Abstract
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy(CADASIL) is an inherited small-artery disease of mid-adulthood caused by mutations of the NOTCH3 gene. The disease is responsible for widespread white-matter Iesions associated with lacunar infarctions in varinus subcortical areas. The disease is responsible for migraine with aura and ischemic strokes, and is associated with various degrees of cognitive impairment and with mood disturbances. CADASIL is considered as a unique model to investigate what is known as "subcortical ischemic vascular dementia. "Recent data suggest that the number of lacunar infarctions and severity of cerebral atrophy are the main magnetic resonance imaging markers associated with cognitive and motor disabilities in this disorder. Mood disturbances are reported in 10% to 20% of patients, most often in association with cognitive alterations. Their exact origin remains unknown; the presence of ischemic lesions within the basal ganglia or the frontal white matter may promote the occurrence of these symptoms. Further studies are needed to better understand the relationships between cerebral lesions and both cognitive and psychiatric symptoms in this small-vessel disease of the brain.
CADASIL (arteriopatía cerebral autonómica dominante con infartos subcorticales y Ieucoencefalopatía) es una enfermedad heredada, que afecta pequeñas arterias durante la adultez media y es causada por mutaciones del gen Notch3. La enfermedad es la responsable de extensas lesiones de la sustancia blanca asociadas con infartos lacunares en varias áreas subcorticales. La enfermedad es responsable de migraña con aura y accidentes vasculares isquémicos, y se asocia con deterioro cognitivo de grado variable y con trastornos del ánimo. CADASIL se considera un modelo único para estudiar lo que se conoce como "demencia vascular isquémica subcortical". Datos recientes sugieren que el número de infartos lacunares y la gravedad de la atrofia cerebral son los principales marcadores en las imágenes de resonancia nuclear magnética que se asocian con las incapacidades cognitivas y motoras en esta enfermedad. Los trastornos del ánimo ocurren en el 10% a 20% de los pacientes, y con gran frecuencia se asocian con alteraciones cognitivas. Aun no se conoce el origen exacto de estos síntomas, pero la presencia de lesiones isquémicas en los ganglios basales o en la sustancia bbanca frontal puede facilitar la aparición de estos. Se requiere de futuros estudios para una mejor comprensión de la relación entre las lesiones cerebrales y los síntomas cognitivos y psiquiátricos en esta enfermedad cerebral de pequeños vasos.
Le CADASIL, ou Cerebral autosomal dominant arteriopathy with subcortical infarcts and Ieukoencephalopathy est une affection héréditaire des petites artères cérébrabes survenant chez l'adulte d'âge moyen, due à des mutations du gène Notch3. La maladie est responsable de lésions diffuses de la substance blanche associées à des infarctus lacunaires au niveau des régions sous-corticales cérébrales. Elle est à l'origine de crises de migraine avec aura, d'accidents ischémiques cérébraux et est associée à différents degrés d'altération cognitive et à des troubles de l'humeur. CADASIL est considéré comme un modébe unique d'étude des «démences sous-corticales d'origine ischémique». Des données récentes suggèrent que le nombre d'infarctus lacunaires et la sévérité de l'atrophie cérébrale sont les principaux marqueurs de la maladie associés au handicap cognitif et moteur de la maladie. Les troubles de l'humeur sont rapportés par 10 à 20% des patients, le plus souvent en association avec des altérations cognitives. Leur origine exacte demeure indéterminée, la présence de lésions ischémiques au niveau des noyaux gris ou au sein de la substance blanche frontale pourrait favoriser l'apparition de ces symptômes. Des études complémentaires sont nécessaires pour mieux comprendre les relations entre les lésions cérébrales et les symptômes cognitifs et psychiatriques observés au cours de cette maladie des petits vaisseaux du cerveau.
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)Citation1 is an inherited small-artery disease of mid-adulthood caused by mutations of the NOTCH3 gene on chromosome 19.Citation2 The exact frequency of CADASIL remains unknown. The disease has been diagnosed in European, Asian, African, and American, as well as in Australian families. In France, Germany, and the United Kingdom, several hundreds of CADASIL families have been identified.Citation3, Citation6 Based on a register for the disease in the West of Scotland, Ravzi et al estimated in 2004 that the prevalence of the NOTCH3 gene mutation was about 4.14 per 100 000 adults in this population.Citation7 This frequency is probably underestimated. CADASIL is still underdiagnosed, and may be one of the most frequent hereditary neurological disorders. It is considered as a model of ”pure“ vascular dementia related to small-vessel disease, and as an archetype of the so-called ”subcortical ischemic vascular dementia,“ CADASIL is also responsible for mood disturbances, most often in association with cognitive impairment.
Pathophysiology
CADASIL is characterized by the presence of whitematter rarefaction and subcortical ischemic lesions of the brain, easily detected using magnetic resonance imaging (MRI). Macroscopic examination of the cerebral tissue shows a diffuse myelin pallor and rarefaction of the hemispheric white matter, sparing the U fibers.Citation8 Lesions predominate in the periventricular areas and centrum semi-ovale. They are associated with lacunar infarcts located in the white matter and basal ganglia (lentiform nucleus, thalamus, caudate).Citation9, Citation10 The most severe hemispheric lesions are the most profound.Citation8,Citation11, Citation12 In the brain stem, the lesions are more marked in the pons, and are similar to the pontine ischemic rarefaction of myelin described by Pullicino et al.Citation13 Small, deep infarcts and dilated Virchow-Robin spaces are also associated with the white-matter lesions.
