Abstract
Antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs), are widely used for the treatment of depression and anxious disorders. The observation that depression is an independent risk factor for cardiovascular mortality and morbidity in patients with ischemic heart disease, the assessment of the central role of serotonin in pathophysiological mechanisms of depression, and reports of cases of abnormal bleeding associated with antidepressant therapy have led to investigations of the influence of antidepressants on hemostasis markers. In this review, we summarize data regarding modifications of these markers, drawn from clinical studies and case reports. We observed an association between the type of antidepressant drug and the number of abnormal bleeding case reports, with or without modifications of hemostasis markers. Drugs with the highest degree of serotonin reuptake inhibition - fluoxetine, paroxetine, and sertraline - are more frequently associated with abnormal bleeding and modifications of hemostasis markers. The most frequent hemostatic abnormalities are decreased platelet aggregability and activity, and prolongation of bleeding time. Patients with a history of coagulation disorders, especially suspected or documented thrombocytopenia or platelet disorder, should be monitored in case of prescription of any serotonin reuptake inhibitor (SRI). Platelet dysfunction, coagulation disorder, and von Willebrand disease should be sought in any case of abnormal bleeding occurring during treatment with an SRI. Also, a non-SSRI antidepressant should be favored over an SSRI or an SRI in such a context. Considering the difficulty in performing platelet aggregation tests, which are the most sensitive in SRI-associated bleeding, and the low sensitivity of hemostasis tests when performed in case of uncomplicated bleeding in the general population, establishing guidelines for the assessment of SRI-associated bleeding complications remains a challenge.
Los antidepresivos, especialmente los inhibidores selectivos de la recaptación de serotonina (ISRS), se utilizan frecuentemente en el tratamiento de la depresión y los trastornos de ansiedad. La consideración de la depresión como factor de riesgo independente que incide en una mayor mortalidad y morbilidad cardiovascular de pacientes con enfermedad cardiaca ischemica, así como la constatación del papel central de la serotonina en los mecanismos fisiopatológicos de la depresión, y el conocimiento de casos de hemorragias anormales producidas durante tratamientos con antidepresivos, han llevado a examinar el efecto de los antidepresivos sobre los medidores de hemostasis. Los fármacos con mayor grado de inhibición de la recaptación de serotonina - fluoxetina, paroxetina y sertralina - son los más frecuentemente asociados con hemorragias anormales y perturbaciones hemostáticas. Los trastornos hemostáticos más frecuentemente observados son la disminución de la aglutinación y de la actividad de las plaquetas, y la prolongación del tiempo de hemorragia. Generalmente, pacientes con antecedentes de trastornos de coagulación, en particular trombocitopenia o disturbio de las plaquetas ya diagnosticadas o sospechadas, deberían estar controlados si se les receta cualquier inhibidor de la recaptación de serotonina. En caso de hemorragias anormales durante un tratamiento con un inhibidor de la recaptación de serotonina (IRS), se debería proceder a la búsqueda de una disfunción de las plaquetas, una coagulopatia y la enfermedad de von Willebrand. Teniendo en cuenta la viabilidad de realizar las pruebas de aglutinación de las plaquetas, que son las más sensibles para casos de hemorragias asociadas con los IRS, y la baja sensibilidad de las pruebas de hemostasis hechas en caso de hemorragia no complicada en la población general, el desarrollo de criterios de guía para casos de hemorragia asociada con los IRS constituye todavía un importante desafío.
Les antidépresseurs, particulièrement les inhibiteurs sélectifs de la recapture de la sérotonine (ISRS), sont largement utilisés dans le traitement des troubles dépressifs et anxieux. La mise en évidence du fait que la dépression est un facteur de risque indépendant pour une mortalité et une morbidité cardio-vasculaire accrue chez les patients souffrant de maladie cardiaque ischémique, celle du rôle central de la sérotonine (5-HT) dans les mécanismes physiopathologiques de la dépression, ainsi que la description de cas de saignements anormaux sous traitement antidépresseur, ont conduit à examiner l'influence des antidépresseurs sur les marqueurs de l'hémostase. Dans cet article, nous avons résumé les données concernant les variations de ces marqueurs à partir de cas et d'études cliniques. Nous avons observé une association entre le type d'antidépresseurs et le nombre de saignements anormaux avec ou sans modification des marqueurs de l'hémostase. Les molécules à haut degré d'inhibition de la recapture de la sérotonine, la fluoxétine, la paroxétine et la sertraline, sont le plus souvent associées à des saignements anormaux et des modifications des marqueurs de l'hémostase. Les anomalies hémostatiques le plus souvent observées sont une diminution de l'agrégabilité et de l'activité plaquettaires, ainsi qu'une prolongation du temps de saignement. De manière générale, les patients avec des antécédents de troubles de la coagulation, en particulier de thrombocytopénie ou de trouble plaquettaire connu ou suspecté, devraient faire l'objet d'une surveillance accrue en cas de prescription de n'importe quel inhibiteur de la recapture de la sérotonine (IRS). Une dysfonction plaquettaire, une coagulopathie et une maladie de Willebrand devraient être recherchées en cas de saignements anormaux au cours d'un traitement avec un IRS auquel on préférera un antidépresseur non IRS dans un tel contexte. Compte tenu de la difficulté de réalisation des tests d'agrégation plaquettaire, qui sont les tests les plus sensibles lors de saignements associés aux IRS, et de la faible sensibilité des tests de l'hémostase réalisés lors de saignements non compliqués dans la population générale, la mise en place de recommandations lors de saignements associés aux IRS reste un défi à relever.
