Abstract
There is increasing knowledge regarding the considerable comorbidity between depression and cardiovascular disease, which are two of the most common disorders in developed countries. The associated vulnerability is not unidirectional, as the presence of cardiovascular disease can also influence mood states. Although this may be the result of psychological factors, common biological mechanisms, including genetic ones, are thought to be responsible for this interaction; we can thus question whether variations in genes could be predisposing factors. Regarding the multiple interactions in the mechanisms between depression and cardiovascular system disorders, eg, dysfunctions in the hypo-thalamic-pituitary-adrenocortical and sympathoadrenal axis and the response to stress, the importance of the sero-tonergic and immune systems, or the impact on the renin-angiotensin system, several candidate genes are being investigated. However, despite the interest in unraveling the potential susceptibility genes for both disorders, most available studies have so far dealt with the impact of polymorphisms in relation to either depression or cardiovascular disease. A few recent studies have now examined the effects of gene-gene or gene-environment interactions, and are investigating the impact of "depression-related" variants on cardiac response to stress. The first promising results were obtained with the serotonin transporter, and it may be hypothesized that this polymorphism interacts via the impact of the S allele on depression and via the effect of the L allele on platelet activation. However, the role played by various other candidate genes remains to be determined, especially regarding the question as to whether they are indicative of common pathophysiological mechanisms, or for identifying a subgroup of patients with somatic disorders that are more closely related to psychiatric symptoms.
Existe un creciente conocimiento relacionado con la considerable comorbilidad entre la depresión y la enfermedad cardiovascular, dos de las enfermedades más comunes en los países desarrollados. La vulnerabilidad asociada no es unidireccional, ya que la presencia de enfermedad cardiovascular también puede afectar el estado de ánimo. Aunque esto puede ser el resultado de factores psicológicos, se piensa que los mecanismos biológicos comunes, incluyendo los de tipo genético, serían responsables de esta interacción y es posible preguntarse si las variaciones en los genes podrían ser factores predisponentes. En relación con las interacciones múltiples en los mecanismos entre la depresión y los trastornos del sistema cardiovascular (como disfunciones en los ejes hipo-tálamo-hipófisis-adrenocortical y simpático-adrenal y la respuesta de estrés, la importancia de la serotonina y el sistema inmune, o el impacto del sistema renina angiotensina) se están estudiando diversos genes candidatos. Sin embargo, a pesar del interés por identificar ciertos potenciales genes de susceptibilidad para ambos trastornos, la mayoría de los estudios disponibles a la fecha han abordado el impacto del polimorfismo en relación con la depresión o con la enfermedad cardiovascular. Unos pocos estudios recientes han examinado los efectos de las interacciones genes-genes o genes-ambiente y están investigando el impacto de las variantes "relacionadas con la depresión" en la respuesta cardíaca al estrés. Los primeros resultados promisorios se obtuvieron con el transportador de serotonina y se puede hipotetizar que este polimorfismo interactúa mediante el impacto del alelo S en la depresión y por el efecto del alelo L en la activación plaquetaria. Sin embargo, el papel que puedan tener otros genes candidatos aun debe ser determinado, especialmente en relación con la pregunta de si acaso ellos representan mecanismos fisiopatológicos comunes y también si permiten identificar un subgrupo de pacientes con trastornos somáticos que tienen mayor relación con síntomas psiquiátricos.
L'importante comorbidité entre la dépression et les maladies cardiovasculaires, deux des maladies les plus répandues dans les pays développés, est de mieux en mieux connue. La présence d'une maladie cardiovasculaire peut également influer sur l'humeur, la vulnérabilité associée n'est donc pas unidirectionnelle. Cette interaction pourrait être due à des mécanismes psychologiques, mais des mécanismes biologiques pourraient constituer des facteurs prédisposants, génétiques par exemple. Plusieurs gènes candidats sont en cours d'analyse se basant sur les nombreuses interactions entre les mécanismes sous-tendant la dépression et les maladies du système cardiovasculaire. Sont ainsi pris en considération les dysfonctionnements des axes hypothalamo-hypophyso-cortico-surrénalien et sympatho-adrénergique, la réponse au stress, l'importance des systèmes sérotoninergique et immun et l'impact du système rénine-angiotensine. La plupart des études disponibles ont traité jusqu'ici l'impact des polymorphismes liés soit à la dépression soit à la maladie cardiovasculaire, malgré l'intérêt de démêler les gènes de susceptibilité potentiels pour les deux maladies. De rares études récentes se sont récemment intéressées aux effets des interactions gène-gène ou gène-environnement, et sont en cours de recherche sur la réponse cardiaque au stress liée à la dépression. Les premiers résultats prometteurs ont été obtenus avec le transporteur de la sérotonine et on peut se demander si ce polymorphisme interagit par l'intermédiaire de l'allèle S sur la dépression et de l'allèle L sur l'activation plaquettaire. Cependant il reste à déterminer le rôle des autres gènes candidats surtout en ce qui concerne leur fonction, soit d'indicateurs de mécanismes physiopathologiques communs, ou d'identifiants pour des sous-groupes de patients souffrant de troubles somatiques plus en rapport avec des symptômes psychiatriques.