In CADASIL, the walls of cerebral and leptomeningeal arterioles are thickened with a significant reduction of the lumen8; thus, penetrating arteries in the cortex and white matter appear stenosed.Citation14, Citation15 Some inconstant features are similar to those reported in patients with hypertensive encephalopathy16: duplication and splitting of internal elastic lamina, adventitial hyalinosis and fibrosis, and hypertrophy of the media. However, a distinctive feature is the presence of a granular material within the media extending into the adventitial.Citation8, Citation11, Citation17-Citation21 The periodic acid Schiff (PAS) positive staining suggested the presence of glycoproteins; staining for amyloid substance and elastin is negative.Citation9, Citation11 Immunohistochemistry does not support the presence of immunoglubulins. In contrast, the endothelium of the vessels is usually spared. Sometimes, the smooth muscle cells are not detectable, and are replaced by collagen fibers.Citation16 On electron microscopy, the smooth muscle cells appear swollen and often degenerated, some of them with multiple nuclei. There is a granular, electron-dense, osmiophilic material (GOM) within the media.Citation22 This material consists of granules of about 10 to 15 nm in diameter. It is localized close to the cell membrane of the smooth muscle cells, where it appears very dense. The smooth muscle cells are separated by large amounts of this unidentified material.
CADASIL is caused by stereotyped mutations of the NOTCH3 gene.Citation2 Unlike other members of the Notch gene family whose expression is ubiquitous, the NOTCH3 gene is expressed only in vascular smooth muscle cellsCitation23 of arterial vessels.Citation24 It encodes a single-pass transmembrane receptor of 2321 amino-acids, with an extracellular domain containing 34 epidermal growth factor-like (EGF) repeats (including 6 cystein residues) and 3 Lin-12 repeats associated with an intracellular and a transmembrane domains.Citation2,Citation25 This cell surface receptor mediates signal transduction with receptor ligands such
as Jagged (Jag) and Delta (D) on neighboring cells which are also type 1 transmembrane receptors.Citation2,Citation25-Citation27 Domenga et al showed that NOTCH3 is required specifically to generate functional arteries in mice by regulating arterial differentiation and maturation of vascular smooth muscle cells.Citation28 The stereotyped mis-sense mutationsCitation2 or deletionsCitation29 responsible for CADASIL are within epidermal-growth-factor-like (EGF-like) repeats and only located in the extracellular domain of the NOTCH3 protein.Citation30-Citation32 All mutations responsible for the disease lead to an uneven number of cystein residues.
The NOTCH3 protein usually undergoes complex proteolytic cleavages, leading to an extracellular and a transmembrane fragment.Citation33 After cleavage, these two fragments form a heterodimer at the cell surface of smooth muscle cells. In CADASIL, the ectodomain of the NOTCH3 receptor accumulates within the vessel wall of affected subjects.Citation23 This accumulation is found near but not within the characteristic granular osmiophilic material seen on electron microscopy It is observed in all vascular smooth mucle cells, and in pericytes within all organs (brain, heart, muscle, lungs, skin). An abnormal clearance of the NOTCH3 ectodomain from the smooth muscle cell surface is presumed to cause this accumulation.Citation23, Citation34-Citation35 The exact mechanisms underlying this phenomenon have not yet been elucidated.
Vascular abnormalities observed in the brain are also detectable in other organs or territories.Citation9, Citation11 The granular and osmiophilic material surrounding the smooth muscle cells as seen with electron microscopy is also present in the media of arteries located in the spleen, liver, kidneys, muscle, and skin, and also in the wall of carotid and aortic arteries.Citation9, Citation11,Citation36 Altered histochemical binding of plant lectins have been recently identified in the vessel walls of peripheral arteries.Citation37 These vascular lesions can be detected by nerve or muscle biopsy.Citation38, Citation39 The presence of the granular osmiophilic material in the skin vessels now allows confirmation of the intra vitam diagnosis of CADASIL using punch skin biopsies,Citation11, Citation40-Citation43 although the sensitivity and specificity of this method have not yet been completely established. In some cases, the vessel changes may be focal, requiring a thorough evaluation of the biopsy specimen.Citation44 Joutel et al proposed using antiNOTCH3 antibodies to reveal the accumulation of NOTCH3 products within the vessel wall in CADASIL patients as an alternative diagnostic method.Citation45
Transgenic mice expressing mutant NOTCH3 develop the vascular alterations characteristic of CADASIL.Citation46
Experimental data show an impaired autoregulation of cerebral blood flow in these mice and suggest a decreased relaxation or increased resistance of cerebral vessels.Citation47 In addition, flow-induced dilation was significantly decreased and pressure-induced myogenic tone significantly increased in these arteries suggestive of impaired vascular mechanotransduction.Citation48
Neuropsychiatric manifestations
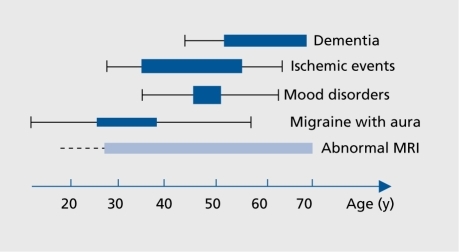
The natural history of CADASIL is summarized in . The first clinical manifestations in CADASIL are attacks of migraine with aura, occurring between the ages of 20 and 40 years.Citation4, Citation41, Citation49 They are observed in 20% to 30% of patients.
Ischemic manifestations, the most frequent clinical manifestations, are reported in 60% to 80% of patients, usually during the fourth and fifth decade.
Neuropsychiatrie manifestations include mood disturbances and various degrees of cognitive impairment. They are observed at all stages of the disorder. A marked decline in cognitive performance is reported in most individuals after age 50 years. Dementia is usually detected at age >60 years, and is found nearly constantly at the end stage of the disorder.Citation4, Citation41, Citation49 Psychiatric symptoms, mainly episodes of mood disturbances, are reported in 10% to 20% of patients during the course of the disease.Citation4, Citation5
Cognitive impairment
Symptomatic patients can remain several years without any neuropsychological decline.Citation50 However, cognitive impairment and dementia represent the second commonest clinical manifestation in CADASIL, after acute ischemic symptoms.
