Abstract
Accumulating evidence suggests that psychotropic agents such as mood stabilizers, antidepressants, and antipsychotics realize their neurotrophic/neuroprotective effects by activating the mitogen activated protein kinaselextracellular signal-related kinase, PI3-kinase, and winglesslglycogen synthase kinase (GSK) 3 signaling pathways. These agents also upregulate the expression of trophic/protective molecules such as brain-derived neurotrophic factor, nerve growth factor, B-cell lymphoma 2, serine-threonine kinase, and Bcl-2 associated athanogene 1, and inactivate proapoptotic molecules such as GSK-3, They also promote neurogenesis and are protective in models of neurodegenerative diseases and ischemia. Most if not all, of this evidence was collected from animal studies that used clinically relevant treatment regimens. Furthermore, human imaging studies have found that these agents increase the volume and density of brain tissue, as well as levels of N-acetyl aspartate and glutamate in selected brain regions. Taken together, these data suggest that the neurotrophic/neuroprotective effects of these agents have broad therapeutic potential in the treatment, not only of mood disorders and schizophrenia, but also neurodegenerative diseases and ischemia.
La evidencia acumulada sugiere que los agentes psicotrópicos como los esiabilizadores del ánimo, los antidepresivos y los antipsicóticos producen sus efectos neurotróficos/neuroproiectores mediante la activación de la quinasa relacionada con la señal de la proteinquinasalextracelular activada por mitógen o, la quinasa PI3 y las vías de señates de la winglesslglicógeno sintelasa quinasa 3 (GSK), Estos agentes también regulan hacia arriba la expresion de moléculas tróficaslprotecioras como el factor neurotrófico cerebral, el factor de crecimiento neural, la proteina 2 del linfoma de células B, la quinasa serina-treonina y el atanogen 1 asociado a Bcl-2, e inactivan moléculas proapoptóticas como la GSK-3. Ellos también promueven la neurogénesis y son protectores en modelos de enfermedades neurodegeneratives e isquemia. La mayor parte, sino toda esta evidencia se recolectó a partir de estudios animales que utilizaron esquemas terapéuticos clínicamente relevantes. Además, en humanos los estudios de imágenes han encontrado que estos fármacos aumentan el volumen y la densidad del tejido cerebral, como también los niveles de N-acetil aspartato y glutamato en determinadas regiones cerebrales. Tomados en conjunto, estos datos sugieren que los efectos neurotróficoslneuroprotectores de estos fármacos tienen un gran potencial terapéutico en el tratamiento no sólo de los trastornos del ánimo y de la esquizofrenia, sino que también en enfermedades neurodegenerativas y en la isquemia.
D'après un nombre croissant d'arguments, les psychotropes, comme les régulateurs de l'humeur, les antidépresseurs et les antipsychotiques, exercent leurs effets neuroirophiques et neuroprotecteurs en activant 3 voies de signalisation: la MAP (mitogen activated protein)/ERK (extracellular signal-related) kinase, la kinase PI3 et la Wnt/GSK (win gless/kinase glycogène synthase). Ces voies régulent également positivement l'expression des molécules irophiques/proiecirices comme le BDNF (brain-derived neurotrophic factor), le NGF (nerve growth factor), la protéine BCL2 (B-cell lymphoma 2), la kinase sérine-thréonine et le BAG-1 (aihanogène 1 associé à BCL-2), et des molécules proapoptotiques inactivées comme la GSK-3. Elles favorisent aussi la neurogenèse et exercent un effet protecteur dans les maladies neurodegeneratives et l'ischémie. La plupart de ces preuves, si ce n'est toutes, sont issues d'études animales utilisant des schémas thérapeutiques cliniquement pertinents. De plus, des études d'imagerie sur l'homme ont montré que ces agents augmentaient le volume et la densité du tissu cérébral ainsi que les taux de N-acétyl aspartate et de glutamate dans les régions cérébrales sélectionnées. Au total, ces données suggèrent que leurs effets neuroirophiques/neuroprotecteurs ont un potentiel thérapeutique large non seulement dans les troubles de l'humeur et la schizophrénie mais aussi dans les maladies neurodegeneratives et l'ischémie.
Historically, psychiatric disorders such as mood disorders and schizophrenia have been conceptualized as neurochemical illnesses. However, accumulating data from both postmortem and brain imaging studies reveal morphological changes in the brains of individuals with these illnesses. These changes include ventricle enlargement, volumetric reduction, attenuation of neuronal viability marker N-acetyl aspartate (NAA), and atrophy or loss of neurons and glial cells in selective cortical and limbic brain regions. Several psychotropic agents - defined as chemical substances that act primarily on the central nervous system (CNS) to alter brain function - are used to treat psychiatric disorders. These psychotropic agents include mood stabilizers, antidepressants, and antipsychotic medications. Many of these drugs exert significant effects on signaling pathways enhancing neurotrophic and neuroprotective cellular mechanisms. Loosely defined, neurotrophic effects can be considered a therapeutic strategy intended to augment proliferation, differentiation, growth, and regeneration, whereas neuroprotective effects slow or halt the progression of neuronal atrophy or cell death following the onset of disease or clinical decline.
In this article, we review evidence from animal and human studies reporting that psychotropic agents affect molecular targets and signaling cascades associated with enhanced neurotrophic and neuroprotective mechanisms, as well as reverse or reduce behavioral deficits associated with preclinical animal models of mania and depression and other psychiatric illnesses. While much of this work has focused on the mood stabilizers lithium and valproate, we will also review the available evidence that antidepressants and antipsychotics exert similar neurotrophic effects.
Mood stabilizers
Mood stabilizers are used to treat bipolar disorder (BPD), which is characterized by mood shifts between mania (characterized by elevated mood, increased energy, impaired judgment, and racing thoughts) and depression (characterized by low mood, anhedonia, etc). These therapeutic agents do not simply target a particular neurotransmitter system or cellular signaling cascade, but diverse targets implicated in many signaling pathways. This may be because mood stabilizers were often designed to treat different disorders, and their use in the treatment of BPD frequently arose through serendipity; for instance, the mood stabilizers carbamazepine and valproate - both used to treat the manic symptoms of BPD - have anticonvulsant properties and were developed for the treatment of epilepsy. In addition, our incomplete understanding of the pathophysiology of BPD, in which both genetic and environmental predispositions may impair cellular resilience and lead to dysfunctional circuits and synapses, further supports the notion that these agents affect diverse targets. Indeed, mood stabilizers may achieve their therapeutic effects by working through these diverse targets to restore cellular resilience; notably, however, chronic treatment is necessary for their neurotrophic and neuroprotective actions to improve functional plasticity in cortical and limbic circuits and synapses.
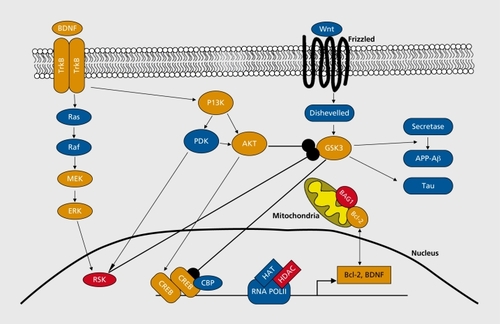
Below we focus on several intracellular signaling pathways targeted by mood stabilizers that may underlie these therapeutic mechanisms: i) the mitogen activated protein kinase/extracellular signal-related kinase (MAPK/ERK) pathway, ii) the phosphatidylinositol 3 kinase (PI3K) pathway, and ii) the wingless/glycogen synthase kinase 3 (Wnt/GSK3) pathway.
Mood stabilizers activate neurotrophic signaling pathways
Mood stabilizers have been reported to activate the intracellular MAPK/ERK signaling pathway (). Citation1-Citation3 This pathway is used by neurotrophins, neurotransmitters, and neuropeptides to exert their neurotrophic and neuroprotective effects by specifically enhancing progenitor cell proliferation and differentiation, neuronal process growth and regeneration, neuronal survival, and long-term synaptic remodeling and plasticity.Citation4-Citation7 The key components of the pathway are three serine/threonine-selective kinases: RAP, MEK, and MAPK/ERK. GTP bond RAS, a small G protein, induces RAF activity. RAF then phosphorylates and activates MEK, which in turn phosphorylates and activates MAPK/ERK. The targets of ERK include protein kinases such as RSK and MNK, ion channel, neurotransmitter receptors, and transcription factors. RSK and MNK are thought to phosphoryiale and activate transcription factor cAMP response element binding (CREB). CREB regulates the expression of many different genes, including B-cell lymphoma 2 (Bcl-2)Citation1,Citation8 and brain-derived neurotrophic factor (BDNF)Citation9 to enhance neuroprotection and neuronal survival mechanisms.
In SH-SY5Y human neuroblastoma cells, the mood stabilizers lithium and valproate activated AP-1 transcription factors, and that activation was blocked by a MEK inhibitor.Citation10 That study also demonstrated that valproate increased levels of activated phospho-ERK and reporter gene expression driven by ELK, an ERK-regulated transcription factor; that activation was further blocked by RAS and RAF functional null mutant.Citation10 Valproate also promoted neurite outgrowth and expression of GAP-43 in these cells, which could be blocked by an ERK pathway inhibitor.Citation10 Taken together, these data indicate that valproate activates the ERK pathway and produces neurotrophic-like cellular effects through this activation. Follow-up studies showed that in cultured cerebral cortical cells, valproate induced ERK pathway activation in a manner that was more sustainable than activation bygrowth factors.Citation3
Valproate-induced activation of the ERK pathway has also been identified in primary cortical neurons,Citation11 cerebral progenitor cells,Citation12 hippocampal progenitor cells,Citation13 and endothelial cells.Citation14 Lithium similarly increased activation-phosphorylation of ERK in SY5Y cells,Citation15 cerebellar granular cells,Citation16,Citation17 hippocampal progenitor cells,Citation18,Citation19 and primary cortical neurons.Citation11 Lithium inhibited the ERK pathway in cultures of serum-deprived, quiescent astrocytes.Citation17 Furthermore, lamotrigine, an anticonvulsant prescribed to prevent recurrences of depression or mania in BPD, did not affect the ERK pathway in SH-SY5Y cellsCitation15 or primary cortical neuronsCitation11; however, lamotrigine still showed neuroprotective effects in models of ischemia and kainate (KA)-induced neurotoxicity, perhaps through glutamate release inhibition.Citation20,Citation21 Taken together, these in vitro data suggest that activation of the ERK pathway is common to only a subgroup of mood stabilizers and is cell-type specific.
