Abstract
An inflammatory pathogenesis has been postulated for schizophrenia and major depression (MD). In schizophrenia and depression, opposing patterns oftype-1 vs type-2 immune response seem to be associated with differences in the activation of the enzyme indoleamine 2,3-dioxygenase and in the tryptophan-kynurenine metabolism, resulting in increased production of kynurenic acid in schizophrenia and decreased production of kynurenic acid in depression. These differences are associated with an imbalance in the glutamatergic neurotransmission, which may contribute to an excessive agonist action of N-methyl-D-aspartate (NMDA) in depression and of NMDA antagonism in schizophrenia. Regarding the neuroprotective function of kynurenic acid and the neurotoxic effects of quinolinic acid (QUIN), different patterns of immune activation may also lead to an imbalance between the neuroprotective and the neurotoxic effects of the tryptophanlkynurenine metabolism. The differential activation of microglia cells and astrocytes may be an additional mechanism contributing to this imbalance. The immunological imbalance results in an inflammatory state combined with increased prostaglandin E2 production and increased cyclo-oxygenase-2 (COX-2) expression. The immunological effects of many existing antipsychotics and antidepressants, however, partly correct the immune imbalance and the excess production of the neurotoxic QUIN, COX-2 inhibitors have been tested in animal models of depression and in preliminary clinical trials, pointing to favorable effects in schizophrenia and in MD.
Se ha postulado una patogénesis inflamatoria para la esquizofrenia y la depresión mayor (DM), En la esquizofrenia y la depresión, patrones opuestos de respuesta inmune tipo 1 versus tipo 2 parecen estar asociados con diferencias en la activación de la enzima indolamina 2,3 dioxigenasa y en el metabolisme triptófano-kinurenina, lo que Ileva a un aumento de la producción de ácido kinurénico en la esquizofrenia y disminución de la producción de este ácido en la depresión. Estas diferencias están asociadas con un desequilibrio en la neuroiransmision glutamatérgica, el cual puede contribuir a una excesiva acción agonista del N-metil-D-aspartato (NMDA) en la depresión y otra antagonisia del NMDA en la esquizofrenia. Respecto a la función neuroprotectora del ácido kinurénico y a los efectos neurotóxicos del ácido quinolínico (QUIN), los diferentes patrones de activación inmune también pueden llevara un desequilibrio entre los efecios neuroprotectores y neurotóxicos del metabolismo triptófano/kinurenina. La activación diferencial de las células de la microglía y los astroctios puede ser un mecanismo adicional que contribuya a este desequilibrio, El desequilibrio inmunológico se traduce en un estado inflamatorio combinado con un aumento de la producción de prostaglandina E2 y aumento de la expresión de ciclo-oxigenasa-2 (COX-2). Sin embargo, los efectos inmunológicos de muchos de los antipsicoticos y antidepresivos exisientes corrigen parcialmente el desequilibrio inmune y el exceso de production del neurotóxico QUIN. Los inhibidores de la COX-2 se han evaluado en modelos animales de depresión y en ensayos clínicos preliminares, y orientan a efectos favorables en la esquizofrenia y en la DM.
L'hypothèse d'une pathogenèse inflammatoire a été avancée pour la schizophrénie et la dépression majeure (DM), Dans la schizophrénie et la dépression, l'opposition des réponses immunes de type 1 vs type 2 semble être associée à des différences dans l'activation de l'enzyme indoleamine 2,3-dioxygénase et dans le métabolisme tryptophanekynurénine, la production d'acide kynurétique étant augmentée dans la schizophrénie et diminuée dans la dépression. Ces différences sont associées à un déséquilibre de la neurotransmission gluiamatergique qui peut entraîner une action agoniste excessive du NMDA (N-méthyl-D aspartate) dans la dépression et à une action antagoniste dans la schizophrénie. En ce qui concerne la fonction neuroprotectrice de l'acide kynurétique et les effets neuroioxiques de l'acide quinolinique (QUIN), différents schémas d'activation immunitaire peuvent aussi conduire à un déséquilibre entre les effets neuroprotecteurs et neurotoxiques du métabolisme tryptophane/kynurénine, auquel peut contribuer l'activation différentielle des cellules de la microglie et des astrocytes. Le déséquilibre immunologique provoque un état inflammatoire associé à une production augmentée de prostaglandine E2 et à une expression augmentée de la COX-2 (cyclo-oxygénase-2). Les effets immunologiques de nombreux antipsychotiques et antidépresseurs existants corrigent cependant en partie ce déséquilibre immunitaire et l'excès de production du neurotoxique QUIN, Les inhibiteurs de la COX-2 ont été testés dans des modèles animaux de dépression et dans des études cliniques préliminaires, montrant des effets favorables dans la schizophrénie et la dépression.
