Abstract
Illustrating the complexity of the stress response and its multifaceted manifestations is the leading idea of this overview of experimental paradigms used for stress induction in laboratory animals. The description of key features of models based on naturalistic stressors, pharmacological challenges, and genomic manipulations is complemented by comprehensive analysis of physiological, behavioral, neurochemical, and endocrine changes and their appropriatness as outcome readouts. Particular attention has been paid to the role of sex and age as determinants of the dynamics of the stress response. Possible translational applications of stress-inducing paradigms as models of disease are briefly sketched.
La complejidad de la respuesta ai estrés y sus manitestaciones polifacéticas son la linea conductora de esta revisión de paradigmas experimentales empleados para inducir estrés en anímales de laboratorio. La descripción de las características fundamentales de los modelos, que están basados en ios eiementos estresantes naturaies, provocaciones farmacológicas y manipulaciones genómicas se completa con un análisis extenso de ios cambios fisiológicos, de comporta mentó, neuroquímicos y endocrinos y su idoneidad como criterios de evaluación. Se ha prestado especia! atención a ia importancia dei sexo y la edad como determinantes de ia dinámica de la respuesta ai estrés. Se esbozan de forma sucinta las posibles aplicaciones translacionaies de los para-digmas inductores del estrés corno modelos de enfermedad.
Cette vue d'ensemble des paradigmes expérimentaux utilisés pour l'induction du stress chez les animaux de laboratoire a pour but d'illustrer la complexité de la réponse au stress et la multiplicité de ses manifestations. La description des caractéristiques clés concernant les différents modèles sont décrits, basés sur des stresseurs nature, sur des tests pharmacologiques ou sur des manipulations du génome, et sont complétés par une analyse détaillée des variations physiologiques, comportementales, neurochimiques et endocriniennes et de leur intérêt pour les résultats qui en découlent. Le rôle du sexe et de l'âge, en tant que déterminants de la dynamique de la réponse au stress, a été particulièrement étudié, La possibilité d'appliquer ces paradigmes d'induction du stress aux modèles! pathologiques est brièvement évoquée.
Stress comprises mobilization of basic physio logical repertoires for coping with adversity and restorIng homeostasis; Inappropriate strain on this arsenal, with respect to either magnitude or duration of the response, precipitates measurable pathological aberrations in several systems of the organism.Citation1-Citation4
After more than six decades of research, virtually every aspect of the organism's responses to stress has been addressed, and numerous end-point parameters have been proposed as descriptors of general and specific reactions to stressful stimuli. Stress4nduced changes in perception, behavior, thermoregulation, social interactions, sleep, cognition, endocrine secretions, neurotransmission, reproductive competence, immune defense, cardiovascular and gastrointestinal function, metabolic outcome, and susceptibility to noxious impact have shown rather concurrent patterns across mammalian species and, there fore, have become reliable indices of both stress exposure and stress coping ability. However, these universal responses to homeostatic disturbance are beset by certain “original sins”: (i) their activation results in over correction of vital parameters that may linger for some time before the status quo is reinstalled; (ii) mobilization of the “full standard repertoire” mostly exceeds the strict demand for the counterbalance of occasional or solitary shifts in homeostasis; (iii) the magnitude and dynamics of response depend not solely on the intensity of the stressful challenge, but also on numerous codeterminant variables, such as stimulus duration and context, sex, age, health condition, and previous experience of the individual, to name only a few.
From the perspective of stress modeling, three important consequences of the temporal dimension should be taken into consideration: the time point of assessment of indicators of the stress, the duration of the stressful challenge, and the phenomenon of habituation. Systems involved in the organism's response to stress have different activation latencies; accordingly, measurable end point changes occur at different intervals upon the challenge. Further, these systems act within physiological limits (described by, eg, synthetic and secretory capacity, feedback regulation within the system, consistency with key vital functions, etc) and cannot indefinitely maintain a maximal level of performance. Thus, changes in measurable end points vary depending on the duration of the stimulus, its perceived homeostatic threat, and the efficacy of the individually selected coping strategy (see below), but also due to output readjustment or exhaustion of the involved system. Finally, repeated exposure to homotypic stressors has been shown to produce gradual decline in the magnitude of several, but not all, commonly used indices of physiological response to stress. The omnipresence of this phenomenon is debatable, though there may be controversy based on species and paradigm differences. Habituation to repeated homotypic stress has a plausible teleological explanation: it is supposed to ensure the ability of a system involved in stress response to discriminate and adequately meet novel incoming challenges. Here, another important feature of the stress response, referred to as cross sensitization, should be mentioned. It has been recognized that, despite habituation to repeated homotypic challenge, stress responsive systems retain and, more importantly, even augment, their ability to react to challenges of a different modality. Several substrates of this phenomenon have been identified,Citation5 and its importance in the pathogenesis of stress related disorders is generally recognized.Citation1,Citation2,Citation4
Experimental modeling of stress requires clear definition of the research objectives, and consideration of numerous factors that may modify individual aspects of the stress response. Investigation of the magnitude and temporal course of a particular stress responsive parameter to a single challenge of limited duration has substantial diagnostic value in several medical disciplines. Ensuring truly “baseline” conditions for the variable of interest by minimization of confounding input from the environment and consideration of sex-and age-related response deviations are usually sufficient prerequisites for obtaining reliable results. However, tasks which aim at the examination of the resistance of a stress responsive physiological system under the influence of long-term or superimposed challenges, pharmacological treatment, or coexisting pathology, are by far more demanding. In such cases, careful evaluation of the condition and response capacity of the targeted system, alterations in its basal function resulting from each individual influence, and the time course of response must be added to the former requirements.
End points for assessment of the response to stress
Stress induces mobilization of a broad array of reactions which involve virtually every physiological system, albeit with different time courses. Accordingly, numerous parameters can be used for response monitoring in models of stress, under the provision that their temporal profiles and the changes possibly occurring in the course of habituation/sensitization are sufficiently defined.
Behavioral end points
The original description of the response to stress as a “fight-or-flight” reaction and evidence that arousal activation is invariably associated with this response implies that observation of general behavior can reliably disclose symptoms of stress. Assessment of the explorative activity by means of well established quantifiable parameters is a frequently used behavioral descriptor of the response to stress in laboratory rodents.Citation6 As in most species exposure to novelty is a stressor perse, monitoring of stressinduced effects in this experimental condition should be preceded by careful baseline definition. Although outcome may vary depending on the characteristics and duration of the challenge, decreased exploratory activity is considered to be a reliable behavioral consequence of stress exposure. In its extreme expression, this response is described as “freezing,” a period of time during which locomotion and exploration are completely abolished. The freezing response is reproducibly evoked in several stress paradigms, and protocols for its quantification have been developed.Citation7 Behavioral deficits known as acquired immobility, behavioral despair, and learned helplessness can be viewed as alterations specifically associated with severe stress; however, a learning component has a leading role in the manifestation of these phenomena.
Behavioral responses to stress are frequently linked with anxiety, and there is a substantial overlap of neurochemical mechanisms activated by stressful challenges and those involved in the control of anxiety. Evaluation of anxiety belongs to the standard arsenal for the assessment of behavioral effects of stress, and offers a direct possibility to disclose stress-associated neuropathological consequences. Since habituation may rapidly occur in some experimental paradigms used for evaluation of anxiety,Citation6 caution applies to their repeated use for the examination of long-term effects.
Elicitation of defensive behavior is a core component of the stress response, and can be perceived as a continuum of altered anxiety Assessment of manifestation of aggression and changes in its prestress degree of expression (especially within an established group hierarchy) is a recommended approach for the monitoring of stress effects,Citation8 and substantial correlation between behavioral and neurochemical end points has been established.