In a series of in vivo studies, Chen and colleagues found that chronic treatment with lithium or valproate increased levels of activated phospho-ERK, phosphoRSK1, and activated phospho-CREB in prefrontal cortex and hippocampus.Citation2,Citation3 Lithium-induced increases in activated phospho-ERKs were also observed in the caudate/putamen of infant mouse brains.Citation22 Another study found that valproate increased levels of activated phospho-ERK and activated phospho-CREB in mice with intracerebral hemorrhage.Citation23 Another study found that valproate did not induce changes in phospho-ERK levels in the nucleus accumbens, and reduced phosphoERK levels in the amygdala,Citation24 suggesting that mood stabilizer-induced ERK pathway activation/inactivation may be brain region-specific.
The phosphatidylinositol 3 kinase (PI3K) pathway - a regulator of neuronal survival and plasticity - is also regulated by growth factors (Figure 1). Citation6,Citation25-Citation27 Upon trophic factor stimulation (Figure 1), the regulatory subunit of PI3K is stimulated by the adapter proteins Grb-2 and Grb-2-associated binding protein 1/2 (Gabl/2), resulting in PI3K activation. The catalytic subunit of PI3K is also stimulated by direct interaction with activated RAS. Activated PI3K converts plasma membrane lipid phosphatidylinositol-4,5-biphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3).PIP3 provides docking sites for phosphoinositide-dependent kinase (PDK) and the serine-threonine kinase Akt (also known as protein kinase B, PKB). Simultaneous binding of PDK and Akt at the PI3K activation site facilitates phosphorylation of Akt by PDK1 and enhances Akt activity. Akt then phosphorylates glycogen synthase kinase-3 (GSK-3), which in contrast to most phosphorylations, leads to the inactivation of this enzyme,Citation28 PI3K, PDK, Akt, and GSK-3 are thought to be the major components of the PI3K pathway.
Mood stabilizers target several of these major components of the PI3K pathway. Acute (minutes to hours) or subacute (several days) lithium treatment of cerebellar granule cells, for instance, increased levels of activated phospho-Akl as well as phospho-GSK-3, a product of Akt-catalyzed phosphorylation.Citation29 Interestingly, similar effects were noted in human SH-SY5Y cells treated with lithium and valproate.Citation1,Citation30 The increases were blocked by PI3K inhibitors, indicating that they required PI3K activation.Citation29 Chronic lithium and valproate treatment also increased levels of phospho-GSK-3p in mouse cerebral cortex and hippocampus.Citation30-Citation32 Lithium injections (200 mg/kg of body weight, IP) significantly increased levels of phospho-Akt, phospho-GSK-3oc and phospho-GSK-3 p in the striatum of dopamine transporter knockout (DAT KO) mice within 30 minutes of administration.Citation33 Valproate increased activated brain phospho-Akt in skeletal muscle in a mouse model of Duchenne's muscular dystrophy,Citation34 as well as in a mouse model of intracerebral hemorrhage.Citation23 These data demonstrate that lithium and valproate stimulate the PI3K pathway in vivo and subsequently inactivate GSK-3.
Mood stabilizers upregulate levels of neurotrophic and neuroprotective molecules
Studies show that lithium and valproate increased mRNA and protein levels of neurotrophins such as BDNF, glial cell-line derived neurotrophic factor (GDNF), neurotrophin 3 (NT-3), and vascular endothelial growth factor (VEGF) in cultured cells and brain regions.Citation2,Citation35-Citation46 Furthermore, lithium increased serum BDNF levels in patients with Alzheimer's disease.Citation47 The effects of mood stabilizers on BDNF levels are thought to be mediated via several different mechanisms. These mechanisms may include enhancing BDNF promoter activationCitation40,Citation43,Citation45 by stimulating the ERK and PI3K pathways using lithium or valproate, leading to CREB activation and CRE-mediated gene transcription of BDNF. Valproate's inhibition of histone deacetylase (HDAC) via an epigenetic mechanism - a molecular process that leads to gene activation and deactivation - may also play a role.Citation40,Citation43,Citation45
In addition to targeting neurotrophic mechanisms, mood stabilizers also target neuroprotective molecules such as Bcl-2. Bcl-2 and its family proteins are the major modulators of apoptosis. Notably, numerous studies have shown that chronic treatment with lithium or valproate upregulates Bcl-2 and Bcl-2 associated athanogene (BAG-1) levels in the brain or nerve tissues.Citation23,Citation32,Citation48-Citation54 This upregulation appears to be partially due to activation of the ERK and PI3K pathways, as well as increased transcriptional activity of CREB.Citation1
Mood stabilizers promote neurogenesis and neuronal process growth
The discovery that mood stabilizers can regulate growth factors and produce neurotrophin-like molecular effects led investigators to explore whether these agents could augment hippocampal neurogenesis. Lithium and valproate were indeed found to promote hippocampal neurogenesis in neuronal cell culture and rodent studies.Citation3,Citation18,Citation19,Citation32,Citation55 In vitro evidence showed that lithium induced neuronal differentiation of hippocampal neural progenitor cells via a phospho-ERK and phospho-CREB dependent pathway.Citation19 An in vivo study showed that lithium increased survival of newborn cells in hippocampus, and that an ERK pathway inhibitor blocked lithium's survival effects.Citation56 Valproate activated the ERK pathway and promoted differentiation of hippocampal neural progenitor cells in culture; however, valproate's differentiation effects were thought to be mediated through FIDAC inhibition, not ERK pathway activation.Citation13,Citation57 Whether valproate uses multiple mechanisms to induce hippocampal neurogenesis in intact animals remains to be elucidated.
Valproate also promoted neurite growth in cultured cells (for reviews see refs 6,7), which was blocked by ERK pathway inhibition.Citation3,Citation10 Animal studies have found that valproate facilitated axonal regeneration and motor function recovery after sciatic nerve axotomy.Citation51,Citation58 Lithium similarly enhanced survival and axonal regeneration of cultured retinal ganglion cells,Citation4 protected retinal ganglion cells following partial optic nerve crush in rats,Citation59 and promoted axonal regeneration of rubrospinal tract (RST) neurons following injury to the spinal cord.Citation53 These neuroprotective findings are, in part, thought to be mediated by Bcl-2. Another recent study showed that lithium facilitated motor function recovery and axonal regeneration after spinal cord injury, and these effects were associated with increased inactivated-phospho-GSK-3. Further support for GSK-3 's role in lithium's neuroprotective effects came from a study where lithium's effects were mimicked by the GSK-3 inhibitor SB415286.Citation60
Another area where lithium exerts neuroprotective effects is in stress-induced morphological alterations. Chronic behavioral stress shortens apical dendrites in the CA3 region of the hippocampus in rodents. Lithium treatment initiated 2 weeks before the stress and continued throughout a 3-week period of stress attenuated these stress-induced reductions in apical dendritic lengths.Citation61 Although the molecular mechanisms of this lithium-induced morphological action are still not fully understood, they are particularly important because social-psychological and behavioral stress cause a variety of brain changes and are key contributing factors to mood disorders.Citation62-Citation65
Evidence from human imaging studies for neurotrophic/neuroprotective actions of mood stabilizers
As noted previously, brain imaging studies show brain ventricular enlargements,Citation66,Citation67 cortical regional morphometric reductions,Citation68-Citation72 and cerebral and hippocampal level reductions of NAACitation72-Citation78 in individuals with mood disorders, especially in unmedicated patients with a family history of mood disorders.
Intrigued by the discovery that Bcl-2 is upregulated by mood stabilizers, investigators used imaging tools to assess the effects of mood stabilizers on brain morphometric and neurochemical measures. In a magnetic resonance imaging (MRI) study, Moore and colleagues found that lithium treatment increased cerebral grey matter volume,Citation79 Similar findings were also obtained in other longitudinal and cross-sectional studies of cerebral grey matter volume,Citation80 left anterior cingulate volume,Citation70 right anterior cingulate volume,Citation81 hippocampal volume,Citation82-Citation86 and amygdala volume,Citation86 A cross-sectional study found that valproate similarly increased left anterior cingulate volume in individuals with BPD.Citation87
One initial longitudinal MRS study found brain regional increases in NAA levels in individuals with BPD and healthy subjects treated for 4 weeks with lithium,Citation79 a finding replicated by other investigators.Citation88-Citation90 NAA levels were also found to be correlated with brain lithium levels in a study of elderly patients with BPD.Citation91 Valproate was similarly found to increase hippocampal NAA levels.Citation72
Mood stabilizers produce neuroprotective effects in animal models of disease
Mood stabilizers are known to protect cultured cells from a variety of insults (for reviews see refs 6,7,92,93). In this section, we review the neuroprotective effects of lithium and valproate in a series of models of brain ischemia, neurodegeneration, and neuroinflammation (eg, cerebral ischemia, Alzheimer's disease (AD), Huntington's disease, amyotrophic lateral sclerosis (ALS), HIV- associated cognitive impairments, and spinocerebellar ataxia).