Depression is considered to be an Independent risk factor for cardiovascular mortality and morbidity in patients with Ischemic heart disease (IHD).Citation1,Citation2The risk of cardiovascular disease is higher in Individuals suffering from depression,Citation3 as is the risk of ischemic stroke.Citation4 Among newly diagnosed patients with coronary heart disease, approximately one in five patients suffers from major depression, with a similar prevalence in patients recovering from acute myocardial infarction.Citation5,Citation6 The proposed mechanisms are either spurious, le, that depression predicts, but is not causally related to, cardiovascular heart disease morbidity and mortality (antidepressant cardiotoxicity, association with cardiac risk factors, sedentary lifestyle), or imply that depression may directly influence the course of cardiac heart disease (nonadherence to cardiac treatment and regimens, dysregulation of autonomic, neuroendocrine, and serotonergic systems).Citation7
Serotonergic neurotransmission dysfunction has been investigated and observed in major depression.Citation8,Citation9 Various findings support the hypothesis that alterations in serotonergic neurons play a role in the pathophysiology of depression,Citation10 and most antidepressants have a direct influence on serotonin (5-HT) transmission and levels. 5-HT is usually a vasodilator, becoming a vasoconstrictor when the endothelium is damaged.Citation11 It is also involved in platelet aggregation. It is taken up from plasma and stored in platelet granules. Upon initiation of platelet aggregation, 5-HT is released into the blood and activates 5-HT2A receptors on the platelet membrane, which enhances the aggregation process. 5-HT per se is a weak activator, but dose-dependently enhances platelet activation induced by adenosine diphosphate (ADP) and, in particular, thrombin in whole blood.Citation12 It also potentiates aggregation in the presence of epinephrine or collagen,Citation13 and potentiates release reactions through a mechanism of amplification by an increase in free cytoplasmic intracellular calcium ion concentration. This induces a shape-change reaction of platelets, priming platelet surfaces for interactions with coagulation factors.Citation14 5-HT may therefore be directly involved in increased cardiovascular mortality and morbidity in depressed patients.
A relationship between depressive symptoms and increased platelet activity has been established in physically healthy depressed patientsCitation15-Citation17 as well as in postmyocardial infarction (MI) depressed patients.Citation8,Citation18 The following mechanisms mediating platelet abnormalities observed in major depression have been proposedCitation19: altered platelet function by increased plasma concentrations of 5-HT and epinephrine, affected platelet function by increased intraplatelet calcium mobilization, upregulation of 5-HT2A receptors or ot-adrenoreceptors, downregulation of 5-HT transporter number, altered second messenger signal transduction, or altered intraplatelet concentrations of monoamines and catecholamines.Citation17
Thus, 5-HT probably plays a role in the pathophysiological mechanisms of depression as well as in primary hemostasis platelet activity, and this neurotransmitter might be a key element in the understanding of the relationship between depression and increased risk of cardiovascular disease. Amplification of platelet aggregation could be altered by antidepressants that inhibit serotonin reuptake, in particular selective 5-HT reuptake inhibitors (SSRIs), because of depletion or decrease in intraplatelet 5-HT levels. The purpose of this review of the literature is to summarize changes in hemostatic function observed during treatment by antidepressants.
We performed a MEDLINE search of the relevant literature, and reviewed prospective and retrospective studies, as well as case reports and reviews of literature related to bleeding side effects and hemostasis laboratory findings, associated with antidepressant treatment in the psychiatric population, in post-MI depressed patients, or in healthy volunteers.
The prothrombotic effect of typical and atypical antipsychotics, as well as the impaired platelet function and thrombocytopenia caused by the mood stabilizer valproate and the possible procoagulant effect of treatment by lithium, are not examined here.
Hemostasis
The process of hemostasis involves four principal stages: first, the initiation and formation of the platelet plug (also called primary hemostasis), second, the coagulation cascade, a series of enzymatic actions on proteins leading to clot formation; third, its termination by antithrombotic control mechanisms; and fourth, the removal of the clot by fibrinolysis. The coagulation cascade leads to formation of fibrin polymers which consolidate the platelet plug formed during primary hemostasis.Citation20 Abnormalities in tests measuring the function of primary hemostasis and clotting cascade (coagulation) have been reported in case reports and cohort studies in patients treated with antidepressants.
During primary hemostasis, the platelets form a plug at a site of injury in order to stop bleeding. This phase, which is the functional response of activated platelets, comprises four different processes: adhesion, aggregation, secretion, and procoagulant activity.Citation21 During the vascular phase, vasoconstriction occurs and procoagulant (von Willebrand factor [vWF], tissue factor) as well as anticoagulant substances prostaglandin I2 [PGI2], thrombomodulin, urokinase, tissue plasminogen activator [tPA], antithrombin, nitric oxide [NO], and endotheliumderived relaxing factor [EDRF]) are secreted by the endothelium. The most potent platelet activators are collagen and thrombin, whereas ADP and epinephrine are weak activators. 5-HT itself is a weak platelet agonist, but it amplifies the effect of other platelet agonists.Citation22 The process comprises successive steps, illustrating the central role of platelets ():
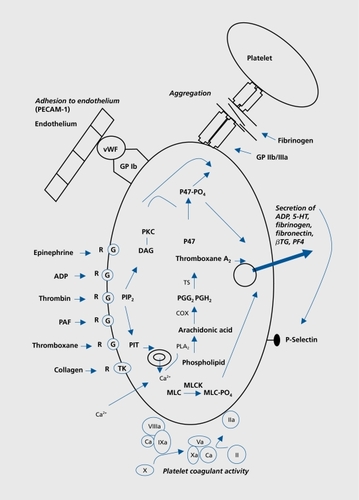
Adhesion: Platelets change shape in response to activation, allowing adhesion to subendothelial matrix. This process is mediated by the binding of platelet surface receptor GPIb/IX/V complex to vWF. Binding of collagen to platelet collagen receptor GPIa/IIa also plays a role in platelet adhesion.
Aggregation: Both conformational and exposure changes in the GPIIb/IIIa on the platelet surface due to activation, result in binding of vWF and fibrinogen.