A large body of evidence has emerged to suggest an extensive comorbidity between depression and cardiovascular disease (CVD). Cross-sectional and prospective analyses have shown that depression may increase mortality and morbidity in patients with heart failure, regardless of its etiology, and independently of traditional cardiovascular factors.Citation1 Although this may be the result of psychological factors such as contemplation of one's mortality and changes in lifestyle and social relationships, there is now compelling evidence that a reciprocal relationship between both disorders exists. The presence of cardiovascular disease can influence mood states,Citation2 and some of the factors associated with depression, especially the multiple alterations associated with acute and chronic stress, may give rise to vascular disorders such as atherosclerosis, microcirculatory endothelial dysfunction, or metabolic conditions such as diabetes and dyslipidemia.Citation3
Concerning the importance of biologic vulnerability factors and environment, it was proposed that a substantial proportion of comorbidities may be attributed to a few underlying liability factors that are applicable to both cardiovascular and depressive disorders.Citation4 Thus, even if both conditions did not affect each other, they might still cosegregate if they shared common underlying factors, including genetic ones. As both disorders are complex and multifactorial in origin, involving multiple genes with interactive or additive effects together with environmental factors, depression and cardiovascular disease could be different manifestations of the same genetic substrate.
Interacting pathophysiological mechanisms
Although the interacting mechanisms have not been fully elucidated, there is much evidence for this interaction. Both disorders have been shown to run in families, and twin studies have provided evidence that this familial aggregation is based on an increased genetic vulnerability.Citation5,Citation6 Interestingly, only one study investigated the association between depressive symptoms, hypertension, and heart disease in male mono- and dizygotic twin pairs to analyze the genetic and/or environmental effects. Thus, Scherrer et alCitation7 found that heart disease and hypertension were significantly associated with up to five symptoms of depression, and concluded that the lifetime co-occurrence of heart disease and depression could best be explained by a substantial genetic contribution, but not by a contribution from the shared environment. The data from this study strongly suggest common genetic influences across depression and CVD.
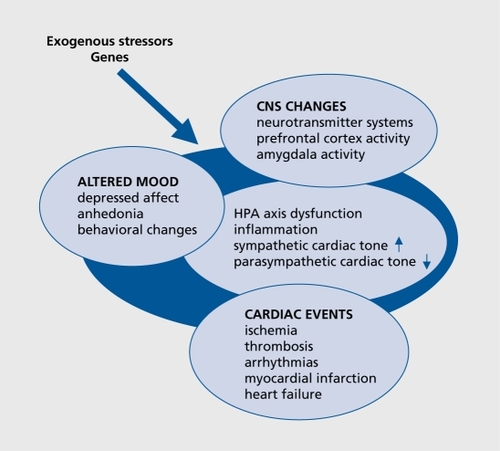
Potential candidate genes may be identified on the basis of the various direct and indirect mechanisms which have been proposed as possible substrates for the interaction between depression and CVD ( ). Among them are hyperactivity of the noradrenergic and hypothalamic-pituitary-adrenal (HPA) systems,Citation8 reduced heart rate variability, myocardial ischemia and ventricular instability in response to psychological stress, and depression-related exaggerated platelet activity, as well as enhanced inflammatory-mediated atherogenesis.Citation8,Citation9 The relationship between the immune, autonomic, neuroendocrine, and neurotransmitter processes is multidirectional; psychological factors act on the neuroendocrine, neurotransmitter, and immune systems and vice versa, thereby influencing vulnerability to affective and cardiovascular disorders. Further impact may be exerted by risk factors common to both disorders, such as smoking, reduced exercise, metabolic disturbances, or antidepressant cardiotoxicity. Citation10 As all these pathways, and even the reaction to environmental stimuli, are genetically regulated, it is plausible that a common genetic vulnerability is likely to account for the observed association between depression and CVD.Citation11 Although genes contributing to the risk for both disorders have so far not been investigated simultaneously as common comorbid factors, this review focuses on the main pathways as an illustration.
Genes of the serotonergic pathway
The neurotransmitter serotonin (5-HT) is essential for a large number of psychological and physiological processes, including the regulation of appetite, mood, anxiety, cognition, and wakefulness, as well as vascular smooth muscle contraction or modulation of platelet aggregation.Citation12 In depression research, a number of alterations have been reported of 5-HT uptake and transporter and receptor binding sites in the brain and periphery,Citation13,Citation14 as well as altered platelet activation.Citation15 Moreover, reduced serotonergic function within the central nervous system (CNS) has been associated with alterations in the HPA response to stress, thus predicting higher rates of coronary heart disease (CHD) and increased mortalityCitation16 With respect to the key position of 5-HT in physiological and psychological processes, it is not surprising that genes coding for the serotonergic pathway have repeatedly been investigated for several years. These include not only a possible association with psychiatric states such as anxiety, hostility, depression, or smoking behavior, but also several characteristics of platelet function or the effect of 5-HT on the vessel wall and induction of atherosclerosis.