There is no doubt that dopaminergic, serotonergic, and/or noradrenergic neurotransmission play an important role in the pathophysiology of major depression (MD) and schizophrenia. Although the roles of dopamine in schizophrenia and of serotonin and noradrenaline in depression have been studied intensively, the exact underlying pathological mechanisms of both disorders are still unclear.
In MD, glutamatergic hyperf unction seems to be closely related to the lack of serotonergic and noradrenergic neurotransmission. Altered glutamate levels have been observed in the plasma, serum, cerebrospinal fluid (CSF), and in imaging and postmortem studies of depressed patients.Citation1 In schizophrenia, in contrast, dopaminergic hyperfunction in the limbic system and dopaminergic hypofunction in the frontal cortex are thought to be the main neurotransmitter disturbances. Recent research provides further insight that glutamatergic hypofunction might be the cause for this dopaminergic dysfunction in schizophrenia,Citation2 whereas glutamatergic hyperfunction acts through low NMDA antagonism in the kynurenine pathway in MD.Citation3 Glutamatergic dysfunction seems to be a common pathway in the neurobiology of schizophrenia and depression. The glutamatergic system is closely related in function to the immune system and to the tryptophankynurenine metabolism, which both seem to play a keyrole in the pathophysiology of schizophrenia and MD.Citation4,Citation5
The immune response and type-1 and type-2 polarization
The innate immune system is phylogenetically the oldest part of the immune response, natural killer (NK) cells and monocytes as the first barrier of the immune system being part of this. The adaptive immune response with the antibody-producing B -lymphocytes, the T-lymphocytes and their regulating “immunotransmitters,” the cytokines, is the specifically acting component of the immune system. (Tables I and II) . Cytokines regulate all types and all cellular components of the immune system, including the innate immune system. Helper T-cells are of two types, T-helper-1 (TH-1) and T-helper-2 (TH-2). TH-1 cells produce the characteristic “type-1” activating cytokines such as interleukin (IL) -2 and interferon (IFN)-γ. However, since not only TH-1 cells, but also certain monocytes/macrophages (M1) and other cell types produce these cytokines, the immune response is called the type-1 immune response. The humoral, antibodyproducing arm of the adaptive immune system is mainly activated by the type-2 immune response. TH-2 or certain monocytes/macrophages (M2) produce mainly IL-4, IL-10, and IL-13.Citation6 Further terminology separates the cytokines into proinflammatory and anti-inflammatory types. Proinflammatory cytokines, such as tumor necrosis factor α (TNF-α) and IL-6 are primarily secreted from monocytes and macrophages, activating other cellular components of the inflammatory response. While TNF-α is an ubiquitiously expressed cytokine mainly activating the type-1 response, IL-6 activates the type-2 response including the antibody production. Anti-inflammatory cytokines such as IL-4 and IL-10 help to downregulate the inflammatory immune response.
Table I. Components of the unspecific “innate” and the specific “adaptive” immune systems in humans.
Table II. Cytokines of the polarized immune response. IL, interleukin; IFN, interferon; TNF, tumor necrosis factor.
The type-1 immune system promotes the cell-mediated immune response directed against intracellular pathogens, whereas the type-2 response helps B-cell maturation and promotes the humoral immune response, including the production of antibodies directed against extracellular pathogens. Type-1 and type-2 cytokines antagonize each other in promoting their own type of response, while suppressing the immune response of the other; therefore the term “polarized” can be used.