Secretion: Substances are secreted from platelet granules upon stimulation. ADP and 5-HT stimulate and recruit other platelets. Fibronectin stabilizes platelet aggregates. Secreted fibrinogen provides a source of fibrinogen at sites of endothelial injury in that present in the plasma. Thromboxane A2 (TXA2, from arachidonic acid [AA] release) stimulates platelet aggregation and causes vasoconstriction. Platelet-derived growth factor (PDGF) mediates tissue reparation.
Procoagulant activity: Exposure of procoagulant phospholipids and the subsequent assembly of the enzyme complexes on the platelet surface represent procoagulant activity.
Among others, the following laboratory tests explore primary hemostasis: platelet count, bleeding time, platelet function analyzer (PFA), platelet functional assessment, medullogram, and vWF (Table I). Normal values are not mentioned, since they are provided by the laboratory when these examinations are requested.
Table I. Laboratory tests of hemostasis. This list does not concern the third and fourth stages of hemostasis; the process is terminated by antithrombotic control mechanisms and fibrinolysis.
The clotting cascade consists of the activation of various proenzymes to active enzymes, resulting in the formation of the red clot. Intrinsic and extrinsic pathways lead to activation of factor X which converts prothrombin to thrombin, the final enzyme of the clotting cascade, which in turns converts fibrinogen into an insoluble fibrin clot. Among others, the following laboratory tests examine the clotting cascade: prothrombin time (PT) and international normalized ratio (INR, extrinsic pathway), partial thromboplastin time (aPTT, intrinsic pathway), fibrinogen, thrombin time (TT), coagulation factors, and inhibitors of coagulation (antithrombin, proteins C and S, Table I).
Influence of antidepressant on hemostasis markers
Numerous prospective open comparative studies,Citation9,Citation16,Citation23-Citation34 randomized double-blind controlled trials,Citation8,Citation35-Citation37 in vitro studies by incubation of the antidepressant compound,Citation38-Citation40 and case reportsCitation41-Citation53 have pointed out changes in laboratory tests assessing function of primary hemostasis and clotting cascade.
Double-blind, randomized, placebo-controlled trials
In a randomized, double-blind, placebo-controlled, twoway crossover trial, Hergovich et al evaluated the potential inhibition of platelet function in 16 healthy male volunteers receiving paroxetine, 20 mg/d over 2 weeks. Paroxetine decreased intraplatelet 5-HT concentration by 83% and therefore prolonged closure time measured by PFA by 31% (in other terms inhibited the plug under shear stress). It also lowered platelet activation in response to thrombin receptor peptide, shown by an 8% decrease in the expression of the platelet activation marker CD63. No changes in plasma concentration of prothrombin fragment, vWF antigen, or soluble Pselectin were observed. This indicated no activation of coagulation, endothelium, or platelet in vivo, underlining a favorable risk:benefit ratio when the drug is used for rehabilitation of post-MI patients.Citation35
In order to Investigate whether depressed post-MI patients have higher markers of platelet activation than nondepressed post-MI patients, and evaluate the effect of mirtazapine on platelet activation, Schins et al con? ducted a randomized, double-blind, placebo-controlled trial in 25 depressed post-MI patients receiving, for 8 weeks, either mirtazapine 30 to 45 mg or placebo. The control group consisted of nondepressed post-MI patients. The markers measured were plasma levels of pthromboglobulin (βTG), platelet factor 4 (PF4), soluble CD40 ligand (sCD40L), whole-blood, and intraplatelet 5-HT. Before treatment, only whole blood and intraplatelet 5-HT levels were significantly higher in depressed patients. Treatment with mirtazapine resulted in a nonsignificant decrease in βTG, PF4, and intraplatelet 5-HT level after 8 weeks. The authors underline the fact that mirtazapine, by lack of inhibitory effect on 5-HT reuptake, has not shown, in contrast with SSRIs, a potentiated risk of bleeding events, when given in combination with nonsteroidal antiinflammatory drugs (NSAIDs).Citation54-Citation56 Therefore, treatment of depressed post-MI patients with mirtazapine may be possible in patients susceptible to bleeding complications, according to the authors.Citation8
In a randomized, double-blind, placebo-controlled trial, Serebruany et al assessed the release of platelet/endotheliai markers in 64 post-MI depressed patients treated with sertraline vs placebo. PF4, βTG, platelet/endothelial cell adhesion molecule-1, P-selectin, thromboxane B2 (TXB2), 6-ketoprostaglandin F1α, vascular cell adhesion molecule-1, and E-selectin were measured by enzymelinked immunosorbent assay (ELIS A). Treatment with sertraline was associated with substantially less release of these markers than treatment with placebo. These differences reached statistical significance for βTG at weeks 6 and 16 and for P-selectin at week 16. Repeatedmeasures ANOVA revealed a significant advantage for sertraline vs placebo for diminishing E-selectin and βTG concentrations across the entire treatment period. Despite previous broad use of aspirin and clopidogrel (though equally distributed between both groups), the authors underline the potential benefit of sertraline treatment of post-MI patients, because of decreased activation of platelets.Citation36
Double-blind, randomized, comparative trial
Pollock et al investigated the influence of a 6-week paroxetine or nortriptyline treatment on platelet activation in 17 depressed patients with IHD, in a randomized double-blind trial. Baseline measurements of βTG and PF4 were significantly elevated in both groups before treatment, compared with those of healthy control subjects. In the paroxetine group, mean βTG and PF4 levels significantly decreased within 1 week of treatment and remained low at the 3- and 6-week measurements. In contrast, the nortriptyline group did not exhibit a significant decrease in βTG and PF4 levels after 1,3, and 6 weeks. A type II error for the nortriptyline group was not excluded; nor was the possible influence of the patient's clinical state on platelet activation. However, according to the authors, the reduction in platelet activation observed after only 1 week of paroxetine treatment is in favor of a pharmacologic effect.Citation37
Prospective open comparative studies
Prospective open comparative studies, conducted in depressed patients, post-MI depressed patients, or healthy volunteers, with comparative measurements of various hemostasis parameters in a healthy control group or in subjects before treatment, demonstrated higher platelet activity in depressed or post-MI depressed patients in comparison with the control group, and/or decrease in platelet activity after antidepressant treatment.