The 5-HT transporter
The 5-HT transporter (5-HTT) clears the synaptic cleft of neurotransmitters and thus limits the duration of 5-HT function. As 5-HTT is not only expressed on neuronal tissues but also on blood platelets, where it is crucial in maintaining the homeostasis of 5-HT,Citation17 this gene has become an important candidate for both disorders. Further, although the expression of 5-HTT is predominantly under genetic control, nongenetic factors, including psychoactive drugs, stress, alcohol, and dietary factors, also regulate its expression.Citation18
One polymorphic site in particular within the 5-HTT gene, located in the promoter region with a deletion/insertion variation of 44 bp, creating short (S) and long (L) alleles, the 5-HTT-linked promoter region (5-HTTLPR), has been the subject of most investigations.Citation19 The presence of the S allele of the HTTLPR was associated with decreased transcriptional activity and decreased 5-HT uptake, which, in turn, results in longer duration of the serotonergic activityCitation20
The 5-HTTLPR has been multiply investigated in psychiatric disorders. Although the results are not conclusive, and differential data were obtained concerning the association of the HTTLPR with depression or suicidality, there is now no doubt that this polymorphism is involved in complex phenomena, such as cognition, temperament, character, and the regulation of emotion.Citation21-Citation23 Following the first observation that individuals carrying at least one S allele of the 5-HTTLPR displayed higher levels of trait anxiety, ”neuroticism,“ ”harm avoidance,“ and depressive symptoms than those with homozygosity for the long allele (L/L),Citation21 many studies followed to con-firm this observation, and recent meta-analyses have demonstrated a significant association between the S allele and increased neuroticism or harm avoidance (for review see refs 24, 25).
The impact of the 5-HTTLPR in CVD has been investigated for several years. In concordance with the increased transcriptional activity of the L allele, it was shown that LL homozygous subjects have higher blood 5-HT concentrations than those with the S allele, presumably due to increased 5-HT uptake and storage.Citation26 These findings are in agreement with the observation in geriatric depressed patients that homozygosity for the long allele was associated with platelet activation, and increased platelet factor 4 and thromboglobulin levels.Citation27 Thus, platelets of persons with the L/L genotype are more efficient in uptake and storage of 5-HT in their dense granules, followed by increased 5-HT release upon activation, which may consequently lead to greater thrombus formation. This hypothesis was underlined in a multicenter study in more than 600 patients with coronary artery disease, as carriers of the LL genotype had a higher risk for myocardial infarction, with an odds ratio of 1.4 (95% CI1.11 to 1.76).Citation28
5-HT not only has effects on platelets, but also has a direct proliferative action on smooth muscle cells. Increased uptake and storage of 5-HT has repeatedly been discussed as a pathophysiological mechanism of pulmonary hypertension, and indeed, an increased incidence of LL homozygotes among patients with pulmonary hypertension was observed; in those patients the pulmonary artery smooth muscle cells showed increased 5-HT uptake and increased proliferation in response to 5-HTCitation29 This is a clinical relationship between a genetic abnormality and a cellular process critical for the development of pulmonary vascular disease.
Interestingly, the S allele was also found to increase the risk for cardiac events via its impact on emotion. In a prospective study investigating patients after acute myocardial infarction, Nakatani and colleagues were able to show that the S allele conferred increased risk for subsequent cardiac events, being mediated by the development of depressive symptoms which were significantly higher among S allele carriers.Citation30 A significant association between the 5-HTTLPR S allele and the incidence of poststroke major depression underlines the importance of the reciprocal relationship on a genetic basis.Citation31 Altogether, these findings might lead to the speculation that the HTTLPR contributes to the risk for CVD with both the S- and L allele, with the L allele working via platelet activation and the S allele contributing via the increased susceptibility for depression.
The 5-HTTLPR and response to stress
The possible impact of the 5-HTTLPR polymorphism on the effects of central 5-HT on cardiovascular reactivity in response to mental stress was investigated in healthy volunteers. Subjects with one or two L alleles had higher cerebrospinal fluid levels of the 5-HT metabolite 5-hydroxyindole-acetic acid (5-HIAA) than those with the S/S genotype, and exhibited increased blood pressure and increased heart rate responses to a mental stress.Citation16
Comparable results were obtained in a further study investigating the cardiovascular response during a psychological challenge in relation to the 5-HTTLPR genotypes. Young healthy male L allele carriers showed increased heart rate reactivity in response to stress, an association that could not be shown in female L allele carriers. This finding could thus at least partly explain the sex differences in heart rate response.Citation32
The link between depression and CVD is strengthened by the recent evidence for a gene-environment interaction. Investigating a large representative cohort in a prospective longitudinal study, Caspi and colleaguesCitation33 were able to show that individuals with one or two copies of the S allele exhibited more depressive symptoms, more diagnosable depression, and more suicidality in relation to stressful life events than individuals homozygous for the L allele. This finding suggests that genetic variants may act to promote resistance to environmental influences. In addition, the study by Grabe et alCitation34 demonstrated this gene-environment interaction in relation to the 5-HTTLPR genotypes in a cohort with severe mental (eg, unemployment, disrupted social network) and physical (eg, myocardial infarction, stroke, diabetes, and degenerative diseases) distress. They found significant interactions between the 5-HTTLPR S allele and unemployment or chronic disease, but only in females. This finding not only confirms previous findings for a significant gene-environment interaction of the S allele, but it also indicates a higher mental vulnerability to social stressors and chronic disease.
The 5-HTTLPR and risk factors for cardiovascular disease
Smoking is one of the unquestioned risk factors for CVD, and dependence on tobacco, like many other drug dependencies, is a complex behavior with both genetic and environmental factors contributing to its variance.Citation35 As there is increasing evidence that chronic nicotine abuse decreases 5-HT levels in the brainCitation36 and selective serotonin reuptake inhibitors (SSRIs), combined with a 5-HT1 receptor antagonist, may reverse the reward deficits during withdrawal in rats,Citation37 the 5-HTT gene has attracted attention. Furthermore, it is well known that smoking behavior is common among depressed patients.
