounds across their membrane to maintain an intracellular milieu distinct from the outer one; this is achieved in part by the membrane transporters, which arc multispecific transport proteins. These transporters display physiologic functions in terms of transporting endogenous compounds, eg, hormones, amino acids, bile acids, and lipids.Citation2-Citation4 Moreover, membrane transporter mutations may lead to severe genetic disorders such as cystic fibrosis (ABCC7),Citation5 immune deficiency (ABCB2 and ABCB3),Citation6 intrahepatic cholestasis of pregnancy (ABCB11),Citation7 persistent hypoglycemia of infancy (ABCC8),Citation8 X-linked adrenoleukodystrophy (ABCD1),Citation9 X-linked ataxia with sideroblastic anemia (ABC.B7),Citation10 and retinal degeneration (ABCA4).Citation11,Citation12
This review focuses on the functional significance of membrane transporters as drug carriers: their role is constantly increasing in current medical practice, as they represent a key factor in clinical outcome.Citation7,Citation13,Citation14
Physiological aspects
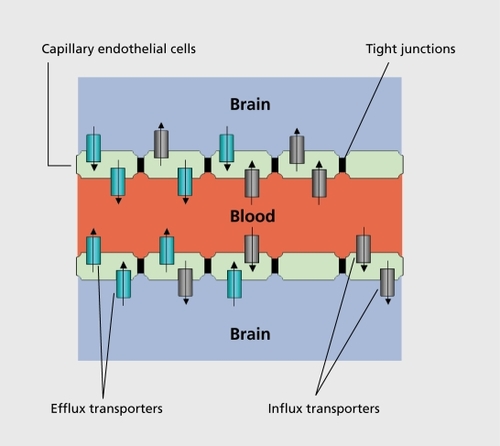
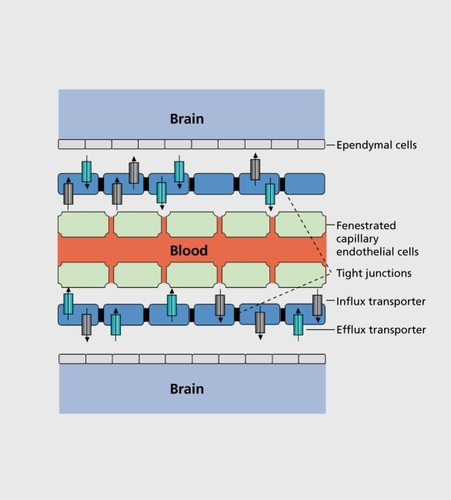
The blood-brain barrier (BBB) is a physical barrier, and maintains a given extracellular environment for neurons and glial cells in the CNS. The BBB is formed by the connection of closely sealed tight junctions between the capillary endothelial cells, which are not fenestrated and which display minimal pinocytosis (). The capillary endothelial cells form a polarized barrier similar to that located in the retina, or in the renal proximal tubule, which regulates diffusion of molecules across the BBB, and limits the entry of xenobiotics via paracellular pathways by intercellular tight junctions.Citation15-Citation17 Not all cerebral blood vessels are closely sealed with tight junctions: fenestrated capillaries are found in the pituitary gland and in the cirumventricular organs such as the area postrema, the lamina terminalis, and the median eminence.Citation18
The choroid plexus is a highly vascularized epithelial organ which secretes the cerebrospinal fluid and regulates its composition through active and selective transport processes; it has an active role in the cleansing of the cerebrospinal fluid of endogenous and exogenous compounds.Citation19 The blood cerebrospinal barrier (CSB) is considered as the second fluid barrier protecting the central nervous system: it is principally formed by epithelial cells of the choroid plexus in the ventricles and the arachnoid membrane. Like the brain capillary endothelial cells, the choroid plexus epithelial cells are connected by high-resistance tight junctions, which closely separate the blood from the cerebrospinal fluid compartment ().Citation20
Together with the BBB and the CSB, the membrane transporter systems represent further gatekeepers to the CNS; these play a critical role in drug disposition.Citation21 Membrane transporters either enhance or restrict drug distribution to the target organs. Depending on their main function, these membrane transporters are divided into two categories: the efflux (export) and the influx (uptake) transporters. Influx transport proteins facilitate and efflux transporters limit drug passage through membrane barriers such as the BBB or the CSB.Citation22 Several membrane transporters are found at the apical and basolateral epithelial cell membrane of the brain capillary and of the choroid plexus endothelial cells ().Citation23
Pharmacological aspects
Drug absorption from the systemic circulation into the CNS was previously considered a passive process that depended on drug physicochemical properties such as molecular size, lipophilicity, and the pKa of a drug. Although the physicochemical properties of a medication do affect its absorption and access to target organs, transporter proteins have a major role in the overall drug distribution process through their targeted expression in tissue such as the brain. Until recently, most pharmacokinetic studies focused only on the role of drugmetabolizing enzymes as a key determinants of drug disposition.Citation24
Phase I enzymatic reactions are mainly represented by the cytochrome P450 mono-oxygenase system, as well as by other enzymes such as pseudocholinesterase or alcohol dehydrogenase. Phase I enzymatic reactions modify the chemical structure of the compound itself with either loss of pharmacological activity or, on the contrary, when prodrugs are administered, enhanced biological activity through biotransformation into an active metabolite. In contrast, Phase II enzymatic reactions are conjugation reactions that lead to the formation of a covalent linkage with an endogenously derived compound such as glucuronic acid, sulfate, glutathione, or amino acids into highly polar conjugates excreted into the bile or urine: Nacetyltransferase 2 or catechol-O-methyltransferase are examples of enzymes that catalyze phase II reactions. Drug membrane transporters enable access of compounds to phase I reactions and further elimination after phase II reactions.