In a seminal study using an animal model of ischemia, Chuang and colleagues found that ischemic infarct size induced by occlusion of the left middle cerebral artery was markedly reduced by lithium treatment administered beforeCitation94 or afterCitation95 the induction of ischemia; these findings have since been replicated by other investigators.Citation96-Citation104 Follow-up studies showed that valproate had similar protective effects on ischemia-induced brain infarction.Citation105,Citation106
ALS is a progressive, lethal neurodegenerative disease with no known cure. Riluzole, which prolongs the survival of patients by several months, is the only FDAapproved treatment for this disease. Interestingly, riluzole itself has been associated with neuroprotective properties.Citation107 SOD1-G93A mice, a model for ALS, carry a high copy number of this transgene with the G93A human SOD1 mutation. Studies show that valproateCitation108 and lithiumCitation109,Citation110 both delay disease onset and prolong lifespan in SOD1-G93A mice. Furthermore, lithium and valproate together produce an additive protective effect in SOD1-G93A mice compared with either treatment alone.Citation110 Notably, a clinical trial found that lithium, compared with riluzole, further delays disease progression and death in individuals with ALS.Citation109
With regards to AD, diverse studies have suggested that lithium's neuroprotective effects may have a potential role in the therapeutics of this disease. AD is a leading cause of dementia in the aging population and the most common neurodegenerative disease without an effective treatment. Briefly, the histological hallmarks of AD include amyloid plaques, neurofibrillary tangles, and neuronal loss. The plaques consist of insoluble deposits of amyloid-beta (Aβ) protein and cellular material outside and around neurons. Aβ protein is derived from amyloid precursor protein (APP) through an endoproteolytic cleavage catalyzed by β- and γ-secretase. Mutations in the genes of presenilins - the core component of γ-secretase, APP, and tau are associated with AD. One series of experiments in cultured cells found that GSK-3α increased Aβ production,Citation111 and that chronic lithium treatment reduced Aβ produced in a genetic mouse model of AD. These mice expressed APP-Swedish (Tg2576) and also carried a knock-in mutation of presenilin-1 (PS1P264L). In a transgenic mouse strain overexpressing mutated (London V717I and Swedish K670M/N671L) human APP (hAPP751), lithium treatment reduced Aβ production, improved performance in the water maze, and preserved dendritic structure in the frontal cortex and hippocampus, all of which are associated with decreased APP phosphorylation and increased levels of phospho-GSK-3β.Citation112 In another animal model of AD where APP23 transgenic mice carried human APP751 cDNA with the Swedish double mutation at positions 670/671, Qing and colleagues observed that valproate treatment decreased Aβ production, reduced neuritic plaque formation, and improved memory deficits; these effects were also associated with increased phospho-GSK-3β.Citation113,Citation114
Neurofibrillary tangles are formed by hyperphosphorylated tau, a microtubule-associated protein. GSK-3 is a major tau kinase and GSK-3β hyperactivity is known to contribute to tau hyperphosphorylation in cell and animal models. Interestingly, lithium treatment reduced tau phosphorylation in the brains of mice overexpressing mutated (London V717I and Swedish K670M/N671L) human APP (hAPP751).Citation112 In another AD model (3xTG-AD), lithium treatment reduced brain tau phosphorylation and increased brain GSK-3α and β phosphorylation at the inhibitory sites; however, it did not improve memory or reduce Aβ protection.Citation115
Given these promising preclinical data, studies began to examine the potential long-term neurotrophic/neuroprotective effects of lithium and valproate in humans. While some studies suggest that naturalistic lithium treatment may indeed be associated with neuroprotective effects in individuals with AD (see, for instance refs 47,116-118), considerably more data are required. Nevertheless, this remains a promising and exciting area for further investigation.
Spinocerebellar ataxia type 1 (SCA1) is a dominantly inherited neurodegenerative disorder characterized by progressive motor and cognitive dysfunction. In a SCA1 mouse model, chronic administration of lithium initiated before or after the deficit onset had a positive effect on multiple behavioral measures and hippocampal neuropathology.Citation119 Indeed, clinical trials of lithium in patients with SCA1 are currently ongoing (see http://clinicaltrials.gov/ for more information).
Finally, the neuroprotective effects of lithium and valproate have also been reported in additional disease and insult models, including animal models of Huntington's disease,Citation120,Citation121 Parkinson's disease,Citation122 HlV-induced encephalitis and dementia,Citation123,Citation124 and aluminum-induced neurodegeneration.Citation50 At least some of these effects are associated with increased Bcl-2 levels.Citation50,Citation120,Citation121,Citation122
Antidepressants
Chemical antidepressants used to treat depressive disorders, or depressive symptoms in other psychiatric disorders, include monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), or selective norepinephrine reuptake inhibitors (SNRIs). These chemical antidepressants act by increasing monoamines (serotonin and/or norepinephrine) in the synaptic cleft, which occurs immediately; however, for most patients, therapeutic effects are observed only after a few days, and often not until 2 weeks or more, This suggests that adaptive changes in cellular signaling cascades may underlie their therapeutic effects.Citation125 Two such pathways that will be considered below include the MAPK/ERK and the Wnt/GSK signaling cascade (Figure 1), which may enhance neurotrophic and neuroprotective mechanisms in addition to neurogenesis. Interestingly, nonchemical antidepressants such as electroconvulsive therapy (ECT) and exercise also target these pathways and may employ similar therapeutic mechanisms.
Antidepressants affect prominent signaling cascades involved in neuronal protection and survival
As noted above, activation of the MAPK/ERK and Wnt/GSK signaling cascades (Figure 1) ultimately targeted by antidepressants may result in enhanced neuroprotective and survival mechanisms. For instance, both chemical antidepressants and ECT increase BDNF levels. In rats, ECT increased BDNF and its receptor (trkB) in the hippocampus.Citation126 A similar effect was also found following chronic (21 days) but not acute treatment with different classes of antidepressants (the MAOI tranylcypromine, the SSRI sertraline, and the TCA desipramine). Furthermore, chronic antidepressant treatment also increased the expression of CREB mRNA in the rat hippocampus,Citation127 suggesting a potential regulatory mechanism for BDNF through CRE-mediated gene transcription.
Exercise has also been reported to upregulate many factors in the MAPK signaling pathway including BDNF, trkB, MEK2, and ERK2.Citation128-Citation133 A recent study found that exercise-induced upregulation of BDNF at the mRNA and protein level and phosphorylation of survival factor Akt both occurred via a CREB-dependent mechanism.Citation134 Interestingly, the SNRI reboxetine also depends on CREB activation (phosphorylation) in order to show similar changes in BDNF and Akt.
In humans, serum levels of BDNF levels are decreased in unmedicated depressed patients compared with depressed patients currently taking antidepressants or healthy controls.Citation135 BDNF serum levels were also found to be negatively correlated with depression scores as assessed by the Hamilton Depression Rating Scale (HDRS). Interestingly, BDNF itself also possesses antidepressant-like effects in rodent models used to screen antidepressants following direct infusion into either the midbrainCitation136 or hippocampus.Citation137 This enhancement in BDNF by antidepressants may help promote mechanisms of neuronal protection and survival key to reducing stress-induced damage.
Antidepressants have also been found to have neuroprotective effects. For instance, the SSRI fluoxetine prevented the neurotoxic effects of ecstasy (3,4-methylenedioxymethamphetamine, MDMA).Citation138,Citation139 Mechanistically, fluoxetine's neuroprotective effects, in addition to restoring serotonin levels, may result from activation of p38 MAPK, BDNF, and GDNF.Citation140
MAOIs (eg, pargyline, nialamide, tranylcypromine) inhibiting both MAO-A and MAO-B protected against l-methyl-4-phenyl-l,2,5,6-tetrahydropyridine (MPTP)induced dopaminergic neural toxicity.Citation141 Interestingly, Ladostigil, a MAOI used to treat both depression and neurodegeneration that has promising neuroprotective effects, reportedly activated Bcl-2 family members and BDNFCitation142 in addition to ERK1/2 (p44/42 MAPK).Citation143
Notably, exercise also possesses neuroprotective effects. Carro and colleagues showed that rodents subjected to treadmill running were protected against various insults ranging from treatment with the neurotoxin domoic acid to inherited neurodegeneration affecting Purkinje cells of the cerebellum.Citation144 These protective effects depended in part on the neurotrophic factor insulin-like growth factor I (IGF-1); infusing a blocking anti-IGF-1 antibody reduced the protective effects of exercise.
Effects of antidepressants on neurogenesis in animals
Antidepressants increase hippocampal adult neurogenesis following chronic but not acute treatment. Chronic treatment with the SSRI fluoxetine, the MAOI tranylcypromine, or the SNRI reboxetine produced an approximately 20% to 40% increase in bromodeoxyuridine BrdU-labeled hippocampal cellsCitation145; at least 2 weeks of fluoxetine treatment was required to enhance neurogenesis. Furthermore, while stress decreases hippocampal neurogenesis, chronic antidepressant treatment prevented these stress-induced changes.Citation146,Citation147 ECT also increased neurogenesis in rodents,Citation148 as well as hippocampal synapse number.Citation149 ECT was similarly found to increase neurogenesis in nonhuman primates,Citation150 and exercise increased hippocampal neurogenesisCitation151 in addition to enhancing hippocampal-dependent learning and long-term potentiation (LTP).Citation151
The molecular mechanisms underlying these antidepressant-induced enhancements in neurogenesis may involve the MAPK/ERK and/or Wnt/GSK-3 pathways. A very recent study found that suppression of the gene disrupted in schizophrenia 1 (DISCI), which has been implicated in BPD, major depressive disorder (MDD), and schizophrenia, decreased neurogenesis by acting through GSK3β.Citation152
Antidepressants and the reversal of stress-induced changes in neuronal plasticity
In terms of clinical implications, a recent meta-analysis found enhanced antidepressant response in the Met variant of the BDNF 66Val/Met polymorphism in individuals with MDD.Citation153 Curiously, 66Met allele carriers have a lower neuronal distribution of BDNF in addition to decreased activity-dependent BDNF secretion. Given the hypothesis that antidepressant effects are partially mediated through enhanced BDNF secretion, it would seem contradictory that 66Met allele carriers, with their attenuated BDNF secretion, have a more robust response to antidepressants. In addition to enhanced antidepressant treatment response, this BDNF polymorphism was also associated with decreased episodic memory performance, lower hippocampal activation (as measured by fMRI), and lower hippocampal NAA levels in humans.Citation154 In a mouse model of the BDNF-Met variant in which BDNF-Met was expressed at normal levels, but regulated secretion from neurons was reduced, fluoxetine was unable to ameliorate a stressinduced anxiety phenotype.Citation155 Taken together, these data suggest a more complicated picture that requires a better understanding of proper BDNF function (and not just its expression); however, normal BDNF function does appear to be important for proper hippocampal function and mood regulation.