The onset of cognitive deficit is usually mild and insidious, and its exact time is often difficult to ascertain. The cognitive changes may appear a long time before transient ischemic attacks (TIAs) or stroke.Citation51 Cross-sectional studiesCitation52, Citation55 have shown that early in the disease, cognitive functions, most frequently attention and executive functions, may be impaired. In a recent series of 42 patients, attention and executive functions were affected in nearly 90% of patients aged between 35 and 50.Citation55 These disturbances are often associated with alterations in attention and memory suggestive of dysfunction within the subcortical-frontal network.Citation52, Citation55, Citation56 In contrast, other functions such as verbal episodic memory and visuopatial abilities are usually preserved, and may remain spared until the late stages of the disease.
Some tests are particularly sensitive to the detection of the early cognitive changes. They include digit span backwards and forwards, the Trail Making Test part B, the Stroop test, and the Wisconsin Card Sorting test. The errors of CADASIL patients may predominantly affect the time measure in timed tasks (Stroop, Trail Making Test, symbol digit, digit cancellation) though errors in monitoring are also observed to a lesser extent.Citation54 Patients may also show poor strategy and planning when completing tasks such as the Wisconsin Card Sorting Test and the Rey-Osterreith memory test. Memory deficit may be associated with executive dysfunction, but its profile is usually distinct from dementias primarily involving the mesiotemporal temporal cortex such as Alzheimer's disease. This is illustrated by procedures such as those used in the Grober and Buschke test. This test allows differentiation of different phases of memory processes, and is likely to show the preservation of the encoding process even though the retrieval is impaired. It is composed of: (1) an encoding phase where 16 words belonging to 16 different semantic categories have to be retrieved; (ii) 3 phases of free recall and cued recall (the last being delayed); and (iii) a recognition test. In CADASIL, this test distinguishes a pattern characterized by low scores in immediate and delayed free recall, improving with cues and associated with relatively intact recognition. Intrusions may occur in the free recall task. This profile supports preservation of the encoding process, and anatomically, of the mesiotemporal cortex. It is still observed in about two thirds of CADASIL patients with dementia.Citation55
With aging, the cognitive decline becomes more homogenous, with significant changes in all cognitive domains. This extension cannot be ascribed solely to the deterioration of executive performances, but appears to be related to additional alterations in instrumental activities, language, and visuospatial abilities, and suggests a diffuse cortical dysfunction well beyond the subcortical-frontal circuits.Citation55 The development of cognitive impairment appears sometimes to be associated with the occurrence of stroke. Nevertheless, a cognitive deficit and even a dementia state may also occur in patients without any clinical history of stroke. The cognitive profile of CADASIL patients was analyzed before and after the occurrence of strokes in two cross-sectional studies, and showed some discrepant results. Amberla et alCitation53 reported that executive functions were more widely affected, with a significant mental slowing in CADASIL patients with a positive history of stroke. Conversely, Buffon et al observed that visuospatial abilities were mostly impaired in patients with stroke.Citation55 The cognitive deficit most often progresses in the total absence of ischemic events, mimicking in some cases a degenerative dementia.Citation5, Citation57, Citation58 The temporal progression of cognitive symptoms varies among subjects from rapid and marked deterioration to stable or even slightly improving performances.Citation59
Dementia is reported in one third of symptomatic patients at the late phase of the disorder. The frequency of dementia increases considerably with age. Thus, about 60% of patients older than 60 years are demented,Citation4 and more than 80% of deceased subjects were reported to be demented before death.Citation5 When dementia is present, the neuropsychological deficit is usually extensive, involving not only executive functions, attention, and memory, but also reasoning and language performances.Citation55 Dementia is often associated with apathy. Conversely, severe aphasia, apraxia or agnosia are rare.Citation55, Citation56 In addition, demented individuals have a relative preservation of recognition and semantic memory.Citation55 Note worthily, two thirds of them present improvement of memory with cues, which suggests that the encoding process is preserved even at the late stage of the disease, in contrast with the pattern of memory impairment in Alzheimer's disease. Dementia is observed in the absence of any other clinical manifestations in 10% of cases.Citation55 The frequency and severity of the cognitive decline are variable in different members of a given family. The variable location and severity of cerebral tissue damage may play a key role in this variability.Citation60, Citation61
Dementia is always associated with pyramidal signs. Gait difficulties are present in 90%, urinary incontinence in 80% to 90%, and pseudobulbar palsy in half of demented individuals. At the end stage of the disorder, CADASIL patients become bedridden. In a large retrospective study in 411 patients, Opherk et al found that the median age at onset for inability to walk without assistance was 59 years in men and 62 in women, and for bedriddenness, 62 years in men and 66.5 years in women.
Psychiatric symptoms
About one fifth of CADASIL patients experienced episodes of mood disturbances. Their frequency is widely variable between families.Citation5, Citation62 Episodes of major depression were reported by 10% of the 80 CADASIL patients investigated by Peters et al. In some cases, antidepressant drugs were found to be inefficient in relieving symptoms during the most severe episodes.