For the Japanese population a relationship between the L allele, myocardial infarction, and smoking was suggested, as the LL and LS genotypes were more frequently observed in male CVD patients, and smoking had a synergistic effect.Citation38 In contrast, in the American population Lerman found no significant difference in the distribution of 5-HTT genotypes among smokers and nonsmokers, but revealed an interaction between the SS genotype and neuroticism in nicotine addiction.Citation39 Recently, in the Caucasian population no association between the 5-HTTLPR genotypes and smoking behavior was found.Citation40 These discrepant findings suggest that nicotine addiction may be influenced by a combination of the 5-HTT gene and anxiety-related personality traits, rather than by each factor alone.Citation35 Furthermore, alcoholism, a known risk factor for hypertension and cerebral hemorrhagic infarction and a common comorbid condition with depression, has been associated with the SS genotype in an American population.Citation41
Integration of the findings with the 5-HTTLPR
As so far no association studies have been carried out with both CVD and depression, it is hard to assess the validity of the separate findings as common genetic risk factors. Nevertheless, the convincing data for the 5HTTLPR as a susceptibility locus for depression and cardiovascular events might be judged as a common mechanism, and could therefore be of theoretical interest, suggesting an impact of the 5-HT transporter. The fact that different alleles of this polymorphism were associated with the different disorders, the S allele with depression and anxiety personality traits and the L allele with vascular events and atherosclerosis, seems contradictory, but might be explained by the complex nature of both disorders. Complex disorders are multifactorial in origin, involving the action of several genes of minor effect together with environmental factors. Thus, an interaction of several genes in particular, each contributing to the risk for one disorder, could increase the liability for both disorders. One example of this might be the observation that the S allele could also increase the risk for cardiac events via its impact on emotion,Citation30 thus inducing a cascade of subsequent stress reactions that themselves have negative input on the vasculature and cardiac function.
Other serotonergic candidates
In contrast to the data with 5-HTT, those for the serotonergic receptor gene polymorphisms are less abundant. The 5-HT2A receptor T102C polymorphism, which was the subject of many pharmacogenetic investigations, was not found to be a susceptibility locus for unipolar or bipolar depression,Citation42,Citation43 and its importance for CVD is completely unresolved.Citation11 Nevertheless, on the basis that depression appears to be associated with an increased susceptibility for 5-HT-mediated platelet activation, mediated via the 5-HT2A receptors, this receptor might contribute to an increased risk of thromboembolic events in patients with depression and CVD.Citation44 Thus, this gene could be seen as important candidate for future investigations.
Concerning a structural variant of the 5-HT2C receptor gene, which gives rise to a cysteine to serine substitution in the N terminal extracellular domain of the receptor protein (Cys23Ser), a significant excess of the 23Ser allele carriers was found among a large European cohort of depressive patients.Citation45 This finding is interesting with respect to the development of diabetes and obesity, since two polymorphisms within the promoter region of the 5-HT2C receptor gene have recently been associated with obesity and/or type 2 diabetes.Citation46
Immune activation in depression and cardiovascular disease
It is now established that a relationship exists between depression and inflammation,Citation47 which might be seen as a process involved in the development or progression of a number of comorbid diseases. A variety of immunologic processes are altered in depression, including those of cellular components and soluble mediators, such as acute-phase proteins and cytokines (eg, interleukin [IL]-1, IL-6, and tumor necrosis factor [TNF]-α).Citation48,Citation49 The most consistent finding in depression, an increase in IL-6 during the acute state, is remarkable, as IL-6 is one of the main stimulators of C-reactive protein (CRP), which was also found to be increased during depression.Citation50
Cytokines induce neuroendocrine and central neurotransmitter alterations that are reminiscent of those seen in depression, and these alterations are exacerbated by stressors.Citation47,Citation49 Both IL-1 and IL-6 stimulate corticotropin-releasing hormone (CRH) secretion, resulting in increased adrenocorticotropic hormone (ACTH) and glucocorticoid release. On the other hand, proinflammatory cytokines, such as IL-1, IL-6, or TNF-α, have profound effects on peripheral and brain serotonergic systems, as they increase extracellular 5-HT concentration within different brain regions or modulate the activity of the 5-HTTCitation51
Inflammation is considered to be involved in most stages of atherosclerosis, from initial recruitment of leukocytes to the formation of atherosclerotic plaques.Citation52 Proatherosclerotic factors acting on the endothelium result in chronic inflammation, with a consequent rise of C-reactive protein, adhesion molecules, and the proinflammatory cytokines TNF-α and IL-6.Citation53,Citation54