Inflammation in schizophrenia and depression
Infection during pregnancy in mothers of offspring who later develop schizophrenia has been repeatedly described, in particular in the second trimester.Citation7,Citation8 The maternal immune response itself, as opposed to any single pathogen, may be related to the increased risk for schizophrenia in the offspring.Citation9 Indeed, increased IL- 8 levels of mothers during the second trimester were associated with an increased risk for schizophrenia in the offspring.Citation7 A fivefold increased risk for developing psychoses later on was detected after infection of the central nervous system (CNS) in early childhood.Citation7,Citation10 These data were confirmed in recent studies.Citation11,Citation12,Citation13
Signs of inflammation were found in schizophrenic brains,Citation14 and the term “mild localized chronic encephalitis” to describe a slight but chronic inflammatory process in schizophrenia was proposed.Citation15
An inflammatory model of MD is “sickness behavior,” the reaction of the organism to infection and inflammation. Sickness behavior is characterized by weakness, malaise, listlessness, inability to concentrate, lethargy, decreased interest in the surroundings, and reduced food intake - all of which are depression-like symptoms. The sicknessrelated psychopathological symptoms during infection and inflammation are mediated by proinflammatorycytokines such as IL-1, IL-6, TNF-α, and IFN-γ. The active pathway of these cytokines from the peripheral immune system to the brain is via afferent neurons and through direct targeting of the amygdala and other brain regions after diffusion at the circumventricular organs and choroid plexus. Undoubtedly, there is a strong relationship between the cytokine and the neurotransmitter systems, but the specific mechanisms underlying the heterogeneous disease MD are not yet fully understood.
In humans, the involvement of cytokines in the regulation of the behavioral symptoms of sickness behavior has been studied by application of the bacterial endotoxin lipoploysaccharide (LPS) to human volunteers.Citation16 LPS, a potent activator of proinflammatory cytokines, was found to induce mild fever, anorexia, anxiety, depressed mood, and cognitive impairment. The levels of anxiety, depression, and cognitive impairment were found to be related to the levels of circulating cytokines.Citation17
Mechanisms that may contribute to inflammation and cause depressive states are:
A direct influence of proinflammatory cytokines on the serotonin and noradrenaline metabolism
An imbalance of the type-1 - type-2 immune response leading to an increased tryptophan and serotonin metabolism by activation of indoleamine 2,3-dioxygenase (IDO) in the CNS, which is associated with:
A decreased availability of tryptophan and serotonin
A disturbance of the kynurenine metabolism with an imbalance in favour of the production of the NMDA receptor agonist quinolinic acid (QUIN)
An imbalance in astrocyte and microglial activation associated with increased production of QUIN.
Effects of antidepressants on the immune function support this view. The mechanisms and the therapeutic implications will be discussed below.
Inflammation, caused by infection or by other mechanisms, seems to play a role in schizophrenia and in MD.
Type-1 and type-2 immune responses in schizophrenia
A well established finding in schizophrenia is the decreased in vitro production of IL-2 and IFN-γ,Citation18,Citation19 reflecting a blunted production of type-1 cytokines. Decreased levels of neopterin, a product of activated monocytes/macrophages, also point to a blunted activation of the type-1 response.Citation20 The decreased response of lymphocytes after stimulation with specific antigens reflects a reduced capacity for a type-1 immune response in schizophrenia, as well.Citation21 intracellular adhesion molecule (ICAM)-l is a type-1 related protein and a celladhesion molecule expressed on macrophages and lymphocytes. Decreased levels of the soluble (s) intercellular adhesion molecule-1 (ICAM-1), as found in schizophrenia, also represent an underactivation of the type-1 immune system.Citation22 Decreased levels of the soluble TNFreceptor p55 - mostly decreased when TNF-α is decreased - were observed, too.Citation23 A blunted response of the skin to different antigens in schizophrenia was observed before the era of antipsychotics.Citation24 This finding could be replicated in unmedicated schizophrenic patients using a skin test for the cellular immune response.Citation25 However, there are some conflicting results regarding increased levels of Thl cytokines in schizophrenia.Citation26 The latest meta-analysis showed dominant proinflammatory changes in schizophrenia but not involving Th2 cytokines.Citation27 After including antipsychotic medication effects into the analysis, only increases of IL1 receptor antagonist serum levels and of IL-6 serum levels were found. Type-1 parameters, hypothesized to be downregulated in schizophrenia, were not included in the meta-analysis, because only a few studies have been performed in unmedicated patients.