Alvarez et al showed that untreated depressed patients have decreased numbers of platelet 5-HT transporter sites and increased levels of platelet inositol triphosphate (PIT), that treatment with both fluoxetine and clomipramine further reduces the density of these transporter sites (more marked in responders to treatment), accompanied by a dramatic decrease in plasma and platelet 5-HT levels, and that, in responders, PIT levels return to normal values.Citation9
Markovitz et al showed a greater platelet secretory response to collagen in depressed patients than in healthy control subjects in baseline comparison measures. They then demonstrated a decrease in collagen-induced platelet secretion after treatment with sertraline for 6 weeks, most other platelet activation measures showing minimal change. These changes were, however, not related to improvement in the Beck Depression Inventory scores, but this finding might be limited by the short treatment duration. Thus, the decreased platelet activation after SSRI treatment could diminish the risk of coronary heart disease among depressed subjects, but the authors underline the need for studies in a large number of patients, placebo controls, and a longer follow-up period.Citation24
In a study by Musselman et al, after 6 weeks of open-label treatment with paroxetine, a normalization of platelet activation occurred in patients with depression. This was shown by a significant decrease in PF4 and of the platelet monoclonal antibodies anti ligand-induced platelet binding site (LIBS) and GA6 (anti P-selectin). Before treatment, these markers were higher among depressed patients compared with the control group. The results of this study could be explained by recovery from depression. Thus, studies with placebo and/or psychotherapy are proposed by the authors as further investigation.Citation25
A decrease in platelet response mediated by the 5-HT2A receptor following effective imipramine treatment was demonstrated by Gomez-Gil et al, shown by a significant decrease in 5-HT-amplified platelet aggregation to ADP, thus suggesting that desensitization or downregulation of platelet 5-HT2A receptor function could be linked to a therapeutic effect of some antidepressants.Citation30
Comparing levels of plasma 5-HT and platelet 5-HT induced aggregation among depressed patients treated with either fluoxetine or amitriptyline and nontreated patients, Menys et al demonstrated a statistically significant decrease in both plasma 5-HT levels and 5-HT induced platelet aggregation, only with fluoxetine. This suggests a higher inhibition of platelet activity by SSRIs than tricyclic antidepressants, and therefore a more suitable treatment for depressed patients with cardiovascular disease.Citation26
Laine-Cessac et al failed to demonstrate a significant effect of a 1-month fluoxetine treatment in 8 depressed patients, on the following primary hemostasis and coagulation tests: PT, aPTT, TT, fibrinogen, platelet count, bleeding time, platelet aggregation induced by ADP, AA, ristocetin, and collagen. The single statistically significant difference, in comparison with values before treatment, was found in a decreased velocity in platelet aggregation induced by epinephrine. The authors conclude that significant platelet dysfunction causing hemorrhagic diathesis is uncommon when fluoxetine is used at a dosage of 20 mg daily.Citation27 However, methodological issues with this publication suggest a high risk of type II error.Citation27
Lederbogen et al measured aPTT, vWF, fibrinogen, fibrin monomer, and prothrombin ratio (Quick) before and after treatment with either amitriptyline or paroxetine. Therapy was effective on depressive symptoms as measured by the Hamilton Depression scale in both groups, and ANOVA revealed prothrombin ratio to increase from start to end of treatment. No effect was seen on the other parameters. The authors conclude that changes observed in prothrombin ratio may be due to nutritional factors, and that bleeding associated with antidepressant therapy is probably not an extreme form of a general influence on the coagulation systems, but rather an idiosyncratic reaction.Citation32
Berk et al studied 10 patients before and after treatment with fluoxetine. No changes in any index of platelet aggregation or coagulation were reported.Citation33 Alderman et al were also unable to demonstrate any changes in primary hemostasis or coagulation parameters after use of fluoxetine or paroxetine for 28 days.Citation28 This was also the case after a fluoxetine trial conducted by Bang et al.Citation34
Interestingly, Tharmapathy et al observed that platelets from six or seven patients undergoing treatment with venlafaxine aggregated spontaneously during a routine centrifugation of platelet-rich plasma. Furthermore, increased baseline platelet activity as measured by P-selectin surface expression was observed during treatment compared with before treatment.Citation29
In vitro studies
The in vitro effects of escalating concentrations of sertraline on human platelets were assessed by Serebruany et al, showing a dose-dependent inhibition of platelet aggregation induced by ADP, collagen, and thrombin, as well as decreased platelet surface expression of CD9, Pselectin, platelet endothelial cell adhesion molecule (PECAM)-I, and glycoproteins Ilb/IIIa and lb. The data from this study, showing a direct inhibitory effect on platelets of therapeutic concentrations of sertraline, suggest that it may account for a substantial portion of the association between depression and adverse outcomes of IHD by a thrombotic mechanism.Citation39
Mohammad and Mason also demonstrated an inhibition of ADP-induced platelet aggregation by the tricyclics imipramine and amitriptyline.Citation38
Case reports (no baseline values)
Among case reports of abnormal bleeding with antidepressant medication, some have revealed abnormalities in hemostasis tests. Alderman et al and De Maistre et al reported cases of abnormal bleeding associated with fluoxetine treatment, accompanied by a decrease in ADP, epinephrine, ristocetin, AA, collagen, and epinephrineinduced platelet aggregation, respectively, which were reversible after discontinuation of the drug (positive dechallenge).Citation41,Citation42
Ottervanger et al reported a decrease in epinephrineinduced platelet aggregation in a patient treated with paroxetine for 4 weeks.Citation45
A decrease in ADP and ristocetin-induced platelet aggregation in a patient treated with fluoxetine was also reported by Evans et al. They also described an increase in bleeding time to more than 25 minutes, with positive dechallenge and rechallenge.Citation46
Increased bleeding time with positive dechallenge was reported by Humphries et al in a patient treated for 2 years with fluoxetine, and by Calhoun and Calhoun for a patient treated for 10 weeks with sertraline.Citation47,Citation48 Ceylan and Alpsan-Omay also described the case of a patient treated with sertraline for 7 days, with prolonged bleeding time and prothrombin time, as well as a decrease in platelet count. All three parameters reversed after discontinuation (positive dechallenge).Citation49
Low platelet count was also noted by Aranth and Lindberg and Leung and Shore in patients treated respectively with fluoxetine and fluvoxamine.Citation43,Citation44
Tham et al, Démet et al, and Tielens reported prolonged aPTT with venlafaxine, mirtazapine, and paroxetine respectivelyCitation50,Citation51,Citation53 in the first two cases, values returned to normal range after discontinuation (positive dechallenge). Tham et al also reported a high antihemophilic factor VIII, whereas Démet et al showed a prolonged prothrombin time and increased INR.Citation50,Citation51
In contradiction with these findings, a case of diminished prothrombin time and aPTT was reported by Hardy and Sirois when trazodone was added to treatment with warfarin after mitral valve replacement, thus decreasing the efficiency of this treatment.Citation52
Table II summarizes the discussed clinical studies on modification of hemostasis markers, while Table III summarizes the case reports.