Few affected subjects have had severe depression of the melancholic type alternating with typical manic episodes suggesting bipolar mood disorder.Citation63 Based on this observation, the potential role of the NOTCH3 gene was thus investigated in familial forms of bipolar disorder, but the results were negative.Citation64 The location of ischemic lesions in basal ganglia and the frontal location of white-matter lesions may play a key role in the occurrence of such mood disturbances in CADASIL patients.Citation65, Citation66
In addition to the mood disorders, a variety of psychiatric manifestations can occur in CADASIL patients. Agoraphobia, addiction to alcohol, and psychotic symptoms have been already reported.Citation4, Citation5,Citation67 The observation of schizophrenia in association with CADASIL appears anecdotal.Citation68
Most often, psychiatric manifestations are observed in patients after diagnosis and a history of ischemic symptoms with signal abnormalities at MRI examination. However these episodes can be inaugural, and may lead to misdiagnosis.Citation5, Citation62, Citation69 Leyhe et al recently reported two cases admitted to a gerontopsychiatric hospital with psychopathological manifestations at the onset of the disorder.Citation70 The first case was a 66-year-old man who was described as a reserved, peaceful, and calm person and who became irritable, started to neglect himself and his duties, and presented a submanic episode which mildly improved after treatment with neuroleptic drugs. The patient started to consume alcohol again after years of abstinence. The second case was a 62-year-old woman with a 2-year episode of depressive symptoms who was initially successfully treated by amitriptyline. She was admitted to hospital because she deteriorated despite medication, developing paranoid ideas and melancholia. The psychopathological symptoms slowly improved on a combination of antidepressant and anxiolytic drugs and neuroleptics. In both cases, the MRI examination and the family history were essential for diagnosis.
Correlations with cerebral tissue lesions
MRI is crucial for the diagnosis of CADASIL, and is much more sensitive than computerized tomography (CT)-scan. It is always abnormal in patients with neurological symptoms other than migraine attacks.Citation1, Citation5, Citation41, Citation71, Citation72 MRI signal abnormalities can also be detected during a presymptomatic period of variable duration. They are observed as early as 20 years of age. After age 35, all subjects having the affected gene have an abnormal MRI.Citation1, Citation71 The frequency of asymptomatic subjects with abnormal MRI decreases progressively with aging, and becomes less than 5% after 60 years.Citation72
MRI shows, on T2-weighted images, widespread areas of increased signal in the white matter associated with focal hyperintensities in basal ganglia, thalamus, and brain stem ( ).Citation72, Citation73 The extent of white-matter signal abnormalities is highly variable. It increases dramatically with age. In subjects under 40 years of age,T2 hypersignals are usually punctuate or nodular with a symmetrical distribution, and predominate in periventricular areas and within the centrum semi-ovale. Later in life, whitematter lesions are diffuse and can involve the whole of white matter, including the U fibers under the cortex.Citation72-Citation75 Scores of severity based on semiquantitative rating scales significantly increase with age, not only in the white matter but also in basal ganglia and brain stem. Frontal and occipital periventricular lesions are constant when MRI is abnormal. The frequency of signal abnormalities in the external capsule (two thirds of cases) and in the anterior part of the temporal lobes (60%) is noteworthy and particularly useful for differential diagnosis with other smallvessel diseases.Citation76, Citation78 T2 hyperintensities can be detected in the corpus callosum.Citation75, Citation79 Brain stem lesions predominate in the pons in areas irrigated by perforating arteries and can involve the mesencephalon.Citation74 In contrast, the medulla is usually spared.
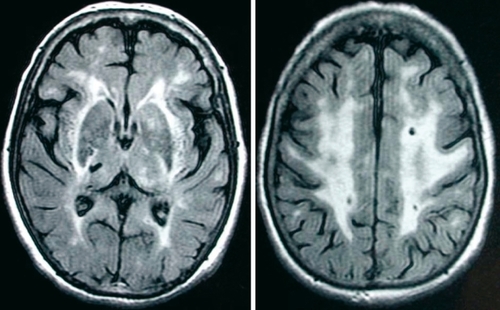
On T1-weighted images, punctiform or larger focal hypointensities are frequent in the same areas and are detected in about two thirds of individuals with T2 hyperintensitiesCitation72 (Figure 2). They are observed both in the white matter and the basal ganglia, but also in the brain stem and correspond mostly to lacunar infarctions. Numerous hypointensities on T1-weighted images may also correspond to Virchow-Robin spaces which are more frequent and extensive in CADASIL than in healthy subjects. MRI signal abnormalities within the temporal white matter in CADASIL and particularly within the subcortical white matter, are considered as a characteristic feature of the disease. They are also caused by a distension of the perivascular space of perforating arteries at the level of the junction of gray and white matter, and by spongiosis in the surrounding parenchyma.Citation80 In contrast with the extent of white-matter hyperintensites weakly associated with the clinical severity,Citation54 the degree of white-matter microstructural damage measured with diffusion tensor imaging (DTI) appears strongly related to the clinical status in CADASIL.Citation81 This is in agreement with the correlations observed between the clinical status and the load of Tl lesions within the white matter, which suggests that the degree of tissue destruction or neuronal loss is crucial for the appearance of disability in CADASIL.Citation60,Citation81, Citation82
The exact mechanisms of cognitive dysfunction in CADASIL remain unknown. The main hypothesis is that accumulation of subcortical lesions may damage in particular the striato-cortical circuits linking basal ganglia to frontal cortical areas, with possible secondary cortical degeneration.Citation60 This hypothesis is supported by evidence of strong correlations between cortical atrophy and the cognitive decline in the disease in both imaging and neuropathological studies. As described previously, severe cortical metabolic depression has indeed been observed by positron-emission tomography (PET) study in association with basal ganglia and thalamic infarcts in a demented patient. The postmortem brain examination of a CADASIL case showed evidence of a diffuse loss of cortical neurons associated with cholinergic denervation.Citation83 In a recent neuropathological study, Viswanathan et al reported the presence of widespread neuronal apoptosis in the cerebral cortices of four CADASIL patients. Semiquantitative analysis suggested that the degree of cortical neuronal apoptosis was related to the extent of white matter lesions and to the intensity of axonal damage in subcortical areasCitation84 and was associated with the severity of cognitive impairment. Therefore, subcortical axonal damage may induce cortical apoptosis through deafferentation and/or retrograde neuronal degeneration in CADASIL.