Analysis of audible and, especially, ultrasonic vocalization is a well-established method for the assessment of stress in pain and fear based paradigms,Citation9 especially in infant rats whose endocrine responses are subject to developmental inconsistency (see below). In juvenile animals, ultrasonic vocalization reliably indicates anxiety, but can be specifically modulated by maternal contact or predator cues.Citation10
Stress exerts profound effects on the acquisition, retention, and retrieval of new behavioral repertoire. As this process is an integral part of the formation of strategies for coping with stress and correlations with morphological and neurochemical measures have been established, assessment of learning and memory can be used for the evaluation of transient and persistent consequences of stress. The emphasis, however, should be put on “persisitent,” as behavioral acquisition is associated with the mobilization of several stress responsive neurochemical mechanisms, and the outcome depends on their “reverberation,” especially considering factors such as stress duration, crosstalk between neurochemical systems, and the organism's adequate coping with the challenge. Several publications on this subject note dichotomous effects: short and controllable stress facilitates acquisition, whereas severe chronic stress interferes with memory consolidation and retrieval. Activation of monoamin-ergic transmission and arousal is a plausible explanation of the former phenomenon, while biphasic effects of glu-cocorticoids, also in conjunction with their secondary influence on neurotransmission, have been implicated in the interpretation of shifts in learning and memory performance under stressful conditions.Citation11 To make this issue even more complicated, significant contribution of sex and age to this outcome should be noted. The concise message in the context of this review is that the impairment of acquisition, consolidation, and retrieval can serve as descriptors of detrimental consequences of poorly controlled chronic stress.
Physiological end points
Cardiovascular responses, such as changes in heart rate and arterial blood pressure, were recognized early as essential components of the response to stress, and are causally associated with the activation of the autonomic nervous system. With the increasing popularity of telemetric recording equipment, monitoring of cardiovascular end points has become a useful research tool in stress models.Citation12
The capacity of stress to trigger pain suppression has been known for a long time, and the involved neurochemical mechanisms have been comprehensively elucidated.Citation13 Measurement of stress-induced analgesia belongs to the standard repertoire of methods for monitoring of stress and pharmacological assessment of involved neurotransmitter and neuromodulator systems.
Transient increase in body core temperature is a wellestablished physiological correlate of stress. Although the proper nature of stress-induced hyperthermia is still a matter of debate, its time course and several contributing neuropharmacological mechanisms have been extensively studied, and the reliability of the method confirmed in various experimental settings.Citation14
Several stressful challenges significantly influence feeding behavior, and investigations of the underlying neurochemical mechanisms have revealed the involvement of some stress-responsive systems in this phenomenon. Changes in the amount and pattern of food intake have been sporadically used for stress monitoring per se, whereas exposure to stress has advanced to a modeling approach of eating disorders.Citation15
Stress-induced changes in sleep architecture in experimental animals have been comprehensively describedCitation16 and used for monitoring in different models; invasive interventions and sophisticated equipment have limited their widespread application.
Metabolic end points
Stress triggers distinct metabolic alterations, most of which are readily discernible. The “prototypic” metabolic response to acute stress consists of rapid and strong elevation of plasma concentrations of glucose, insulin, glycerol, and ketone bodies. The latter effects probably reflect the stimulation of adipose tissue lipase by circulating catecholamines. Activation of the autonomic nervous system has been also associated with stress-induced stimulation of glucagon secretion. Changes associated with repeated stress are also of catabolic nature, but less dramatic and, in some aspects (insulin) inconsistent. Both acute and chronic stress regimens decrease triacylglycerol levels, whereas reports on changes in cholesterol fractions are controversial.Citation17
Neurochemical end points
Increased sympathoadrenal outflow in the periphery and activation of monoaminergic neurotransmission in the brain were among the first described neurochemical correlates of the stress response, and their importance for the elicitation of several allostatic reactions in the organism is beyond doubt. Measurement of circulating levels of catecholamines and/or their metabolites, as well as their content, release, and biosynthesis in discrete brain regionsCitation18 have become standard approaches for stress response monitoring. Continuous microdialysis of discrete projection areas, in combination with morphological and histochemical techniques, has provided comprehensive description of the neuronal populations and pathways affected by stress, as well as of their distinct responsiveness to specific stressors.Citation3 Meticulous studies on the role of catecholamines in stress have shown that the morphofunctional heterogeneity of peripheral and central monoaminergic systems ensures discriminative responses to individual stress modalities.
Early experimental evidence for stress-induced changes in serotonergic neurotransmission has been extensively corroborated in subsequent pharmacological studies.Citation19 Monitoring of serotonin synthesis, release, and receptor expression have provided valuable insight into the role of this transmitter in certain aspects of the behavioral and neuroendocrine response to stress and the pathogenesis of stress-related disorders.
Evidence for global activation of dopaminergic neuro-transmission under stressful conditions and links to stress-related pathology suggests possible use of changes in this system for stress monitoring. These include morphological and functional heterogeneity of dopaminergic pathways, intricate involvement of dopaminergic transmission in selective information transfer, and motivation, integration, and adjustment of central nervous system (CNS) responses to novelty and aversion20; how-ever, the appropriateness of dopamine-related end points in stress research requires careful evaluation. It should be noted that individual dopaminergic projections display differential degree of activation following stress, with the mesoprefrontal pathway being particularly vulnerable,Citation21 and the character of changes in dopaminergic transmission might heavily depend on the context of stress and cross-modulation by multiple convergent neurotransmitter input and endocrine variables. Stressinduced changes in reward-mediating neurotransmitters and their interaction with other neurohumoral constituents of the stress response entail the possibility of using liability to addiction as a measure for the assessment of behavioral impact of stress.
Activation of cerebral cholinergic transmission by stress has been documented, and its established roles in arousal, motivation, and cognition are suggestive of an involvement in the processing of stressful stimuli. Probably due to differential regional and temporal release patterns, as well as discordant observations on their coincidence with other physiological end points,Citation22 changes in acetylcholine release are less frequently used as end points for stress evaluation.
Dramatic stress-induced increase in extracellular levels of glutamate, the major excitatory amino acid transmitter, have been reported in numerous brain regions. Glutamate efflux in the prefrontal cortex has been implicated in the modulation of the dopamine response to stress, and an array of potential pathological consequences was outlined.Citation23 Interactions between adrenocortical secretions and glutamate signaling in the hippocampus have prompted strong interest in the role of this neurotransmitter in long-term consequences of stress and their projections to various aspects of neuro and psychopathology, as well as therapeutic strategies.Citation24
Measurements of the synthesis and release of γ-aminobutyric acid (GABA) in the course of stress response have a long history; however, results are burdened by contro-versy, and the relevance of this end point in stress monitoring has been questioned.Citation25 On the other hand, pharmacological modification of GABA-ergic transmission and measurement of changes in GABA receptor properties convincingly demonstrate a substantial involvement of GABA in the control of the stress response. The importance of GABA has been increasingly associated with anxiety and related defensive responses, as well as regulation of stress-specific neuroendocrine circuits.Citation26 It is pertinent to note that several aspects of GABA-ergic neurotransmission can be obscured by endogenous steroid hormone derivatives, which act as allosteric lig-ands of the GABA-A receptor, and whose synthesis is increased following stress. These compounds have been shown to influence several aspects of the behavioral and neuroendocrine response to stress.
Antinociceptive effects of endocannabinoids, evidence for stress-related changes in their release in discrete brain areas, and localization of cannabinoid receptors in neuronal populations that participate in the behavioral and endocrine response to stress have stimulated the interest in monitoring the activity of this system. Although the current prevailing view is that endocannabinoids play a pivotal role in the modulation of the stress response and neuroprotection, several contentious issues on the dynamics of these modulatory effects remain to be resolved.Citation27
The causal involvement of endogenous opioids in stress-induced analgesia has been the starting point for extensive research on the global role of opioidergic transmission in stress. Ample evidence supports the view that opioidergic systems are profoundly affected by stress, and their secretory products participate in several aspects of the organism's response. Alterations in the endogenous opioid tone are implicated in stress-related endocrine and autonomic responses.Citation28 Anatomical and neurochemical heterogeneity of endogenous opioidergic systems, however, has made pharmacological paradigms a preferential approach for the investigation of stress-related changes in opioid neurotransmission.
Observations of rapid induction of proto-oncogenes in distinct brain regions by various stress modalities led to the adoption of c-fos expression as a firm morpho-functional marker of stress exposure. Monitoring of c-fos induction is a reliable tool for the identification of neuronal populations affected by stress,Citation29 and has significantly contributed to the delineation of neural pathways involved in the stress response.Citation3 The applicability of this method is, however, restricted to post-mortem examination; it should be also noted that signs of habituation of this response have been described, and controversy exists as to whether its magnitude reflects the stressfulness and intensity of the challenge. Nonetheless, monitoring of proto-oncogene induction may become an essential approach to the elucidation of spatiotemporal patterns in novel and less familiar models of stress.
