Abstract
Animals respond to stress by activating a wide array of behavioral and physiological responses that are collectively referred to as the stress response. Corticotropin-releasing factor (CRF) plays a central role in the stress response by regulating the hypothalamic-pituitary-adrenal (HPA) axis. In response to stress, CRF initiates a cascade of events that culminate in the release of glucocorticoids from the adrenal cortex. As a result of the great number of physiological and behavioral effects exerted by glucocorticoids, several mechanisms have evolved to control HPA axis activation and integrate the stress response. Glucocorticoid feedback inhibition plays a prominent role in regulating the magnitude and duration of glucocorticoid release. In addition to glucocorticoid feedback, the HPA axis is regulated at the level of the hypothalamus by a diverse group of afferent projections from limbic, mid-brain, and brain stem nuclei. The stress response is also mediated in part by brain stem noradrenergic neurons, sympathetic andrenornedullary circuits, and parasympathetic systems. In summary, the aim of this review is to discuss the role of the HPA axis in the integration of adaptive responses to stress. We also identify and briefly describe the major neuronal and endocrine systems that contribute to the regulation of the HPA axis and the maintenance of homeostasis in the face of aversive stimuli.
Los animales responden al estrés, activando una amplia gama de respuestas comportamentales y fisiológicas que se conocen, de forma genérica, como respuesta al estrés. El factor liberador de corticotro-pina (CRF) desempeña una misión cardinal en la respuesta al estrés, al regular e! eje hipotálamo-hipófisis-suprarrenal (HHS). En respuesta al estrés, el CRF inicia una cascada de acontecimientos gue culminan con la liberación de glucocorticoides por la corteza suprarrenal. Como consecuencia del elevado número de efectos fisiológicos y conductuales inducidos por los glucocorticoides, han surgido varios mecanismos para controlar la activación del eje HHS e integrar la respuesta al estrés. La inhibición por retroaIimentación de los glucocorticoides contribuye decisivamente a regular la magnitud y la duración de su liberación. Además de esta reiroalimentación glucocorticoidea, el eje HHS está regulado en el hipotálamo por un grupo diverso de proyecciones aferente de los núcleos límbícos, mesencefálicos y del tronco cerebral. La respuesta al estrés está mediada también, en parte, por las neuronas noradrenérgicas del tronco cerebral, los circuitos adrenomedulares simpáticos y los sistemas parasimpáticos. En resumen, el objetivo de esta revisión es exponer la importancia del eje HHS en la integración de las respuestas adaptativas al estrés. Asimismo, se señalan y describen brevemente los principales sistemas neuronales y endocrinos que contribuyen a la regulación del eje HHS y al mantenimiento de la homeostasis frente a tos estímulos adversos.
Les animaux répondent au stress en activant un large panel de réponses comportementales et physiologiques, collectivement considérés comme constituant la réponse au stress. Le facteur de libération de corticotrophine (CRF) joue un rôle central dans la réponse au stress en régulant l'axe hypothalamo-hypophyso-surrénalien (HPA). Dans la réponse au stress, le CRF déclenche une cascade d'événements qui aboutissent à la libération de glucocorticoïdes à partir du cortex surrénalien. Etant donné le grand nombre d'effets physiologiques et comportementaux produits par les glucocorticoïdes, plusieurs mécanismes se sont développés afin de contrôler l'activation de l'axe HPA et intégrer les réponses au stress. Le rétrocontrôle inhibiteur des glucocorticoïdes joue un rôle essentiel dans l'ampleur et la durée de leur libération. En plus de ce rétro-contrôle, l'axe HPA est régulé au niveau hypothalamique par différentes projections afférentes provenant du système limbique, du mésencéphale et des noyaux du tronc cérébral. La réponse au stress est également transmise en partie par les neurones noradrénergiques du tronc cérébral, les circuits sympathiques adrénomédullaires et le système parasympathique. En résumé, cet article a pour but d'examiner le rôle de l'axe HPA dans l'intégration des réponses adaptatives au stress. Nous avons aussi identifié et brièvement décrit les principaux systèmes neuronaux et endocriniens qui participent à la régulation de l'axe HPA et au maintien de l'homéostasie face à des agressions.
Stress is commonly defined as a state of real or perceived threat to homeostasis. Maintenance of homeostasls In the presence of averslve stimuli (stressors) requires activation of a complex range of responses involving the endocrine, nervous, and immune systems, collectively known as the stress response.Citation1,Citation2 Activation of the stress response initiates a number of behavioral and physiological changes that improve an individual's chance of survival when faced with homeostatic challenges. Behavioral effects of the stress response include increased awareness, improved cognition, euphoria, and enhanced analgesia.Citation1,Citation3 Physiological adaptations initiated by activation of this system include increased cardiovascular tone, respiratory rate, and intermediate metabolism, along with inhibition of general vegetative functions such as feeding, digestion, growth, reproduction, and immunity.Citation4,Citation5 Due to the wide array of physiologic and potentially pathogenic effects of the stress response, a number of neuronal and endocrine systems function to tightly regulate this adaptive process.
Anatomy of the stress response
The anatomical structures that mediate the stress response are found in both the central nervous system and peripheral tissues. The principal effectors of the stress response are localized in the paraventricular nucleus (PVN) of the hypothalamus, the anterior lobe of the pituitary gland, and the adrenal gland. This collection of structures is commonly referred to as the hypothalamic-pituitary-adrenal (HPA) axis (). In addition to the HPA axis, several other structures play important roles in the regulation of adaptive responses to stress. These include brain stem noradrenergic neurons, sympathetic andrenomedullary circuits, and parasympathetic systems.Citation5-Citation7
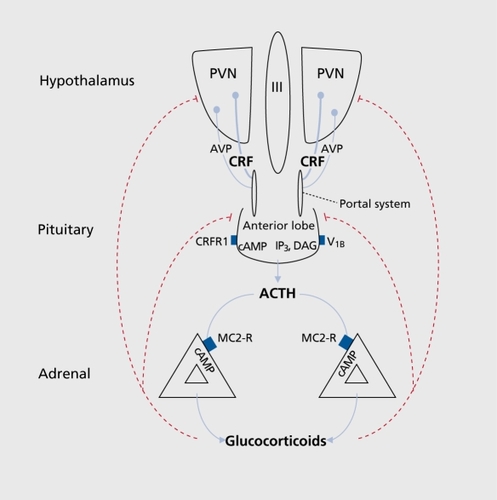
The HPA axis