Disruption of cortical connections may affect striatocortical circuits relaying to the thalamus and basal ganglia as well as cortical networks. This is supported by recent DTI findings from Sullivan et al, who observed: (1) a strong correlation between mean diffusivity measured in the thalamus (which could reflect either direct pathological damage or secondary degeneration due to disruption of white matter tracts relaying in this structure) and executive dysfunction85; (ii) executive performances also correlated with mean diffusivity in the anteroposterior fasciculus of the cingulum bundle which connects the dorsolateral prefrontal lobe with more posterior cortical regions including the hippocampal formation.Citation86
Other clinical manifestations
In contrast with migraine without aura, whose frequency is identical to that estimated in the general population, migraine with aura is reported in 20% to 40% of CADASIL patients, a frequency 4- to 5-fold higher than in the general population. Among pedigrees, this frequency appears extremely variable. The mean age at onset is between 28 to 30 years,Citation49, Citation87 with a wide range from 6 to 48 years. In the largest series, that of Vahedi et al, the frequency of attacks appears extremely variable among affected individuals, from two per week to one to every 3 to 4 years.Citation87 Triggering factors of migraine with aura are similar to those of migraine in the general population (stress, flashing lights, fatigue, physical exercise, head trauma, strong smells, etc).Citation87 The most frequent symptoms are visual, sensory, or aphasie. Motor symptoms are reported in one fifth of CADASIL patients who have attacks of migraine with aura. In contrast with the aura symptoms reported in the general population, more than half of patients have a history of atypical aura such as basilar, hémiplégie, or prolonged aura (International Headache Society criteriaCitation88). A few patients even suffer from severe attacks with unusual symptoms such as confusion, fever, meningitis or coma,Citation89-Citation91 exceptionally reported in migraine with aura.Citation92, Citation93
Ischemic manifestations are the most frequent clinical events in CADASIL: 60% to 85% of patients have had TIAs or completed strokes.Citation4-Citation6, Citation94 They occur at a mean age of 45 to 50 years (extreme limits from 20 to 70 years).Citation4, Citation5, Citation20, Citation41 Age of onset does not differ between men and women. In a recent follow-up study, Peters et al estimated the incidence rate of stroke at 10.4 per 100 person-years.Citation59 Two thirds of them are classical lacunar syndromes: pure motor stroke, ataxic hemiparesis, pure sensory stroke, sensory-motor stroke.Citation5 Other focal neurologic deficits of abrupt onset are less frequent: dysarthria, either isolated or associated with motor or sensory deficit, monoparesis, paresthesiae of one limb, isolated ataxia, nonfluent dysphasia, hemianopia.Citation5
Five percent to 10% of CADASIL patients experience seizures, either focal or generalized.Citation4, Citation20, Citation95 They are usually reported in patients with a positive history of stroke. Epilepsy is usually well-controlled by current antiepileptic drugs.
Other neurological manifestations have occasionally been reported in CADASIL. Parkinsonism has been diagnosed in a a few patients whose clinical presentation can mimic, in rare cases, progressive supranuclear palsyCitation96 Deafness of acute or rapid onset has been reported in a few subjects, but its exact frequency remains unknown.Citation71 Rufa et al reported an acute unilateral visual loss secondary to a nonarteritic ischemic optic neuropathy in a single 60-year-old case who was demented, but this had occurred 33 years earlier at age 27.Citation97
The lack of cranial nerve palsy, spinal cord disease, and symptoms of muscular origin is noteworthy in CADASIL. The exact cause of the radiculopathy reported in one case by Ragno et al remains undetermined.Citation98 Recently, several cases belonging to Italian and Chinese families with clinical and electrophysiological signs of peripheral sensorimotor neuropathy were described.Citation99, Citation100
Conclusion
Neuropsychiatrie manifestations are common in CADASIL, a genetic small-vessel disease leading to “subcortical ischemic vascular dementia.” Cognitive alterations are frequent, and can be detected at the early stages of the disorder, as early as the third decade. They can remain insidious for several years, mainly involving executive functions and attention. A decline in cognitive performances is usually observed after the fifth decade, in association with the recurrence of ischemic manifestations, which leads progressively to dementia associated with pseudobulbar plasy, gait disturbances, and motor impairment.
Psychiatric episodes may also occur during the course of the disorder, rarely before 40 years, most frequently after the occurrence of ischemic events during the fifth or sixth decade. Episodes of mood disorders, the most frequent psychiatric symptoms, are rarely isolated and are often associated with executive dysfunction. When they are inaugural, different features such as their resistance to antidepressant drugs, the association with neurological signs (pyramidal symptoms, cognitive alterations), and the detection of white-matter MRI abnormalities, as well as a positive family history of stroke and dementia, are helpful for raising the diagnosis of CADASIL.
CADASIL is a unique model to investigate the relationships between subcortical ischemic lesions and the cognitive and psychiatric status in small vessel diseases. Further studies are needed to better understand the exact impact of cerebral tissue lesions, and the role of their distribution or of their severity on the occurrence of cognitive and psychiatric symptoms in this disorder.