Notably, severely depressed patients show elevated levels of the stress hormone Cortisol, which is thought to result from a dysfunctional hypothalamic-pituitaryadrenal (HPA) axis negative feedback circuit,Citation156,Citation157 and which may ultimately contribute to the hippocampal damage and volumetric changes reported in the literature, Subjects with MDD were found to have significantly smaller hippocampal volumes, and these reductions correlated with total duration of depression but not with age,Citation158,Citation159 suggesting that the stress associated with depression may have contributed to these volumetric changes. Further support for this notion comes from studies reporting that individuals with post-traumatic stress disorder (PTSD) had impaired hippocampal function (deficits in short term memory, total recall, longterm storage, and retrieval) but no overall IQ differences when compared with controlsCitation160; MRI studies found that these PTSD patients had an 8% smaller right hippocampus than controls.Citation161 In addition, the polymorphism in the BDNF gene (val(66)met) has also been associated with reduced hippocampal volume.Citation162
Interestingly, antidepressants can reverse some of these changes. In tree shrews, the selective serotonin reuptake enhancer (SSRE) tianeptine prevented the decreased brain metabolites (NAA, creatine, phosphocreatine), suppressed neurogenesis, and reduced hippocampal volume associated with chronic psychosocial stress.Citation147 In another study, chronic treatment with antidepressants induced hippocampal neurogenesis, blocked inescapable foot shock stress-induced decreases in hippocampal neurogenesis, and normalized corticosterone levels and behavioral deficits.Citation145,Citation163 Finally, repetitive transcranial magnetic stimulation (rTMS) has also shown putative neurotrophic properties in patients with MDD. In one study, rTMS improved refractory depression by augmenting catecholamines and BDNF,Citation164 while another study found that rTMS augmented BDNF in drug-resistant patients.Citation165
Antipsychotics
Antipsychotic medications are traditionally categorized as typical (also known as traditional, conventional, or classic neuroleptics) or atypical (second generation). Several typical antipsychotics have a higher dopamine D2 receptor affinity than atypical antipsychotics, which bind to a broader group of receptors, including dopamine, serotonin, glutamate, histamine, α-adrenergic, and muscarinic receptors.Citation166 While antipsychotics can have an immediate impact on symptoms such as agitation, it often takes weeks before improvement is seen in other symptoms, such as delusions; however, recent findings suggest these improvements may emerge more rapidly than previously believed.Citation167,Citation168 As with mood stabilizers and antidepressants, it is likely that these drugs improve many facets of psychosis through mechanisms beyond their fundamental interaction with dopaminergic, serotonergic, muscarinic, and other receptor families.
Chronic treatment with conventional antipsychotics can lead to adverse extrapyramidal side effects (EPS), which mimic the neurodegenerative disorder Parkinson's disease, as well as the potentially irreversible condition known as tardive dyskinesia.Citation169 These effects are less common with atypical antipsychotics, which also have improved efficacy in treating the negative symptoms associated with schizophrenia, though their overall benefit is still unclearCitation170; atypical antipsychotics also have their own adverse metabolic side effects like weight gain and diabetes.Citation171 As highlighted below, these two classes of antipsychotics show markedly different profiles for activating neuroplasticity cascades, and for enhancing neuroprotection and neurogenesis in both animal studies and patient-based studies.
Antipsychotics alter the expression of prominent intracellular cascades and influence neuroplasticity and neuroprotection in animal models
Studies conducted in rodents and cell lines have demonstrated that some antipsychotics can induce significant changes in intracellular cascades that are involved in neuroplasticity and neuroprotection against excitotoxic insults, including ERK/MAPK, Akt, Bcl-2, and BDNF pathways. Acute treatment with the atypical antipsychotic clozapine led to increased levels of active (phosphorylated) MEK1/2 in rat prefrontal cortex,Citation172 while chronic treatment with the atypical antipsychotic olanzapine increased pERK1/2 levels in rat prefrontal cortex (PFC).Citation173 Interestingly, Browning and colleagues observed decreases in pERK1/2 following either a single injection of olanzapine or haloperidol (a typical antipsychotic), but chronic haloperidol did not alter pERK1/2 levels. Multiple studies in phenochromocytoma (PCI 2) cells have also noted upregulation of pERK1/2,pAkt, and PI3K following olanzapine treatment.Citation174,Citation175 Antipsychotics have also been shown to influence other prominent cascades discussed above, including Bcl-2,Citation176 GSK-3,Citation177 and CREB.Citation178
Many studies have assessed the effects of antipsychotics on neurotrophic factors such as BDNF and nerve growth factor (NGF), and have noted significant differences between typical and atypical antipsychotics. Typical antipsychotics such as haloperidol tend to reduce BDNF expression in regions of the hippocampusCitation179-Citation181 and striatum.Citation182 Atypical antipsychotics do not consistently downregulate BDNF, and their more diverse set of responses make critical evaluations more challenging (see ref 183). One recent study noted that, after chronic (90-day) treatment with haloperidol, transitioning to the atypical antipsychotics olanzapine or risperidone appeared to rescue BDNF expression back to near baseline levels.Citation182,Citation184
Studies have demonstrated that chronic or high doses of typical antipsychotics, like haloperidol and reserpine, can be neurotoxic, inducing apoptosis and reducing cell viability. Though the mechanism remains unclear, high doses of haloperidol induced apoptosis in the striatum and substantia nigra of rats treated via acute intraperitoneal injection.Citation185 In vivo investigations have further noted that brain regions like the striatum, hypothalamus, and limbic structures were some of the most drasticallyaltered cytoarchitecturally by conventional antipsychotics.Citation186 Macaque monkeys treated for 17 to 27 months with therapeutic levels of either haloperidol or olanzapine had reduced brain volumes by ~10%, most prominently in the parietal and frontal brain lobes.Citation187 Other studies found the opposite effect, that chronic treatment of rats with haloperidol increased striatal volume.Citation188
In contrast, atypical antipsychotics appear to have some neuroprotective functions. For example, pretreatment with the atypical antipsychotics clozapine, quetiapine, or risperidone prevented PC12 cell death following serum withdrawal,Citation189 while olanzapine reduced cell death in PC12, SH-SY5Y, and 3T3 cells following a number of death-inducing treatments.Citation174 Neuroprotective properties have also been demonstrated for the atypical antipsychotic olanzapine against various insults, such as oxidative stressorsCitation190 and ischemia.Citation191 Olanzapine also upregulated the expression of Bcl-2 in rat frontal cortex and the hippocampus, as well as the expression of BDNF in the hippocampus.Citation176,Citation181 Studies have suggested that other atypical antipsychotics, such as risperidone and quetiapine, have neuroprotective properties that might be relevant to their clinical efficacy.Citation192,Citation193 For instance, one study found that the effects of stress-induced decreases of BDNF could be prevented by pretreatment with quetiapine,Citation194
Overall, the findings suggest that antipsychotics can alter prominent intracellular cascades and ultimately induce neurotrophic or neurotoxic responses in vivo and in vitro, depending upon the drug conditions, time course, and brain region under consideration.
Effects of antipsychotics on neurogenesis in animals
Initial studies detected increased neurogenesis in the gerbil hippocampus following haloperidol treatment,Citation195 but not in the rat hippocampus.Citation145 Two more recent studies found that haloperidol did not affect neurogenesis,Citation196,Citation197 although a study that used osmotic pumps (instead of daily intraperitoneal injections or delivery in drinking water) found that haloperidol increased neural stem cell (NSC) proliferation in the adult rat forebrain.Citation198 Furthermore, the researchers demonstrated that this proliferation was mediated through D2 receptor stimulation in vitro, suggesting that under certain conditions, haloperidol could promote neurogenesis through its suppression of D2-mediated pathways that normally prevent NSC proliferation.