Several reports described increased serum IL-6 levels in schizophrenia.Citation28 IL-6 serum levels might be especially high in patients with an unfavorable course of the disease.Citation29 IL-6 is a product of activated monocytes, and some authors refer to it as a marker of the type-2 immune response. Moreover, several other signs of activation of the type-2 immune response are described in schizophrenia, including increased Th2 type of lymphocytes in the blood,Citation30 increased production of immunoglobuiinE (IgE), and an increase in IL-10 serum levels.Citation31,Citation32 In the CSF, IL-10 levels were found to be related to the severity of the psychosis.Citation32
The key cytokine of the type-2 immune response is IL4. Increased levels of IL-4 in the CSF of juvenile schizophrenic patients have been reported,Citation33 which indicates that the increased type-2 response in schizophrenia is not only a phenomenon of the peripheral immune response.
However, the data show that the immune response in schizophrenia can be confounded partly by factors specific to the disease such as its duration, chronicity, or therapy response, and partly by other factors such as antipsychotic medication, smoking, etc.
Increased proinflammatory type-1 cytokines in major depression
Characteristics of the immune activation in MD include increased numbers of circulating lymphocytes and phagocytic cells, upregulated serum levels of indicators of activated immune cells (neopterin, soluble IL-2 receptors), and higher serum concentrations of positive acute phase proteins (APPs), coupled with reduced levels of negative APPs, as well as increased release of proinflammatory cytokines, such as IL-1, IL-2, TNF-α and IL-6 through activated macrophages and IFN-γ through activated T-cells.Citation32-Citation39 Increased numbers of peripheral mononuclear cells in MD have been described by different groups of researchers.Citation40
Neopterin is a sensitive marker of the cell-mediated type-1 immunity. The main sources of neopterin are monocytes/macrophages. In accordance with the findings of increased monocytes/macrophages, an increased secretion of neopterin has been described by several groups of researchers.Citation41,Citation42
The increased plasma concentrations of the proinflammatory cytokines IL-1 and IL-6 observed in depressed patients was found to correlate with the severity of depression and with measures of the hypothalamus-pituitary-adrenal (HPA)-axis hyperactivity.Citation43,Citation44 As genetics plays a role in MD, the genetics of the immune system in relation to MD has also been investigated. Particular cytokine gene polymorphisms, eg, in genes coding for IL1 and TNF-α may confer a greater susceptibility to develop MD, although studies are conflicting.Citation45,Citation46
The production of IL-2 and IFN-γ is the typical marker of a type-1 immune response. In contrast to schizophrenia, IFN-γ is produced in greater amounts by lymphocytes of patients with MD than of healthy controls.Citation42,Citation45 Higher plasma levels of IFN-γ in depressed patients, accompanied by lower plasma tryptophan availability were described,Citation42 and the IFNγ/IL-4 ratio, a marker for Thl/Th2 balance is also higher in depressed patients.Citation45 Data on IL-2 in MD are mainly restricted to the estimation of its soluble receptor sIL-2R in the peripheral blood. Increased sIL-2R levels reflect an increased production of IL-2. The blood levels of sIL-2R were repeatedly found to be increased in MD patients.Citation39
Increased expression of ICAM-1 is observed in inflammatory processes, and promotes the influx of peripheral immune cells through the blood-brain barrier.Citation47 By this mechanism, macrophages and costimulatory lymphocytes can invade the central nervous system (CNS), further increasing the proinflammatory immune response. The plasma levels and CNS expression of ICAM-1 are associated with depressive symptoms in patients treated with IFN-γ. Increased sICAM-1 levels were observed in patients with more depressive symptoms,Citation48 and increased expression of ICAM-1 was found in the prefrontal cortex of elderly depressed patients.Citation49 In late -life depression, however, there are conflicting results.Citation50
Since different pathologies may underlie the syndrome of depression, different immunological states might be involved. Indeed, different types of MD were observed to exhibit different immune profiles: the subgroup of melancholic depressed patients showed a decreased type-1 activation - as observed in schizophrenic patientsCitation40 - while the nonmelancholic depressed patients showed signs of inflammation such as increased monocyte count and increased levels of α2-macroglobulin.Citation40 Suicidality, observed in a very high proportion of depressed patients, seems to be an example of the immune activation pattern in depression, since clinical studies have observed higher levels of type-1 cytokines in suicidal patients. In a small study, distinct associations between suicidality and type-1 immune response and a predominance of type-2 immune parameters in nonsuicidal patients were observed.Citation51 An epidemiological study hypothesized that high IL-2 levels are associated with suicidality.Citation52 Increased levels of serum sIL-2R have been described in medication-free suicide attempters, irrespective of the psychiatric diagnosis,Citation53 and treatment with high-dose IL-2 has been associated with suicide in a case report.Citation54
These data show that possible different immune states within the category of MD need to be better differentiated. The predominant proinflammatory, type-1 dominated immune state described in MD may be a kind of model state state restricted to a majority of patients suffering from MD. Therefore, these and other methodological concerns have to be considered carefully in future studies.