REFERENCES
- Tournier-LasserveE.JoutelA.MelkiJ.et al.Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy maps to chromosome 19q12.Nat Genet199332562598485581
- JoutelA.CorpechotC.DucrosA.et al.Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia.Nature.19963837077108878478
- OpherkC.PetersN.HerzogJ.LuedtkeR.DichgansM.Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients.Brain.2004127 (Pt 11)2533253915364702
- DichgansM.MayerM.UttnerI.et al.The phenotypic spectrum of CADASIL: clinical findings in 102 cases.Ann Neurol.1998447317399818928
- ChabriatH.VahediK.Iba-ZizenMT.et al.Clinical spectrum of CADASIL: a study of 7 families. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.Lancet.19953469349397564728
- SinghalS.BevanS.BarrickT.RichP.MarkusHS.The influence of genetic and cardiovascular risk factors on the CADASIL phenotype.Brain.20041272031203815229130
- RazviSS.DavidsonR.BoneI.MuïrKW.The prevalence of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) in the west of Scotland.J Neurol Neurosurg Psychiatry.20057673974115834040
- BaudrimontM.DubasF.JoutelA.Tournïer-LasserveE.BousserMG.Autosomal dominant leukoencephalopathy and subcortical ischemic stroke. Aclinicopathological study.Stroke.1993241221258418535
- RuchouxMM.MaurageCA.CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.J Neuropathol Exp Neurol.1997569479649291937
- RuchouxMM.BrulinP.BrillaultJ.DehouckMP.CecchelliR.BataillardM.Lessons from CADASILAnn N Y Acad Sci.200297722423112480754
- RuchouxMM.GuerouaouD.VandenhauteB.PruvoJP.VermerschP.LeysD.Systemic vascular smooth muscle cell impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.Acta Neuropathol (Berl).1995895005127676806
- DavousP.Fallet-BiancoC.[Familial subcortical dementia with arteriopathic leukoencephalopathy. Aclinico-pathological case].Rev Neurol (Paris).19911473763841853035
- PullicinoP.OstowP.MillerL.SnyderW.MuschauerF.Pontine ischemic rarefaction.Ann Neurol.1995374604667717682
- OkedaR.ArimaK.KawaiM.Arterial changes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) in relation to pathogenesis of diffuse myelin loss of cerebral white matter: examination of cerebral medullary arteries by reconstruction of serial sections of an autopsy case.Stroke.2002332565256912411643
- MiaoQ.PalonevaT.TuominenS.et al.Fibrosis and stenosis of the long penetrating cerebral arteries: the cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.Brain Pathol.20041435836415605982
- ZhangWW.MaKC.AndersenO.SouranderP.TollessonPO.OlssonY.The microvascular changes in cases of hereditary multi-infarct disease of the brain.Acta Neuropathol (Berl).1994873173248009965
- GrayF.RobertF.LabrecqueR.et al.Autosomal dominant arteriopathic leuko-encephalopathy and Alzheimer's disease.Neuropathol Appl Neurobiol.19942022308208337
- MikolJ.HeninD.BaudrimontM.et al.[Atypical CADASIL phenotypes and pathological findings in two new French families].Rev Neurol (Paris).200115765566711458185
- BergmannM.EbkeM.YuanY.BruckW.MuglerM.SchwendemannG. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL): a morphological study of a German family.Acta Neuropathol (Berl).1996923413508891065
- DesmondDW.MoroneyJT.LynchT.et al.CADASIL in a North American family: clinical, pathologic, and radiologic findings.Neurology.1998518448499748037
- FilleyCM.ThompsonLL.SzeCI.SimonJA.PaskavitzJF.Kleinschmidt-DeMastersBK.White matter dementia in CADASIL.J Neurol Sci.199916316316710371078
- Gutierrez-MolinaM.Caminero RodriguezA.Martinez Garcia C.Arpa GutierrezJ.Morales BastosC.AmerG.Small arterial granular degeneration in familial Binswanger's syndrome.Acta Neuropathol (Berl).199487981058140899
- JoutelA.AndreuxF.GaulïsS.et al.The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients.J Clin invest.200010559760510712431
- VillaN.WalkerL.LïndsellCE.GassonJ.Iruela-ArispeML.WeinmasterG.Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels.Mech Dev.200110816116411578869
- JoutelA.CorpechotC.DucrosA.et al.Notch3 mutations in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), a mendelian condition causing stroke and vascular dementia.Ann N Y Acad Sci.19978262132179329692
- JoutelA.Tournier-LasserveE.Notch signalling pathway and human diseases.Semin Cell Dev Biol.1998961962510075489
- GrayGE.MannRS.MïtsiadïsE.et al.Human ligands of the Notch receptor.Am J Pathol.19991547859410079256
- DomengaV.FardouxP.LacombeP.et al.Notch3 is required for arterial identity and maturation of vascular smooth muscle cells.Genes Dev.2004182730273515545631
- JoutelA.ChabriatH.VahediK.et al.Splice site mutation causing a seven amino acid Notch3 in-frame deletion in CADASIL.Neurology.2000541874187510802807
- JoutelA.VahediK.CorpechotC.et al.Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients.Lancet.1997350151115159388399
- DottiMT.FedericoA.MazzeiR.et al.The spectrum of Notch3 mutations in 28 Italian CADASIL families.J Neurol Neurosurg Psychiatry.20057673673815834039
- PetersN.OpherkC.BergmannT.CastroM.HerzogJ.DichgansM.Spectrum of mutations in biopsy-proven CADASIL: implications for diagnostic strategies.Arch Neurol.2005621091109416009764
- BlaumuellerCM.QiH.ZagourasP.Artavanis-TsakonasS.Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane.Cell.1997902812919244302