Hypophysiotropic neurons localized in the medial parvocellular subdivision of the PVN synthesize and secrete corticotropin-releasing factor (CRF), the principle regulator of the HPA axis.Citation8,Citation9 In response to stress, CRF is released into hypophysial portal vessels that access the anterior pituitary gland. Binding of CRF to its receptor on pituitary corticotropes induces the release of adrenocorticotropic hormone (ACTH) into the systemic circulation. The principal target for circulating ACTH is the adrenal cortex, where it stimulates glucocorticoid synthesis and secretion from the zona fasciculata. Glucocorticoids are the downstream effectors of the HPA axis and regulate physiological changes through ubiquitously distributed intracellular receptors.Citation10,Citation11 The biological effects of glucocorticoids are usually adaptive; however, inadequate or excessive activation of the HPA axis may contribute to the development of pathologies.Citation10,Citation12
The CRF family of peptides
Corticotropin-releasing factor is a 41 amino acid peptide that was originally isolated from ovine hypothalamic tissue in 1981. Citation8 Since this initial identification, CRF has been shown to be the primary regulator of ACTH release from anterior pituitary corticotropesCitation9 and has also been implicated in the regulation of the autonomic nervous system, learning and memory, feeding, and reproductionrelated behaviors.Citation13-Citation19 CRF is widely expressed through-out the central nervous system (CNS) and in a number of peripheral tissues. In the brain, CRF is concentrated in the medial parvocellular subdivision of the PVN and is also localized in the olfactory bulb, bed nucleus of the stria terminalis (BNST), medial preoptic area, lateral hypothalamus, central nucleus of the amygdala, Barington's nucleus, dorsal motor complex, and inferior olive.Citation20 In the periphery, CRF has been detected in the adrenal gland, testis, placenta, gastrointestinal tract, thymus, and skin.Citation21-Citation23
Three additional members of the CRF peptide family have recently been identified. These include urocortin (Ucn) lCitation24 and the recently cloned Ucn 2Citation25 and Ucn 3,Citation26 which are also known as stresscopin-related peptide and stresscopin,Citation27 respectively In the mammalian brain, Ucn 1 is predominantly expressed in the Edinger-Westphal nucleusCitation24 and Ucn 2 expression is restricted to the PVN and locus coeruleus.Citation25 Ucn 3 has a wider distribution in the brain and is localized in the perifornical area of the hypothalamus, BNST, lateral septum (LS), and amygdala.Citation28 The widespread anatomical distribution of CRF and the urocortins correlates well with the diverse array of physiological functions associated with this peptide family
CRF receptors
The physiological actions of the CRF family of peptides are mediated through two distinct receptor subtypes belonging to the class B family of G-protein coupled receptors.Citation29 The CRF type 1 receptor (CRFR1) gene encodes one functional variant (α) in humans and rodents along with several nonfunctional splice variants.Citation30-Citation32 The CRF type 2 receptor (CRFR2) has three functional splice variants in human (α, β, and γ) and two in rodents (α and β) resulting from the use of alternate 5' starting exons.Citation33,Citation34
CRFR1 is expressed at high levels in the brain and pituitary and low levels in peripheral tissues. The highest levels of CRFR1 expression are found in the anterior pituitary, olfactory bulb, cerebral cortex, hippocampus, and cerebellum. In peripheral tissues, low levels of CRFR1 are found in the adrenal gland, testis, and ovary.Citation35,Citation36 In contrast, CRFR2 is highly expressed in peripheral tissues and localized in a limited number of nuclei in the brain.Citation37 In rodents, the CRF type 2α splice variant is preferentially expressed in the mammalian brain and is localized in the lateral septum, BNST, ventral medial hypothalamus, and mesencephalic raphe nuclei.Citation36 The CRF type 2β variant is expressed in the periphery and is concentrated in the heart, skeletal muscle, skin, and the gastrointestinal tract.Citation29,Citation38,Citation39
Radioligand binding and functional assays have revealed that CRFR1 and CRFR2 have different pharmacological profiles. CRF binds to the CRFR1 with higher affinity than to CRFR2.Citation29,Citation33 Ucnl has high affinity for both CRFR1 and CRFR2 and is more potent than CRF on CRFR2.Citation24,Citation33 Ucn 2 and Ucn 3 are highly selective for CRFR2 and exhibit low affinities for CRFR1. In addition, Ucn 2 and Ucn 3 minimally induce cyclic adenosine monophosphate (cAMP) production in cells expressing either endogenous or transfected CRFRl.Citation25-Citation27
The neuroendocrine properties of CRF are mediated through CRFRl in the anterior pituitary. Binding of CRF to the type 1 receptor results in the stimulation of adenylate cyclase and a subsequent activation of cAMP pathway events that culminate with the release of ACTH from pituitary corticotropes.Citation29,Citation39,Citation40 The integral role of CRFRl in the regulation of ACTH release was confirmed by the phenotype of CRFRl -deficient mice. Mice deficient for CRFRl have a severely attenuated HPA response to stress and display decreased anxietylike behaviors.Citation41,Citation42 The role of CRFR2 in the regulation of the HPA axis and adaptive responses to stress is less clear. Mice deficient for CRFR2 have an amplified HPA response to stress and display increased anxiety-like behaviors.Citation43-Citation45 However, administration of CRFR2 agonists and antagonists into discrete brain regions reveal both anxiolytic and anxiogenic roles for CRFR2.Citation45
Vasopressin
Vasopressin (AVP) is a nonapeptide that is highly expressed in the PVN, supraoptic (SON), and suprachiasmatic nuclei of the hypothalamus.Citation46,Citation47 Magnocellular neurons of the PVN and SON project to the posterior lobe of the pituitary and release AVP directly into the systemic circulation to regulate osmotic homeostasis.Citation48,Citation49
In addition to magnocellular neurons, parvocellular neurons of the PVN synthesize and release AVP into the portal circulation, where this peptide potentiates the effects of CRF on ACTH release from the anterior pituitary.Citation7,Citation50,Citation51
The synergistic effects of AVP on ACTH release are mediated through the vasopressin V1b (also known as V3) receptor on pituitary corticotropes.Citation52 Binding of AVP to the V1b receptor activates phospholipase C by coupling to Gq proteins. Activation of the phospholipase C stimulates protein kinase C, resulting in the potentiation of ACTH release.Citation53 Several investigators have reported that the expression of AVP in parvocellular neurons of the PVN and V1b receptor density in pituitary corticotropes is significantly increased in response to chronic stress.Citation54-Citation58
These findings support the hypothesis that AVP plays an important role in the stress response by maintaining ACTH responsiveness to novel stressors during periods of chronic stress.