With respect to the fact that the expression and function of cytokines is highly genetically influenced, several studies have been performed to investigate the influence of genes coding for cytokines on CVD and diabetes or obesity. The most extensively studied polymorphism of the IL-6 gene is a G/C conversion in the promoter region (-174G/C), which alters the cyclic AMP (cAMP) binding site and affects the transcription and plasma level of IL-6.Citation54 Though some negative results were also reported in patients with coronary artery disease and previous myocardial infarction, there is now a consensus that, at least in man, the -174C allele is associated with increased plasma CRP and IL-6 concentrations,Citation55 with higher systolic blood pressure,Citation56 especially above a body mass index of 25, and with an increased risk of coronary heart disease.Citation57 Smoking appears to have an additive effect on these associations, as both an increase in blood pressure and a detrimental effect on endothelial function was observed in -174CC carriers.Citation58 Obesity (an expansion of the adipose tissue closely related to insulin resistance) was also shown to be related to the IL-6 -174C allele, as the GG genotype is more common among lean male subjects with low concentrations of either insulin or glucose.Citation59 A relationship was shown between alcohol consumption and clinical cardiovascular events and carotid atherosclerosis, as in subjects with a daily alcohol consumption of >30 g and with the CC genotype, there were significantly higher plasma IL-6 levels and carotid artery intima-media thickness.Citation60
The data with TNF-α gene polymorphisms are less abundant. TNF-α is involved in the inflammation process of atherosclerosis and lipid metabolism. A common polymorphism in the promoter region (-308 G/A) regulates the TNF-α production and was shown to be a candidate gene for the development of both obesity and insulin resistance.Citation61 In an Australian population, it was shown that subjects who were homozygous for the -308 A allele had higher fasting insulin levels, higher systolic blood pressure, and lower high-density-lipoprotein (HDL) levels than subjects homozygous for the -308G allele. Thus, this variant conveys an increased risk for the development of insulin resistance in obese subjects, and low HDL levels further increase the risks associated with insulin resistance in A allele carriers.Citation62
Unfortunately, to date only a few inflammation genes related to CVD have also been examined in depression. For the IL-1BC to T substitution at position -511 (C-511T), there is literature suggesting that the -511C allele is more prevalent among patients with dysthymia,Citation63 and might be associated with the severity of depressive symptoms,Citation64 even in patients with Alzheimer's disease.Citation65 For the TNF-α gene polymorphism, the -308A allele, which was associated with enhanced inflammatory response after cardiac surgeryCitation66 was proposed to influence depressive symptoms.Citation67 The genes of other inflammatory markers have not yet been examined in depression.
Angiotensin-converting enzyme gene
Angiotensin-converting-enzyme (ACE) is a membrane-bound endopeptidase which is involved in the metabolism of many small peptides, such as the generation of angiotensin II or bradykinin, both being important for the regulation of vascular tone and cardiac functions. In addition, angiotensin II has proinflammatory actions in the vascular wall, inducing the production of IL-6 and adhesion molecules, and thus augmenting vascular inflammation, inducing endothelial dysfunction and enhancing the atherogenesis process.Citation68 However, the effects of this enzyme are not restricted to the vasculature, as the renin-angiotensin system (RAS) exists within the CNS, with multiple effects on different systems.Citation69
The most extensively investigated polymorphism of ACE is the insertion (I) or deletion (D) of a 287 bp Alu repeat sequence within intron 16 of the gene, and the D allele is associated with increased levels of circulating ACE.Citation70
There has been considerable interest in the potential associations of this polymorphism with CVD, including myocardial infarction, hypertension, and left ventricular hypertrophy, but the associations were not consistent for all disorders. A review of the literature revealed a moderate degree of increased risk for myocardial infarction associated with the ACE DD genotype in most populations, especially in the Japanese.Citation71
There was also one study suggesting that the ACE genotypes confer susceptibility to depression, with an over-representation of the DD genotype in Japanese depressed patients,Citation72 a finding which could not be replicated in an European population.Citation73 However, we have observed that patients with the I/I genotype responded more rapidly to antidepressant treatment.Citation73,Citation74 Furthermore we were able to demonstrate the interaction with the HPA axis, as patients with the DD genotype had higher basal and stimulated Cortisol levels in the combined dexamethasone-CRH suppression test.Citation75 Recently the impact of the ACE DD genotype on myocardial infarction was re-evaluated using the paradigm of gene-gene interaction. In patients with or with-out coronary artery disease, the ACE insertion/deletion polymorphism was investigated, together with a functional polymorphism in the G-protein β-3 subunit (C825T), which had earlier been associated with hyper-tension and obesity.Citation76 This analysis revealed the highest odds ratio (OR=7.5) in patients also with the combined homozygous Gβ3 825TT and ACE DD genotypes, suggesting a significant interaction of the Gp3 825T and the ACE D alleles as possible contributing factors for myocardial infarction.Citation76 Based on our own previous results concerning an association of the Gβ3 825T allele with affective disordersCitation77 and a possible influence of the ID polymorphism of the ACE gene polymorphism on therapeutic outcome in affective disorders,Citation73 we studied the interaction of both genes in 201 patients with unipolar major depression and 161 ethnically matched controls. Interestingly, in depressed patients we also observed a combined action of ACE and GB3 genotypes, as ACE-ID and DD/Gβ3-TT carriers were more than four times more frequent among the patient group compared with controls.Citation78 As our study was carried out in depressed patients with no serious CVD, we are presently unable to predict whether this combined action of the ACE ID/DD-Gβ3 TT genotype increases the risk for both disorders. Nevertheless, our study reports for the first time that the same allelic combination of two genes that have been shown to increase the risk for myocardial infarctionCitation79 also increase the vulnerability for depression, and this could be a link to explain the comorbidity of depression with CVD.