It should be mentioned that several neuropeptide systems in the brain are substantially affected by stressCitation30 and, upon characterization of their distinct expression patterns in the selected paradigm, might eventually enrich the palette of neurochemical indicators.
Endocrine end points
Activation of the limbic-hypothalamo-pituitary-adrenal (LHPA) neuroendocrine axis is not only a “constant companion” of the stress response, but also provides the most reliable neurohumoral substrate for the assessment of its magnitude, dynamics and, ultimately, the capacity of the organism to overcome the present and meet sub-sequent challenges. As comprehensive work of reference has addressed the structural and functional organization and the regulation of the LHPA axis under stressful conditions,Citation31 here we will focus on the conclusiveness of individual measures of its activity in models of stress.
Input from stress-responsive neural circuits onto the hypothalamic paraventricular nucleus (PVN) induces the release of neuropeptide secretagogues of adrenocorti-cotropin (ACTH). Although stress-related fluctuations in corticotropin-releasing hormone (CRH) blood levels have been reported, its measurement in the systemic circulation has not attained widespread appreciation in laboratory animals. Monitoring of CRH concentrations in hypophyseal portal blood and, especially, perfusates and dialysates from defined brain regions is considered more reliable, and enables the distinction of CRH release from individual neuronal populations.Citation3 The most popular approach, however, is the direct assessment of CRH neurons by either the “output” of the hypophyseotropic population to the median eminence or the “steady state” of the CRH gene expression. The latter gained importance also in view of evidence for multiple neurotropic effects of intracerebral projections of CRH neurons, beyond those involved in the neuroendocrine response to stress.Citation32 CRH-coding transcripts in the parvocellular compartment of the PVN are a good descriptor of LHPA axis activity under basal and stress-related conditions.
Measurements of circulating vasopressin (AVP) levels have been used for assessment of stress responses; however, caution applies to their interpretation, due to the heterogeneity of the neuronal populations that produce AVP found in the circulation.Citation33 Peripheral AVP originates mainly from the posterior pituitary terminals of magnocellular neurons of the supraoptic and the posterior lateral portion of the paraventricular nucleus, and the involvement of these neuronal populations in the control of the LHPA axis is ambivalent.Citation34 Thus, quantification of AVP expression in anatomically defined neuronal clusters, which make up the adenohypophyseal projection of the PVN, appears to be the method of choice for assessement of the contribution of vasopressin to the endocrine response to stress. Extensive research in the past has shown that stress-associated changes in CRH and AVP expression in the PVN follow distinct temporal patterns, with AVP “coming into action” with certain delay or in the course of chronic stress load.Citation35
Oxytocin and angiotensin also deserve mention as auxiliary peptidergic ACTH secretagogues. Like AVP, oxytocin is produced in heterogeneous neuronal populations, and is released in response to various stressors in the systemic and adenohypophyseal portal circulation. Induction of oxytocin synthesis and secretion have been documented in various stress paradigms, and its role seems to extend beyond that of mere “booster” of CRH and AVP. However, while oxy-tocin is clearly a stress-responsive hormone, the interpretation of its “net” effect compels consideration of dissociated secretory activity of hypophyseotropic and intracerebral projections, subject's sex and physiological condition, stress modality, and other interacting factors.Citation36 Changes in angiotensin secretion represent an established component of the neuroendocrine response to stress, with multiple involvements in several aspects of allostasis.Citation37
Increased concentrations of ACTH in the systemic circulation and its precursor peptide pro-opiomelanocortin (POMC) in the anterior pituitary are a typical consequence of stress exposure. While in acute stress ACTH responses fairly reflect the activity level of CRH neurons, chronic stress and continuous CRH hypersecretion result in desensitization of pituitary CRH receptors and blunted ACTH release. This dissociation between CRH hyperactivity and refractory corticotrophin responsiveness is a pathognomonic feature of stress-associated neu-roendocrine dysregulation.
Systemic glucocorticoid levels under quiescent conditions (eg, at the nadir and zenith of circadian activity), the amplitude of the acute stress-induced increase (albeit influenced by sex, age and diurnal time point of examination), and the sensitivity of the hypothalamo-pituitary unit for glucocorticoids (as defined by the swiftness of reinstatement of basal secretions after stress cessation or the capacity of exogenously administered glucocorticoids to subdue the diurnal secretory peak) comprehensively characterize the status of the LHPA axis ()
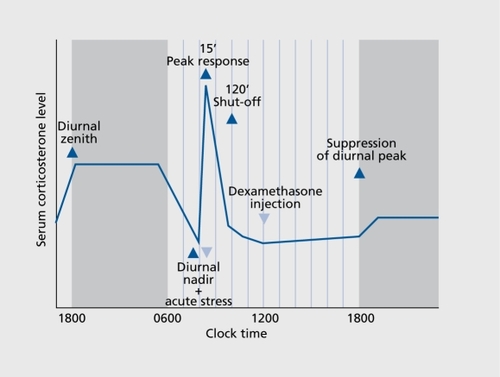
Stress profoundly affects reproductive function and gonadal secretions; however, changes in sex hormone levels following acute stress are not among the widely used monitoring end points. While there is unambiguous evidence that stress exposure impairs gonadal function and reproductive activity, the reserved use of measurements of gonadal secretions for the assessment of acute stress consequences is based on the complexity of neural mechanisms which control the key variable, the pulsatile discharge of gonadotropin-releasing hormone (GnRH)-producing neurons.Citation38 On the other hand, decreased gonadotropin levels, suppressed secretion of gonadal steroids, disruption of the ovarian cycle, and inhibition of sexual behavior are consistent outcomes of chronic and insuperable stress.Citation39 Circulating prolactin levels promptly increase with acute stressCitation40 and are a reliable endocrine end point, even if one abstains from reflective élaboration on the multiplicity of pathophysiological projections of stress-related hyperprolactinemia. Growth hormone secretion is altered by stress40; however, the pattern of changes may vary depending on the stress modality and require sophisticated evaluation.
Alterations in thyroid axis function and hormone secretion following stress exposure have been described in various experimental settings. The reported consequences of acute stress are somewhat contradictory, as both activation and inhibition have been described. Suppression by chronic or uncontrollable stressCitation41 is in line with the prevailing view of thyroid axis hypofunction in stress-related disorders; however, conflicting data exist also on this aspect.
Immunological end points
The immune system is unequivocally influenced by stress, and changes in various aspects of the inflammatory/immune response have been extensively documented. Exposure to infectious agents or antigenic challenge are stressful stimuli per se, and trigger a cascade of reactions within an intricate network which encompasses several components of the humoral stress response. The changes in immunological parameters following nonimmune stressful stimuli, however, are mostly considered consequences of the activation of two fast-acting stress-responsive systems, the sympatho-adrenomedullary and the hypothalamo-pituitary-adrenocortical.Citation42,Citation43 In general, immunosuppression is an obvious and understandable effect of acute stress, whereas persistent activation of the LHPA axis under the condition of chronic stress is accompanied with substantial shift in the quality of the immune response.
Experimental approach to stress induction
Physiological responses directed to restoration of the homeostasis and encompassing changes in several of the above-listed end points can be elicited by a myriad of environmental challenges and perturbations of the milieu intérieur. For the purpose of modeling, however, it is essential to demonstrate that a given challenge engenders traceable changes in (preferably, more than one) end points indicative of the occurrence of an allostatic response.
The most widely used classification of stress-inducing paradigms operates with two principal categories: systemic (physical) and neurogenic (psychoemotional), with conscious processing of the stimulus being the leading separation criterion.Citation31 While adhering to this taxonomy, we will take the liberty to introduce, for didactic reasons, subcategories based upon the procedural features of the stress model
Naturalistic models of survival threat
Deprivation paradigms
Food deprivation (not to be confused with caloric restriction) produces alterations in numerous descriptors of the humoral and behavioral response to stress. While demonstration of rapid-onset responses requires consideration of species-specific circadian activity patterns, prolonged food deprivation produces long-term consequences which are compatible with those seen in chronic exposure to stress.Citation44
Water deprivation and ensuing dehydration has been shown to elicit humoral changes suggestive of stressinduced LHPA axis activation.Citation45 Similar effects can be rapidly triggered by osmotic challenge using intraperitoneal injections of hypertonic saline. Osmotic challenge is a reliable paradigm of stress induction, and repeated application is reportedly not accompanied by signs of response desensitization. Since dehydration selectively activates neuronal populations with a primary role in osmoregulation and only auxiliary contributions to the LHPA axis stimulation, explanation of mechanisms involved in the hormonal response suffers from a certain inconsistency.