Adrenocorticotropic hormone
Pro-opiomelanocortin (POMC) is a prohormone that is highly expressed in the pituitary and the hypothalamus. POMC is processed into a number of bioactive peptides including ACTH, β-endorphin, β-lipotropic hormone, and the melanocortins.Citation59-Citation61 In response to CRF, ACTH is released from pituitary corticotropes into the systemic circulation where it binds to its specific receptor in the adrenal cortex. ACTH binds to the melanocortin type 2 receptor (MC2-R) in parenchymal cells of the adrenocortical zona fasciculata. Activation of the MC2-R induces stimulation of cAMP pathway events that induce steroidogenesis and the secretion of glucorticoids, mineralcorticoids, and androgenic steroids.Citation62,Citation63 Specifically, ACTH promotes the conversion of cholesterol into 5-5 pregnenolone during the initial step of glucocorticoid biosynthesis.Citation61,Citation64
Glucocorticoids
Glucocorticoids, Cortisol in humans and corticosterone in rodents, are a major subclass of steroid hormones that regulate metabolic, cardiovascular, immune, and behavioral processes.Citation3,Citation4 The physiological effects of glucocorticoids are mediated by a 94kD cytosolic protein, the glucocorticoid receptor (GR).The GR is widely distributed throughout the brain and peripheral tissues. In the inactive state, the GR is part of a multiprotein complex consisting of several different molecules of heat shock proteins (HSP) that undergo repeated cycles of dissociation and ATP-dependent reassociation.Citation11,Citation65,Citation66 Ligand binding induces a conformational change in the GR, resulting in the dissociation of the receptor from the HSP complex and translocation into the nucleus. Following translocation, the GR homodimer binds to specific DNA motifs termed glucocorticoid response elements (GREs) in the promoter region of glucocorticoid responsive genes and regulates expression through interaction with transcription factors.Citation11,Citation67,Citation68 The GR has also been shown to regulate activation of target genes independent of GRE-binding through direct protein-protein interactions with transcription factors including activating protein 1 (AP-1) and nuclear factor-βB (NF-βB).Citation69-Citation71
Endocrine regulation of the HPA axis
Activation of the HPA axis is a tightly controlled process that involves a wide array of neuronal and endocrine systems. Glucocorticoids play a prominent role in regulating the magnitude and duration of HPA axis activation.Citation72 Following exposure to stress, elevated levels of circulating glucocorticoids inhibit HPA activity at the level of the hypothalamus and pituitary. The HPA axis is also subject to glucocorticoid independent regulation. The neuroendocrine effects of CRF are also modulated by CRF binding proteins that are found at high levels in the systemic circulation and in the pituitary gland.Citation73,Citation74
Glucocorticoid negative feedback
The HPA axis is subject to feedback inhibition from circulating glucocorticoids.Citation72 Glucocorticoids modulate the HPA axis through at least two distinct mechanisms of negative feedback. Glucocorticoids have traditionally been thought to inhibit activation of the HPA axis through a delayed feedback system that is responsive to glucocorticoid levels and involves genomic alterations. There is increasing evidence for an additional fast nongenomic feedback system that is sensitive to the rate of glucocorticoid secretion; however, the exact mechanism that mediates rapid feedback effects has not yet been characterized.Citation11,Citation72,Citation75
The delayed feedback system acts via transcriptional alterations and is regulated by GR localized in a number of stress-responsive brain regions.Citation76 Following binding of glucocorticoids, GRs modulate transcription of HPA components by binding to GREs or through interactions with transcription factors.Citation11,Citation72 Glucocorticoids have a low nanomolar affinity for the GR and extensively occupy GRs during periods of elevated glucocorticoid secretion that occur following stress.Citation77 Mineralocorticoid receptors (MRs) have a subnanomolar affinity for glucocorticoids, a restricted expression pattern in the brain, and bind glucocorticoids during periods of basal secretion.Citation76,Citation77 The distinctive pharmacologies of these two receptors suggest that MRs regulate basal HPA tone while GRs mediate glucocorticoid negative feedback following stress.Citation75,Citation78,Citation79
GRs are widely expressed in the brain, and thus the precise anatomical locus of glucocorticoid negative feedback remains poorly defined. However, two regions of the brain appear to be key sites for glucocorticoid feedback inhibition of the HPA axis. High levels of GR are expressed in hypophysiotropic neurons of the PVN, and local administration of glucocorticoids reduce PVN neuronal activity and attenuate adrenalectomy-induced ACTH hypersecretion.Citation80-Citation83 These findings suggest that the PVN is an important site for glucocorticoid feedback inhibition of the HPA axis. The hippocampus has been implicated as a second site for glucocorticoid negative feedback regulation of the HPA axis. The hippocampus contains a high concentration of both GR and MR, and infusion of glucocorticoids into this structure reduces basal and stress induced glucocorticoid release.Citation84-Citation86
CRF binding proteins
Two soluble proteins have been identified that bind the members of the CRF family of peptides with high affinity. The CRF binding protein (CRF-BP) is a highly conserved 37kD glycoprotein that binds both CRF and Ucn 1 with high affinityCitation74,Citation87,Citation88 The CRF-BP was originally identified in maternal plasma where it functions to inhibit HPA axis activation stemming from the elevated circulating levels of placenta-derived CRF.Citation89,Citation90 The CRF-BP is highly expressed in the pituitary, and recombinant CRF-BP attenuates CRF-induced ACTH release from dispersed anterior pituitary cells in culture.Citation74 These findings suggest the CRF-BP may function to sequester CRF at the level of the pituitary and reduce CRFR activity.
Our laboratory has recently identified a transcript that encodes a soluble splice variant of the CRFR2 receptor (sCRFR2α) in the mouse brain.Citation73 Soluble CRFR2α is a predicted 143 amino acid protein generated from a predicted 143 amino acid protein generated from exons 3-5 of the extracellular domain of CRFR2α gene and a unique 38 amino acid hydrophilic C-terminal tail. High levels of sCRFR2α expression are found in the olfactory bulb, cortex, and midbrain regions that have been shown to express CRFRl.Citation36 Recombinant sCRFR2α binds CRF with low nanomolar affinity and inhibits cellular responses to both CRF and Ucn 1 in signal transduction assays,Citation73 suggesting that sCRFR2α may function as a decoy receptor for the CRF family of peptides.
Neuronal regulation of the HPA axis
Hypophysiotropic neurons in the PVN are innervated by a diverse constellation of afferent projections from multiple brain regions. The majority of afferent inputs to the PVN originate from four distinct regions: brain stem neurons, cell groups of the lamina terminalis, extra-PVN hypothalamic nuclei, and forebrain limbic structures.Citation20,Citation91
These cell groups integrate and relay information regarding a wide array of sensory modalities to influence CRF expression and release from hypophysiotropic neurons of the PVN ().
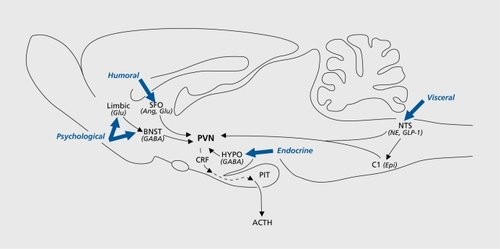
Brain stem neurons
Brain stem catecholaminergic centers play an important role in the regulation of the HPA axis. Neurons of the nucleus of the solitary tract (NTS) relay sensory information to the PVN from cranial nerves that innervate large areas of thoracic and abdominal viscera. The NTS also receives projections from limbic structures that regulate behavioral responses to stress including the medial prefrontal cortex and the central nucleus of the amygdala.Citation92 Accordingly, neuronal populations in the NTS are activated following lipopoly saccharide injection,Citation93,Citation94 hypotension,Citation95 forced swim, and immobilization stress paradigms.Citation96
Stress-receptive neurons in the A2/C2 region of the NTS densely innervate the medial parvocellular subdivision of the PVN.Citation97,Citation98 Findings from both in vivo and in vitro studies demonstrate that catecholaminergic input represents a major excitatory drive on the HPA axis and induces CRF expression and protein release through an α-1 adrenergic receptor-dependent mechanism.Citation99-Citation101
Nonaminergic NTS neurons also innervate the PVN and contribute to HPA axis regulation. Glucagon-like peptide 1 containing neurons in the NTS are activated by physiological stressors and have been shown to induce ACTH release in vivo.Citation102,Citation103 The neuropeptides somatostatin, substance P, and enkephalin are also expressed in NTS neurons that innervate the PVN and have been shown to have regulatory effects on the HPA axis.Citation104-Citation106
The lamina terminals
A series of interconnected cell groups including the subfornical organ (SFO), median preoptic nucleus (MePO), and the vascular organ of the lamina terminalis are localized on the rostral border of the third ventricle and make up the lamina terminalis.Citation107 Cell groups of the lamina terminalis lie outside of the blood-brain barrier and relay information concerning the osmotic composition of blood to the PVN.Citation108 The medial parvocellular subdivision of the PVN receives rich innervation from the SFO and to a lesser extent from the OVLT and MePO.Citation109 Neurons in the SFO that project to the PVN are angiotensinergic, and promote CRF secretion and biosynthesis.Citation110,Citation111 This afferent pathway has parallel input to the magnocellular division of the PVN, and had been hypothesized to serve as a link between HPA and neurohypophysial activation.Citation112,Citation114
Hypothalamus
The medial parvocellular subdivision of the PVN receives afferent projections from y-aminobutyric acid (GABA)-ergic neurons of the hypothalamus.Citation115 Hypophysiotropic neurons of the PVN express GABA-A receptor subunitsCitation116 and hypothalamic injection of the GABA-A receptor agonists inhibit glucocorticoid secretion following exposure to stressors.Citation117,Citation118 These studies suggest that GABA plays a prominent role in hypothalamic stress integration.