The findings with the ACE gene were further extended in our recent study, where we were able to associate one single-nucleotide polymorphism (SNP) in the promoter region of the ACE gene (rs4291) with an A/T transition in two independent large case-control samples of patients suffering from major depression. We have further observed that the T allele of this variant was not only associated with depression, but also with the serum ACE concentration and with hypercortisolism during the acute state of the disorder.Citation80 This finding suggests that the SNP rs4291 might therefore represent a common pathophysiological link for depression and CVD. This hypothesis is supported by another observation from our group, as we found that in a proportion of patients the initially increased serum CRP levels did not decrease during effective antidepressant treatment, and these constantly increased CRP levels can be associated to both, the ACE D allele and the RS4291T allele.Citation81
Summary and conclusions
Despite the interesting and promising genetic findings on depression and cardiovascular disorders, and despite the considerable overlap in the pathophysiological mechanisms of these disorders, up to now few studies have been carried out to investigate a possible combined genetic mechanism. Considering the fact that both disorders are complex traits resulting from multiple genotypes, and from gene-gene as well as gene-environment interactions, the identification of a liability gene for either disorder is difficult. Some multifactorial disorders, rather than resulting from variations in many genes of small effect, may result from variations in fewer genes whose effects are conditional on exposure to environmental risks.Citation33 In recent years, many studies have investigated polymorphisms in candidate genes in relation to functional characteristics of central or peripheral mechanisms which are involved in the development of CVD. The fact that many of these genes are also discussed as liability genes for depression raises the intriguing question whether an interactive or synergistic effect is responsible for the bidirectional relationship.
Prospective studies in a reasonable number of patients, including intensive clinical characterization, the investigation of biological markers in both patient groups, and genotyping for relevant polymorphisms in genes which are assumed to be involved in both disorders will have to be carried out to shed more light on the interaction between depression and CVD.
Selected abbreviations and acronyms
| 5-HT | = | serotonin |
| 5-HTT | = | serotonin transporter |
| 5-HTTLPR | = | serotonin transporter promoter region |
| ACE | = | angiotensin-converting enzyme |
| HPA | = | hypothalamic-pituitary-adrenal |
| IL | = | interleukin |
REFERENCES
- JiangW.KrishnanRR.O'ConnorCM.Depression and heart disease: evidence of a link, and its therapeutic implications.CNS Drugs.20021611112711825102
- RooseSP.GlassmanAH.SeidmanSN.Relationship between depression and other medical illnesses.JAMA.20012861687169011594878
- PlanteGE.Depression and cardiovascular disease: a reciprocal relationship.Metabolism.200554454815877313
- NeelemanJ.OrmelJ.BijlRV.The distribution of psychiatric and somatic 111 health: associations with personality and socioeconomic status.Psychosom Med.20012001;63247
- ZdravkovicS.WienkeA.PedersenNL.MarenbergME.YashinAI.de FaireU.Genetic influences on CHD-death and the impact of known risk factors: comparison of two frailty models.Behav Genet.20043458559215520515
- SullivanPF.NealeMC.KendlerKS.Genetic epidemiology of major depression: review and meta-analysis.Am J Psychiatry.20001571552156211007705
- ScherrerJF.XianH.BucholzKK.et al.A twin study of depression symptoms, hypertension, and heart disease in middle-aged men.Psychosom Med.20036554855712883104
- CarneyRM.FreedlandKE.VeithRC.et al.Major depression, heart rate, and plasma norepinephrine in patients with coronary heart disease.Biol Psychiatry.19994545846310071718
- MusselmanDL.EvansDL.NemeroffCB.The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment.Arch Gen Psychiatry.1998555805929672048
- LettHS.BlumenthalJA.BabyakMA.et al.Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment.Psychosom Med.20046630531515184688
- McCafferyJM.Frasure-SmithN.et al.Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin.PsychosomMed. 200668187200
- KroezeWK.KristiansenK.RothBL.Molecular biology of serotonin receptors structure and function at the molecular level.Curr Top Med Chem.2002250752812052191
- StockmeierCA.Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter.J Psychiatr Res.20033735737312849929
- HrdinaPD.BakishD.ChudzikJ.RavindranA.LapierreYD.Serotonergic markers in platelets of patients with major depression: upregulation of 5-HT2 receptors.J Psychiatry Neurosci.19952011197865496
- NemeroffCB.MusselmanDL.Are platelets the link between depression and ischemic heart disease?.Am Heart J.2000140576211011349
- WilliamsRB.MarchukDA.GaddeKM.et al.Central nervous system serotonin function and cardiovascular responses to stress.Psychosom Med.20016325
- OwensMJ.NemeroffCB.Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter.Clin Chem.1994402882957508830
- RamasubbuR.Serotonin transporter gene functional polymorphism: a plausible candidate gene for increased vascular risk in depression.Med Hypotheses.200361364412781638