Table II. Clinical studies on modifications of hemostasis markers. DB, double-blind; PC, placebo-controlled; POC, prospective open comparative study; PO, prospective open; CS,cross-sectional; DEP, depression; SS, statistically significant; MAB, monoclonal antibodies; NA, non-available; IHD, ischemic heart disease; βTG, β-thromboglobulin; PF4, platelet factor 4; ANOVA, analysis of variance; PECAM, platelet endothelial cell adhesion molecule; 5-HT, serotonin; CI, confidence interval; PIT, platelet inositol triphosphate; LIBS, ligand-induced platelet binding site; aPTT, partial thromblastin time; INR, international normalized ratio; TT, thrombin time; AA, arachidonic acid; ADP, adenosine diphosphate; PT, prothtrombin time
Table III Case reports of modifications of hemostasis markers. AA, arachidonic acid; ADP, adenosine diphosphate; aPTT, partial thromboplastin time; INR, international normalized ratio
Discussion
In this review, we have presented the modifications of hemostasis markers caused by antidepressants. The most frequent modifications are decreased platelet aggregability and activity, and prolongation of bleeding time (primary hemostasis); they are more likely to occur with antidepressants such as fluoxetine, sertraline, or paroxetine. Other antidepressants such as venlafaxine, fluvoxamine, amitriptyline, imipramine, and even mirtazapine, can also influence hemostasis. Modifications of platelet count, as well as PT and PTT (coagulation cascade) are much less frequent. It is not known whether there might be laboratory modifications in the last two stages of hemostasis, ie, the termination by antithrombotic control mechanisms and the removal of the clot by fibrinolysis.
Antidepressants that inhibit platelet reuptake of 5-HT cause a platelet 5-HT depletion. This can inhibit 5-HTinduced platelet aggregation amplification. Patients suffering from bleeding complications during antidepressant treatment may have a mild pre-existing platelet disorder or a modified platelet serotonergic response amplified by depletion of 5-HT stocksCitation57; autoimmune mechanisms may also be involved.Citation50
Different types of studies were performed, from case reports to epidemiological studies and prospective laboratory studies comparing subjects and controls receiving antidepressants. These studies did not lead to the same conclusions.
A causal association between use of antidepressants, especially SSRIs, and abnormal bleeding or need for transfusion during surgical procedures has been found in retrospective studies.Citation54-Citation56,Citation58-Citation61 The main observation concerns a relationship between the type of antidepressant drug and the risk of bleeding complications. The risk of upper gastrointestinal bleeding was twice as high for SSRIs than for other antidepressant drugs.Citation55 The risk of upper gastrointestinal bleeding in elderly and depressed patients increased with antidepressants having the greatest extent of inhibition of 5-HT reuptake.Citation56 Similarly, a significant association between the degree of 5-HT reuptake Inhibition by antidepressants and the risk of hospital admission for abnormal bleeding as primary diagnosis was found.Citation59 in these studies, antidepressants were classified according to their degree of 5-HT reuptake Inhibition according to pharmacological studies.Citation62,Citation63 Blood transfusion require? ments during surgery was Increased for SRI antidepressant users compared with nonusers, which was not the case for nonserotonerglc antidepressant users.Citation60 Upper gastrointestinal bleeding risk was found to be 12.2 times greater than expected when there was a concomitant use of SSRIs and NSAIDs.Citation54
In the prospective laboratory studies mentioned, the results are heterogenous. Indeed, some studies found changes in given laboratory tests which were normal in other studies. Some studies failed to show any modlflca? tion in measured hemostasis markers.Citation28,Citation33,Citation34 Otherwise, decrease in platelet/plasma 5-HT level and diminution of 5-HT-lnduced aggregation are the markers which were more often modified upon antldepresslve treatment, in line with the central role of this neurotransmitter in primary hemostasis.Citation9,Citation26,Citation30 Thus, prospective studies clearly Indicate that antidepressants modify primary hemostasis. However, the configuration and the extent of these changes remains unspecified.
Most case reports of abnormal bleeding associated with the use of antidepressants that have failed to demonstrate perturbations in hemostasis concern the use of antidepressants with high degree of Inhibition of 5-HT reuptake,Citation45,Citation57,Citation64,Citation76 or, to a lesser extent, antidepressants with a mild degree of Inhibition of 5-HT reuptake.Citation77,Citation80 This was also the case for abnormal bleeding case reports with perturbation of hemostasis markers. In accordance with classification of antidepressants by their degree of 5-HT reuptake Inhibition, the drugs most frequently associated with abnormal bleeding are the SSRIs fluoxetine, sertra? line and paroxetine, thus confirming that 5-HT may directly be involved in the pathophysiology of bleeding side effects in patients undergoing antidepressant treatment, and may therefore be a more potent platelet activator in vivo than in vitro. Interestingly, in those case reports where coagulation markers were measured, about half of the patients showed no modification of hemostasis markers. This is coherent with the observa? tion that hemostasis tests, when performed in the general population, in cases of uncomplicated bleeding such as bruising, have a low sensitivity, ie, show normal results.