Hypothalamus: DMH and POA
GABAergic neurons in the dorsomedial hypothalamic nucleus (DMH) and preoptic area (POA) project to the medial parvocellular division of the PVN, and are activated following exposure to stressors.Citation115,Citation117 Lesions of hypothalamic regions encompassing the DMH and the POA amplify HPA responses to stress.Citation119,Citation120 Furthermore, glutamate microstimulation of DMH neurons produces inhibitory postsynaptic potentials in hypophysiotropic neurons of the PVN,Citation121 and stimulation of the POA attenuates the excitatory effects of medial amygdalar stimulation of glucocorticoid release.Citation122 The POA is a potential site of integration between gonadal steroids and the HPA axis. Accordingly, neurons of the POA are activated by gonadal steroids and express high levels of androgen, estrogen, and progesterone receptors.Citation123,Citation124
Hypothalamus: feeding centers
Hypothalamic centers involved in the regulation of energy homeostasis directly innervate PVN neurons. Neurons in the arcuate nucleus are sensitive to circulating levels of glucose, insulin, and leptin These cells also synthesize neuropeptide Y (NPY), agouti-related peptide (AGRP), αmelanocyte stimulating hormone (αMSH), and cocaineand amphetamine-regulated transcript (CART) which play critical roles in the regulation of feeding behaviors.Citation125,Citation127 In addition to their roles in energy homeostasis, arcuate neuropeptides have significant effects on HPA axis activity.
Central injection of the orexigenic factor NPY results in HPA axis activationCitation128,Citation129 and infusion of AGRP significantly increases CRF release from hypothalamic expiants.Citation130 The anorectic peptides αMSH and CART have been reported to increase circulating levels of ACTH and corticosterone,Citation130,Citation132 induce cAMP binding protein phosphorylation in CRF neurons,Citation133 and stimulate CRF release from hypothalamic neurons.Citation130,Citation134 These studies suggest that the HPA axis is activated in response to positive and negative states of energy balance.
The limbic system
Limbic structures of the f orebrain contribute to the regulation of the HPA axis. Neuronal populations in the hippocampus, prefrontal cortex, and amygdala are the anatomical substrates for memory formation and emotional responses, and may serve as a link between the stress system and neuropsychiatrie disorders.Citation86,Citation135 The hippocampus, prefrontal cortex, and amygdala have significant effects on glucocorticoid release and behavioral responses to stress.Citation84,Citation136,Citation137 However, these limbic structures have a limited number of direct connections with hypophysiotropic neurons of the PVN and are thought to regulate HPA axis activity through intermediary neurons in the BNST, hypothalamus, and brain stem.Citation20,Citation138,Citation139
Limbic system: hippocampus
The hippocampus plays an important role in the terminating HPA axis responses to stress.Citation84,Citation139 Stimulation of hippocampal neurons decreases neuronal activity in the parvocellular division of the PVN and inhibits glucocorticoid secretion.Citation140,Citation142 Hippocampal lesions produce elevated basal levels of circulating glucocorticoids,Citation143,Citation144 increase parvocellular CRF and AVP expression,Citation145 and prolong ACTH and corticosterone release in response to stress.Citation141,Citation146
The regulatory effects of the hippocampus on the HPA axis are mediated through a multisynaptic pathway and appear to be stressor-specific.Citation139 Hippocampal outflow to the hypothalamus originates in the ventricle subiculum and CA1 regions of the hippocampus.Citation139,Citation147 These regions send afferent projections to GABAergic neurons of BNST and the peri-PVN region of the hypothalamus that directly innervate the parvocellular division of the PVN.Citation139,Citation147,Citation148 Hippocampal lesions encompassing the ventral subiculum produce exaggerated HPA responses to restraint and open field exposure, but not to hypoxia or ether exposure, suggesting that hippocampal neurons respond to distinct stress modalities.Citation146,Citation149,Citation150
Limbic system: prefrontal cortex
The prefrontal cortex also regulates HPA responses to stress. Neurons of the medial prefrontal cortex are activated and release catecholamines following exposure to acute and chronic stressors.Citation117,Citation151,Citation152 Bilateral lesions of the anterior cingulate and prelimbic cortex increase ACTH and glucocorticoid responses to stress,Citation85,Citation153 demonstrating that the prefrontal cortex has inhibitory effects on the HPA axis. Anatomic tracing studies reveal that the there is an intricate topographic organization of prefrontal cortex output to HPA regulatory circuits. Afférents from the infralimbic cortex project extensively to the BNST, amygdala, and the NTS.Citation154,Citation155 In contrast, the prelimbic/anterior cingulate cortex projects to the POA and the DMH but fails to synapse with the BNST, NTS, or amygdalar neurons.Citation139,Citation154,Citation155
The prefrontal cortex may also play a role in glucocorticoid feedback inhibition of the HPA axis. High densities of GR are expressed in layers II, III, and VI of the prefrontal cortex.Citation156 Infusion of glucocorticoids into the medial prefrontal cortex attenuates ACTH and corticosterone responses to restraint stress, but has no significant effect on HPA responses to ether.Citation85,Citation157 Similarly to the hippocampus, it appears that neurons of the prefrontal cortex are subject to modality-specific regulation of glucocorticoid feedback inhibition of the HPA axis.Citation139
Limbic system: amygdala
In contrast to the hippocampus and the prefrontal cortex, the amygdala is thought to activate the HPA axis. Stimulation of amygdalar neurons promotes glucocorticoid synthesis and release into the systemic circulation.Citation158,Citation159 The medial (Me A) and central (Ce A) nuclei of the amygdala play a key role in HPA axis activity and contribute the majority of afferent projections from the amygdala to cortical, midbrain, and brain stem regions that regulate adaptive responses to stress.Citation160,Citation161 The MeA and CeA respond to distinct stress modalities and are thought to have divergent roles in HPA regulation.Citation139
Neurons in the MeA are activated following exposure to “emotional” stressors including predator, forced swim, social interaction, and restraint stress paradigms.Citation117,Citation162-Citation165 In contrast, the CeA appears to be preferentially activated by “physiological” stressors, including hemorrhage and immune challenge.Citation166,Citation167
The CeA exerts its regulatory effects on the HPA axis through intermediary neurons in the brain stem.Citation139 Afferent projections from the CeA densely innervate the NTS and parabrachial nucleus.Citation92,Citation168 The MeA sends a limited number of direct projections to the parvocellular division of the PVNCitation169; however, this subnucleus innervates a number of nuclei that directly innervate the PVN. Neurons of the MeA project to the BNST, MePO, and ventral premammillary nucleus.Citation169
The amygdala is a target for circulating glucocorticoids and the CeA and MeA express both GR and MR. In contrast to the effects on hippocampal and cortical neurons, glucocorticoids increase expression of CRF in the CeA and potentiate autonomic responses to chronic stressors. Glucocorticoid infusion into the CeA does not acutely effect HPA activation but may play a feed-forward role to potentiate HPA responses to stress.Citation139,Citation157,Citation170
Sympathetic circuits and the stress response
Activation of brain stem noradrenergic neurons and sympathetic andrenomedullary circuits further contribute to the body's response to stressful stimuli. Similarly to the HPA axis, stress-evoked activation of these systems promotes the mobilization of resources to compensate for adverse effects of stressful stimuli.Citation3,Citation171 The locus coeruleus (LC) contains the largest cluster of noradrenergic neurons in the brain and innervates large segments of the neuroaxis.Citation172 The LC has been implicated in a wide array of physiological and behavioral functions including emotion, vigilance, memory, and adaptive responses to stress.Citation173-Citation175 A wide array of stressful stimuli activate LC neurons, alter their electrophysiological activity, and induce norepinephrine release.Citation176-Citation178 Stimulation of the LC elicits several stressassociated responses including ACTH release,Citation179 anxiogenic-like behaviors,Citation180 and suppression of immune functions.Citation181 In addition, there are interactions between CRF and NE neurons in the CNS. Central administration of CRF alters activity of LC neurons and NE catabolism in terminal regions.Citation13,Citation182 Finally, dysfunction of catecholamergic neurons in the LC has been implicated in the pathophysiology of affective and stress-related disorders.Citation183,Citation184
Conclusions
Maintenance of homeostasis in the presence of real or perceived challenges requires activation of a complex range of responses involving the endocrine, nervous, and immune systems, collectively known as the stress response. Inappropriate regulation of the stress response has been linked to a wide array of pathologies including autoimmune disease, hypertension, affective disorders, and major depression. In this review we briefly discussed the major neuronal and endocrine systems that contribute to maintenance of homeostasis in the presence of stress. Clearly deciphering the role of each of these systems and their regulatory mechanisms may provide new therapeutic targets for treatment and prophylaxis of stress-related disorders including anxiety, feeding, addiction, and energy metabolism.