Thus, drug uptake transporters deliver the drug to an intracellular enzymatic detoxification system, whereas drug efflux transporters decrease the intracellular load from the detoxification system. Inhibition of transmembrane transporters may lead to a lower substrate uptake with a poorer intracellular access of the drug to the enzymatic systems.Citation25
This synergy between metabolizing enzymes and drug transporters accounts for the distribution to brain tissues, and determines the pharmacokinetic profile of CNS drugs.Citation26 Furthermore, substrate similarity is well documented between membrane transporters and cytochrome P450 enzymes. For instance, substrates of the PGP multidrug resistance protein (MDR1) are usually substrates of the CYP3A4, digoxin and fexofenadine excepted.Citation27
Characterization of drug transporters
Many drug transporters are members of the adenosine triphosphate (ATP)-binding cassette (ABC) transporter superfamily or the solute-linked carrier (SLC) class. The ABC superfamily (ABC transporters) comprises the main efflux membrane transporters for drug elimination, and the solute -linked carriers (SLC transporters) are invlolved with influx and efflux transport function for drug uptake and export.Citation28
ABC transporters
The ABC transporters are transmembranous proteins found in all animal species; they require energy from ATP hydrolysis to actively remove compounds from the cell, often against a high concentration gradient. They are composed of two transmembrane domains that form a pathway through which the substrates move, and by two nucleotide-binding domains located at the cytoplasmatic face of the membrane, providing ATP hydrolysis to allow substrate translocation across cellular membranes.Citation29 Members of the ABC superfamily are classified as such according to consensus sequences including both domains (Walkers A and Walkers B ATP-binding motifs), as well as the ABC signature (C motif).Citation30 To date, 49 human ABC family members have been identified, divided into seven different subfamilies.
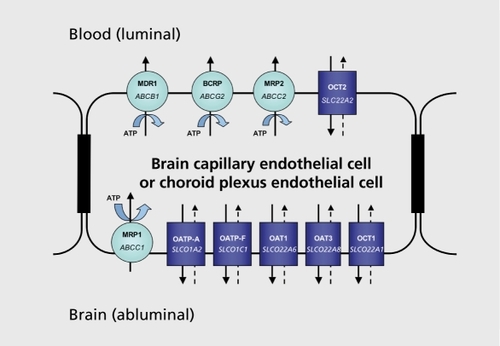
The ABC transporter superfamily includes medically important members such as the multidrug resistanceassociated protein 1 (MRP1) located at the basolatcral plasma membrane domain (abluminal),the multidrug resistance-associated protein 2 (MRP2), the breast cancer resistance protein (BCRP), and MDR1 (also known as PGP) localized at the apical membrane (luminal). MDR1 (encoded ABCB1), BCRP (encoded ABCG2), MRP1 (encoded ABCC1), and MRP2 (encoded ABCC2) were identified in the BBB at the luminal side of the capillary endothelial cells, M.RP1 excepted (Figure 3).
The substrates handled by the ABC transporters include a wide range of endogenous and exogenous compounds and diverse type of molecules, from organic cations and anions to larger molecules such as large polypeptides or therapeutic agents. For instance, MRP1 has preferential transport of anionic compounds such as sulfate conjugates or glutathione, whereas MDR1 shows broader substrate specificity.Citation31,Citation32
Variation of ABC transporter activity by drug-drug interactions, genetic polymorphisms, and overexpression is considered as a major cause of treatment failure, interindividual variability, and adverse drug reactions.Citation25,Citation33
However, randomized controlled studies on these issues with antidepressants, antipsychotics, or mood stabilizers in humans are still lacking.