- LeschKP.BallingU.GrossJ.et al.Organization of the human serotonin transporter gene.J Neural Transm Gen Sect.1994951571627865169
- HeilsA.TeufelA.PetriS.et al.Allelic variation of human serotonin transporter gene expression.J Neurochem.199666262126248632190
- LeschKP.BengelD.HeilsA.et al.Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region.Science.1996274152715318929413
- AnguelovaM.BenkelfatC.TureckiG.A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: I. Affective disorders.Mol Psychiatry.2003857459112851635
- BondyB.BuettnerA.ZillP.Genetics of suicide.Mol Psychiatry.20061133635116462816
- HaririAR.HolmesA.Genetics of emotional regulation: the role of the serotonin transporter in neural function.Trends Cogn Sci.20061018219116530463
- MunafoMR.ClarkT.FlintJ.Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? A meta-analysis.Mol Psychiatry.20051041541915599377
- HannaGL.HimleJA.CurtisGC.et al.Serotonin transporter and seasonal variation in blood serotonin in families with obsessive-compulsive disorder.Neuropsychopharmacol.1998 18 102111
- WhyteEM.PollockBG.WagnerWR.MulsantBH.FerrellRE.MazumdarS.et alInfluence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression.Am J Psychiatry.20011581216
- FumeronF.BetoulleD.NicaudV.et al.Serotonin transporter gene polymorphism and myocardial infarction: Etude Cas-Temoins de l'Infarctus du Myocarde (ECTIM).Circulation.20021052943294512081984
- EddahibiS.MorrellN.d'OrthoMP.NaeijeR.AdnotS.Pathobiology of pulmonary arterial hypertension.Eur Respir J.2002201559157212503718
- NakataniD.SatoH.SakataY.et al.Influence of serotonin transporter gene polymorphism on depressive symptoms and new cardiac events after acute myocardial infarction.Am Heart J.200515065265816209960
- RamasubbuR.TobiasR.BuchanAM.Bech-HansenNT.Serotonin transporter gene promoter region polymorphism associated with poststroke major depression.J Neuropsychiatry Clin Neurosci.200618969916525076
- McCafferyJM.BleilM.Pogue-GeileMF.FerrellRE.ManuckSB.Allelic variation in the serotonin transporter gene-linked polymorphic region (5HTTLPR) and cardiovascular reactivity in young adult male and female twins of European-American descent.Psychosom Med.20036572172814508012
- CaspiA.SugdenK.MoffittTE.et al.Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene.Science.200330138638912869766
- GrabeHJ.LangeM.WolffB.et al.Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden.Mol Psychiatry.20051022022415263905
- BatraV.PatkarAA.BerrettiniWH.WeinsteinSP.LeoneFT.The genetic determinants of smoking.Chest.20031231730173912740294
- BenwellME.BalfourDJ.AndersonJM.Smoking-associated changes in the serotonergic systems of discrete regions of human brain.Psychopharmacology (Berl).199010268721697418
- HarrisonAA.LiemYT.MarkouA.Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats.Neuropsychopharmacol.2001255571
- IshikawaH.OhtsukiT.IshiguroH.et al.Association between serotonin transporter gene polymorphism and smoking among Japanese males.Cancer Epidemiol Biomarkers Prev.1999883183310498403
- LermanC.ShieldsPG.AudrainJ.et al.The role of the serotonin transporter gene in cigarette smoking.Cancer Epidemiol Biomarkers Prev.199872532559521442
- TrummerO.KoppelH.WascherTC.et al.The serotonin transporter gene polymorphism is not associated with smoking behavior.Pharmacogenomics J.2006639740016702982
- ReichT.HinrichsA.CulverhouseR.BierutL.Genetic studies of alcoholism and substance dependence.Am J Hum Genet.19996559960510441565
- MassatI.SoueryD.LippO.et al.A European multicenter association study of HTR2A receptor polymorphism in bipolar affective disorder.Am J Med Genet.20009613614010893484
- MinovC.BaghaiTC.SchuleC.et al.Serotonin-2A-receptor and -transporter polymorphisms: Lack of association in patients with major depression.Neurosci Lett.200130311912211311507
- SchinsA.HonigA.CrijnsH.BaurL.HamulyakK.Increased coronary events in depressed cardiovascular patients: 5-HT2A receptor as missing link?Psychosom Med.20036572973714508013
- LererB.MacciardiF.SegmanRH.et al.Variability of 5-HT2C receptor cys23ser polymorphism among European populations and vulnerability to affective disorder.Mol Psychiatry.2001657958511526472
- YuanX.YamadaK.Ishiyama-ShigemotoS.KoyamaW.NonakaK.Identification of polymorphic loci in the promoter region of the serotonin 5-HT2C receptor gene and their association with obesity and type II diabetes.Diabetologia.20004337337610768099
- MillerGE.RohlederN.StetlerC.KirschbaumC.Clinical depression and regulation of the inflammatory response during acute stress.Psychosom Med.20056767968716204423
- MaesM.Evidence for an immune response in major depression: a review and hypothesis.Prog Neuropsychopharmacol Biol Psychiatry.1995 1911387708925
- PasicJ.LevyWC.SullivanMD.Cytokines in depression and heart failure.Psychosom Med.20036518119312651985
- MillerGE.StetlerCA.CarneyRM.FreedlandKE.BanksWA.Clinical depression and inflammatory risk markers for coronary heart disease.Am J Cardiol.2002901279128312480034
- HonigA.MaesM.Psychoimmunology as a common pathogenetic pathway in myocardial infarction, depression and cardiac death.Curr Opin Psychiatry.200013661664
- LibbyP.RidkerPM.Inflammation and atherosclerosis: role of C-reactive protein in risk assessment.Am J Med.2004116(suppl 6A)9S16S15050187
- RossR.Atherosclerosis is an inflammatory disease.Am Heart J.1999138S419S42010539839