Selected abbreviations and acronyms
| ACTH | = | adrenocorticotropic hormone |
| BNST | = | bed nucleus of stria terminalis |
| cAMP | = | cyclic adenosine monophosphate |
| CeA | = | central nuclei of amygdala |
| CNS | = | central nervous system |
| CRF | = | corticotropin-releasing factor |
| DMH | = | dorsomedial hypothalamic nucleus |
| GR | = | glucocorticoid receptor |
| HPA | = | hypothalamic-pituitary-adrenal |
| LC | = | locus coeruleus |
| LS | = | lateral septum |
| MeA | = | medial nuclei of the amygdala |
| NTS | = | nucleus of solitary tract |
| POA | = | preoptic area |
| PVN | = | paraventricular nucleus |
| SFO | = | subfornical organ |
This work is supported by NIDDK Program Project Grant DK26741 and by the Clayton Medical Research Foundation, Inc. Wylie Vale is a Senior Clayton Medical Research Foundation Investigator.
REFERENCES
- ChrousosGP.GoldPW.The concepts of stress and stress system disorders. Overview of physical and behavioral hmeostasis.JAMA.1992267124412521538563
- CarrascoGA.Van de KarLD.Neuroendocrine pharmacology of stress.Eur J Pharmacol.200346323527212600714
- CharmandariE.TsigosC.ChrousosG.Endocrinology of the stress response.Annu Rev Physiol.20056725928415709959
- SapolskyRM.RomeroLM.MunckAU.How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions.Endocr Rev.200021558910696570
- HabibKE.GoldPW.ChrousosGP.Neuroendocrinology of stress.Endocrinol Metab Clin North Am.20013069572811571937
- ChrousosGP.Regulation and dysregulatïon of the hypothalamic-pïtuitary-adrenal axis. The corticotropin-releasing hormone perspective.Endocrinol Metab Clin North Am.1992218338581486878
- WhitnallMH.Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system.Prog Neurobiol.1993405736298484004
- ValeW.SpiessJ.RivierC.RivierJ.Characterization of a 41 -residue ovine hypothalamic peptide that stimulates secretion of corticotropin and betaendorphin.Science.1981213139413976267699
- RivierC.ValeW.Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin.Nature.19833053253276312319
- MunckA.GuyrePM.HolbrookNJ.Physiological functions of glucocorticoids in stress and their relation to pharmacological actions.Endocr Rev.1984525446368214
- BambergerCM.SchulteHM.ChrousosGP.Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids.Endocr Rev.1996172452618771358
- McEwenBS.StellarE.Stress and the individual. Mechanisms leading to disease.Arch Intern Med.1993153209321018379800
- ValentinoRJ.FooteSL.Aston-JonesG.Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus.Brain Res.19832703633676603889
- ValentinoRJ.FooteSL.Corticotropin-releasing hormone increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats.J Neurosci.19888101610253258021
- ChattertonRT.The role of stress in female reproduction: animal and human considerations.IntJ Fertil.1990358131968447
- PetraglïaF.FlorïoP.GallinelliA.et al.Secretion and putative role of activin and CRF in human parturition.Ann N Y Acad Sci.19947343803867978940
- ContarinoA.DelluF.KoobGF.et al.Dissociation of locomotor activation and suppression of food intake induced by CRF in CRFR1 -deficient mice.Endocrinology.20001412698270210875276
- CroisetG.NïjsenMJ.KamphuisPJ.Role of corticotropin-releasing factor, vasopressin and the autonomic nervous system in learning and memory.Eur J Pharmacol.200040522532411033330
- RichardD.LinQ.TimofeevaE.The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance.Eur J Pharmacol.200244018919712007535
- SawchenkoPE.ImakiT.PotterE.KovacsK.ImakiJ.ValeW.The functional neuroanatomy of corticotropin-releasing factor.Ciba Found Symp.1993172521;discussion 21298491094
- BruhnTO.EngelandWC.AnthonyEL.GannDS.JacksonIM.Corticotropin-releasing factor in the adrenal medulla.Ann N Y Acad Sci.19875121151283502062
- AudhyaT.HollanderCS.SchlesingerDH.HutchinsonB.Structural characterization and localization of corticotropin-releasing factor in testis.Biochim BiophysActa.19899951016
- BaleTL.ValeWW.CRF and CRF receptors: role in stress responsivity and other behaviors.Annu Rev Pharmacol Toxicol.20044452555714744257
- VaughanJ.DonaldsonC.BittencourtJ.et al.Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor.Nature.19953782872927477349
- ReyesTM.LewisK.PerrinMH.et al.Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors.Proc Natl Acad Sci U SA 20019828433848
- LewisK.LiC.PerrinMH.et al.Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor.Proc Natl Acad Sci U S A.2001987570757511416224
- HsuSY.HsuehAJ.Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor.Nat Med.2001760561111329063
- LiC.VaughanJ.SawchenkoPE.ValeWW.Urocortin lll-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophinreleasing factor receptor expression.J Neurosci.200222991100111826127
- PerrinMH.ValeWW.Corticotropin releasing factor receptors and their ligand family.Ann N Y Acad Sci.199988531232810816663
- ChenR.LewisKA.PerrinMH.ValeWW.Expression cloning of a human corticotropin-releasing-factor receptor.Proc Natl Acad Sci U S A.199390896789717692441
- VitaN.LaurentP.LefortS.et al.Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors.FEBS Lett.1993335158243652
- ChangCP.PearseRV.2ndO'ConnellS.RosenfeldMG.Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain.Neuron.199311118711958274282
- PerrinM.DonaldsonC.ChenR.et al.Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart.Proc Natl Acad Sci U S A.199592296929737708757
- StenzelP.KestersonR.YeungW.ConeRD.RittenbergMB.StenzelPooreMP.Identification of a novel murine receptor for corticotropin-releasing hormone expressed in the heart.Moi Endocrinol.199596376457565810
- PotterE.SuttonS.DonaldsonC.et al.Distribution of corticotropinreleasing factor receptor mRNA expression in the rat brain and pituitary.Proc Natl Acad Sci U S A.99491877787818090722
- Van PettK.ViauV.BittencourtJC.et al.Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse.J Comp Neurol.200042819121211064361
- KishimotoT.PearseRV II.LinCR.RosenfeldMG.A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle.Proc Natl Acad Sci U S A.199592110811127755719
- DautzenbergFM.KilpatrickGJ.HaugerRL.MoreauJ.Molecular biology of the CRH receptors-in the mood.Peptides.20012275376011337088
- DautzenbergFM.HaugerRL.The CRF peptide family and their receptors: yet more partners discovered.Trends Pharmacol Sci.200223717711830263
- BilezikjianLM.ValeWW.Glucocorticoids inhibit corticotropin-releasing factor-induced production of adenosine 3',5'-monophosphate in cultured anterior pituitary cells.Endocrinology.19831136576626191964
- SmithGW.AubryJM.DelluF.et al.Corticotropin releasing factor receptor 1 -deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development.Neuron.199820109311029655498
- TimplP.SpanagelR.SillaberI.et al.Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1.Nat Genet.1998191621669620773
- BaleTL.ContarinoA.SmithGW.et al.Mice deficient for corticotropinreleasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress.Nat Genet.20002441041410742108
- CosteSC.KestersonRA.HeldweïnKA.et al.Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropinreleasing hormone receptor-2.Nat Genet.20002440340910742107