ABC transporters are considered as the most relevant determinants of efflux transport and provide multiple barriers in the brain capillary and choroid plexus endothelial cells.Citation34 A multidrug resistance feature is associated with a poorer clinical outcome in several CNS disorders.Citation24 Furthermore, several ABC transporters were directly implicated in drug delivery to brain neoplasms and in the response to therapeutic agents.Citation35 For instance, the expression of the ABC transporters ABCC4 and ABCC5 was associated with an astrocytic phenotype with higher chemoresistance of astrocytic tumors compared with oligodendrogliomas.Citation36 Recently, the ABC transporters were also found to be associated with pharmacoresistance to anticonvulsant drugs in patients with intractable mesial temporal lobe epilepsyCitation37
MDR1 activity significantly decreases during aging with, consecutively, an increased brain exposure to drugs and toxins in elderly subjects.Citation38 Furthermore, impaired MDR1 function is reported as a predisposing factor in the development of neurodegenerative diseases such as Parkinson's disease or sporadic Alzheimer's dementia.Citation39 Pathologic accumulation of amyloid β in Alzheimer's disease may result from an impaired MDR1 activity, as amyloid β is considered as a substrate for MDR1.Citation13
SLC transporters
The SLC class of solute carriers consists of specific membrane transporters that mediate sodium-independent transmembrane solute transport: it is divided into 43 human families based upon amino acid homology of at least 25% between family members. To date, nearly 300 genes have been identified.Citation40 The Human Genome Organization (HUGO) Nomenclature Committee Database provides information about new transporter families of the SLC gene series (SLC transporters-gene nomenclature, SLCO).Citation41 Members of the SCLO superfamily are not only expressed in the BBB and in the choroid plexus, but also in the small intestine, the liver, the kidney, the blood-testis barrier, and the placenta.Citation42,Citation43
The SLCO superfamily of solute carriers includes carriers, such as the organic anion transporting protein family (OATPs), the organic anion transporter family (OATs, SLCO22A), and the organic cation transporter family (OCTs, SLCO22A). The organic cation/camitine transporter (OCTN, SLCO22A), the monocarboxylate transporter family (MCTs, SLCO16), and the peptide transporter (PEPT, SLCO15A) may represent further important. SLC families, and their function as CNS barriers is currently under investigation.Citation44,Citation45 For example, α-hydroxybutyrate (GHB), a therapeutic agent for catalepsy with narcolepsy, undergoes passive diffusion through the BBB but also the MCT1 carrier-mediated process, that is saturable and can be inhibited.Citation46 Proof-of-concept studies are being conducted to provide better insights into GHB therapy and GHB toxicity by means of transport inhibitors.Citation47
Several in vitro and in vivo data indicate that OATP1A2, OATP1C1, and OATP2B1 (members of the SLCO21 A family), and OAT1, OAT2, OCT1, OCT2, and OCT3 (members of the SLCO22A family) are expressed in the murine and human brain, and mediate drug transport through the CNS barriers.Citation2-Citation4,Citation36,Citation41,Citation48,Citation49
The SLCO21A family is referred to as the OATP family: these transporters consist of 12 transmembrane domain proteins, whose substrates are anionic amphipathic highmolecular-weight molecules that bind to albumin.Citation40 Ihe transport mechanism is based upon anion exchange coupled to cellular uptake of organic compounds with the efflux of bicarbonate, glutathione, and conjugates.
The SLC22A transporters include OCTs including OCTN, and OATs that also consist of 12 transmembrane domain proteins, but with different substrate specificity. Indeed, OATPs not only mediate uptake of anionic, but also neutral and cationic, compounds. OCT members are mainly unidirectional porters, whereas OAT members act as anion exchangers. 'Ihe organic anion transporting proteins (OATPs), OATs, and the OCTs represent the major uptake transport systems that mediate organic compound transport activities at the apical and the basolateral plasma membrane domains.
Drug transporter polymorphisms
The expression of transport proteins localized in the membranes of various organs are significant determinants of the pharmacokinetics of therapeutic agent including at the level of the CSB and the BBB.Citation33,Citation50-Citation53
There is genetic polymorphism of drug transporters in the structure of genes and in the number of alleles. The MDR1 gene has been particularly well investigated and several MDR1 polymorphisms have been found: many of them determine membrane transporters expression in the BBB and in the CNS with variable drug transport activity.Citation26,Citation54,Citation55 Such medically relevant polymorphisms are called nonsynonymous polymorphisms, as they directly condition the drug transporter function with potentially variable clinical outcomes.
The functional significance of different MDR1 expression for drug disposition was mainly studied with MDR1 knockout, mice. For instance, MDR1 knockout mice are 50- to 100-fold more sensitive to the neurotoxic pesticide ivermectine, and the accumulation of this drug in the CNS was 80- to 100-fold greater when compared with control mice.Citation56 In humans, several mutations resulting in several MDR1 single nucleotide polymorphisms (SNPs) have been identified, but only those at positions 2677 and 3435 seem to be associated with changes in PGP expression and function.Citation25,Citation57,Citation58 In contrast to the P450 drug-metabolizing enzymes such as CYP2C9, CYP2C19, and CYP2D6, for which loss of function mutations or gene amplification manifests as distinct phenotypes in the population (eg, poor, intermediate, extensive, or ultrarapid metabolizers), the impact of MDR1 polymorphisms on pharmacokinetics is moderate: no definite MDR1 phenotype is recognized in humans.Citation59 There is no complete loss of transport function when polymorphisms are present: the genotyperelated differences in the MDR1 expression between, eg, the 3435 genotype, remains moderate with substantial overlap.Citation59 However, the difference between clinical outcomes may be in some conditions very impressive: patients with drug-resistant epilepsy were much more likely to have the CC genotype at ABCB1 3435 than the TT genotype (odds ratio: 2.66) .Citation60 Furthermore, ABC transporter polymorphisms are not only associated with resistance to treatment or failure, for example, for anticonvulsants, cytostatics, or antibiotics, but they also determine the incidence of adverse drug events.Citation50,Citation53,Citation57,Citation60-Citation63 Some examples of clinical effects and potential implications associated with human drug transporter polymorphisms are listed in Table I.