Deprivation of rapid eye movement (REM) sleep by different procedures is a recognized method of stress induction. There is firm evidence that prolonged sleep deprivation affects several physiological parameters in a fashion indicative of severe stress.Citation46 In this paradigm initial responses can be largely ascribed to the encounter with a highly adverse and novel environment, whereas changes seen in the course of long-term exposure also reflect progressive exhaustion of adaptation-relevant systems.
Restriction of the freedom of locomotion and exploration, better known and referred to as restraint or immobilization, is probably the most widespread method of stress induction (as judged by its reported use in more than 2000 publications). In any mode of application (single short-term, intermittent, chronic), restraint is perceived as a severe stressor, and robustly induces the entire spectrum of known allostatic responses.Citation47
Exposure to adverse environmental stimuli
Cold exposure (also cold-water swimming) causes noticeable activation of several stress-responsive systems.Citation48 The magnitude of some changes suggests that cold environment is not a powerful stressor in adult rats, but is a reliable method of stress induction in neonates. Cold stress is consistently associated with activation of the thyroid axis, which probably serves thermogenesis.
Significant neurochemical and endocrine responses have been documented in laboratory rodents following exposure to a hot environment.Citation49 While the magnitude of changes seems to correlate with the abruptness of transition and the ambient temperature, their temporal dynamics is rather sluggish.
Acute hemorrhage is a powerful signal for the activation of allostatic mechanisms. Induction of neurohumoral and endocrine responses by this systemic stressor has been extensively documented,Citation50 whereas behavioral and metabolic alterations have not been systematically examined. Even if not associated with specific adverse stimuli, exposure to novel environment is a well-recognized naturalistic stressor, and changes in brain catecholamines and pituitary and adrenal secretions have been demonstrated. Less congruous are data concerning the dynamics of the hormonal response following repeated exposure and the direction of changes in hypothalamic peptide stimulators of ACTH release.Citation51,Citation52
Several environmental signals acting through different sensory modalities (auditory, visual, tactile) have been shown to elicit stress responses. Audiogenic stress (noise exposure) is a well-characterized paradigm, with response profiles of individual parameters having been thoroughly examined.Citation53 Exposure to bright light or abrupt alteration of illumination rhythms are naturalistic stressors in laboratory rodents, and endocrine responses have been documented,Citation54 though some mechanisms require elucidation. Responses induced by modification of the illumination regimen may be obscured by interference with established circadian and ultradian activity patterns of the involved physiological systems.
The capacity of olfactory stimuli to elicit pronounced stress reactions is best exemplified by studies employing the paradigm of exposure to odors originating from either a predator or a stressed cospecific individual. Odor-induced stress responses do not completely overlap with those seen after realistic encounter with a predator.Citation55 The importance of olfactory stressors in experimental routine should be taken into consideration: whenever animals are sequentially stressed, the odor of the “predecessor” must be eliminated after completion of the test.
Pain paradigms
Nociceptive stimuli are among the most powerful inducers of stress responses. Although concerns of animal welfare have gradually diminished the use of pain-based paradigms, painful manipulations, such as electric footshock, tail pinch, and pharmacologically-induced hyperalgesia (formalin, carrageenan), have served for decades as fundamental approaches for stress induction and dependable manifestation of most of the known stress-associated reactions of the organism. Chronic pain of inflammatory or neuropathic origin produces consequences that show extensive similarities and share several mediators with chronic stress.Citation56
Fear-and anxiety-based paradigms
Exposure to a predator is a prototypic example for fearmediated stress induction, and the response profiles of several systems have been comprehensively elucidated.Citation55 Intriguingly, repeated predator stress appears to promote a homotypic sensitization of neuroendocrine response mechanisms, with little evidence for a primary involvement of hypothalamic corticotropin secretagogue-producing neuronal populations.Citation57
Albeit with certain exaggeration, the generic term neophobia summarizes the anxiogenic potential of a host of stimuli emerging from either the natural environment or the laboratory settingCitation58 and their capacity to evoke measurable behavioral, neurochemical, endocrine, and metabolic stress responses. This intrinsic conflict between the drive for exploration of a novel environment and the assessment of the threatening potential of nonfamiliar stimuli is exploited for the generation of standard methods of fear- and anxiety-based stress induction.Citation59 Conditioned anticipation of fearful experience is also a powerful tool for the induction of stress responses, and there is substantial overlapping of the anatomical substrates involved in unconditioned and conditioned fear. However, quantitative and, to a lesser degree, qualitative differences in the activation of distinct neural populations have been revealed,Citation60 and the LHPA axis appears to have a crucial role in the emergence of conditioned fear. It should be mentioned that the degree of stress response resulting from the first (and, sometimes, also subsequent) exposure to experimental devices and procedures must be meticulously characterized and, if possible, minimized by handling, in order to avoid bias while measuring the “proper” outcome of a stress model.
Models of social conflict and disruption
Interactions within a cospecific group (population) are probably the most persistent source of stressful stimuli; however, in a colony of highly domesticated laboratory animals their impact often remains unaccounted, especially when using them as subjects in stress experiments. The baseline characteristics and the response profiles of end points used for stress assessment may critically depend on the individual's status within the rapidly formed social group hierarchy and/or his or her previous experience in this environment. Models based on social conflicts exploit either the aggravation of existing, or the de novo creation of, stressful interactions in the course of establishing and maintaining of hierarchic relationships of dominance or subordination. Specific conflict-producing experimental settings, such as territory defense (resident-intruder paradigm, colony overcrowding), hierarchy formation (social defeat, visible burrow system), offspring protection, and social instability are comprehensively reviewed.Citation61 These paradigms produce strong alterations in several indicators of the stress response and, upon chronic application, the outcome may mimic the features of human pathological conditions. In rats there are pronounced sex differences in the liability to social stress, with females being generally refractory to paradigms of hierarchy formation, but responsive to conditions of social instability.Citation62
Social isolation (solitary housing) has been considered an appropriate method for stress induction63; however, some caveats of this model merit consideration. Social isolation implies long-term deprivation of the familiar environment and, accordingly, immediate effects of separation can be ascribed to novelty and experimental procedures (eg, handling, restraint). Most consequences of social isolation become manifest after longer exposure periods. Finally, alterations in stress-related end points may be indicative of increased sensitivity to superim posed challenges rather than persistent activation of stress-responsive systems.
Disruption of social contacts during early ontogeny, mostly referred to as maternal separation/deprivation, is a powerful stressor in several species. The reputation of this paradigm is based on its capacity to evoke long-lasting alterations in the function of several adaptation-relevant systems and their susceptibility to stress.Citation64 A few marginal notes appear appropriate with regard to the practical use of this model. While immediate behavioral correlates (eg, vocalization) have been routinely used for monitoring the effects of maternal separation, the time course of endocrine responses to this stressor indicates that significant changes become apparent only after 2 to 4 hours of exposure, and their amplitude may vary depending on the age of the animals.Citation65 Thus, although maternal deprivation is a recognized stressor, caution applies to the selection of parameters and timepoints for the assessment of its early consequences.
Pharmacological models
Accumulation of knowledge on neurohumoral systems, which participate in the processing of stressful stimuli and induction of related physiological reactions, enables the use of appropriate pharmacological agents to modify the activity of individual response cascade fragments and bring about changes in end-point indicators even in the absence of a prototypic stressor. Conceivably, druginduced alterations in the initial “links” of stress-reactive chains would result in a broader spectrum of “downstream” responses; however, as systems of allostatic regulation operate through closed-loop mechanisms, pharmacological modifications that interfere with feedback circuits are also capable of changing the activity level of several interconnected response cascades.