Therapeutic mechanisms and the type-1/type-2 imbalance in schizophrenia and depression
Schizophrenia: antipsychotic drugs correct the type-litype-2 imbalance
In-vitro studies show that the blunted IFN-γ production becomes normalized after therapy with neuroleptics.Citation18 An increase of “memory cells” (CD4+CD45RO+) cells - one of the main sources of IFN-γ production - during antipsychotic therapy with neuroleptics was observed by different groups.Citation55 Additionally, an increase of sIL-2R - the increase reflects an increase of activated, IL-2 bearing T-cells - during antipsychotic treatment was described.Citation56 The reduced sICAM-1 levels show a significant increase during short-term antipsychotic therapy,Citation22 and the ICAM-1 ligand leukocyte function antigen-1 (LFA-1) shows a significantly increased expression during antipsychotic therapy.Citation57 The increase of TNF-α and TNF-α receptors during therapy with clozapin was observed repeatedly.Citation58 Moreover, the blunted reaction to vaccination with Salmonella typhii was not observed in patients medicated with antipsychotics.Citation59 An elevation of IL-18 serum levels was described in medicated schizophrenics.Citation60 Since IL-18 plays a pivotal role in the type-1 immune response, this finding is consistent with other descriptions of type-1 activation during antipsychotic treatment.
Regarding the type-2 response, several studies point out that antipsychotic therapy is accompanied by a functional decrease of the IL-6 system.Citation19,Citation61 These findings provide further evidence that antipsychotics have a “balancing” effect on cytokines.
Therapeutic techniques in depression are associated with downregulation of the proinflammatory immune response
Antidepressant pharmacotherapy
A modulatory, predominantly inhibitory effect of selective serotonin reuptake inhibitors (SSRIs) on activation of proinflammatory immune parameters was demonstrated in animal experiments.Citation62,Citation63
Several antidepressants seem to be able to induce a shift from type 1 to type 2, in other words from a proinflammatory to an anti-inflammatory immune response, since the ability of three antidepressants (sertraline, clomipramine, and trazodone) to greatly reduce the IFN-γ/IL-10 ratio was shown in vitro. These drugs reduced the IFN-γ production significantly, while sertraline and clomipramine additionally raised the IL-10 production.Citation61 Regarding other in-vitro studies, a significantly reduced production of IFN-γ, IL-2, and sIL-2R was found after antidepressant treatment compared with pretreatment values.Citation63 A downregulation of the IL-6 production was observed during amitriptyline treatment; in treatment responders, the TNF-α production decreased to normal.Citation66 There are also studies, however, showing no effect of antidepressants to the in-vitro stimulation of cytokines (overview, ref 67) but methodological issues have to be taken into account. There is significant evidence suggesting that antidepressants of different classes induce downregulation of the type 1 cytokine production in vitro,Citation67 including noradrenaline reuptake inhibitorsCitation68 and the ”dual“ serotonin and noradrenalin reuptake inhibitors.Citation69 Several researchers have observed a reduction of IL-6 during treatment with the serotonin reuptake inhibitor fluoxetine.Citation70 A decrease of IL-6 serum levels during therapy with different antidepressants has been observed by other researchers.Citation71 The shift of imbalanced IFNγ/IL-4 towards normal after 6 weeks' antidepressant treatment has also been reported.Citation41 On the other hand, other groups did not find any effect of some antidepressants on serum levels of different cytokines.Citation61,Citation72
Since IL-6 stimulates PGE2 and antidepressants inhibit IL-6 production, an inhibiting action of antidepressants on PGE2 would be expected, too.Citation73 Over 30 years ago it was suggested that antidepressants inhibit PGE2.Citation74 A recent invitro study showed that both tricyclic antidepressants and selective serotonin inhibitors attenuated cytokine-induced PGE2 and nitric oxide production by inflammatory cells.Citation75
Nonpharmacological therapies: electroconvulsive therapy and sleep deprivation
Electroconvulsive therapy (ECT) was found to downregulate increased levels of the proinflammatorycytokine TNF-α in patients with MD.Citation76
An immune analysis during sleep showed an increase in the type-1 monocyte derived cytokines TNF-α and IL-12 and a decrease of the type-2 IL-10 producing monocytes.Citation77 In contrary, continuous wakefulness blocked the increase of type-1 and decrease of type-2 cytokines (T. Lange and S. Dimitrov, personal communication). Thus, sleep deprivation may exert therapeutic effects through a low suppression of type-1 cytokines.