Table I. Examples of genetic polymorphisms in human drug transporters. ABC, adenosine triphosphate-binding cassette; MDR, multi-drug resitance; BCRP, breast cancer resistance protein; SLC, solute-linked carriers; OATP, organic anion transporting peptide; OAT, organic ion transporter; OCT, organic cation transporter; CNS, central nervous system.
Interestingly, the clinical impact of single nucleotide polymorphisms on genetic variability of expression and function of the multidrug resistance-associated proteins (MRPs, ABCC transporter) is to date rather limited as compared with eg, MDR1.Citation73 Outside the CNS, multiple but rare familial mutations in, eg, the ABCC2 gene (MRP2) are responsible for the recessive inherited Dubin-Johnson syndrome: although hepatic function is normal, patients with this syndrome have an increased risk of drug-induced liver toxicity.Citation74
Although SLCO transporters are genetically extensively characterized, relevant clinical data about the impact of polymorphisms are still limited. Genetic variants of uptake transporters have predominantly been investigated for OATPs, but a large number of single nucleotide polymorphisms in the OCT1 (SLCO22A1) and OCT2 (SLCO22A2) gene were also found, altering the transport function in vitro.Citation25,Citation75 As OATP1A2 is predominantly localized in the capillary endothelial cells of the brain, genetic variability and polymorphisms of this drug uptake carrier may represent a future pathway for CNS drugs as it is a determinant of brain toxicity.Citation23
Drug transporter interactions
Membrane transporters can undergo inhibition or induction, which respectively slow down or accelerate their transporter activity.Citation76-Citation78
In 1951, indirect clinical evidence already suggested the role of specific transport systems at the level of renal cell membranesCitation79: coadministration of probenecid with penicillin resulted in decreased renal clearance, prolonged half-life, and elevated plasma level of penicillin, enabling a substantial reduction in antibiotic dose. The mechanism of this interaction was found several years later: the active penicillin secretion was reduced by OAT inhibition in the basolateral membrane of renal proximal tubule.Citation80 Similarly, coadministration of probenecid with HIV antiviral drugs or with antihypertensive drugs such as the angiotensin-converting enzyme inhibitors also causes a reduction in renal clearance, a prolonged halflife, and elevated plasma, levels.Citation81 In humans, digoxin is a high-affinity substrate for MDR1, and most interacting drugs are either inductors, or, more frequently inhibitors, of MDR1.Citation82 Significant MDR1 inhibition, by administrating atorvastatin, clarithromycin, or verapamil as MDR1 inhibitors, was associated with a significant increase in the serum digoxin concentration, ie, more than twice the upper therapeutic limit.Citation76,Citation78,Citation83,Citation84 Another striking and clinically relevant effect, of the PGP-associated interactions was demonstrated by giving healthy volunteers loperamide, an opiate that is not absorbed from the gut, simultaneously with quinidine, a potent MDRl inhibitor: coadministration of this antidiarrheal agent, with quinidine resulted in central opioid effect such as respiratory depression and euphoria,Citation85,Citation86 confirming in vivo a major MDR1 inhibition in the intestinal and in the BBB gatekeeper function.Citation52,Citation87
Recently, a population pharmacokinetic analysis of drug-drug interactions between risperidone, bupropion, and sertraline in rodents suggested that sertraline produces significant inhibitory effects on MDR1 transport at the BBB, increasing the brain entry of risperidone and its metabolite 9-OH-risperidone.Citation88 'Ihe order of magnitude was high, and could be clinically significant, for humans: sertraline did not change the plasma concentration of risperidone and of its metabolite, but increased the brain area under the plasma concentration curve of risperidone and 9-hydroxy-risperidone 1.5-fold (P<0.05) and 5-fold (P<0.01), respectively.Citation88
Interestingly, another study with rodents showed that the MDR1 localized in the BBB is more resistant, to inhibition than in other tissues.Citation51 In vivo studies in humans are needed to assess the clinical relevance of such differential sensitivity to inhibition.