Several pharmacological challenges are able to activate individual stress-responsive systems (eg, the LHPA axis). However, since stress is a complex and multipronged response, the list of pharmacological agents that can simultaneously influence several systems is rather short. The concomitant occurrence of pharmacologically induced responses in multiple systems involved in adaptation is exemplified by the effects of ether inhalation. This stressor produces behavioral agitation (before anesthesia takes place) and affects brain monoamine metabolism, and CRH and AVP biosynthesis and release. Likewise, glucoprivation induced by either insulin or 2-deoxyglucose administration results in distinct stress-like behavioral, neurochemical, and neuroendocrine alterations.
Abundant experimental evidence shows that pharmacological modulation of the major neurotransmitter systems that inaugurate the response to stressful stimuli can mimic several behavioral and endocrine responses to stress. Approaches aiming at the activation of distinct aspects of monoaminergic neurotransmission have been impressively summarizedCitation66 and their efficacy convincingly demonstrated. The established role of GABA-ergic signaling as a major tonic inhibitor of stress responses provides plausible explanation for the capacity of GABA/benzodiazepine antagonists to induce several behavioral and endocrine correlates of stress or augment the responsiveness to systemic and emotional challenges.Citation67
Although endogenous opioids definitely contribute to several aspects of the response to stress, divergent effects of opioid administration on neuroendocrine parameters, also due to intricate interactions with other neurotransmitter systems, appear to be somewhat at odds with the reigning opinion that opioids tonically suppress the LHPA axis.Citation68 It is thus helpful to consider that the issue discussed herein concerns pharmacological effects with abrupt onset, which are not expected to produce immediately dramatic shifts in what is called “opioidergic tone.” An abridged statement in the context of this paper summarizes that (i) acute administration of morphine or receptor-selective opioid agonists results in distinct stresslike changes of neuroendocrine end points and (ii) similar phenomena occur after spontaneous or antagonistprecipitated withdrawal from chronic opioid treatment. As with several other opioid-sensitive systems, development of tolerance is accompanied by attenuated responsiveness of the LHPA axis to subsequent opioid administration. The effects of psychomotor stimulants, as exemplified by cocaineCitation69 and amphetamine,Citation70 include stress-like symptoms of behavioral disruption and defensive withdrawal and stimulation of hypothalamo-pituitary-adrenal secretions. Most of these effects and the stress-contrasting suppression of prolactin release are ascribed to their agonistic influence on central monoaminergic transmission. Elevation of circulating ACTH and glucocorticoid concentrations has been demonstrated following intracerebral cannabinoid treatment; however, the involvement of drug-specific signaling mechanisms remains unclear, as specific cannabinoid receptor antagonists have produced biphasic effects. Alcohol administration powerfully stimulates the LHPA axisCitation71 and potentiates defensive responses. As with opioids, endocrine changes in the course of chronic treatment are suggestive of the development of selective tolerance.
In view of its essential role in the initiation and integration of behavioral, autonomic, and endocrine responses to stress, exogenous CRH dependably mimics several consequences of stressful stimuli. It should be added, however, that the stressogenic action of CRH is warranted following intracerebral administration, while some divergence (eg, in cardiovascular effects) may occur following systemic application.Citation72 Despite compelling evidence for the involvement of vasopressin in several aspects of the stress response,Citation73 administration of exogenous vasopressin has produced, at best, modest stress-like symptoms. Concerning the endocrine response, these observations are in agreement with the auxiliary role of vasopressin in the control of the LHPA axis. Continuing interest in the involvement of neuropeptides other than ACTH secretagogues in stress and emerging availability of selective analogues suggests novel possibilities for the use of such agents in pharmacological stress modeling.Citation30,Citation74
Persistent hypercorticalism has been shown to result in deterioration of neuroendocrine circuits that control the basal activity of the LHPA axis and its responsiveness to stressful challenges.Citation4 This outcome can be brought about pharmacologically by long-term administration of supraphysiological doses of glucocorticoids. Although this approach is confined to the LHPA axis and manifestation of stress-related symptoms in other systems has not been meticulously examined, distinct signs of basal hyperactivity and exaggerated endocrine responses to stress persist in this model for several weeks upon cessation of the glucocorticoid treatment.Citation75
A typical example of pharmacologically induced activation of several stress-reactive systems is represented by peptide mediators/integrators of the inflammatory and immune responses. The most frequently used agents are tumor necrosis factor a, interleukin-1 and interleukin6, or their sequential releaser, bacterial lipopolysaccharide (LPS). Endotoxinor cytokine-induced effects involve a complex of typical defensive behavioral responses, referred to as “sickness behavior,” with vagal afferentation playing an essential role.Citation76 Alterations in central and peripheral neurotransmission largely resemble those evoked by physical and neurogenic stress modalities,Citation77 and activation of the LHPA axis is a firmly established consequence.Citation78 Suppression of reproductive functions as part of the “sickness behavior,” and in terms of endocrine secretionsCitation79 has been demonstrated; it seems that cytokine-mediated disruption of the gonadal axis employs mechanisms which are independent of those involved in the general stress response. The reports on changes in growth hormone and prolactin secretion upon cytokine challenge are ambivalent.
The list of drugs with stressogenic properties becomes considerably longer if LHPA axis activation is considered a solitary symptom of stress. Association of thyreotoxicosis with symptoms of hypercorticalism has prompted experimental studies showing that chronic administration of thyroid hormones leads to activation of the LHPA axis.Citation80 Increased secretion of ACTH and glucocorticoids has been also seen following treatment with cholinomimetics, adenosine and histamine agonists, phosphodiesterase inhibitors, free fatty acids, and a high-fat diet. However, convincing evidence is still lacking that these agents are able to elicit a full-scale stress response.
Genetic models
Since stress is a transient condition, and its enduring presence is incompatible with survival, the following subject should be understood as models of increased stress responsiveness resulting from genetic manipulations or selective breeding.
Breeding strategies aiming at the consolidation of behavioral traits suggestive of increased vulnerability to stress have yielded interesting models; however, concordant changes in multiple end points were not always observable. Thus, several rat strains which are typified by enhanced anxiety and dysproportionate behavioral responsiveness to stress displayed inconsistent signs of increased (Fawn-Hooded, Maudsley reactive, Roman high avoidance) or, even, paradoxically subdued (Syracuse low avoidance) LHPA axis activity. The behavioral repertoire of the Flinders Sensitive line reveals several symptoms of aberrant responsiveness, but abnormal hormonal reactions could be evoked only by specific pharmacological challenges. Similarly, animals selected for their predisposition to learned helplessness upon stress exposure are fulfilling several behavioral and neurochemical criteria,Citation81 but establishment of endocrine correlates seems to depend on additional challenges during early ontogeny. Recent reports indicate that selective breeding based on the manifestation of enhanced anxiety produces a phenotype that is characterized by dominance of defensive responses to novelty, increased ultrasonic vocalization, and amplified endocrine reactivity. In this rat line, increased activity of the LHPA axis appears to result from vasopressin overexpression and hypersecretion, and the phenotype apparently correlates with distinct signs of polymorphism in the vasopressin gene promoter.Citation82
The most advanced approach to stress liability modeling is the targeted modifications of the expression of genes encoding individual components of stress-responsive cascades. Overexpression of monoamine-synthesizing enzymes, even in brain regions of specific importance, was not associated with a stress-prone phenotype.Citation83 More successful were genetic modifications of mechanisms involved in the control of endogenous catecholamine release and metabolism. Genomic disruption of γ2-adrenoceptors resulted in behavioral and neurochemical phenotypes that resemble those seen following stress exposure or pharmacological interventions,Citation84 but copresent endocrine alterations have not been reported. Similarly, increased behavioral responsiveness to stressful stimulin animals deficient for monoamine oxidase ACitation85 and catechol-o-methyltransferaseCitation86 is not accompanied by corresponding changes in endocrine end points. Overexpression of inflammatory cytokines (interleukin6, leukemia inhibitory factor) and growth hormone has resulted in distinct symptoms of LHPA axis activitation which, however, have been ascribed to either altered adrenocortical sensitivity or improper pituitary development.