The platelet aggregation tests are the most sensitive tests when modifications of hemostasis markers are suspected during treatment with antidepressants; however, they are time-consuming or, unfortunately, not performed in routine laboratory studies. Furthermore, these tests sometimes give negative results because of genetic factors, leading to the absence of platelet aggregation. This is explained by inherited differences in platelet aggregation (PAR)-l thrombin receptor levels, and may be clinically relevant for subjects at increased risk of bleeding.Citation81
Several pertinent questions remain about the clinical relevance of hemostasis modifications by antidepressants. Should antidepressants be contraindicated in patients receiving anticoagulation treatment, or suffering from gastric ulcer, from von Willebrand disease, or from hemo? philia? Should hemostasis tests be performed in all patients treated with SSRIs and undergoing surgery?Citation82
What is the correct course to take in case of abnormal bleeding in a patient treated with an antidepressant? in our opinion, the above questions are highly relevant to clinical practice, but it remains difficult to provide straightforward answers for several reasons. In most of the publications mentioned, the authors propose no guidelines for SRI? or SSRI-associated bleeding complications, arguing that complementary investigations are still requested. Members of hematological societies are best equipped to establish guidelines on the above issues. Such guidelines will help clinicians. In the meantime, we can nevertheless propose the following general comments. In case of abnormal bleeding in a patient treated with an SSRI, the pharmacological treatment should be stopped, and replaced if needed by a non-SSRI antidepressant. Patients with a medical history of coagulation disorders, especially suspected or documented thrombo? cytopenia or platelet disorder, should be monitored in case of prescription of any SRI. Platelet dysfunction, coagulation disorders, and von Willebrand disease (characterized by prolongation of bleeding time combined with decreased factor VIII procoagulant activity) should be sought in case of abnormal bleeding occurring during treatment with any SRI. Platelet aggregation measurement by PFA is the most sensitive laboratory test in these situations, and should be considered if hemostasis tests are requested, for example before surgery. Non-SSRI antidepressants should be preferred to SSRIs or SRIs in cases of von Willebrand disease, hemophilia, gastric ulcer, and anticoagulation treatment.
Selected abbreviations and acronyms
| 5-HT | = | 5-hydroxytryptamine (serotonin) |
| AA | = | arachidonic acid |
| ADP | = | adenosine diphosphate |
| aPTT | = | partial thromboplastin time |
| βTG | = | β-thromboglobulin |
| IHD | = | ischémie heart disease |
| INR | = | international normalized ratio |
| MI | = | myocardial infarction |
| NSAID | = | nonsteroidal anti-inflammatory drug |
| PDGF | = | platelet-derived growth factor |
| PF4 | = | platelet factor 4 |
| PFA | = | platelet function analyzer |
| PIT | = | platelet inositol triphosphate |
| PT | = | prothrombin time (Quick) |
| SRI | = | serotonin reuptake inhibitor |
| SSRI | = | sélective serotonin reuptake inhibitor |
| TT | = | thrombin time |
| TXA2 | = | thromboxane A2 |
| vWF | = | von Willebrand factor |
REFERENCES
- BushDE.ZiegelsteinRC.TaybackM.et al.Even minimal symptoms of depression increase mortality risk after acute myocardial infarction.Am J Cardiol.20018833734111545750
- BarefootJC.SchroIIM.Symptoms of depression, acute myocardial infarction, and total mortality in a community sample.Circulation.199693197619808640971
- PenninxBW.BeekmanAT.HonigA.et al.Depression and cardiac mortality: results from a community-based longitudinal study.Arch Gen Psychiatry.20015822122711231827
- OhiraT.IsoH.SatohS.et al.Prospective study of depressive symptoms and risk of stroke among Japanese.Stroke.20013290390811283390
- CarneyRM.FreedlandKE.RichMW.JaffeAS.Depression as a risk factor for cardiac events in established coronary heart disease: a review of possible mechanisms.Ann Behav Med.19951714214918425665
- GlassmanAH.ShapiroPA.Depression and the course of coronary heart disease.Am J Psychiatry.1998155104111
- CarneyRM.FreedlandKE.MillerGE.JaffeAS.Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms.J Psychosom Res.20025389790212377300
- SchinsA.HamulyakK.ScharpeP.et al.Whole blood serotonin and platelet activation in depressed post-myocardial infarction patients.Life Sci.200424;76637650
- AlvarezJC.GluckD.ArnulfI.et al.Decreased platelet serotonin transporter sites and increased platelet inositol triphosphate levels in patients with unipolar depression: effects of clomipramine and fluoxetine.Clin Pharmacol Ther.19996661762410613618
- OwensMJ.NemeroffCB.Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter.Clin Chem.1994402882957508830
- GolinoP.PiscioneF.WillersonJT.et al.Divergent effects of serotonin on coronary-artery dimensions and blood flow in patients with coronary atherosclerosis and control patients.N Engl J Med.19913246416481994246