Antidepressant pharmacotherapy, but also other antidepressant therapeutic agents or techniques, have a downregulating effect on proinflammatory cytokines.
Divergent effects of type-1 type-2 immune activation are associated with different effects on the kynurenine metabolism in schizophrenia and depression
Schizophrenia
The only known naturally occurring NMDA receptor antagonist in the human CNS is kynurenic acid (KYNA). KYNA is one of the several neuroactive intermediate products of the kynurenine pathway ( .). Kynurenine (KYN) is the primary major degradation product of tryptophan (TRP). While the excitatory KYN metabolites 3-hydroxy kynurenine (3HK) and QUIN are synthesized from KYN in the process toward NAD formation, KYNA is formed in a dead-end side arm of the pathway.Citation78
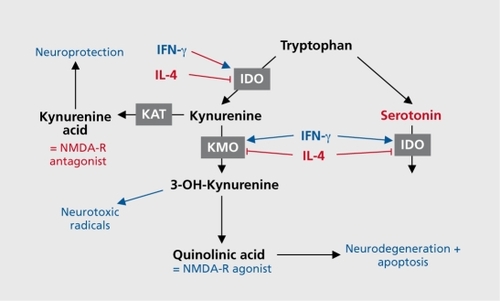
KYNA acts both as a blocker of the glycine coagonistic site of the NMDA receptor and as a noncompetitive inhibitor of the α7 nicotinic acetylcholine receptor.Citation79 The production of KYN metabolites is partly regulated by IDO and tryptophan 2,3-dioxygenase (TDO). Both enzymes catalyze the first step in the pathway, the degradation from tryptophan to kynurenine. Type-1 cytokines, such as IFN-γ and IL-2, stimulate the activity of IDO.Citation80 There is a mutual inhibitory effect of TDO and IDO: a decrease in TDO activity occurs concomitantly with IDO induction, resulting in a coordinate shift in the site (and cell types) of tryptophan degradation.Citation81 While it has been known for a long time that IDO is expressed in different types of CNS cells, TDO was thought for manyyears to be restricted to liver tissue. It is known today, however, that TDO is also expressed in CNS cells, probably restricted to astrocytes.Citation82
The type-2 or Th-2 shift in schizophrenia may result in a downregulation of IDO through the inhibiting effect of Th2 cytokines. TDO, on the other hand, was shown to be overexpressed in postmortem brains of schizophrenic patients.Citation82 The type-l/type-2 imbalance with type-2 shift is therefore associated with overexpression of TDO. The type 1/type 2 imbalance is associated with the activation of astrocytes and an imbalance in the activation of astrocytes/microglial cells.Citation83 The functional excess of astrocytes may lead to a further accumulation of KYNA. Indeed, a study referring to the expression of IDO and TDO in schizophrenia showed exactly the expected results. An increased expression of TDO compared with IDO was observed in schizophrenic patients and the increased TDO expression was found, as expected, in astrocytes, not in microglial cells.Citation82
However, it is necessary to note that the above proposed mechanism would fit only for the subpopulation of schizophrenic patients with Th2 dominant immune response. In those schizophrenics with Th1 dominant immune response, the kynurenine pathway changes would be more similar to those changes in MD.Citation84,Citation85
Major depression
Two directing enzymes of the kynurenine metabolism, IDO and kynurenine monoxygenase (KMO), are induced by the type-1 cytokine IFN-γ. The activity of IDO is an important regulatory component in the control of lymphocyte proliferation, the activation of the type-1 immune response, and the regulation of the tryptophan metabolism.Citation85 It induces a halt in the lymphocyte cell cycle due to the catabolism of tryptophan:Citation87 In contrast to the type-1 cytokines, the type-2 cytokines IL-4 and IL-10 inhibit the IFN-γ-induced IDO-mediated tryptophan catabolism.Citation87 IDO is located in several cell types, including monocytes and microglial cells.Citation88 An IFN-γ-induced, IDO-mediated decrease of CNS tryptophan availability may lead to a serotonergic deficiency in the CNS, since tryptophan availability is the limiting step in serotonin synthesis. Other proinflammatory