The most compelling data have been obtained in studies with transgenics overexpressing CRH. The phenotype of these animals recapitulates most of the effects seen following CRH administration, such as increased anxiety and defensive behavior, impaired autonomic functions, immunosuppression, reproductive impairment, and LHPA axis hyperactivity under basal and post-challenge conditions.Citation87 Genetic elimination of the CRH-binding protein resulted in behavioral symptoms compatible with increased CRH bioavailability, but failed to alter pituitary-adrenal secretions under basal and stress-related conditions.Citation88
The crucial role of glucocorticoid receptor (GR) signaling in the tonic restraint and dynamic feedback control of the magnitude and duration of the neuroendocrine stress response, as well as its involvement in virtually every aspect of allostasis and adaptation,Citation43 has prompted numerous investigations on the outcome of GR genetic modifications. The results have produced more questions than answers, thus illustrating the intricacy of neuroendocrine control of stress responsiveness. Partial or complete disruption of GR expression in the brain has consistently led to increased LHPA axis output; however, surprisingly, this was not accompanied by behavioral alterations (as disclosed by measures of anxiety)89; some signs of coincident behavioral and neuroendocrine impairment following targeted GR disruption were reported only recentlyCitation90 Brain-specific overexpression of GR had anxiogenic effects, but failed to alter the activity of the LHPA axis under both basal and stressful conditions.Citation91 An elegant explanation of these confounding observations suggests that proper GR signaling in the brain not only controls the expression of stressogenic neuropeptides, but also ensures the correct detection of stress-induced adrenocortical output and its translation into defensive behavioral responses.Citation92
The importance of sex and age
Sex-related dichotomy has been recognized and extensively studied with regard to virtually every aspect of the stress response. Sympathoadrenal responses to stressCitation93 and basal or stress-induced LHPA axis activity are higher in females, as long as physiological gonadal secretions are maintained (for review see ref 94). The neurobiological foundations for this dichotomy appear to be laid down during early ontogeny under the organizing influence of perinatal sex hormone levels.Citation95 Glucocorticoid-sensing mechanisms in the female brain operate at lower discrimination thresholds, and female sex steroids seem to deflect the loss of sensitivity induced by autologous downregulation.Citation94 Most of the listed differences are abolished by gonadectomy and reinstalled by hormone replacement, thus underlining the role of activating effects of physiological gonadal secretions.Citation94,Citation96
Interestingly, sex-specific differences in the magnitude of neurochemical and neuroendocrine responses do not correlate with the expression of defensive behavior. Several studies using various experimental paradigms indicate that stress-induced behavioral suppression and anxiety are rather a “male privilege.” Experimental data on sex differences in stress-related analgesia reveal that this phenomenon is predominantly expressed in males, and generally matches gender differences seen in the responsiveness to analgesic drugs. The abovementioned sex differences in neuroendocrine responses to stress are not necessarily in accordance with observations in humans. Data from clinical studies are suggestive of stronger responsiveness in males,Citation97 and these sex-specific profiles persisted under the condition of simulated hypogonadism.Citation98
The robust female-specific response to stress in laboratory rodents is significantly attenuated during pregnancy, parturition, and lactation. Extensive research in the past has elucidated the joint causal contribution of various neurochemical and neuroendocrine mechanisms to this stress-protective phenomenon.Citation99
During a defined phase of early ontogeny (between postnatal days 3 and 14) rats and mice display blunted pituitary-adrenal responsiveness to several stressors that are perfectly effective in adult animals. The mechanisms underlining this stress-hyporesponsive period have been exhaustively elucidated. Briefly, subdued hormonal secretions following stress are believed to reflect the immaturity of pituitary corticotropin synthesis,Citation100 sluggish mobilization of adrenocortical steroidogenesis, and tight, pituitary-focused glucocorticoid-mediated control of the LHPA axis.Citation101 Stress hyporesponsiveness during early ontogeny is not absolute, as it can be breached by cytokine, endotoxin, and pharmacological challenges or pre-exposure to maternal separation. There are changes in proto-oncogene expression in relevant areas, and the neonatal brain reacts to several stressful stimuli,Citation102 but neuronal activation is apparently not translated into commensurate endocrine responses. The behavioral repertoire in infant animals is relatively poor, and does not provide many end point choices for the assessment of the stress response. Nonetheless, ultrasonic vocalization, a reliable sign of behavioral distress, is manifest also during the stress-hyporesponsive period.
The LHPA axis function in senescent animals displays aberrations that are attributed to dwindling efficacy of GR-mediated feedback control. While age-dependent differences in the magnitude of the stress-induced secretory response occasionally become apparent after a single challenge, deficits in its termination can be readily disclosed in both acute and chronic paradigms. Impaired signal discrimination in glucocorticoid-sensing mechanisms is considered the principal cause for protracted duration of the secretory response to stress in aged animals. A few debatable issues affecting the use of aged subjects in models of stress should be mentioned. Data on LHPA function under basal conditions are contradictory,Citation103,Citation104 and there is little evidence that disinhibition of this endocrine axis becomes apparent during its circadian acrophase. Age-associated changes in the adrenocortical sensitivity and expression/secretion of CRH and AVP are also arguable. Although some discordance exists as to the response profiles of the sympatho-adrenomedullary system and brain monoamines in aged animals, the majority of published data suggests exaggerated and, in some cases, protracted increases, with possible aberrations depending on the stressor modality.Citation105 Observations of reduced neophobia and anxiety (but also locomotion and exploration) in aged rodentsCitation106 is a further illustration of the difficulties on the way to an all-embracing view of age-associated control of stress responsiveness.
Translational aspects: models of stress as models of diszase
Assessment of individual aspects of the response to acute stress provides valuable information on the integrity of the major systems of vital importance for adaptation, as well as on the perception of a stimulus as a homeostatic threat. Usually, response deficiency is interpreted as a clue for the search of organic damage in the challenged system or, alternatively, a sign of negligible aversive property/hazard potential of the stressful stimulus. Rather than by its magnitude, the physiological dimension of a response to stress is defined by the organism's ability to terminate it upon cessation of the stimulus or by the implementation of adequate means to control it or avoid repeated exposure. Elimination of the latter prerequisites is readily achieved in stress paradigms employing enduring, variable, and nonpredictable challenges, whose common outcome is persistent activation and, ultimately, insuperable allostatic load. Rheostasis (set-point shifting) may postpone, but not prevent, exhaustion of adaptive capacity, and is probably the best indicator of the transition from norm to pathology. Achievement of persistent shift in set points of signal reading and thresholds of response initiation, and the resulting formation of self-potentiating vicious circuits describes the objectives of the generation of stress-based models of disease. These objectives can be achieved in several paradigms under the conditions of chronic, unpredictable, and uncontrollable exposure, but also by exploiting sex- and age-dependent set-point differences or their pharmacological or genetic modification.
The list of stress-related models that have been successfully used to establish approximate correlates of human disease is long and steadily growing. Evidence for the role of stress as (at the minimum) precipitating factor in depression and has encouraged the extensive transfer of stress paradigms into models of this disease. Posttraumatic stress disorder is another major area for the translational application of experimental stress models. Stress-based paradigms have a firm place in the arsenal of methods for realistic modeling of alcohol and drug addiction, withdrawal, and relapse. Knowledge accumulated in stress research has been implicated in models of eating disorders, aggression, and self-destructive behavior. Increasing understanding of specific stress-related consequences in vital physiological systems has opened new possibilities for the modeling of cardiovascular, gastrointestinal, and, more recently, metabolic conditions. The profound projections of stress to the regulation of the immune responsiveness and reproduction form a solid rationale for the use of stress paradigms in investigations of the pathogenesis of inflammatory/immune disorders and reproductive disturbance.
Conclusions: the perfect model
Under laboratory conditions, stress can be readily emulated through numerous modalities. Nevertheless, stress modeling is associated with considerable problems casting doubts on the quality of results and the validity of conclusions.
Several essential features of allostatic responses, such as variable amplitude, sensitization, and habituation, and complex interactions between their mechanisms preclude the existence of perfect models. Besides adherence to general precautions that guarantee the reproducibility of experimental data (eg, animal strain, sex, age, source, ambient conditions, staff skills, etc), preemptive consideration of certain issues may improve the design and performance of animal models of stress. What is the temporal profile of the selected outcome? Is the stressor capable of eliciting coincident changes in several systems? Are there confounding interactions between simultaneously activated responses? Can effects be obscured by physiological oscillations of the baseline of the selected parameter? Are the responses of interest subject to rapidly evolving habituation or cross-sensitization? What are the physiological limits of the system used for response monitoring? This catalogue can be extended depending on the experimental objective and investigator's concerns.