- KishimotoT.RadulovicJ.RadulovicM.et al.Deletion of CRFR2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2.Nat Genet.20002441541910742109
- SwansonLW.SawchenkoPE.Hypothalamic integration: organization of the paraventricular and supraoptic nuclei.Annu Rev Neurosci.198362693246132586
- BrownsteinMJ.Biosynthesis of vasopressin and oxytocin.Annu Rev Physiol.1983451291356847162
- BrownsteinMJ.RussellJT.GainerH.Synthesis, transport, and release of posterior pituitary hormones.Science.19802073733786153132
- VerbalisJG.Osmotic inhibition of neurohypophysial secretion.Ann N Y Acad Sci.19936891461608373011
- RivierC.ValeW.Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo.Endocrinology.19831139399426307672
- AntoniFA.Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age.Front Neuroendocrinol.199314761228387436
- HernandoF.SchootsO.LolaitSJ.BurbachJP.Immunohistochemical localization of the vasopressin V1 b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin.Endocrinology.20011421659166811250948
- BirnbaumerM.Vasopressin receptors.Trends Endocrinol Metab.20001140641011091117
- SawchenkoPE.Adrenalectomy-induced enhancement of CRF and vasopressin immunoreactivity in parvocellular neurosecretory neurons: anatomic, peptide, and steroid specif icity.J Neurosci.19877109311063553442
- KovacsKJ.SawchenkoPE.Regulation of stress-induced transcriptional changes in the hypothalamic neurosecretory neurons.J Mol Neurosci.199671251338873896
- KovacsKJ.SawchenkoPE.Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons.J Neurosci.1996162622738613792
- AguileraG.Rabadan-DiehlC.Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation.Regul Pept200096232911102648
- AguileraG.Rabadan-DiehlC.Regulation of vasopressin V1 b receptors in the anterior pituitary gland of the rat.Exp Physiol.200085 Spec No19S26S10795903
- ChangAC.CochetM.CohenSN.Structural organization of human genomic DNA encoding the pro-opiomelanocortin peptide.Proc Natl Acad Sci USA.98077489048946254047
- Lacaze-MasmonteïlT.de KeyzerY.LutonJP.KahnA.BertagnaX.Characterization of proopiomelanocortin transcripts in human nonpituitary tissues.Proc Natl Acad Sci U S A.198784726172653478693
- Raffin-SansonML.de KeyzerY.BertagnaX.Proopiomelanocortin, a polypeptide precursor with multiple functions: from physiology to pathological conditions.Eur J Endocrinol.2003149799012887283
- MountjoyKG.RobbinsLS.MortrudMT.ConeRD.The cloning of a family of genes that encode the melanocortin receptors.Science.19922571248 12511325670
- ConeRD.LuD.KoppulaS.et al.The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation.Recent Prog Horm Res.199651287317; discussion 3188701084
- SimpsonER.WatermanMR.Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH.Annu Rev Physiol.1988504274402837136
- GiguereV.HollenbergSM.RosenfeldMG.EvansRM.Functional domains of the human glucocorticoid receptor.Cell.1986466456523742595
- CadepondF.Schweizer-GroyerG.Segard-MaurelI.et al.Heat shock protein 90 as a critical factor in maintaining glucocorticosteroid receptor in a nonfunctional state.J Biol Chem.1991266583458412005120
- PrattWB.The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor.J Biol Chem.19932682145521488407992
- HollenbergSM.EvansRM.Multiple and cooperative trans-activation domains of the human glucocorticoid receptor.Cell.1988558999063191531
- Yang-YenHF.ChambardJC.SunYL.et al.Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction.Cell.199062120512152169352
- SchuleR.RangarajanP.KliewerS.et al.Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor.Cell.199062121712262169353
- RayA.PrefontaineKE.Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor.Proc Natl Acad Sci U S A.1994917527568290595
- Keller-WoodME.DallmanMF.Corticosteroid inhibition of ACTH secretion.Endocr Rev.198451246323158
- ChenA.PerrinM.BrarB.et al.Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids.Mol Endocrinol.20051944145815514029
- WestphalNJ.SeasholtzAF.CRH-BP: the regulation and function of a phylogenetically conserved binding protein.Front Biosci.2006111878189116368564
- De KloetER.VreugdenhilE.OitzlMS.JoelsM.Brain corticosteroid receptor balance in health and disease.Endocr Rev.1998192693019626555
- ReulJM.de KloetER.Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis.J Steroid Biochem.1986242692723702410
- ReulJM.de KloetER.Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation.Endocrinology.1985117250525112998738
- DallmanMF.LevinN.CascioCS.AkanaSF.JacobsonL.KuhnRW.Pharmacological evidence that the inhibition of diurnal adrenocorticotropin secretion by corticosteroids is mediated via type I corticosterone-preferring receptors.Endocrinology.1989124284428502542001
- RatkaA.SutantoW.BloemersM.de KloetER.On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation.Neuroendocrinology.1989501171232550833
- SawchenkoPE.Evidence for a local site of action for glucocorticoids in inhibiting CRF and vasopressin expression in the paraventricular nucleus.Brain Res.19874032132233493829
- KovacsKJ.MakaraGB.Corticosterone and dexamethasone act at different brain sites to inhibit adrenalectomy-induced adrenocorticotropin hypersecretion.Brain Res.19884742052102850089
- KovacsKJ.FoldesA.SawchenkoPE.Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons.J Neurosci.2000203843385210804224
- WattsAG.Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback.Front Neuroendocrinol.20052610913016289311
- JacobsonL.SapolskyR.The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis.Endocr Rev.1991121181342070776
- DiorioD.ViauV.MeaneyMJ.The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress.J Neurosci.199313383938478396170
- McEwenBS.The neurobiology of stress: from serendipity to clinical relevance.Brain Res.200088617218911119695
- PotterE.BehanDP.FischerWH.LintonEA.LowryPJ.ValeWW.Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins.Nature.19913494234261846945
- HuisingMO.FlikG.The remarkable conservation of corticotropinreleasing hormone (CRH)-binding protein in the honeybee (Apis mellifera) dates the CRH system to a common ancestor of insects and vertebrates.Endocrinology.20051462165217015718273
- LintonEA.WolfeCD.BehanDP.LowryPJ.A specific carrier substance for human corticotrophin releasing factor in late gestational maternal plasma which could mask the ACTH-releasing activity.Clin Endocrinol (Oxf).1988283153242844451
- McLeanM.SmithR.Corticotrophin-releasing hormone and human parturition.Reproduction.200112149350111277868
- HermanJP.FigueiredoH.MuellerNK.et al.Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitaryadrenocortical responsiveness.Front Neuroendocrinol.20032415118014596810
- SchwaberJS.KappBS.HîggïnsGA.RappPR.Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus.J Neurosci.19822142414386181231
- EricssonA.KovacsKJ.SawchenkoPE.A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons.J Neurosci.1994148979138301368
- LacroixS.RïvestS.Functional circuitry in the brain of immune-challenged rats: partial involvement of prostaglandins.J Comp Neurol.19973873073249336231