In vitro techniques for the assessment of drug-drug interactions involving membrane transporters are currently under development.Citation89 Inhibition on the MDR1 was investigated in vitro with recent, antidepressants, displaying different, interaction potentials: whereas paroxetine is regarded as a strong PGP inhibitor, citalopram and venlafaxine are considered as antidepressants with low interaction potential with membrane transporters.Citation77,Citation90 A case report confirmed the ability of paroxetine to inhibit the PGP in the BBB and in the kidney, causing digitalis intoxication with delirium, visual hallucinations, and disorientation.Citation78 However, specific data from in vivo studies, substrate specificity, and inhibition or induction potential of psychiatric and neurological medication are still lacking. Examples related to drugdrug interaction at the membrane transporter level are illustrated in Table II.
Table II. Examples of drug-drug interactions involving drug transporters. MDR, multidrug resistance; CNS, centra! nervous system; OCT, organic cation transporter; OATP, organic anion transporting peptide; OAT, organic ion transporter.
Discussion
In vitro and in vivo studies show that, drug carriers are expressed in the BBB and in the CSB. They represent major determinants of toxicity and clinical outcome related to drug response. Understanding the functional significance of membrane transporters in the BBB and in the CSB provides further opportunities to improve drug delivery to the CNS.
We propose that the role of transporter proteins should be studied at, an early stage of CNS drug development, as there are in vitro methods such as cell cultures to achieve this purpose. Knockout, animals are valuable in vivo models, but, in vivo methods in humans are few. Direct in vivo determinations of drug concentration and effective transporter function into the brain remain particularly challenging, as invasive techniques are necessary. Neuroirmaging techniques should be helpful, since molecules can be measured by positron emission tomography (PET) or by magnetic resonance imaging spectrometry. For example, the latter can be used to assess the pharmacokinetics of some fluoride-containing molecules in the brain. Although several members of the membrane transporters present in the BBB have been characterized in detail, numerous questions remain open. Firstly, the determination of detailed tissue expressions and in vivo studies of carriers with better specificity are required to target more efficiently therapeutic agents into the CNS and into other organs. Secondly, in order to enhance the potential clinical implications of drug transporter polymorphisms and interactions, the development of specific inductors and inhibitors may represent, promising strategies.
Thirdly, future delivery procedures include the use of prodrugs, drug-targeting vector conjugates, or liposomes tagged with targeting vectors to elude physiological barriers.
Drug transporter protein studies provide insight into the mechanisms of resistance, treatment failure, and interindividual response to neurological and psychiatric medication. Membrane transporter proteins arc not only CNS gatekeepers, but represent determinant partners in CNS drug development, strategies. Exploring the functional significance of membrane transporters in drug delivery to the CNS is essential for the treatment, of neurological and psychiatric disorders.
Selected abbreviations and acronyms
| ABC | = | adenosine triphosphate-binding cassette |
| ATP | = | adenosine triphosphate |
| BBB | = | blood-brain barrier |
| BCRP | = | breast cancer resistance protein |
| CNS | = | central nervous system |
| CSB | = | (blood) cerebrospinal barrier |
| MDR | = | multidrug resistance |
| MRP | = | multidrug resistance-associated protein |
| OAT | = | organic ion transporter |
| OATP | = | organic anion transporting peptide |
| OCT | = | organic cation transporter |
| SLC | = | solute-linked carriers |
REFERENCES
- TamaiI.TsujiA.Transporter mediated permeation of drugs across the blood brain barrier.J Pharrn Sci.20008913711388
- Kullak UblickGA.FischT.OswaldM.et al.Dehydroepiandrosterone sulfate (DHEAS): identification of a carrier protein in human liver and brain.FEBS Lett.19984241731769539145
- KusuharaH.SugiyamaY.Efflux transport systems for drugs at the blood brain barrier and blood-cerebrospinal fluid barrier (Part 1).Drug Discov Today.2001615015611165188
- KusuharaH.SugiyamaY.KusuharaH.SugiyamaY.Efflux transport systems for drugs at the blood-brain barrier and blood-cerebrospinal fluid barrier (Part 2).Drug Discov Today.2001620621211173268