- LiN.WallenNH.LadjevardiM.HjemdahlP.Effects of serotonin on platelet activation in whole blood.Blood Coagul Fibrinolysis.199785175239491270
- SkopBP.BrownTM.Potential vascular and bleeding complications of treatment with selective serotonin reuptake inhibitors.Psychosomatics.19963712168600488
- TroyGC.An overview of hemostasis.Vet Clin North Am Small Anim Pract.1988185203282384
- SchinsA.HonigA.CrijnsH.BaurL.HamulyakK.Increased coronary events in depressed cardiovascular patients: 5-HT2A receptor as missing link?Psychosom Med.20036572977714508013
- PiletzJE.ZhuH.MadakasiraS.et al.Elevated P-Selectin on platelets in depression: response tobupropion. J Psych iatr Res.200034397404
- MusselmanDL.TomerA.ManatungaAK.et al.Exaggerated platelet reactivity in major depression.Am J Psych ia try.199615313131317
- Laghrissi-ThodeF.WagnerWR.PollockBG.JohnsonPC.FinkelMS.Elevated platelet factor 4 and p-thromboglobulin plasma levels in depressed patients with ischemic heart disease.Biol Psychiatry.1997422902959270907
- NemeroffCB.MusselmanDL.Are platelets the link between depression and ischemic heart disease?Am Heart J.2000140 (4 suppl)576211011349
- Leung LawrenceLK.Overview of hemostasis. Available at: www.uptodate.com Accessed November 2006
- ColmanRW.ClowesAW.GeorgeJN.et al.Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Fifth ed. Philadelphia, Pa: Lippincott Williams & Wilkins;2006
- De ClerckF.The role of serotonin in thrombogenesis.Clin Physiol Biochem.19908(suppl 3)40491966729
- ButlerJ.LeonardBE.The platelet serotonergic system in depression and following sertraline treatment.Int Clin Psychopharmacol.198833433473235819
- MarkovitzJH.ShusterJL.ChitwoodWS.MayRS.TolbertLC.Platelet activation in depression and effects of sertraline treatment: an open-label study.Am J Psychiatry.20001571006100810831484
- MusselmanDL.MarzecUM.ManatungaA.et al.Platelet reactivity in depressed patients treated with paroxetine: preliminary findings.Arch Gen Psychiatry.20005787588210986551
- MenysVC.SmithCC.LewinsP.FarmerRD.NobleMl.Platelet 5-hydroxytryptamine is decreased in a preliminary group of depressed patients receiving the 5-hydroxytryptamine re-uptake inhibiting drug fluoxetine.Clin Sci (Lond).9969187928774265
- Laine-CessacP.ShoaayI.GarreJB.GlaudV.TurcantA.AllainP.Study of haemostasis in depressive patients treated with fluoxetine.Pharmacoepidemiol Drug Saf.19987(suppl 1)S54S5715073961
- AldermanCP.SeshadriP.Ben-TovimDl.Effects of serotonin reuptake inhibitors on hemostasis.Ann Pharmacother.199630123212348913401
- TharmapathyP.SelheimF.OdegaardK.LundA.HolmsenH.Venlafaxine treatment stimulates blood platelet activity.J Clin Psychopharmacol.20002058959011001252
- Gomez-GilE.GastoC.CarreteroM.et al.Decrease of the platelet 5HT2A receptor function by long-term imipramine treatment in endogenous depression.Hum Psychopharmacol.20041925125815181653
- LederbogenF.GillesM.MarasA.et al.Increased platelet aggregability in major depression?Psychiatry Res.20012425526111440776
- LederbogenF.WeberB.CollaM.HeuserH.DeuschleM.DempfleCE.Antidepressant treatment and global tests of coagulation and fibrinolysis.J Clin Psychiatry.2001622
- BerkM.JacobsonBF.HurlyE.Fluoxetine and hemostatic function: a pilot study.J Clin Psychiatry.19955614167836333
- BangNU.Acquired abnormalities of platelet function [letter].N Engl J Med.199132416722030725
- HergovichN.AignerM.EichlerHG.EntlicherJ.DruckerC.JilmaB.Paroxetine decreases platelet serotonin storage and platelet function in human beings.Clin Pharmacol Ther.20006843544211061584
- SerebruanyVL.GlassmanAH.MalininAI.et al.Endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) platelet substudy.Circulation.200310893994412912814
- PollockBG.Laghrissi-ThodeF.WagnerWR.Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment.J Clin Psychopharmacol.20002013714010770450
- MohammadSF.MasonRG.Inhibition of human platelet-collagen adhesion reaction by amitriptyline and impramine.Proc Soc Exp Biol Med.1974145110611134206514
- SerebruanyVL.GurbelPA.O'ConnorCM.Platelet inhibition by sertraline and N-desmethylsertraline: a possible missing link between depression, coronary events, and mortality benefits of selective serotonin reuptake inhibitors.Pharmacol Res.20014345346211394937
- BondurantT.DarrellMJ.El AsyoutyS.et al.Effect of fluoxetine on prothrombin time.Psychosomatics.1998392962989664780
- AldermanCP.MoritzCK.Ben-TovimDl.Abnormal platelet aggregation associated with fluoxetine therapy.Ann Pharmacother.199226151715191482806
- De MaistreE.AllartC.LecompteT.BollaertPE.Severe bleeding associated with use of low molecular weight heparin and selective serotonin reuptake inhibitors.Am J Med.200211353053212427509
- AranthJ.LindbergC.Bleeding, a side effect of fluoxetine.Am J Psychiatry.19921494121536285
- LeungM.ShoreR.Fluvoxamine-associated bleeding.Can J Psychiatry.1996416046058946088
- OttervangerJP.StriekerBH.HulsJ.WeedaJN.Bleeding attributed to the intake of paroxetine.Am J Psychiatry.19941517817828166328
- EvansTG.BuysSS.RodgersGM.Acquired abnormalities of platelet function [letter].N Engl J Med.19913241671
- HumphriesJE.WhebyMS.VandenbergSR.Fluoxetine and the bleeding time.Arch Pathol Lab Med.19901147277282363631