Atypical antipsychotics have shown a more consistent profile of enhancing neurogenesis, but do not necessarily increase neuronal survival or differentiation into adult neurons. Chronic treatment of rats with clozapine or olanzapine, for example, augmented the number of BrdU-labeled cells in the dentate gyrusCitation196 or prefrontal cortex and dorsal striatum.Citation197 Although both studies detected increased proliferation of precursor cells, neither found a significant difference in the number of BrdU-positive, mature neurons in the weeks following treatment with antipsychotics. Quetiapine has also been shown to reverse the inhibition of hippocampal neurogenesis caused by chronic restraint stress, and significantly increase the number of BrdU-labeled immature neurons detected compared with vehicle-treated, stressed rats.Citation199
Effect of antipsychotics on NAA levels, brain volume, and density in patients
Studies conducted with schizophrenic patients have examined NAA measures and volumetric brain changes using 1H-MRS and MRI, respectively, to elucidate the effects of chronic antipsychotic treatment. Patients treated with atypical antipsychotics had higher NAA measures in the frontal lobesCitation200 and anterior cingulate gyrusCitation201 than those treated with typical antipsychotics. Another study measured NAA changes during antipsychotic treatment and after cessation for at least 2 weeks in individual patients using a within-subject design and found significant decreases (~9%) in NAA levels in the dorsolateral prefrontal cortex after ending antipsychotic treatment; no differences were found in other brain regions.Citation202
Schizophrenia, the disorder most often treated with antipsychotics, is well-known to be associated with reduced regional volumes, increased ventricle size,Citation203 and deteriorating course,Citation204 making it difficult to distinguish volumetric changes induced by antipsychotic treatment. Overall, studies suggest that there are differences in the brain volumes of patients treated with antipsychotics compared with controls, or within groups of patients treated chronically with typical versus atypical antipsychotics; for a thorough analysis, see ref 186. One study of patients with first-episode psychosis found that treatment with haloperidol reduced grey matter volume; in contrast, olanzapine-treated patients showed no significant reductions compared with controls.Citation205 Another recent study found that olanzapine increased NAA in the prefrontal cortex of remitted adolescent patients with mania compared with nonremitted patients.Citation206 Although this suggests a possible in vivo neurotrophic effect, this finding needs further replication because the primary aim of the study - a NAA increase following olanzapine treatment, independent from clinical change - was negative. In fact, it is possible that the NAA increase seen in responders was more closely related to improved mood than to olanzapine's neurotrophic properties.
Closing remarks
The growing data from molecular, cellular, animal, and human studies described in this review support the notion that psychotropic agents used to treat the major psychiatric disorders - especially mood stabilizers - are associated with significant neurotrophic/neuroprotective effects. These effects may enhance cellular resilience and plasticity in dysfunctional synapses and neural circuitry implicated in psychiatric disorders. The crux of such research is that, in addition to their proven ability to treat psychiatric disorders, these agents may be useful in the treatment of neurodegenerative illnesses and ischemia. Similarly, psychotropic agents developed for the treatment of neurodegenerative illnesses may be beneficial as therapeutics for major psychiatric illnesses. Currently, several clinical trials are being conducted to evaluate the feasibility of using lithium and valproate to treat a variety of neurodegenerative diseases. Indeed, neuroprotection is the most consistent biological outcome associated with lithium treatment. There is hope that these clinically safe and widely used agents will slow disease progression, and perhaps produce functional improvements. Furthermore, because lithium and valproate stimulate the ERK and PI3K pathways, increase BDNF, Bcl-2, and BAG-1 expression, block HDAC activity (valproate only), and inhibit GSK-3 alpha and beta activities, continued study of these agents may elucidate other clinically relevant targets, ultimately leading to improved treatments for these devastating disorders. Additional data are also needed to understand whether the neurotrophic and neuroprotective effects of mood stabilizers, antidepressants, and antipsychotics are cell-type or circuitry specific, and to what extent their neurotrophic/neuroprotective effects contribute to their therapeutic action. Finally, gaining insight into rapid-acting versus long-term compensatory changes facilitated by these psychotropic agents will pave the way for the next generation of therapeutics, whose dual nature will provide both a rapid treatment response to restore function, as well as support long-term changes to maintain successful treatment and prevent relapse.
Selected abbreviations and acronyms
| BDNF | = | brain-derived neurotrophic factor |
| CREB | = | cAMP response element binding |
| ERK | = | extracellular signal-related kinase |
| MAPK | = | mitogen activated protein kinase |
| NAA | = | N-acetyl aspartate |
| P13K | = | FI3-kinase |
| Wnt/GSK | = | wingless/glycogen synthase kinase |
Funding for this work was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH). The authors have no conflicts of interest, financial or otherwise, to disclose.
REFERENCES
- CresonTK.YuanP.ManjiHK.ChenG.Evidence for involvement of ERK, PI3K, and RSK in induction of Bcl-2 by valproate.J Mol Neurosci.20093712313418677583
- EinatH.YuanP.GouldTD.et al.The role of the extracellular signal-regulated kinase signaling pathway in mood modulation.J Neurosci.2003237311731612917364
- HaoY.CresonT.ZhangL.et al.Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis.J Neurosci.2004246590659915269271
- HuangX.WuDY.ChenG.ManjiH.ChenDF.Support of retinal ganglion cell survival and axon regeneration by lithium through a Bcl-2dependent mechanism.Invest Ophthalmol Vis Sci.20034434735412506095
- SweattJD.Mitogen-activated protein kinases in synaptic plasticity and memory.Curr Opin Neurobiol.20041431131715194111
- ChenG.CresonT.SharonE.HaoY.WangG.Neurotrophic actions of mood-stabilizers: a recent research discovery and its potential clinical applications.Curr Psych Rev.20051173185
- ChenG.ManjiHK.The extracellular signal-regulated kinase pathway: an emerging promising target for mood stabilizers.Curr Opin Psychiatry.20061931332316612219
- RiccioA.AhnS.DavenportCM.BlendyJA.GintyDD.Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons.Science.19992862358236110600750
- TaoX.FinkbeinerS.ArnoldDB.ShaywitzAJ.GreenbergME.Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism.Neuron.1998207097269581763
- YuanPX.HuangLD.JiangYM.GutkindJS.ManjiHK.ChenG.The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth.J Biol Chem.2001276316743168311418608
- Di DanielE.MudgeAW.MaycoxPR.Comparative analysis of the effects of four mood stabilizers in SH-SY5Y cells and in primary neurons.Bipolar Disord.20057334115654930
- JungGA.YoonJY.MoonBS.et al.Valproic acid induces differentiation and inhibition of proliferation in neural progenitor cells via the betacatenin-Ras-ERK-p21Cip/WAF1 pathway.BMC Cell Biol.200896619068119
- HsiehJ.NakashimaK.KuwabaraT.MejiaE.GageFH.Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells.Proc Natl Acad Sci U S A.2004101166591666415537713
- MichaelisM.SuhanT.MichaelisUR.et al.Valproic acid induces extracellular signal-regulated kinase 1/2 activation and inhibits apoptosis in endothelial cells.Cell Death Differ.20061344645316167071
- MaiL.JopeRS.LiX.BDNF-mediated signal transduction is modulated by GSObeta and mood stabilizing agents.J Neurochem.200282758312091467
- KopniskyKL.Chalecka-FranaszekE.Gonzalez-ZuluetaM.ChuangDM.Chronic lithium treatment antagonizes glutamate-induced decrease of phosphorylated CREB in neurons via reducing protein phosphatase 1 and increasing MEK activities.Neuroscience.200311642543512559097
- PardoR.AndreolottiAG.RamosB.PicatosteF.ClaroE.Opposed effects of lithium on the MEK-ERK pathway in neural cells: inhibition in astrocytes and stimulation in neurons by GSK3 independent mechanisms.J Neurochem.20038741742614511119
- SonH.YuIT.HwangSJ.et al.Lithium enhances long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus.J Neurochem.20038587288112716419
- KimJS.ChangMY.YuIT.et al.Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo.J Neurochem.20048932433615056276
- MajR.FarielloRG.UkmarG.et al.PNU-151774E protects against kainate-induced status epilepticus and hippocampal lesions in the rat.Eur J Pharmacol.199835927329831289
- WiardRP.DickersonMC.BeekO.NortonR.CooperBR.Neuroprotective properties of the novel antiepileptic lamotrigine in a gerbil model of global cerebral ischemia.Stroke.1995264664727886726
- YoungC.StraikoMM.JohnsonSA.CreeleyC.OlneyJW.Ethanol causes and lithium prevents neuroapoptosis and suppression of pERK in the infant mouse brain.Neurobiol Dis.20083135536018595723
- SinnDI.KimSJ.ChuK.et al.Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation.Neurobiol Dis.20072646447217398106
- CasuMA.SannaA.SpadaGP.FalzoiM.MongeauR.PaniL.Effects of acute and chronic valproate treatments on p-CREB levels in the rat amygdala and nucleus accumbens.Brain Res.20071141152417270156
- CantleyLC.The phosphoinositide 3-kinase pathway.Science.20022961655165712040186