- VerganiP.BassoC.MenseM.NairnAC.GadsbyDC.Control of the CFTR channel's gates.Biochem Soc Trans.200533(Pt 5)1003100716246032
- HergetM.TampeR.Intracellular peptide transporters in human - com partmentalization of the “peptidome.”Pfluge's Arch.2006 5 18 [Epub ahead of print]
- Pauli MagnusC.LangT.MeierY.et al.Sequence analysis of bile salt export pump (ABCB11) and multidrug resistance p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy.Pharmacogenetics.2004149110215077010
- Fernandez-MarmiesseA.SalasA.VegaA.Fernandez-LorenzoJR.BarreiroJ.CarracedoA.Mutation spectra of ABCC8 gene in Spanish patients with hyperinsulinism of infancy (HI).Hum Mutat.20062721416429405
- GueugnonF.VolodinaN.TaouilJE.et al.A novel cell model to study the function of the adrenoleukodystrophy-related protein.Biochem Biophys Res Commun.200634115015716412981
- BekriS.KispalG.LangeH.et al.Human ABC7 transporter: gene structure and mutation causing X-linked sideroblastic anemia with ataxia with disruption of cytosolic iron-sulfur protein maturation.Blood.2000963256326411050011
- StenirriS.BattistellaS.FermoI.et al.De novo deletion removes a conserved motif in the C-terminus of ABCA4 and results in cone-rod dystrophy.Clin Chem Lab Med.20064453353716681420
- MichaelidesM.HardcastleAJ.HuntDM.MooreAT.Progressive cone and cone-rod dystrophies: phenotypes and underlying molecular genetic basis.Surv Ophthalmol.20065123252816644365
- CirritoJR.DeaneR.FaganAM.et al.P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model.J Clin Invest.20051153285329016239972
- StefkovaJ.PoledneR.HubacekJA.ATP-binding cassette (ABC) transporters in human metabolism and diseases.Physiol Res.20045323524315209530
- RedzicZB.BiringerJ.BarnesK.et al.Polarized distribution of nucleoside transporters in rat brain endothelial and choroid plexus epithelial cells.J Neurochem.2005941420142616111480
- SteuerH.JaworskiA.ElgerB.et al.Functional characterization and comparison of the outer blood-retina barrier and the blood-brain barrier.Invest Ophthalmol Vis Sci.2005461047105315728564
- HawkinsRA.PetersonDR.VinaJR.The complementary membranes forming the blood-brain barrier.IUBMB Life.20025410110712489636
- GanongWF.Circumventricular organs: definition and role in the regulation of endocrine and autonomic function.Clin Exp Pharmacol Physiol.20002742242710831247
- StrazielleN.KhuthST.Ghersi-EgeaJF.Detoxification systems, passive and specific transport for drugs at the blood-CSF barrier in normal and pathological situations.Adv Drug Deliv Rev.2004561717174015381331
- EmerichDF.VasconcellosAV.ElliottRB.SkinnerSJ.BorlonganCV.The choroid plexus: function, pathology and therapeutic potential of its transplantation.Expert Opin Biol Ther.20044119120115268655
- ltoK.SuzukiH.HorieT.SugiyamaY.Apical/basolateral surface expression of drug transporters and its role in vectorial drug transport.Pharm Res.2005221559157716180115
- Ghersi-EgeaJF.StrazielleN.Choroid plexus transporters for drugs and other xenobiotics.J Drug Target.20021035335712164384
- HagenbuchB.GaoB.MeierPJ.Transport of xenobiotics across the bloodbrain barrier.News Physiol Sci.20021723123412433976
- LoscherW.PotschkaH.Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases.Prog Neurobiol.200576227616011870
- KerbR.Implications of genetic polymorphisms in drug transporters for pharmacotherapy.Cancer Lett.200623443316504381
- TaylorEM.The impact of efflux transporters in the brain on the development of drugs for CNS disorders.Clin Pharmacokinet.200241819211888329
- WacherVJ.WuCY.BenetLZ.Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy.Mol Carcinog.1995131291347619215
- KusuharaH.SugiyamaY.Active efflux across the blood-brain barrier: role of the solute carrier family.NeuroRx.20052738515717059
- OswaldC.HollandIB.SchmittL.The motor domains of ABC-transporters. What can structures tell us?Naunyn Schmiedebergs Arch Pharmacol.200637238539916541253
- LeslieEM.DeeleyRG.ColeSP.Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense.Toxicol Appl Pharmacol.200520421623715845415
- eierI.JedlitschkyG.BuchholzU.ColeSP.DeeleyRG.KepplerD.The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates.J Biol Chem.199426927807278107961706
- LeierI.JedlitschkyG.BuchholzU.et al.ATP-dependent glutathione disulphide transport mediated by the MRP gene-encoded conjugate export pump.Biochem J.1996314(Pt 2)4334378670053
- HoRH.KimRB.Transporters and drug therapy: implications for drug disposition and disease.Clin Pharmacol Ther.20057826027716153397
- SoontommalaiA.VlamingML.FritschyJM.Differential, strain-specific cellular and subcellular distribution of multidrug transporters in murine choroid plexus and blood-brain barrier.Neuroscience.200613815916916361063