- KrukoffTL.MacTavishD.HarrisKH.JhamandasJH.Changes in blood volume and pressure induce c-fos expression in brainstem neurons that project to the paraventricular nucleus of the hypothalamus.Brain Res Mol Brain Res.199534991088750865
- SawchenkoPE.LiHY.EricssonA.Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms.Prog Brain Res.2000122617810737051
- CunninghamET.Jr Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus.J Comp Neurol.198827460762458397
- CunninghamET.Jr Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat.J Comp Neurol.19902926516672324319
- PlotskyPM.Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catechol mi nergic pathways or central norepinephrine injection.Endocrinology.19871219249303497798
- WidmaierEP.PlotskyPM.SuttonSW.ValeWW.Regulation of corticotropin-releasing factor secretion in vitro by glucose.Am J Physiol.1988255E287E2922901813
- PlotskyPM.CunninghamET.Jr Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion.Endocr Rev.1989104374582558876
- inamanL.Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus.Am J Physiol.1999277R582R59010444567
- KinzigKP.D'AlessïoDA.HermanJP.et al.CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors.J Neurosci.2003236163617012867498
- awchenkoPE.BenoitR.BrownMR.Somatostatin 28-immunoreactive inputs to the paraventricular and supraoptic nuclei: principal origin from non-aminergic neurons in the nucleus of the solitary tract.J Chem Neuroanat.1988181942908291
- SawchenkoPE.AriasC.BittencourtJC.Inhibin beta, somatostatin, and enkephalin immunoreactivities coexist in caudal medullary neurons that project to the paraventricular nucleus of the hypothalamus.J Comp Neurol.19902912692801967618
- SaphierD.WelchJE.FarrarGE.et al.Interactions between serotonin, thyrotropin-releasing hormone, and substance P in the CNS regulation of adrenocortical secretion.Psychoneuroendocrinology.1994197797977527566
- BerkML.FinkelsteinJA.Afferent projections to the preoptic area and hypothalamic regions in the rat brain.Neuroscience.19816160116247266881
- JohnsonAK.CunninghamJT.ThunhorstRL.Integrative role of the lamina terminalis in the regulation of cardiovascular and body fluid homeostasis.Clin Exp Pharmacol Physiol.1996231831918819650
- SawchenkoPE.SwansonLW.The organization of forebrain afférents to the paraventricular and supraoptic nuclei of the rat.J Comp Neurol.19832181211446886068
- PlotskyPM.SuttonSW.BruhnTO.FergusonAV.Analysis of the role of angiotensin II in mediation of adrenocorticotropin secretion.Endocrinology.19881225385452828001
- AguileraG.YoungWS.KissA.BathiaA.Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin II.Neuroendocrinology.1995614374447783857
- LindRW.SwansonLW.GantenD.Angiotensin II immunoreactive pathways in the central nervous system of the rat: evidence for a projection from the subfornical organ to the paraventricular nucleus of the hypothalamus.Clin Exp HypertensA.1984619151920
- LindRW.SwansonLW.GantenD.Angiotensin II immunoreactivity in the neural afférents and efferents of the subfornical organ of the rat.Brain Res.19843212092156388733
- EngelmannM.LandgrafR.WotjakCT.The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited.Front Neuroendocrinol.20042513214915589266
- RolandBL.SawchenkoPE.Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat.J Comp Neurol.19933321231437685780
- CullinanWE.GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study.J Comp Neurol.200041934435110723009
- CullinanWE.HelmreichDL.WatsonSJ.Fos expression in forebrain afférents to the hypothalamic paraventricular nucleus following swim stress.J Comp Neurol.199636888998725295
- CullmanWE.WolfeTJ.Chronic stress regulates levels of mRNA transcripts encoding beta subunits of the GABA(A) receptor in the rat stress axis.Brain Res.200088711812411134596
- BealerSL.Corticosteroids and plasma restitution after hemorrhage and hypothalamic lesions.Am J Physiol.1986250R18R233510569
- ViauV.MeaneyMJ.The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area.J Neurosci.199616186618768774455
- BoudabaC.SzaboK.TaskerJG.Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus.J Neurosci.199616715171608929424
- FeldmanS.ConfortiN.SaphierD.The preoptic area and bed nucleus of the stria terminalis are involved in the effects of the amygdala on adrenocortical secretion.Neuroscience.1990377757792247223
- GrecoB.AllegrettoEA.TetelMJ.BlausteinJD.Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment.Endocrinology.20011425172518111713212
- SimerlyRB.ChangC.MuramatsuM.SwansonLW.Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study.J Comp Neurol.199029476952324335
- SahuA.Minireview: a hypothalamic role in energy balance with special emphasis on leptin.Endocrinology.20041452613262015044360
- HiguchiH.HasegawaA.YamaguchiT.Transcriptional regulation of neuronal genes and its effect on neural functions: transcriptional regulation of neuropeptide Y gene by leptin and its effect on feeding.J Pharmacol Sci.20059822523116006740
- ButlerAA.The melanocortin system and energy balance.Peptides.20062728129016434123
- WahlestedtC.SkagerbergG.EkmanR.HeiligM.SundlerF.HakansonR.Neuropeptide Y (NPY) in the area of the hypothalamic paraventricular nucleus activates the pituitary-adrenocortical axis in the rat.Brain Res.198741733383040184
- LeibowitzSF.SladekC.SpencerL.TempelD.Neuropeptide Y, epinephrine and norepinephrine in the paraventricular nucleus: stimulation of feeding and the release of corticosterone, vasopressin and glucose.Brain Res Bull.1988219059123224284
- DhilloWS.SmallCJ.SealLJ.et al.The hypothalamic melanocortin system stimulates the hypothalamo-pituitary-adrenal axis in vitro and in vivo in male rats.Neuroendocrinology.20027520921611979051
- VrangN.LarsenPJ.ClausenJT.KristensenP.Neurochemical characterization of hypothalamic cocaine-amphetamine-regulated transcript neurons.J Neurosci.199919RC510234051
- SmithSM.VaughanJM.DonaldsonCJ.et al.Cocaine- and amphetamine- regulated transcript activates the hypothalamic-pituitary-adrenal axis through a corticotropin-releasing factor receptor-dependent mechanism.Endocrinology.20041455202520915271883
- SarkarS.WittmannG.FeketeC.LechanRM.Central administration of cocaine- and amphetamine-regulated transcript increases phosphorylation of cAMP response element binding protein in corticotropin-releasing hormone-producing neurons but not in prothyrotropin- releasing hormoneproducing neurons in the hypothalamic paraventricular nucleus.Brain Res.200499918119214759497
- StanleySA.SmallCJ.MurphyKG.et al.Actions of cocaine- and amphetamine-regulated transcript (CART) peptide on regulation of appetite and hypothalamo-pituitary axes in vitro and in vivo in male rats.Brain Res.200189318619411223006
- RaisonCL.MillerAH.When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders.Am J Psychiatry.20031601554156512944327
- FeldmanS.ConfortiN.WeidenfeldJ.Limbic pathways and hypothalamic neurotransmitters mediating adrenocortical responses to neural stimuli.Neurosci Biobehav Rev.1995192352407630579
- ForrayMl.GyslingK.Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis.Brain Res Brain Res Rev.20044714516015572169