- FishmanD.FauldsG.JefferyR.et al.The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic- onset juvenile chronic arthritis.J Clin Invest.2000102136913769769329
- JennyNS.TracyRP.OggMS.et al.In the elderly, interleukin-6 plasma levels and the -174G>C polymorphism are associated with the development of cardiovascular disease.Arterioscler Thromb Vase Biol.20022220662071
- LositoA.KalidasK.SantoniS.JefferyS.Association of interleukin-6 174G/C promoter polymorphism with hypertension and left ventricular hypertrophy in dialysis patients.Kidney Int.20036461662212846758
- HumphriesSE.LuongLA.OggMS.HaweE.MillerGJ.The interleukin-6 174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men.Eur Heart J.2001222243225211728144
- BrullDJ.LeesonCP.MontgomeryHE.et al.The effect of the lnterleukin-6-174G > C promoter gene polymorphism on endothelial function in healthy volunteers.Eur J Clin Invest.20023215315711895465
- BerthierMT.ParadisAM.TchernofA.et al.The interleukin 6-174G/C polymorphism is associated with indices of obesity in men.J Hum Genet.200348141912560873
- Jerrard-DunneP.SitzerM.RisleyP.et al.Interleukin-6 promoter polymorphism modulates the effects of heavy alcohol consumption on early carotid artery atherosclerosis: the Carotid Atherosclerosis Progression Study (CAPS).Stroke.20033440240712574550
- PihlajamakiJ.YlinenM.KarhapaaP.VauhkonenI.LaaksoM.The effect of the -308a allele of the TNF-alpha gene on insulin action is dependent on obesity.Obes Res.20031191291712855762
- DalzielB.GosbyAK.RichmanRM.BrysonJM.CatersonID.Association of the TNF-alpha -308 G/A promoter polymorphism with insulin resistance in obesity.Obes Res.20021040140712006640
- FertuzinhosSM.OliveiraJR.NishimuraAL.et al.Analysis of IL-1 alpha, IL-1beta, and IL-1RA [correction of IL-RA] polymorphisms in dysthymia.J Mol Neurosci.20042225125614997019
- YuYW.ChenTJ.HongCJ.ChenHM.TsaiSJ.Association study of the interleukin-1beta (c-511t) genetic polymorphism with major depressive disorder, associated symptomatology, and antidepressant response.Neuropsychopharmacol.200328 11821185
- McCulleyMC.DayIN.HolmesC.Association between interleukin 1-beta promoter (-511) polymorphism and depressive symptoms in Alzheimer's disease.Am J Med Genet B Neuropsychiatr Genet.2004 124505314681913
- TomasdottirH.HjartarsonH.RickstenA.WasslavikC.BengtssonA.RickstenSE.Tumor necrosis factor gene polymorphism is associated with enhanced systemic inflammatory response and increased cardiopulmonary morbidity after cardiac surgery.Anesth Analg.20039794494914500138
- JunTY.PaeCU.HoonH.et al.Possible association between -G308A tumour necrosis factor-alpha gene polymorphism and major depressive disorder in the Korean population.Psychiatr Genet.20031317918112960751
- BrasierAR.RecinosA.EledrisiMS.Vascular inflammation and the reninangiotensin system.Arterioscler Thromb Vasc Biol.2002221257126612171785
- KramerMS.CutlerN.FeighnerJ.et al.Distinct mechanism for antidepressant activity by blockade of central substance P receptors.Science.1998281164016459733503
- RigatB.HubertC.AlhencGF.CambienF.CorvolP.SoubrierF.An insertion/deletion polymorphism in the angiotensin l-converting enzyme gene accounting for half the variance of serum enzyme levels.J Clin Invest.199086134313461976655
- MoskowitzDW.Is angiotensin l-converting enzyme a “master” disease gene?Diabetes Technol Ther.2002468371112458570
- ArinamiT.LiL.MitsushioH.ItokawaM.HamaguchiH.ToruM.An insertion/deletion polymorphism in the angiotensin converting enzyme gene is associated with both brain substance P contents and affective disorders.Biol Psychiatry.199640112211278931914
- BaghaiT.SchuleC.ZwanzgerP.et al.Possible influence of the insertion/deletion polymorphism in the angiotensin l-converting enzyme gene on therapeutic outcome in affective disorders.Moi Psychiatry.200162585911326291
- BaghaiTC.SchuleC.ZillP.et al.The angiotensin I converting enzyme insertion/deletion polymorphism influences therapeutic outcome in major depressed women, but not in men.Neurosci Lett.2004363384215157992
- BaghaiTC.SchuleC.ZwanzgerP.et al.Hypothalamic-pituitary-adrenocortical axis dysregulation in patients with major depression is influenced by the insertion/deletion polymorphism in the angiotensin l-converting enzyme gene.Neurosci Lett.200232829930312147330
- NaberCK.HusingJ.WolfhardU.ErbelR.SiffertW.Interaction of the ACE D allele and the GNB3 825T allele in myocardial infarction.Hypertension.20003698698911116112
- ZillP.BaghaiTC.ZwanzgerP.et al.Evidence for an association between a G-protein beta3-gene variant with depression and response to antidepressant treatment.Neuroreport.2000111893189710884039
- BondyB.BaghaiTC.ZillP.et al.Combined action of the ACE D- and the G-protein β3-allele in major depression: a possible link to cardiovascular disorder?Mol Psychiatry.200271120112612476328
- NaberCK.HusingJ.WolfhardU.ErbelR.SiffertW.Interaction of the ACE D allele and the GNB3 825T allele in myocardial infarction.Hypertension.2000369868911116112
- BaghaiTC.BinderEB.SchuleC.et al.Polymorphisms in the angiotensinconverting enzyme gene are associated with unipolar depression, ACE activity and hypercortisolism.Mol Psychiatry.2006111003101516924268
- HaefnerS.BaghaiTC.BedaridaG.et al.C-reactive protein is associated with polymorphisms of the angiotensin converting enzyme gene in major depressed patients.J Psychiatr Res. In press.