- JoutelA.FrancoisA.ChabriatH.et al.[CADASIL: genetics and physiopathology].Bull Acad Natl Med.200018415351542; discussion 1542-154411261257
- JoutelA.Tournier-LasserveE.[Molecular basis and physiopathogenic mechanisms of CADASIL: a model of small vessel diseases of the brain].J Soc Biol.200219610911512134625
- RobinsonW.GalettaSL.McCluskeyL.FormanMS.BalcerLJ.Retinal findings in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (cadasil).Surv Ophthalmol.20014544544811274696
- Brulin-FardouxP.GodfrainC.MaurageCA.et al.Glycohistochemical characterization of vascular muscle cell destruction in CADASIL subjects by lectins, neoglycoconjugates and galectin-spedfic antibodies.Neuropathol Appl Neurobioi.200329400410
- SchroderJM.SellhausB.JorgJ.Identification of the characteristic vascular changes in a sural nerve biopsy of a case with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL).Acta Neuropathol (Berl).1995891161217732783
- GoebelHH.MeyermannR.RosinR.SchloteW.Characteristic morphologic manifestation of CADASIL, cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy, in skeletal muscle and skin.Muscle Nerve.1997206256279140375
- RuchouxMM.ChabriatH.BousserMG.BaudrimontM.TournierLasserveE.Presence of ultrastructural arterial lesions in muscle and skin vessels of patients with CADASIL.Stroke.199425229122927974561
- ChabriatH.JoutelA.VahediK.Iba-ZizenMT.Tournier-LasserveE.BousserMG.[CADASIL. cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalophathy].Rev Neurol (Paris).1997153(67)3763859684003
- EbkeM.DichgansM.BergmannM.et al.CADASIL: skin biopsy allows diagnosis in early stages.Acta Neurol Scand.1997953513579228269
- JenJ.CohenAH.YueQ.et al.Hereditary endotheliopathy with retinopathy, nephropathy, and stroke (HERNS).Neurology.199749132213309371916
- SchultzA.SantoïannïR.Hewan-LoweK.Vasculopathïc changes of CADASIL can be focal in skin biopsies.Ultrastruct Pathol.19992324124710503743
- JoutelA.FavroleP.LabaugeP.et al.Skin biopsy ïmmunostaïnïng with a Notch3 monoclonal antibody for CADASIL diagnosis.Lancet.20013582049205111755616
- RuchouxMM.DomengaV.BrulïnP.et al.Transgenic mice expressing mutant Notch3 develop vascular alterations characteristic of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.Am J Pathol.200316232934212507916
- LacombeP.OligoC.DomengaV.Tournier-LasserveE.JoutelA.Impaired cerebral vasoreactivity in a transgenic mouse model of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy arteriopathy.Stroke.2005361053105815817893
- DubrocaC.LacombeP.DomengaV.et al.Impaired vascular mechanotransduction in a transgenic mouse model of CADASIL arteriopathy.Stroke.20053611311715569862
- DesmondDW.MoroneyJT.LynchT.ChanS.ChinSS.MohrJP.The natural history of CADASIL: a pooled analysis of previously published cases.Stroke.1999301230123310356105
- TrojanoL.RagnoM.MancaA.CarusoG.A kindred affected by cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). A 2-year neuropsychological follow-up.J Neurol.19982452172229591223
- Lesnik ObersteinSA.van den BoomR.MiddelkoopHA.et al.Incipient CADASIL.Arch Neurol.20036070771212756134
- TailliaH.ChabriatH.KurtzA.et al.Cognitive alterations in nondemented CADASIL patients.CerebrovascDis. 1998897101
- AmberlaK.WaljasM.TuominenS.et al.Insidious cognitive decline in CADASIL.Stroke.2004351598160215143298
- HoltmannspotterM.PetersN.OpherkC.et al.Diffusion magnetic resonance histograms as a surrogate marker and predictor of disease progression in CADASIL: a two-year follow-up study.Stroke.2005362559256516269644
- BuffonF.PorcherR.HernandezK.et al.Cognitive profile in CADASILJ Neurol Neurosurg Psychiatry.20067717518016421118
- PetersN.OpherkC.DanekA.BallardC.HerzogJ.DichgansM.The pattern of cognitive performance in CADASIL: a monogenic condition leading to subcortical ischemic vascular dementia.Am J Psychiatry20051622078208516263847
- HederaP.FrïedlandRP.Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: study of two American families with predominant dementia.J Neurol Sci.199714627339077492
- ChabriatH.Tournier-LasserveE.VahediK.et al.Autosomal dominant migraine with MRI white-matter abnormalities mapping to the CADASIL locus.Neurology.199545108610917783868
- PetersN.HerzogJ.OpherkC.DichgansM.A two-year clinical followup study in 80 CADASIL subjects: progression patterns and implications for clinical trials.Stroke.2004351603160815155961
- MolkoN.PappataS.ManginJF.et al.Diffusion tensor imaging study of subcortical gray matter in cadasil.Stroke.2001322049205411546896
- ZekryD.DuyckaertsC.BelmïnJ.et al.The vascular lesions in vascular and mixed dementia: the weight of functional neuroanatomy.Neurobiol Aging.20032421321912498955
- ChabriatH.JoutelA.VahediK.Iba-ZizenMT.Tournier-LasserveE.BousserMG.[CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy): clinical features and neuroimaging].Bull Acad Natl Med.200018415231531; discussion 1531-1533.11261256
- KumarSK.MahrG.CADASIL presenting as bipolar disorder.Psychosomatics.1997383973989217412
- AhearnEP.SpeerMC.ChenYT.et al.Investigation of Notch3 as a candidate gene for bipolar disorder using brain hyperintensities as an endophenotype.Am J Med Genet.200211465265812210282
- AylwardED.Roberts-WillieJV.BartaPE.et al.Basal ganglia volume and white matter hyperintensities in patients with bipolar disorder.Am J Psychiatry.199456876938166310