- HuangEJ.ReichardtLF.Trk receptors: roles in neuronal signal transduction.Annu Rev Biochem.20037260964212676795
- SegalRA.Selectivity in neurotrophin signaling: theme and variations.Annu Rev Neurosci.20032629933012598680
- CrossDA.AlessiDR.CohenP.AndjelkovichM.HemmingsBA.Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B.Nature.19953787857898524413
- Chalecka-FranaszekE.ChuangDM.Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons.Proc Natl Acad Sci U S A.1999968745875010411946
- De SarnoP.LiX.JopeRS.Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium.Neuropharmacology.2002431158116412504922
- KozlovskyN.AmarS.BelmakerRH.AgamG.Psychotropic drugs affect Ser9-phosphorylated GSK-3 beta protein levels in rodent frontal cortex.Int J Neuropsychopharrnacol.20069337342
- YazlovitskayaEM.EdwardsE.ThotalaD.et al.Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation.Cancer Res.200666111791118617145862
- BeaulieuJM.SotnikovaTD.YaoWD.et al.Lithium antagonizes dopamlne-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade.Proc Natl Acad Sci U S A.20041015099510415044694
- GurpurPB.LiuJ.BurkinDJ.KaufmanSJ.Valproic acid activates the PBK/Akt/mTOR pathway in muscle and ameliorates pathology in a mouse model of Duchenne muscular dystrophy.Am J Pathol.2009174999100819179609
- FukumotoT.MorinobuS.OkamotoY.KagayaA.YamawakiS.Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain.Psychopharmacoiogy (Berl).2001158100106
- AngelucciF.AloeL.Jimenez-VasquezP.MatheAA.Lithium treatment alters brain concentrations of nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor in a rat model of depression.Int J Neuropsychopharrnacol.20036225231
- JacobsenJP.MorkA.The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels.Brain Res.2004102418319215451381
- CastroLM.GallantM.NilesLP.Novel targets for valproic acid: up-regulation of melatonin receptors and neurotrophic factors in C6 glioma cells.J Neurochem.2005951227123616313512
- FreyBN.AndreazzaAC.CereserKM.et al.Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania.Life Sci.20067928128616460767
- ChenPS.PengGS.LiG.et al.Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes.Mol Psychiatry.2006111116112516969367
- WalzJC.FreyBN.AndreazzaAC.et al.Effects of lithium and valproate on serum and hippocampal neurotrophin-3 levels in an animal model of mania.J Psychiatr Res.20084241642117512948
- BredyTW.WuH.CregoC.ZellhoeferJ.SunYE.BaradM.Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear.Learn Mem.20071426827617522015
- YasudaS.LiangMH.MarinovaZ.YahyaviA.ChuangDM.The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons.Mol Psychiatry.200914515917925795
- OmataN.MurataT.TakamatsuS.et al.Neuroprotective effect of chronic lithium treatment against hypoxia in specific brain regions with upregulation of cAMP response element binding protein and brain-derived neurotrophic factor but not nerve growth factor: comparison with acute lithium treatment.Bipolar Disord.20081036036818402624
- WuX.ChenPS.DallasS.et al.Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons.Int J Neuropsychopharrnacol.20081111231134
- GuoS.AraiK.StinsMF.ChuangDM.LoEH.Lithium upregulates vascular endothelial growth factor in brain endothelial cells and astrocytes.Stroke.20094065265518974377
- LeyheT.EschweilerGW.StranskyE.et al.Increase of BDNF serum concentration in lithium treated patients with early Alzheimer's disease.J Alzheirners Dis.200916649656
- WlodarczykBC.CraigJC.BennettGD.CalvinJA.FinnellRH.Valproic acid-induced changes in gene expression during neurulation in a mouse model.Teratology.1996542842979098922
- ChenG.ZengWZ.YuanPX.et al.The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS.J Neurochem.1999728798829930766
- GhribiO.HermanMM.SpauldingNK.SavoryJ.Lithium inhibits aluminum-induced apoptosis in rabbit hippocampus, by preventing cytochrome c translocation, Bcl-2 decrease, Bax elevation and caspase-3 activation.J Neurochem.20028213714512091474
- CuiSS.YangCP.BowenRC.et al.Valproic acid enhances axonal regeneration and recovery of motor function after sciatic nerve axotomy in adult rats.Brain Res.2003975(1-2)22923612763612
- ZhouR.GrayNA.YuanP.et al.The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers.J Neurosci.2005254493450215872096
- YickLW.SoKF.CheungPT.WuWT.Lithium chloride reinforces the regeneration-promoting effect of chondroitinase ABC on rubrospinal neurons after spinal cord injury.J Neurotrauma.20042193294315307905
- KagaS.ZhanL.AltafE.MaulikN.Glycogen synthase kinase-3beta/betacatenin promotes angiogenic and anti-apoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium.J Mol Cell Cardiol.20064013814716288908
- ChenG.RajkowskaG.DuF.Seraji-BozorgzadN.ManjiHK.Enhancement of hippocampal neurogenesis by lithium.J Neurochem.2000751729173410987856
- YanXB.HouHL.WuLM.LiuJ.ZhouJN.Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia.Neuropharmacology.20075348749517686496
- YuIT.ParkJY.KimSH.LeeJS.KimYS.SonH.Valproic acid promotes neuronal differentiation by induction of proneural factors in association with H4 acetylation.Neuropharmacology.20095647348019007798
- WuF.XingD.PengZ.RaoT.Enhanced rat sciatic nerve regeneration through silicon tubes implanted with valproic acid.J Reconstr Microsurg.20082426727618496777
- SchuettaufF.RejdakR.ThalerS.et al.Citicoline and lithium rescue retinal ganglion cells following partial optic nerve crush in the rat.Exp Eye Res.2006831128113416876158
- DillJ.WangH.ZhouF.LiS.Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS.J Neurosci.2008288914892818768685
- WoodGE.YoungLT.ReaganLP.ChenB.McEwenBS.Stress-induced structural remodeling in hippocampus: prevention by lithium treatment.Proc Natl Acad Sci U S A.20041013973397815001711
- HeimC.NemeroffCB.The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies.Biol Psychiatry.2001491023103911430844
- McEwenBS.Mood disorders and allostatic load.Biol Psychiatry.2003542002712893096
- ShelineYl.Neuroimaging studies of mood disorder effects on the brain.Biol Psychiatry.20035433835212893109
- BownCD.WangJF.YoungLT.Attenuation of N-methyl-D-aspartate-mediated cytoplasmic vacuolization in primary rat hippocampal neurons by mood stabilizers.Neuroscience.200311794995512654346
- McDonaldC.ZanelliJ.Rabe-HeskethS.et al.Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder.Biol Psychiatry.2004 Sep 155641141715364039
- KemptonMJ.GeddesJR.EttingerU.WilliamsSC.GrasbyPM.Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder.Arch Gen Psychiatry.2008651017103218762588
- DrevetsWC.PriceJL.SimpsonJR.Jr.et al.Subgenual prefrontal cortex abnormalities in mood disorders.Nature.19973868248279126739
- HirayasuY.ShentonME.SalisburyDF.et al.Subgenual cingulate cortex volume in first-episode psychosis.Am J Psychiatry.19991561091109310401458
- SassiRB.BrambillaP.HatchJP.et al.Reduced left anterior cingulate volumes in untreated bipolar patients.Biol Psychiatry.20045646747515450781
- CampbellS.MarriottM.NahmiasC.MacQueenGM.Lower hippocampal volume in patients suffering from depression: a meta-analysis.Am J Psychiatry.200416159860715056502
- AtmacaM.YildirimH.OzdemirH.OgurE.TezcanE.Hippocampal 1H MRS in patients with bipolar disorder taking valproate versus valproate plus quetiapine.Psychol Med.20073712112917094813
- BertolinoA.FryeM.CallicottJH.et al.Neuronal pathology in the hippocampal area of patients with bipolar disorder: a study with proton magnetic resonance spectroscopic imaging.Biol Psychiatry.20035390691312742678
- WinsbergME.SachsN.TateDL.AdalsteinssonE.SpielmanD.KetterTA.Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder.Biol Psychiatry.20004747548110715353
- SoaresJC.Contributions from brain imaging to the elucidation of pathophysiology of bipolar disorder.Int J Neuropsychopharrnacol.20036171180
- CecilKM.DelBelloMP.SellarsMC.StrakowskiSM.Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders.J Child Adolesc Psychopharmacol.20031354555514977467
- Yildiz-YesilogluA.AnkerstDP.Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: a systematic review of the in vivo proton magnetic resonance spectroscopy findings.Prog Neuropsychopharrnacol Biol Psychiatry.200630969995
- OlveraRL.CaetanoSC.FonsecaM.et al.Low levels of N-acetyl aspartate in the left dorsolateral prefrontal cortex of pediatric bipolar patients.J Child Adolesc Psychopharmacol.20071746147317822341
- MooreGJ.BebchukJM.WildsIB.ChenG.ManjiHK.MenjiHK.Lithium-induced increase in human brain grey matter.Lancet.20003561241124211072948
- SassiRB.NicolettiM.BrambillaP.et al.Increased gray matter volume in lithium-treated bipolar disorder patients.Neurosci Lett.200232924324512165422
- BeardenCE.ThompsonPM.DalwaniM.et al.Greater cortical gray matter density in lithium-treated patients with bipolar disorder.Biol Psychiatry.20076271617240360
- BeyerJL.BurchittB.GersingK.KrishnanKR.Patterns of pharmacotherapy and treatment response in elderly adults with bipolar disorder.Psychopharmacol Bull.20084110211418362874
- BeardenCE.ThompsonPM.DuttonRA.et al.Three-dimensional mapping of hippocampal anatomy in unmedlcated and lithium-treated patients with bipolar disorder.Neuropsychopharmacology.2008331229123817687266
- YucelK.McKinnonMC.TaylorVH.et al.Bilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: a longitudinal MRI study.Psychopharmacology (Berl).200719535736717705060
- YucelK.TaylorVH.McKinnonMC.et al.Bilateral hippocampal volume increase in patients with bipolar disorder and short-term lithium treatment.Neuropsychopharmacology.20083336136717406649
- FolandLC.AltshulerLL.SugarCA.et al.Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium.Neuroreport.20081922122418185112