Research areas with a long and successful history, such as the biology of stress, persuade scientists to rely unreservedly on the validity and reliability of frequently used “hallmark” techniques and experimental models. One of our intentions was to underline that the complexity of the stress response may produce variable outcomes, even in models that have been established for decades. Thus, adherence to the rule Sapiens nihil affirmat quod non probat may prove more useful than recommendations in favor of, or dissuasion from, the use of specific models and end points.
Selected abbreviations and acronyms
| ACTH | = | adrenocorticotropic hormone |
| AVP | = | vasopessin |
| CRH | = | corticotropin-releasing hormone |
| DMH | = | dorsomedial hypothalamic nucleus |
| GABA | = | γ-aminobutyrc acid |
| GR | = | glucocorticoid receptor |
| LHPA | = | limbic-hypothalamic-pituitary-adrenal |
| POMC | = | pro-opiomelanocortin |
| PVN | = | paraventricular nucleus |
Please note that the reference 1st below is an abridged list; a full list of the references used for this article can be obtained by contacting the author: [email protected]
REFERENCES
- ChrousosGP.GoldPW.The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA.1992267124412521538563
- De KloetER.JoelsM.HolsboerF.Stress and the brain: from adaptation to disease. Nat Rev Neurosci.2005646347515891777
- PacakK.PalkovitsM.Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev.20012250254811493581
- McEwenBS.Protective and damaging effects of stress mediators. N Engl J Med.19983381711799428819
- BhatnagarS.DallmanM.Neuroanatomical basis for facilitation of hypothalamic pituitary adrenal responses to a novel stressor after chronic stress. Neuroscience.199884102510399578393
- FileSE.Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res.200112515115711682106
- BlanchardDC.GriebelG.BlanchardRJ.The Mouse Defense Test Battery:pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol.20034639711612600704
- WoodGE.YoungLT.ReaganLP.McEwenBS.Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm Behav.20034320521312614651
- SanchezC.Stress-induced vocalisation in adult animals. A valid model of anxiety? Eur J Pharmacol.200346313314312600706
- HoferMA.Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology.1996212032178774063
- ConradCD.LupienSJ.McEwenBS.Support for a bimodal role for type ll adrenal steroid receptors in spatial memory. Neurobiol Learn Mem.199972394610371714
- SgoifoA.BuwaldaB.RoosM.CostolïT.MeratiG.MeerloP.Effects of sleep deprivation on cardiac autonomic and pituitary-adrenocortical stress reactivity in rats. Psychoneuroendocrinology.20063119720816154708
- AmitZ.GalinaZH.Stress-induced analgesia: adaptive pain suppression. Physiol Rev.198666109111202876446
- OlivierB.ZethofT.PattïjT.et al.Stress-induced hyperthermia and anxiety: pharmacological validation. Eur J Pharmacol.200346311713212600705
- CorwinRL.Buda-LevinA.Behavioral models of binge-type eating. Physiol Behav.20048212313015234600
- PapaleLA.AndersenML.AntunesIB.AlvarengaTA.TufikS.Sleep pattern in rats under different stress modalities. Brain Res.20051060475416226230
- DallmanMF.StrackAM.AkanaSF.et al.Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol.1993143033478258378
- StanfordSC.Central noradrenergic neurones and stress. Pharmacol Ther.1995682973428719972
- ChaouloffF.Serotonin, stress and corticoids. J Psychopharmacol.20001413915110890308
- GraceAA.Dopamine. In: Davis KL, Charney D, Coyle JT, Nemeroff C, eds. Neuropsychopharmacology: the Fifth Generation of Progress. Philadelphia, Pa: Lippincott Williams SWilkins.2002120132
- HorgerBA.RothRH.The role of mesoprefrontal dopamine neurons in stress. Crit Rev Neurobiol.1996103954188978988
- ImperatoA.Puglisi-AIIegraS.CasoliniP.AngelucciL.Changes in brain dopamine and acetylcholine release during and following stress are independent of the pituitary-adrenocortical axis. Brain Res.19915381111172018923
- MoghaddamB.Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry.20025177578712007451
- SwansonCJ.BuresM.JohnsonMP.LindenAM.MonnJA.SchoeppDD.Metabotropïc glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov.2005413114415665858
- TïmmermanW.WesterinkBH.Brain microdialysis of GABA and glutamate: what does it signify? Synapse.1997272422619329159
- HermanJP.MuellerNK.FigueiredoH.Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann N Y Acad Sci.20041018354515240350
- ViverosMP.MarcoEM.FileSE.Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav.20058133134215927244
- DroletG.DumontEC.GosselinI.KinkeadR.LaforestS.TrottierJF.Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry.20012572974111383975
- HoffmanGE.SmithMS.VerbalisJG.c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol.1993141732138349003
- HolmesA.HeiligM.RupnïakNM.StecklerT.GrïebelG.Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol Sci.20032458058814607081
- SawchenkoPE.LiHY.EricssonA.Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res.2000122617810737051
- HeinrichsSC.KoobGF.Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther.200431142744015297468
- AntoniFA.Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol.199314761228387436
- WotjakCT.LudwigM.EbnerK.et al.Vasopressin from hypothalamic magnocellular neurons has opposite actions at the adenohypophysis and in the supraoptic nucleus on ACTH secretion. Eur J Neurosci.20021647748512193191
- VolpïS.Rabadan-DïehlC.AguileraG.Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation. Stress.20047758315512850
- NeumannID.Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res.200213914716212436933
- AguileraG.KissA.LuoX.AkbasakBS.The renin angiotensin system and the stress response. Ann N Y Acad Sci.19957711731868597396
- LevineJE.Bauer-DantoinAC.BeseckeLM.et al.Neuroendocrine regulation of the luteinizing hormone-releasing hormone pulse generator in the rat. Recent Prog Horm Res.199147971511745827
- DobsonH.GhumanS.PrabhakarS.SmithR.A conceptual model of the influence of stress on female reproduction. Reproduction.200312515116312578529
- ReichlinS.Prolactin and growth hormone secretion in stress. Adv Exp Med Biol.19882453533763067562
- JoskoJ.Liberation of thyrotropin, thyroxine and triiodothyronine in the controllable and uncontrollable stress and after administration of naloxone in rats. J Physiol Pharmacol.1996473033108807557
- ElenkovIJ.WilderRL.ChrousosGP.ViziES.The sympathetic nervean integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev.20005259563811121511
- SapolskyRM.RomeroLM.MunckAU.How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev.200021558910696570
- DallmanMF.AkanaSF.BhatnagarS.et al.Starvation: early signals, sensors, and sequelae. Endocrinology.19991404015402310465271
- AguileraG.LightmanSL.KissA.Regulation of the hypothalamic-pituitary-adrenal axis during water deprivation. Endocrinology.19931322412488380375
- AndersenML.MartinsPJ.D'AlmeidaV.BignottoM.TufikS.Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res.200514839015743338
- GlavinGB.PareWP.SandbakT.BakkeHK.MurisonR.Restraint stress in biomedical research: an update. Neurosci Biobehav Rev.1994182232498058215
- FukuharaK.KvetnanskyR.CizzaG.et al.Interrelations between sympathoadrenal system and hypothalamo-pituitary-adrenocortical/thyroid systems in rats exposed to cold stress. J Neuroendocrinol.199685335418843022
- CureM.Plasma corticosterone response in continuous versus discontinuous chronic heat exposure in rat. Physiol Behav198945111711222554346
- DarlingtonDN.BarracloughCA.GannDS.Hypotensive hemorrhage elevates corticotropin-releasing hormone messenger ribonucleic acid (mRNA) but not vasopressin mRNA in the rat hypothalamus. Endocrinology.1992130128112881311234
- RomeroLM.PlotskyPM.SapolskyRM.Patterns of adrenocorticotropin secretagog release with hypoglycemia, novelty, and restraint after colchicine blockade of axonal transport. Endocrinology.19931321992047678213
- WotjakCT.KubotaM.LïebschG.et al.Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: a novel mechanism of regulating adrenocorticotropic hormone secretion? J Neurosci.199616772577328922428
- CampeauS.WatsonSJ.Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol.199795775889283046
- Vernikos-DanellisJ.WingetCM.HetheringtonNW.Diurnal rhythm of the pituitary-adrenocortical response to stress: effect of constant light and constant darkness. Life Sci Space Res.1970824024611826885
- AdamecRE.BlundellJ.BurtonP.Neural circuit changes mediating lasting brain and behavioral response to predator stress. Neurosci Biobehav Rev.2005291225124116099042
- Blackburn-MunroG.Blackburn-MunroRE.Chronic pain, chronic stress and depression: coincidence or consequence? J Neuroendocrinol.2001131009102311722697
- FigueiredoHF.BodieBL.TauchiM.DolgasCM.HermanJP.Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitaryadrenocortical axis. Endocrinology.20031445249525812960031
- DallmanMF.AkanaSF.BellME.et al.Warning! Nearby construction can profoundly affect your experiments. Endocrine.19991111111310709756
- FileSE.LippaAS.BeerB.LippaMT.Animal tests of anxiety. In: Crawley JN, Gerfen CR, Rogawski MA, Sibley DR, Skolnïck P, Wray S, eds. Current Protocols in Neuroscience. Vol. 2. New York, NY: John Wiley & Sons.19978.3.1.