- HermanJP.MuellerNK.FigueiredoH.Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration.Ann N Y Acad Sci.20041018354515240350
- HermanJP.OstranderMM.MuellerNK.FigueiredoH.Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis.Prog Neuropsychopharmacol Biol Psychiatry.2005291201121316271821
- RubinRT.MandellAJ.CrandallPH.Corticosteroid responses to limbic stimulation in man: localization of stimulus sites.Science.19661537677685940897
- SapolskyRM.KreyLC.McEwenBS.Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response.Proc Natl Acad Sci U S A.198481617461776592609
- SaphierD.FeldmanS.Effects of septal and hippocampal stimuli on paraventricular nucleus neurons.Neuroscience.1987207497553601063
- KniggeKM.Adrenocortical response to stress in rats with lesions in hippocampus and amygdala.Proc Soc Exp Biol Med.1961108182114457232
- apolskyRM.Zola-MorganS.SquireLR.Inhibition of glucocorticoid secretion by the hippocampal formation in the primate.J Neurosci.199111369537041744687
- HermanJP.CullinanWE.YoungEA.AkilH.WatsonSJ.Selective forebrain fiber tract lesions implicate ventral hippocampal structures in tonic regulation of paraventricular nucleus corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) mRNA expression.Brain Res.19925922282381333341
- HermanJP.CullinanWE.MoranoMl.AkilH.WatsonSJ.Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis.J Neuroendocrinol.199574754827550295
- CullinanWE.HermanJP.WatsonSJ.Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis.J Comp Neurol.19933321207685778
- KohlerC.Subicular projections to the hypothalamus and brainstem: some novel aspects revealed in the rat by the anterograde Phaseolus vulgaris leukoagglutinin (PHA-L) tracing method.Prog Brain Res.19908359692392571
- HermanJP.DolgasCM.CarlsonSL.Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors.Neuroscience.1998864494599881860
- MuellerNK.DolgasCM.HermanJP.Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses.Endocrinology.2004145376337815142982
- inlayJM.ZigmondMJ.AbercrombïeED.Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam.Neuroscience.1995646196287715775
- JedemaHP.SvedAF.ZigmondMJ.FinlayJM.Sensitization of norepinephrine release in medial prefrontal cortex: effect of different chronic stress protocols.Brain Res.199983021121710366677
- FigueiredoHF.BruestleA.BodieB.DolgasCM.HermanJP.The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor.Eur J Neurosci.2003182357236414622198
- SesackSR.DeutchAY.RothRH.BunneyBS.Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutininJ Comp Neurol.19892902132422592611
- HurleyKM.HerbertH.MogaMM.SaperCB.Efferent projections of the infralimbic cortex of the rat.J Comp Neurol.19913082492761716270
- AhimaRS.HarlanRE.Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system.Neuroscience.1990395796041711170
- AkanaSF.ChuA.SorianoL.DallmanMF.Corticosterone exerts site- specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots.J Neuroendocrinol.20011362563711442777
- MathesonGK.BranchBJ.TaylorAN.Effects of amygdaloid stimulation on pituitary- adrenal activity in conscious cats.Brain Res.1971321511674329647
- Van de KarLD.BlairML.Forebrain pathways mediating stress-induced hormone secretion.Front Neuroendocrinol.1999201489882535
- PetrovichGD.SwansonLW.Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit.Brain Res.1997763247549296566
- DongHW.PetrovichGD.SwansonLW.Topography of projections from amygdala to bed nuclei of the stria terminalis.Brain Res Brain Res Rev.20013819224611750933
- CullinanWE.HermanJP.BattagliaDF.AkilH.WatsonSJ.Pattern and time course of immediate early gene expression in rat brain following acute stress.Neuroscience.1995644775057700534
- Kollack-WalkerS.WatsonSJ.AkilH.Social stress in hamsters: defeat activates specific neurocircuits within the brain.J Neurosci.199717884288559348352
- Kollack-WalkerS.DonC.WatsonSJ.AkilH.Differential expression of c- fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat.J Neuroendocrinol.19991154755910444312
- igueiredoHF.BodieBL.TauchiM.DolgasCM.HermanJP.Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo- pituitaryadrenocortical axis.Endocrinology.20031445249525812960031
- SawchenkoPE.BrownER.ChanRK.et al.The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress.Prog Brain Res.19961072012228782521
- ThrivikramanKV.SuY.PlotskyPM.Patterns of fos-immunoreactivity in the CNS induced by repeated hemorrhage in conscious rats: correlations with pituitary-adrenal axis activity.Stress.199721451589787263
- van der KooyD.KodaLY.McGintyJF.GerfenCR.BloomFE.The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat.J Comp Neurol.19842241246715573
- CanterasNS.SimerlyRB.SwansonLW.Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat.J Comp Neurol.19953602132458522644
- DallmanMF.PecoraroN.AkanaSF.et al.Chronic stress and obesity: a new view of comfort food“. Proc Natl Acad Sci U S A.2003100116961170112975524
- SvedAF.CanoG.PasserinAM.RabinBS.The locus coeruleus, Barrington#s nucleus, and neural circuits of stress.Physiol Behav.20027773774212527028
- FooteSL.BloomFE.Aston-JonesG.Nucleus locus ceruleus: new evidence of anatomical and physiological specificity.Physiol Rev.1983638449146308694
- Aston-JonesG.EnnisM.PieriboneVA.NickellWT.ShipleyMT.The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network.Science.19862347347373775363
- Aston-JonesG.ShipleyMT.ChouvetG.et al.Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology.Prog Brain Res.19918847751687622
- ValentinoRJ.CurtisAL.PageME.PavcovichLA.Florin-LechnerSM.Activation of the locus ceruleus brain noradrenergic system during stress: circuitry, consequences, and regulation.Adv Pharmacol.1998427817849328014
- AbercrombïeED.JacobsBL.Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats II. Adaptation to chronically presented stressful stimuli.J Neurosci.1987728442883625276
- PasserinAM.CanoG.RabinBS.DelanoBA.NapierJL.SvedAF.Role of locus coeruleus in foot shock-evoked Fos expression in rat brain.Neuroscience.20001011071108211113356
- DayasCV.BullerKM.CraneJW.XuY.DayTA.Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups.Eur J Neurosci.2001141143115211683906
- WardDG.GrizzleWE.GannDS.Inhibitory and facilitatory areas of the rostral pons mediating ACTH release in the cat.Endocrinology.19769912201228186252
- ButlerPD.WeissJM.StoutJC.NemeroffCB.Corticotropin- releasing factor produces fear- enhancing and behavioral activating effects following infusion into the locus coeruleus.J Neurosci.1990101761832299391
- RassnickS.SvedAF.RabinBS.Locus coeruleus stimulation by corticotropin-releasing hormone suppresses in vitro cellular immune responses.J Neurosci.199414603360407931560
- LavickyJ.DunnAJ.Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis.J Neurochem.1993606026127678287
- SouthwickSM.BremnerJD.RasmussonA.MorganCA III.ArnstenA.CharneyDS.Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder.Biol Psychiatry.1999461192120410560025
- SullivanGM.CoplanJD.KentJM.GormanJM.The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias.Biol Psychiatry.1999461205121810560026