- AtmacaM.OzdemirH.CetinkayaS.et al.Cingulate gyrus volumetry in drug free bipolar patients and patients treated with valproate or valproate and quetiapine.J Psychiatr Res.20074182182716950400
- SilverstonePH.WuRH.O'DonnellT.UlrichM.AsgharSJ.HanstockCC.Chronic treatment with lithium, but not sodium valproate, increases cortical N-acetyl-aspartate concentrations in euthymic bipolar patients.Int Clin Psychopharmacol.200318737912598817
- BrambillaP.StanleyJA.NicolettiMA.et al.1H magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in bipolar disorder patients.J Affect Disord.200586616715820271
- BrambillaP.StanleyJA.SassiRB.et al.1H MRS Study of dorsolateral prefrontal cortex in healthy individuals before and after lithium administration.Neuropsychopharmacology.2004291918192415257303
- ForesterBP.FinnCT.BerlowYA.WardropM.RenshawPF.MooreCM.Brain lithium, N-acetyl aspartate and myo-inositol levels in older adults with bipolar disorder treated with lithium: a lithium-7 and proton magnetic resonance spectroscopy study.Bipolar Disord.20081069170018837863
- ChuangDM.Neuroprotective and neurotrophic actions of the mood stabilizer lithium: can it be used to treat neurodegenerative diseases?Crit Rev Neurobiol.200416839015581403
- ChuangDM.ManjiHK.In search of the Holy Grail for the treatment of neurodegenerative disorders: has a simple cation been overlooked?Biol Psychiatry.2007624617572175
- NonakaS.ChuangDM.Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats.Neuroreport.19989208120849674597
- RenM.SenatorovW.ChenRW.ChuangDM.Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model.Proc Natl Acad Sci U S A.20031006210621512732732
- XuJ.CulmanJ.BlumeA.BrechtS.GohlkeP.Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death.Stroke.2003341287129212677021
- XuXH.HuaYN.ZhangHL.et al.Greater stress protein expression enhanced by combined prostaglandin A1 and lithium in a rat model of focal ischemia.Acta Pharmacol Sin.2007281097110417640469
- XuXH.ZhangHL.HanR.GuZL.QinZH.Enhancement of neuroprotection and heat shock protein induction by combined prostaglandin A1 and lithium in rodent models of focal ischemia.Brain Res.2006110215416216797496
- MaJ.ZhangGY.LiuY.YanJZ.HaoZB.Lithium suppressed Tyr-402 phosphorylation of proline-rich tyrosine kinase (Pyk2) and interactions of Pyk2 and PSD-95 with NR2A in rat hippocampus following cerebral ischemia.Neurosci Res.20044935736215236860
- MaJ.ZhangGY.Lithium reduced N-methyl-D-aspartate receptor subunit 2A tyrosine phosphorylation and its interactions with Src and Fyn mediated by PSD-95 in rat hippocampus following cerebral ischemia.Neurosci Lett.200334818518912932824
- SasakiT.HanF.ShiodaN.MoriguchiS.et al.Lithium-induced activation of Akt and CaM kinase II contributes to its neuroprotective action in a rat microsphere embolism model.Brain Res.200611089810616843447
- YanXB.WangSS.HouHL.JiR.ZhouJN.Lithium improves the behavioral disorder in rats subjected to transient global cerebral ischemia.Behav Brain Res.200717728228917210190
- HanR.GaoB.ShengR.et al.Synergistic effects of prostaglandin E1 and lithium in a rat model of cerebral ischemia.Acta Pharmacol Sin.2008291141114918817617
- MastroiacovoF.BuscetiCL.BiagioniF.et al.Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia.J Cereb Blood Flow Metab.20092926427618827832
- RenM.LengY.JeongM.LeedsPR.ChuangDM.Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction.J Neurochem.2004891358136715189338
- KimHJ.RoweM.RenM.HongJS.ChenPS.ChuangDM.Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action.J Pharmacol Exp Ther.200732189290117371805
- ZarateCA.QuirozJ.PayneJ.ManjiHK.Modulators of the glutamatergic system: implications for the development of improved therapeutics in mood disorders.Psychopharmacol Bull.200236358312858143
- SugaiF.YamamotoY.MiyaguchiK.et al.Benefit of valproic acid in suppressing disease progression of ALS model mice.Eur J Neurosci.2004203179318315579172
- FornaiF.LongoneP.CafaroL.et al.Lithium delays progression of amyotrophic lateral sclerosis.Proc Natl Acad Sci U S A.20081052052205718250315
- FengHL.LengY.MaCH.ZhangJ.RenM.ChuangDM.Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model.Neuroscience.200815556757218640245
- PhielCJ.WilsonCA.LeeVM.KleinPS.GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides.Nature.200342343543912761548
- RockensteinE.TorranceM.AdameA.et al.Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer's disease are associated with reduced amyloid precursor protein phosphorylation.J Neurosci.2007271981199117314294
- De FerrariGV.ChaconMA.BarriaMl.et al.Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by betaamyloid fibrils.Mol Psychiatry.2003819520812610652
- QingH.HeG.LyPT.et al.Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer's disease mouse models.J Exp Med.20082052781278918955571
- CaccamoA.OddoS.TranLX.LaFerlaFM.Lithium reduces tau phosphorylation but not A beta or working memory deficits in a transgenic model with both plaques and tangles.Am J Pathol.20071701669167517456772
- AngstJ.GammaA.Gerber-WerderR.ZarateCA.Jr.ManjiHK.Does long-term medication with lithium, clozapine or antidepressants prevent or attenuate dementia in bipolar and depressed patients?Int J Psychiatry Clin Practice.20071128
- KessingLV.SondergardL.FormanJL.AndersenPK.Lithium treatment and risk of dementia.Arch Gen Psychiatry.2008651331133518981345
- NunesPV.ForlenzaOV.GattazWF.Lithium and risk for Alzheimer's disease in elderly patients with bipolar disorder.Br J Psychiatry.200719035936017401045
- WataseK.GatchelJR.SunY.et al.Lithium therapy improves neurological function and hippocampal dendritic arborization in a spinocerebellar ataxia type 1 mouse model.PLoS Med.20074e18217535104
- WeiH.QinZH.SenatorovVV.et al.Lithium suppresses excitotoxicityinduced striatal lesions in a rat model of Huntington's disease.Neuroscience.200110660361211591460
- SenatorovVV.RenM.KanaiH.WeiH.ChuangDM.Short-term lithium treatment promotes neuronal survival and proliferation in rat striatum infused with quinolinic acid, an excitotoxic model of Huntington's disease.Mol Psychiatry.2004937138514702090
- YoudimMB.ArrafZ.Prevention of MPTP (N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) dopaminergic neurotoxicity in mice by chronic lithium: involvements of Bcl-2 and Bax.Neuropharmacology.2004461130114015111020
- EverallIP.BellC.MalloryM.et al.Lithium ameliorates HIV-gp120-mediated neurotoxicity.Mol Cell Neurosci.20022149350112498789
- DouH.BirusinghK.FaraciJ.et al.Neuroprotective activities of sodium valproate in a murine model of human immunodeficiency virus-1 encephalitis.J Neurosci.2003239162917014534250
- D'SaC.DumanRS.Antidepressants and neuroplasticity.Bipolar Disord.2002418319412180273
- NibuyaM.MorinobuS.DumanRS.Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments.J Neurosci.199515753975477472505
- NibuyaM.NestlerEJ.DumanRS.Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus.J Neurosci.199616236523728601816
- HunsbergerJG.NewtonSS.BennettAH.et al.Antidepressant actions of the exercise-regulated gene VGF.Nat Med.2007131476148218059283
- BerchtoldNC.KesslakJP.CotmanCW.Hippocampal brain-derived neurotrophic factor gene regulation by exercise and the medial septum.J Neurosci Res.20026851152112111841
- FarmerJ.ZhaoX.van PraagH.WodtkeK.GageFH.ChristieBR.Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo.Neuroscience.2004124717914960340
- NeeperSA.Gomez-PinillaF.ChoiJ.CotmanCW.Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain.Brain Res.199672649568836544
- VaynmanS.YingZ.Gomez-PinillaF.Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition.Eur J Neurosci.2004202580259015548201
- YingZ.RoyRR.EdgertonVR.Gomez-PinillaF.Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury.Exp Neurol.200519341141915869943
- ChenMJ.Russo-NeustadtAA.Running exercise-induced up-regulation of hippocampal brain-derived neurotrophic factor is CREB-dependent.Hippocampus.2009. In press
- ShimizuE.HashimotoK.OkamuraN.et al.Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants.Biol Psychiatry.200354707512842310
- SiuciakJA.LewisDR.WiegandSJ.LindsayRM.Antidepressant-like effect of brain-derived neurotrophic factor (BDNF).Pharmacol Biochem Behav.1997561311378981620
- ShirayamaY.ChenAC.NakagawaS.RussellDS.DumanRS.Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression.J Neurosci.2002223251326111943826
- AzmitiaEC.MurphyRB.Whitaker-AzmitiaPM.MDMA (ecstasy) effects on cultured serotonergic neurons: evidence for Ca2(+)-dependent toxicity linked to release.Brain Res.1990510971031969761
- PanHS.WangRY.MDMA: further evidence that its action in the medial prefrontal cortex is mediated by the serotonergic system.Brain Res.19915393323361675911
- MercierG.LennonAM.RenoufB.et al.MAP kinase activation by fluoxetine and its relation to gene expression in cultured rat astrocytes.J Mol Neurosci.20042420721615456934
- HeikkilaRE.ManzinoL.CabbatFS.DuvoisinRC.Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors.Nature.19843114674696332989
- WeinrebO.AmitT.Bar-AmO.Yogev-FalachM.YoudimMB.The neuroprotective mechanism of action of the multimodal drug ladostigil.Front Biosci.2008135131513718508575
- Yogev-FalachM.AmitT.Bar-AmO.WeinstockM.YoudimMB.Involvement of MAP kinase in the regulation of amyloid precursor protein processing by novel cholinesterase inhibitors derived from rasagiline.FASEB J.2002161674167612206996
- CarroE.TrejoJL.BusiguinaS.Torres-AlemanI.Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy.J Neurosci.2001215678568411466439
- MalbergJE.EischAJ.NestlerEJ.DumanRS.Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus.J Neurosci.2000209104911011124987
- HitoshiS.MarutaN.HigashiM.KumarA.KatoN.IkenakaK.Antidepressant drugs reverse the loss of adult neural stem cells following chronic stress.J Neurosci Res.2007853574358517668856