- CalatozzoloC.GelatiM.CiusaniE.et al.Expression of drug resistance proteins Pgp, MRP1, MRP3, MRP5 and GST-pi in human glioma.J Neurooncol.20057411312116193381
- BrongerH.KonigJ.KopplowK.et al.ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier.Cancer Res.200565114191142816357150
- KubotaH.IshiharaH.LangmannT.et al.Distribution and functional activity of P-glycoprotein and multidrug resistance-associated proteins in human brain microvascular endothelial cells in hippocampal sclerosis.Epilepsy Res.20066821322816361082
- ToornvlietR.van BerckelBN.LuurtsemaG.et al.Effect of age on functional P-glycoprotein in the blood-brain barrier measured by use of (R)-[C]verapamil and positron emission tomography.Clin Pharmacol Ther.20067954054816765142
- SasongkoL.LinkJM.MuziM.et al.Imaging P-glycoprotein transport activity at the human blood-brain barrier with positron emission tomography.Clin Pharmacol Ther.20057750351415961982
- HagenbuchB.MeierPJ.Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties.Pflugers Arch.200444765366514579113
- edigerMA.RomeroMF.PengJB.RolfsA.TakanagaH.BrufordEA.The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins. Introduction.Pflugers Arch.200444746546814624363
- HagenbuchB.MeierPJ.The superfamily of organic anion transporting polypeptides.Biochim Biophys Acta.2003160911812507753
- PizzagalliF.HagenbuchB.StiegerB.KlenkU.FolkersG.MeierPJ.Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter.Mol Endocrinol.2002162283229612351693
- GalicS.SchneiderHP.BroerA.DeitmerJW.BroerS.The loop between helix 4 and helix 5 in the monocarboxylate transporter MCT1 is important for substrate selection and protein stability.Biochem J.2003376(Pt 2)41342212946269
- HalestrapAP.MeredithD.The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond.PflugersArch.2004447619628
- EnersonBE.DrewesLR.Molecular features, regulation, and function of monocarboxylate transporters: implications for drug delivery.J Pharm Sci.2003921531154412884241
- BhattacharyaI.BojeKM.GHB (gamma-hydroxybutyrate) carrier-mediated transport across the blood-brain barrier.J Pharmacol Exp Ther.2004311929815173314
- BuschAE.KarbachU.MiskaD.et al.Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine.Mol Pharmacol.1998543423529687576
- van MontfoortJE.MullerM.GroothuisGM.MeijerDK.KoepsellH.MeierPJ.Comparison of “type I” and “type II” organic cation transport by organic cation transporters and organic anion-transporting polypeptides.J Pharmacol Exp Ther.200129811011511408531
- EvansWE.McLeodHL.Pharmacogenomics - drug disposition, drug targets, and side effects.N Engl J Med.200334853854912571262
- ChooEF.KurnikD.MuszkatM.et al.Differential in vivo sensitivity to inhibition of P-glycoprotein located in lymphocytes, testes, and the blood-brain barrier.J Pharmacol Exp Ther.20063171012101816537797
- LeeW.GlaeserH.SmithLH.et al.Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry.J Biol Chem.20052809610961715632119
- O'KaneDJ.WeinshilboumRM.MoyerTP.Pharmacogenomics and reducing the frequency of adverse drug events.Pharmacogenomics.200341412517278
- LinJH.YamazakiM.Clinical relevance of P-glycoprotein in drug therapy.Drug Metab Rev.20033541745414705869
- LinJH.YamazakiM.Role of P-glycoprotein in pharmacokinetics: clinical implications.Clin Pharmacokinet.200342599812489979
- SchinkelAH.SmitJJ.van TellingenO.et al.Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs.Cell.1994774915027910522
- SchwabM.EichelbaumM.FrommMF.Genetic polymorphisms of the human MDR1 drug transporter.Annu Rev Pharmacol Toxicol.20034328530712359865
- BabaogluMO.BayarB.AynaciogluAS.et al.Association of the ABCB1 3435C>T polymorphism with antiemetic efficacy of 5-hydroxytryptamine type 3 antagonists.Clin Pharmacol Ther.20057861962616338277
- EichelbaumM.FrommMF.SchwabM.Clinical aspects of the MDR1 (ABCB1) gene polymorphism.Ther Drug Monit.20042618018515228162
- SiddiquiA.KerbR.WealeME.et al.Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1 .N Engl J Med.20033481442144812686700
- WeinshilboumR.Inheritance and drug response.N Engl J Med.200334852953712571261
- CrivoriP.PoggesiI.Computational approaches for predicting CYP-related metabolism properties in the screening of new drugs.Eur J Med Chem.2006 4 24 [Epub ahead of print]
- KonigJ.SeithelA.GradhandU.FrommMR.Pharmacogenomics of human OATP transporters.Naunyn Schmiedebergs Arch Pharmacol.200637243244316525793
- HaasDW.SmeatonLM.ShaferRW.et al.Pharmacogenetics of long-term responses to antiretroviral regimens containing efavirenz and/or nelfinavir: an adult AIDS Clinical Trials Group Study.J infect Dis.20051921931194216267764
- VerstuyftC.SchwabM.SchaeffelerE.et al.Digoxin pharmacokinetics and MDR1 genetic polymorphisms.Eur J Clin Pharmacol.20035880981212698307