- CampeauS.FallsWA.CullinanWE.HelmreichDL.DavisM.WatsonSJ.Elicitation and reduction of fear: behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience.199778108711049174076
- BlanchardRJ.McKïttrïckCR.BlanchardDC.Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav.20017326127111438351
- HallerJ.FuchsE.HalaszJ.MakaraGB.Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res Bull.199950333910507469
- WeissIC.PryceCR.Jongen-ReloAL.Nanz-BahrNI.FeldonJ.Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res.200415227929515196796
- KuhnCM.SchanbergSM.Responses to maternal separation: mechanisms and mediators. Int J Dev Neurosci.1998162612709785122
- SchmidtM.EnthovenL.van WoezikJH.LevineS.de KloetER.OitzlMS.The dynamics of the hypothalamic-pituitary-adrenal axis during maternal deprivation. J Neuroendocrinol.200416525714962076
- Van de KarLD.BlairML.Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol.1999201489882535
- JungME.LaiH.GatchMB.The discriminative stimulus effects of pentylenetetrazol as a model of anxiety: recent developments. Neurosci Biobehav Rev.20022642943912204190
- PechnickRN.Effects of opioids on the hypothalamo-pituitary-adrenal axis. Annu Rev Pharmacol Toxicol.1993333533828494344
- GoedersNE.A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology.1997222372599226728
- SwerdlowNR.KoobGF.CadorM.LorangM.HaugerRL.Pituitaryadrenal axis responses to acute amphetamine in the rat. Pharmacol Biochem Behav.1993456296378392732
- RivierC.Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res.1996202402548730214
- De SouzaEB.GrigoriadisDE.Corticotropin-releasing factor: physiology, pharmacology and role in central nervous system disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C, eds. Neuropsychopharmacology: the Fifth Generation of Progress. Philadelphia, Lippincott Williams & Wilkins.2002 91107
- LandgrafR.WotjakCT.NeumannID.EngelmannM.Release of vasopressin within the brain contributes to neuroendocrine and behavioral regulation. Prog Brain Res.199811920122010074790
- CarrascoGA.Van de KarLD.Neuroendocrine pharmacology of stress. Eur J Pharmacol.200346323527212600714
- KonakchievaR.MitevY.AlmeidaOF.PatchevVK.Chronic melatonin treatment counteracts glucocorticoid-induced dysregulation of the hypothalamicpituitary-adrenal axis in the rat. Neuroendocrinology.1998671711809630434
- DantzerR.Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun.20011572411259077
- DunnAJ.WangJ.AndoT.Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv Exp Med Biol.199946111712710442171
- TurnbullAV.RivierC.Regulation of the HPA axis by cytokines. Brain Behav Immun.199592532758903845
- RivestS.RivierC.The role of corticotropin-releasing factor and interleukin-1 in the regulation of neurons controlling reproductive functions. EndocrRev.199516177199
- KamilarisTC.DeBoldCR.JohnsonEO.et al.Effects of short and long duration hypothyroidism and hyperthyroidism on the plasma adrenocorticotropin and corticosterone responses to ovine corticotropin-releasing hormone in rats. Endocrinology.1991128256725761850357
- HennFA.VollmayrB.Stress models of depression: forming genetically vulnerable strains. Neurosci Biobehav Rev.20052979980415925700
- LandgrafR.WiggerA.Born to be anxious: neuroendocrine and genetic correlates of trait anxiety in HAB rats. Stress.2003611111912775330
- KanedaN.SasaokaT.KobayashiK.et al.Tissue-specific and high-level expression of the human tyrosine hydroxylase gene in transgenic mice. Neuron.199165835941673055
- SchrammNL.McDonaldMP.LïmbïrdLE.The alpha(2a)-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J Neurosci.20012148754882
- ShinJC.ChenK.RiddMJ.Monoamine oxidase: from genes to behavior. Annu Rev Neurosci.19992219721710202537
- GogosJA.MorganM.LuineV.et al.Catechol-O-methyltransferase-defïcient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A.199895999199969707588
- BakshiVP.KalinNH.Corticotropin-releasing hormone and animal models of anxiety: gene-environment interactions. Biol Psychiatry.2000481175119811137059
- KarolyiIJ.BurrowsHL.RameshTM.et al.Altered anxiety and weight gain in corticotropin-releasing hormone-binding protein-deficient mice. Proc Natl Acad Sci U S A.199996115951160010500222
- TroncheF.KellendonkC.KretzO.et al.Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet.1999239910310471508
- BoyleMP.BrewerJA.FunatsuM.et al.Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A.200510247347815623560
- WeiQ.LuXY.LiuL.et al.Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci USA.2004101118511185615280545
- AkilH.Stressed and depressed. Nat Med.20051111611815692589
- CurtisAL.BetheaT.ValentinoRJ.Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology.20063154455416123744
- PatchevVK.AlmeidaOF.Gender specificity in the neural regulation of the response to stress: new leads from classical paradigms. Mol Neurobiol.19981663779554702
- PatchevVK.HayashïS.OrikasaC.AlmeidaOFX.Ontogeny of genderspecific responsiveness to stress and glucocorticoids in the rat and its determination by the neonatal gonadal steroid environment. Stress.19993415419016192
- SealeJV.WoodSA.AtkinsonHC.HarbuzMS.LightmanSL.Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocrinol.20041698999815667454
- KajantieE.PhillipsDl.The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology.20063115117816139959
- RocaCA.SchmidtPJ.DeusterPA.et al.Sex-related differences in stimulated hypothalamic-pituitary-adrenal axis during induced gonadal suppression. J Clin Endocrinol Metab.20059042244231 15886244
- NeumannID.Alterations in behavioral and neuroendocrine stress coping strategies in pregnant, parturient and lactating rats. Prog Brain Res.200113314315211589127
- VazquezDM.Stress and the developing limbic-hypothalamic-pituitaryadrenal axis. Psychoneuroendocrinology.1998236637009854741
- WalkerCD.SapolskyRM.MeaneyMJ.ValeWW.RivierCL.Increased pituitary sensitivity to glucocorticoid feedback during the stress nonresponsive period in the neonatal rat. Endocrinology.1986119181618213019645
- SmithMA.KimSY.van OersHJ.LevineS.Maternal deprivation and stress induce immediate early genes in the infant rat brain. Endocrinology.1997138462246289348187
- HermanJP.LarsonBR.SpeertDB.SeasholtzAF.Hypothalamo-pituitaryadrenocortical dysregulation in aging F344/Brown-Norway F1 hybrid rats. Neurobiol Aging.20012232333211182482
- CizzaG.CalogeroAE.BradyLS.et al.Male Fischer 344/N rats show a progressive central impairment of the hypothalamic-pituitary-adrenal axis with advancing age. Endocrinology.1994134161116208137722
- MabryTR.GoldPE.McCartyR.Age-related changes in plasma catecholamine responses to chronic intermittent stress. Physiol Behav.19955849567667427
- orras-GarcïaM.Costa-MiserachsD.Coll-AndreuM.Portell-CortesI.Decreased anxiety levels related to aging. Exp Brain Res.200516417718415856210