- CzehB.MichaelisT.WatanabeT.et al.Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine.Proc Natl Acad Sci U S A.200198127961280111675510
- MadsenTM.TreschowA.BengzonJ.BolwigTG.LindvallO.TingstrornA.Increased neurogenesis in a model of electroconvulsive therapy.Biol Psychiatry.2000471043104910862803
- ChenF.MadsenTM.WegenerG.NyengaardJR.Repeated electroconvulsive seizures increase the total number of synapses in adult male rat hippocampus.Eur Neuropsychopharrnacol.200919329338
- PereraTD.CoplanJD.LisanbySH.et al.Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates.J Neurosci.2007274894490117475797
- van PraagH.ChristieBR.SejnowskiTJ.GageFH.Running enhances neurogenesis, learning, and long-term potentiation in mice.Proc Natl Acad Sci U S A.199996134271343110557337
- MaoY.GeX.FrankCL.MadisonJM.et al.Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling.Cell.20091361017103119303846
- KatoM.SerrettiA.Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder.Mol Psychiatry. In press
- EganMF.KojimaM.CallicottJH.et al.The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function.Cell.200311225726912553913
- ChenZY.JingD.BathKG.et al.Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior.Science.200631414014317023662
- CarrollCurtisGC.MendelsJ.Neuroendocrine regulation in depression. I. Limbic system-adrenocortical dysfunction.Arch Gen Psychiatry.19763310391044962488
- CarrollBJ.CurtisGC.MendelsJ.Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients.Arch Gen Psychiatry.19763310511058962489
- ShelineYl.SanghaviM.MintunMA.GadoMH.Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression.J Neurosci.1999195034504310366636
- ShelineYl.WangPW.GadoMH.CsernanskyJG.VannierMW.Hippocampal atrophy in recurrent major depression.Proc Natl Acad Sci U S A.199693390839138632988
- BremnerJD.ScottTM.DelaneyRC.et al.Deficits in short-term memory in posttraumatic stress disorder.Am J Psychiatry.1993150101510198317569
- BremnerJD.RandallP.ScottTM.et al.MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder.Am J Psychiatry.19951529739817793467
- BuellerJA.AftabM.SenS.Gomez-HassanD.BurmeisterM.ZubietaJK.BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects.Biol Psychiatry.20065981281516442082
- MalbergJE.DumanRS.Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment.Neuropsychopharmacology.2003281562157112838272
- YukimasaT.YoshimuraR.TamagawaA.et al.High-frequency repetitive transcranial magnetic stimulation improves refractory depression by influencing catecholamine and brain-derived neurotrophic factors.Pharmacopsychiatry.200639525916555165
- ZanardiniR.GazzoliA.VentrigliaM.et al.Effect of repetitive transcranial magnetic stimulation on serum brain derived neurotrophic factor in drug resistant depressed patients.J Affect Disord.200691838616448701
- NasrallahHA.Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles.Mol Psychiatry.200813273517848919
- KapurS.ArenovichT.AgidO.ZipurskyR.LindborgS.JonesB.Evidence for onset of antipsychotic effects within the first 24 hours of treatment.Am J Psychiatry.200516293994615863796
- AgidO.KapurS.ArenovichT.ZipurskyRB.Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected.Arch Gen Psychiatry.2003601228123514662555
- CaseyDE.Tardive dyskinesia and atypical antipsychotic drugs.Schizophr Res.199935 (suppl)S61S6610190226
- JonesPB.BarnesTR.DaviesL.et al.Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1).Arch Gen Psychiatry.2006631079108717015810
- EderU.MangwethB.EbenbichlerC.et al.Association of olanzapineinduced weight gain with an increase in body fat.Am J Psychiatry.20011581719172211579009
- FumagalliF.FrascaA.SpartaM.DragoF.RacagniG.RivaMA.Longterm exposure to the atypical antipsychotic olanzapine differently up-regulates extracellular signal-regulated kinases 1 and 2 phosphorylation in subcellular compartments of rat prefrontal cortex.Mol Pharmacol.2006691366137216391238
- BrowningJL.PatelT.BrandtPC.YoungKA.HolcombLA.HicksPB.Clozapine and the mitogen-activated protein kinase signal transduction pathway: implications for antipsychotic actions.Biol Psychiatry.20055761762315780848
- LuXH.BradleyRJ.DwyerDS.Olanzapine produces trophic effects in vitro and stimulates phosphorylation of Akt/PKB, ERK1/2, and the mitogenactivated protein kinase p38.Brain Res.20041011586815140644
- LuXH.DwyerDS.Second-generation antipsychotic drugs, olanzapine, quetiapine, and clozapine enhance neurite outgrowth in PC12 cells via PI3K/AKT, ERK, and pertussis toxin-sensitive pathways.J Mol Neurosci.200527436416055946
- BaiO.ZhangH.LiXM.Antipsychotic drugs clozapine and olanzapine upregulate bcl-2 mRNA and protein in rat frontal cortex and hippocampus.Brain Res.20041010818615126120
- EnglJ.LaimerM.NiederwangerA.et al.Olanzapine impairs glycogen synthesis and insulin signaling in L6 skeletal muscle cells.Mol Psychiatry.2005101089109616130009
- PozziL.HakanssonK.UsielloA.et al.Opposite regulation by typical and atypical anti-psychotics of ERK1/2, CREB and Elk-1 phosphorylation in mouse dorsal striatum.J Neurochem.20038645145912871586
- LipskaBK.KhaingZZ.WeickertCS.WeinbergerDR.BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs.Eur J Neurosci.20011413514411488957
- Chlan-FourneyJ.AsheP.NylenK.JuorioAV.LiXM.Differential regulation of hippocampal BDNF mRNA by typical and atypical antipsychotic administration.Brain Res.2002954112012393228
- BaiO.Chlan-FourneyJ.BowenR.KeeganD.LiXM.Expression of brainderived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs.J Neurosci Res.20037112713112478621
- PillaiA.TerryAV.Jr.MahadikSP.Differential effects of long-term treatment with typical and atypical antipsychotics on NGF and BDNF levels in rat striatum and hippocampus.Schizophr Res.2006829510616442781
- DeanCE.Antipsychotic-associated neuronal changes in the brain: toxic, therapeutic, or irrelevant to the long-term outcome of schizophrenia?Prog Neuropsychopharrnacol Biol Psychiatry.200630174189
- ParikhV.KhanMM.MahadikSP.Olanzapine counteracts reduction of brain-derived neurotrophic factor and TrkB receptors in rat hippocampus produced by haloperidol.Neurosci Lett.200435613513914746882
- MitchellIJ.CooperAC.GriffithsMR.CooperAJ.Acute administration of haloperidol induces apoptosis of neurones in the striatum and substantia nigra in the rat.Neuroscience.2002109899911784702
- NavariS.DazzanP.Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings.Psychol Med. In press
- Dorph-PetersenKA.PierriJN.PerelJM.SunZ.SampsonAR.LewisDA.The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys.Neuropsychopharmacology.2005301649166115756305
- ChakosMH.ShirakawaO.LiebermanJ.LeeH.BilderR.TammingaCA.Striatal enlargement in rats chronically treated with neuroleptic.Biol Psychiatry.1998446756849798070
- BaiO.WeiZ.LuW.BowenR.KeeganD.LiXM.Protective effects of atypical antipsychotic drugs on PC12 cells after serum withdrawal.J Neurosci Res.2002692788312111809
- QingH.XuH.WeiZ.GibsonK.LiXM.The ability of atypical antipsychotic drugs vs. haloperidol to protect PC12 cells against MPP+-induced apoptosis.Eur J Neurosci.2003171563157012752374
- YulugB.YildizA.HudaogluO.KilicE.CamE.SchabitzWR.Olanzapine attenuates brain damage after focal cerebral ischemia in vivo.Brain Research Bulletin.20067129630017113959
- BianQ.KatoT.MonjiA.et al.The effect of atypical antipsychotics, perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-gamma.Prog Neuropsychopharrnacol Biol Psychiatry.2007324248
- YulugB.YildizA.GuzelO.KilicE.SchabitzWR.KilicE.Risperidone attenuates brain damage after focal cerebral ischemia in vivo.Brain Research Bulletin.20066965665916716834
- XuH.QingH.LuRB.et al.Quetiapine attenuates the immobilization stress-induced decrease of brain-derived neurotrophic factor expression in rat hippocampus.Neurosci Lett.2002321656811872258
- DawirsRR.HildebrandtK.Teuchert-NoodtG.Adult treatment with haloperidol increases dentate granule cell proliferation in the gerbil hippocampus.J Neural Transm.19981053173279660110
- HalimND.WeickertCS.McClintockBW.WeinbergerDR.LipskaBK.Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology. 20042910631069
- WangHD.DunnavantFD.JarmanT.DeutchAY.Effects of antipsychotic drugs on neurogenesis in the forebrain of the adult rat.Neuropsychopharmacology.2004291230123815085089
- KippinTE.KapurS.van der KooyD.Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs.J Neurosci.20052558152315958748
- LuoC.XuH.LiXM.Quetiapine reverses the suppression of hippocampal neurogenesis caused by repeated restraint stress.Brain Res.20051063323916271709
- HeimbergC.KomoroskiRA.LawsonWB.CardwellD.KarsonCN.Regional proton magnetic resonance spectroscopy in schizophrenia and exploration of drug effect.Psychiatry Res.1998831051159818736
- BrausDF.EndeG.Weber-FahrW.DemirakcaT.TostH.HennFA.Functioning and neuronal viability of the anterior cingulate neurons following antipsychotic treatment: MR-spectroscopic imaging in chronic schizophrenia.Eur Neuropsychopharrnacol.200212145152
- BertolinoA.CailicottJH.MattayVS.et al.The effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophrenia.Biol Psychiatry.200149394611163778
- LawrieSM.AbukmeilSS.Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies.Br J Psychiatry.19981721101209519062
- LiebermanJA.Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective.Biol Psychiatry.19994672973910494440
- LiebermanJA.TollefsonGD.CharlesC.et al.Antipsychotic drug effects on brain morphology in first-episode psychosis.Arch Gen Psychiatry.20056236137015809403
- DelBelloMP.CecilKM.AdlerCM.DanielsJP.StrakowskiSM.Neurochemical effects of olanzapine in first-hospitalization manic adolescents: a proton magnetic resonance spectroscopy study.Neuropsychopharmacology.2006311264127316292323