- CalhounJW.CalhounDD.Prolonged bleeding time in a patient treated with sertraline.Am J Psychiatry.19961534438610842
- CeylanME.Alpsan-OmayMH.Bleeding induced by SSRIs.Eur Psychiatry.20052057057116337893
- ThamCJ.TrewM.BragerN.Abnormal clotting and production of factor VIII inhibitor in a patient treated with venlafaxine.Can J Psychiatry.19994492392410584166
- DemetMM.MizrakS.Esen-DanaciA.Mirtazapine-induced arthralgia and coagulopathy: a case report.J Clin Psychopharmacol.20052539539616012290
- HardyJL.SiroisA.Reduction of prothrombin and partial thromboplastin times with trazodone.CMAJ.1986 1513513723779574
- TielensJA.Vitamin C for paroxetine - and fluvoxamine - associated bleeding.Am J Psychiatry.1997 1548838849167526
- DaltonSO.JohansenC.MellemkjoerL.NorgardB.SorensenHT.OlsenJH.Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding.Arch Intern Med.2003163596412523917
- de AbajoFJ.Garcia RodriguezLA.MonteraD.Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study.BMJ.19993191106110910531103
- van WalravenC.MamdaniMM.WellsPS.WilliamsJL.Inhibition of serotonin reuptake by antidepressants and upper gastrointestinal bleeding in elderly patients: retrospective cohort study.BMJ.20013231611440920
- PaiVB.KellyMW.Bruising associated with the use of fluoxetine.Ann Pharmacother.1996307867888826562
- LaytonD.ClarkDW.PearceGL.ShakirSA.Is there an association between selective serotonin reuptake inhibitors and risk of abnormal bleeding?Eur J Clin Pharmacol.20015716717611417450
- MeijerWE.HeerdinkER.NolenWA.HeringsRM.LeufkensHG.EgbertsAC.Association of risk of abnormal bleeding with degree of serotonin reuptake inhibition by antidepressants.Arch Intern Med.20041642367237015557417
- MovigKL.JanssenMW.de Waal MalefijtJ.KabelPJ.LeufkensHG.EgbertsAC.Relationship of serotonergic antidepressants and need for blood transfusion in orthopedic surgical patients.Arch Intern Med.20031632354235814581256
- TataLJ.FortunPJ.HubbardRB.et al.Does concurrent prescription of selective serotonin reuptake inhibitors and non-steroidal anti-inflammatory drugs substantially increase the risk of upper gastrointestinal bleeding?Aliment Pharmacol Ther.20052217518116091054
- TatsumiM.GroshanK.BlakelyRD.RîchelsonE.Pharmacological profile of antidepressants and related compounds at human monoamine transporters.Eur J Pharmacol.19973402492589537821
- RichelsonE.Pharmacology of antidepressants: clinical relevance of effects on neurotransmitter systems and their receptors. In: Den Boer JA, Westenberg HGM, eds.Antidepressants: Selectivity or Multiplicity. Amsterdam, the Netherlands: Benecke NI;20014360
- NelvaA.GuyC.Tardy-PoncetB.et al..Syndromes hémorragiques sous antidépresseurs inhibiteurs sélectifs de la recapture de la serotonine (ISRS). A propos de sept cas et revue de la littérature.Rev Méd Interne.200021152160
- VandelP.VandelS.KantelipJP.SSRI-induced bleeding: two case reports.Thérapie.200156445447
- WilmhurstPT.KumarAV.Subhyaloid haemorrhage with fluoxetine.Eye.19961014150
- GunzbergerDW.MartinezD.Adverse vascular effects associated with fluoxetine.Am J Psychiatry199214917511443256
- Yaryura-TobiasJA.KirschenH.NimanP.MosbergHJ.Fluoxetine and bleeding in obsessive-compulsive disorder.Am J Psychiatry.19911489492053641
- MhannaMJ.BennetJB.IzattSD.Potential fluoxetine chloride (Prozac) toxicity in a newborn.Pediatrics.19971001581599229710
- SmithM.RobinsonD.Sertraline and vaginal bleeding: a possible association [letter].J Am Geriatr Soc.20025020020112028271
- LakeMB.BirmaherB.WassickS.MathosK.YelovichAK.Bleeding and selective serotonine reuptake inhibitors in childhood and adolescence.J Child Adolesc Psychopharmacol.2000 10353810755580
- ShenW.SwartzC.CalhounJ.Is inhibition of nitric oxide synthase a mechanism for SSRI-induced bleeding? [letter].Psychosomatics.19994026828910341542
- HolzerL.HalfonO.Sertraline and gastrointestinal bleeding in an adolescent girl.J Child Adolesc Psychopharmacol.2006161216553521
- CooperTA.ValcourVG.GibbonsRB.O'Brien-FallsK.Spontaneous ecchymoses due to paroxetine administration.Am J Med.19981041971989528740
- DuijvestijnYC.KalmeijerMD.PassierAL.DahlemP.SmiersF.Neonatal intraventricular haemorrhage associated with maternal use of paroxetine.Br J Clin Pharmacol.20035658158214651736
- BergC.CouturierF.GrassF.AujoulatO.GuillardD.StoeckelC.Saignements sous inhibiteurs spécifiques de la recapture de la sérotonine: à propos d'un cas clinique.Thérapie.2001566567
- KohnS.LabbateLA.Venlafaxine and ecchymoses.Can J Psychiatry.199742919040933
- LinneburSA.SaseenJJ.PaceWD.Venlafaxine-associated vaginal bleeding.Pharmacotherapy.20022265265512013367
- RubellEB.Does imipramine (Tofranil) cause oral bleeding?Pediatrics.1969431441455304344
- BenazziF.Hemorrhages during escitalopram- venlafaxine-mirtazapine combination treatment of depression.Can J Psychiatry.20055018415830829
- DupontA.FontanaP.Bachelot-LozaC.et al.An intronic polymorphism in the PAR-1 gene is associated with platelet receptor density and the response to SFLLRN.Blood.20031011833184012406873
- SewnathME.van HillegersbergR.KoopmanMM.LeviMM.GoumaDJ.Increased perioperative blood loss during treatment with paroxetine.Ned Tijdschr Geneeskd.20021461800180212369443