- RobertsRL.JoycePR.MulderRT.BeggEJ.KennedyMA.A common P-glycoprotein polymorphism is associated with nortriptyline-induced postural hypotension in patients treated for major depression.Pharmacogenomics J.2002219119612082591
- IllmerT.SchulerUS.ThiedeC.et al.MDR1 gene polymorphisms affect therapy outcome in acute myeloid leukemia patients.Cancer Res.2002624955496212208746
- YamauchiA.leiriI.KataokaY.et al.Neurotoxicity induced by tacrolimus after liver transplantation: relation to genetic polymorphisms of the ABCB1 (MDR1) gene.Transplantation.20027457157212352921
- SparreboomA.GelderblomH.MarshS.et al.Dif lomotecan pharmacokinetics in relation to ABCG2 421C>A genotype.Clin Pharmacol Ther.200476384415229462
- FujitaT.BrownC.CarlsonEJ.et al.Functional analysis of polymorphisms in the organic anion transporter, SLC22A6 (OAT1).Pharmacogenet Genomics.20051520120915864112
- KerbR.BrinkmannU.ChatskaiaN.et al.Identification of genetic variations of the human organic cation transporter hOCT1 and their functional consequences.Pharmacogenetics.20021259159512439218
- LeabmanMK.HuangCC.KawamotoM.et al.Polymorphisms in a human kidney xenobiotic transporter, OCT2, exhibit altered function.Pharmacogenetics .20021239540512142729
- DallasS.MillerDS.BendayanR.Multidrug resistance-associated proteins: expression and function in the central nervous system.Pharmacol Rev.20065814016116714484
- ElferinkRO.GroenAK.Genetic defects in hepatobiliary transport.Biochim Biophys Acta.2002158612914511959455
- MarzoliniC.TironaRG.KimRB.Pharmacogenomics of the OATP and OAT families.Pharmacogenomics.2004527328215102542
- WakasugiH.YanoI.ItoT.et al.Effect of clarithromycin on renal excretion of digoxin: interaction with P-glycoprotein.Clin Pharmacol Ther1998641231289695727
- WeissJ.DormannSM.Martin-FacklamM.KerpenCJ.Ketabi-KiyanvashN.HaefeliWE.Inhibition of P-glycoprotein by newer antidepressants.J Pharmacol Exp Ther.200330519720412649369
- Yasui-FurukoriN.KanekoS.Digitalis intoxication induced by paroxetine co-administration.Lancet.200636778816517279
- BurnellJM.KirbyWM.Effectiveness of a new compound, benemid, in elevating serum penicillin concentrations.J Clin Invest.19513069770014850548
- JariyawatS.SekineT.TakedaM.et al.The interaction and transport of beta-lactam antibiotics with the cloned rat renal organic anion transporter 1.J Pharmacol Exp Ther.199929067267710411577
- AyrtonA.MorganP.Role of transport proteins in drug absorption, distribution and excretion.Xenobiotica.20013146949711569523
- ParkerRB.YatesCR.SobermanJE.LaizureSC.Effects of grapefruit juice on intestinal P-glycoprotein: evaluation using digoxin in humans.Pharmacotherapy.20032397998712921244
- AszalosA.ThompsonK.YinJJ.RossDD.Combinations of P-glycoprotein blockers, verapamil, PSC833, and cremophor act differently on the multidrug resistance associated protein (MRP) and on P-glycoprotein (Pgp).Anticancer Res.1999191053106410368654
- BoydRA.SternRH.StewartBH.et al.Atorvastatin coadministration may increase digoxin concentrations by inhibition of intestinal P-glycoprotein-mediated secretion.J Clin Pharmacol.200040919810631627
- EnerothA.AstromE.HoogstraateJ.et al.Evaluation of a vincristine resistant Caco-2 cell line for use in a calcein AM extrusion screening assay for P-glycoprotein interaction.Eur J Pharm Sci.20011220521411113639
- FrohlichM.AlbermannN.SauerA.Walter-SackI.HaefeliWE.WeissJ.In vitro and ex vivo evidence for modulation of P-glycoprotein activity by progestins.Biochem Pharmacol.2004682409241615548387
- SadequeAJ.WandelC.HeH.ShahS.WoodAJ.Increased drug delivery to the brain by P-glycoprotein inhibition.Clin Pharmacol Ther.20006823123711014404
- WangJS.DeVaneCL.GibsonBB.DonovanJL.MarkowitzJS.ZhuHJ.Population pharmacokinetic analysis of drug-drug interactions among risperidone, bupropion, and sertraline in CF1 mice.Psychopharmacology(Berl). 200618349049916283256
- KeoghJP.KuntaJR.Development, validation and utility of an in vitro technique for assessment of potential clinical drug-drug interactions involving P-glycoprotein.Eur J Pharm Sci.20062754355416406207
- KorenG.WoodlandC.ItoS.Toxic digoxin-drug interactions: the major role of renal P-glycoprotein.Vet Hum Toxicol.19984045469467211
- LeabmanMK.HuangCC.KawamotoM.et al.Polymorphisms in a human kidney xenobiotic transporter, OCT2, exhibit altered function.Pharmacogenetics.20021239540512142729
- DresserGK.SchwarzUl.WilkinsonGR.KimRB.Coordinate induction of both cytochrome P4503A and MDR1 by St John's wort in healthy subjects.Clin Pharmacol Ther.200373415012545142
- DresserGK.SpenceJD.BaileyDG.Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition.Clin Pharmacokinet.200038415710668858