- BhatiaK.MarsdenC.The behavioural and motor consequences of focal lesions of the basal ganglia in man.Brain.19941178598767922471
- VerïnM.RollandY.LandgrafF.et al.New phenotype of the cerebral autosomal dominant arteriopathy mapped to chromosome 19: migraine as the prominent clinical feature.J Neurol Neurosurg Psychiatry.1995595795857500094
- LagasPA.JuvonenV.Schizophrenia in a patient with cerebral autosomally dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL disease).Nord J Psychiatry.200155414211827605
- ThomasN.MathewsT.LoganathanA.Cadasil: presenting as a mood disorder.Scott Med J.200247363712058662
- LeyheT.WïendlH.BuchkremerG.WormstallH.CADASIL: underdiagnosed in psychiatric patients?Acta Psychiatr Scand.2005111392396; discussion 396-39715819734
- Tournier-LasserveE.Iba-ZizenMT.RomeroN.BousserMG.Autosomal dominant syndrome with strokelike episodes and leukoencephalopathy.Stroke.199122129713021926242
- ChabriatH.LevyC.TailliaH.et al.Patterns of MRI lesions in CADASILNeurology.1998514524579710018
- DichgansM.FïlïppïM.BruningR.et al.Quantitative MRI in CADASIL: correlation with disability and cognitive performance.Neurology.1999521361136710227618
- ChabriatH.MrissaR.LevyC.et al.Brain stem MRI signal abnormalities in CADASILStroke.1999304574599933287
- CoulthardA.BlankSC.BushbyK.KalariaRN.BurnDJ.Distribution of cranial MRI abnormalities in patients with symptomatic and subclinical CADASIL.Br J Radiol.20007325626510817040
- AuerDP.PutzB.GosslC.ElbelG.GasserT.DichgansM.Differential lesion patterns in CADASIL and sporadic subcortical arteriosclerotic encephalopathy: MR imaging study with statistical parametric group comparison.Radiology.200121844345111161160
- O'SullivanM.JaroszJM.MartinRJ.DeasyN.PowellJF.MarkusHS.MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL.Neurology.20015662863411245715
- MarkusHS.MartinRJ.SimpsonMA.et al.Diagnostic strategies in CADASILNeurology.2002591134113812395806
- IwatsukiK.MurakamiT.ManabeY.et al.Two cases of Japanese CADASIL with corpus callosum lesion.Tohoku J Exp Med.200119513514011846209
- van Den BoomR.Lesnik ObersteinSA.van DuinenSG.et al.Subcortical lacunar lesions: an MR imaging finding in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.Radiology.200222479179612202716
- ChabriatH.PappataS.PouponC.et al.Clinical severity in CADASIL related to ultrastructural damage in white matter: in vivo study with diffusion tensor MRI.Stroke.1999302637264310582990
- ChabriatH.Diffusion histograms in CADASILStroke.2005362526XX16269643
- MesulamM.SiddiqueT.CohenB.Cholinergic denervation in a pure multi-infarct state: observations on CADASILNeurology.2003601183118512682331
- ViswanathanA.GrayF.BousserMG.BaudrimontM.ChabriatH.Cortical neuronal apoptosis in CADASILStroke.2006372690269517008611
- O'SullivanM.SinghalS.CharltonR.MarkusHS.Diffusion tensor imaging of thalamus correlates with cognition in CADASIL without dementia.Neurology.20046270270715007117
- O'SullivanM.BarrickTR.MorrisRG.ClarkCA.MarkusHS.Damage within a network of white matter regions underlies executive dysfunction in CADASILNeurology.2005651584159016301485
- VahediK.ChabriatH.LevyC.JoutelA.Tournier-LasserveE.BousserMG.Migraine with aura and brain magnetic resonance imaging abnormalities in patients with CADASIL.Arch Neurol.2004611237124015313840
- Society. IH. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain.Cephalalgia.1988 suppl78
- SchonF.MartinRJ.PrevettM.CloughC.EnevoldsonTP.MarkusHS.“CADASIL coma”: an underdiagnosed acute encephalopathy.J Neurol Neurosurg Psychiatry.20037424925212531961
- Le BerI.CarluerL.DeracheN.LaleveeC.LedozeF.DeferGL.Unusual presentation of CADASIL with reversible coma and confusion.Neurology.2002591115111612370482
- FeuerhakeF.VolkB.OstertagCB.et al.Reversible coma with raised intracranial pressure: an unusual clinical manifestation of CADASIL.Acta Neuropathol (Berl).200210318819211810186
- RequenaI.IndakoetxeaB.LemaC.SantosB.Garcia-CastineiraA.AriasM.[Coma associated with migraine].Rev Neurol.1999291048105110637870
- FitzimonsRB.WolfendenWH.Migraine coma. Meningitic migraine with cerebral oedema associated with a new form of autosomal dominant cerebellar ataxia..Brain.1991108555577
- BousserMG.Tournier-LasserveE.Summary of the proceedings of the First International Workshop on CADASIL. Paris, May 19-21, 1993.Stroke.1994257047077907448
- MalandriniA.CarreraP.CiacciG.et al.Unusual clinical features and early brain MRI lesions in a family with cerebral autosomal dominant arteriopathy.Neurology.199748120012039153443
- Van GerpenJA.AhlskogJE.PettyGW.Progressive supranuclear palsy phenotype secondary to CADASIL.Parkinsonism Relat Disord.2003936736912853237
- RufaA.De StefanoN.DottiMTet al.Acute unilateral visual loss as the first symptom of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.Arch Neurol.20046157758015096408
- RagnoM.Tournier-LasserveE.FiorïMG.et al.An Italian kindred with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL).Ann Neurol.1995382312367654071
- LvH.YaoS.ZhangW.et al.[Clinical features in 4 Chinese families with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL).].Beijing Da Xue Xue Bao.20043649650015489930
- SicurelliF.DottiMT.De StefanoN.et al.Peripheral neuropathy in CADASILJ Neurol.20052521206120915827866