molecules such as PGE2 or TNF-α, however, induce synergistically with IFN-γ the increase of IDO activity.Citation89 Therefore, not only IFN-γ and type-1 cytokines, but also other proinflammatory molecules induce IDO activity. Since increased levels of PGE2 and TNF-α were described in MD, other proinflammatory molecules also contribute to IDO activation and tryptophan consumption, (eg, ref 39). An imbalance between the NMDA antagonist action by KYNA and the NMDA agonist action by QUIN has been proposed to be involved in the pathophysiology of MDCitation90; a recent study demonstrated this imbalance in patients with MD.Citation3 Accordingly, since the activity of the enzyme kynurenine 3 mono-oxygenase (KMO), directing the production of QUIN, is inhibited by type-2 cytokines but activated by proinflammatory type-1 cytokines,Citation91 an increased production of QUIN in depressive states would be expected. The role of QUIN in depression is discussed in more detail below.
One of the more consistent findings is that patients with low5-hydroxyindoleacetic acid (5-HIAA), the metabolite of serotonin, in CSF are prone to commit suicide.Citation92,Citation93 This gives further indirect evidence for a possible link between the type-1 cytokine IFN-γ and the IDO-related reduction of serotonin availability in the CNS of suicidal patients.
A study in patients suffering from hepatitis C showed that immunotherapy with IFN-γ was followed by an increase of depressive symptoms and serum kynurenine concentrations on the one hand, and a decrease in serum concentrations of tryptophan and serotonin on the other hand.Citation94 The kynurenine/tryptophan ratio, which reflects the activity of IDO, increased. Changes in depressive symptoms were significantly positively correlated with kynurenine and negatively correlated with serotonin concentrations.Citation94 This study and othersCitation95 clearly show that the IDO activity is increased by IFN, leading to an increased kynurenine production and a depletion of tryptophan and serotonin. The further metabolism of kynurenine, however, seems to play an additional crucial role for the psychopathological states.
In addition to the effects of the proinflammatory immune response on the serotonin metabolism, other neurotransmitter systems, in particular the catecholaminergic system, are involved in depression, too. Although the relationship of immune activation and changes in catecholaminergic neurotransmission has not been well studied, an increase in monoamino-oxidase (MAO) activity, which leads to decreased noradrenergic neurotransmission, might be an indirect effect of the increased production of kynurenine and QUIN;Citation45
The proinflammatory immune state in MD leads on the one hand to a lack of serotonin and on the other hand to an overproduction of the neurotoxic and depressiogenic metabolite QUIN by induction of the directing enzymes of the kynurenine metabolism. Two depressiogenic components result from the IDO activation.
Astrocytes, microglia, and type-1/type-2 response
The cellular sources for the immune response in the CNS are astrocytes and microglia cells. Microglial cells, deriving from peripheral macrophages, secrete preferentially type-1 cytokines such as IL-12, while astrocytes inhibit the production of IL-12 and ICAM-1 and secrete the type-2 cytokine IL-10.Citation96 Therefore, the type-1/type-2 imbalance in the CNS seems to be represented by the imbalance in the activation of microglial cells and astrocytes, although it has to be taken into consideration that the production of cytokines by astrocytes and microglial cells depends on activation conditions. The hypothesis of an overactivation of astrocytes in schizophrenia is supported by the finding of increased CSF levels of S100B - a marker of astrocyte activation - independent of the medication state of the schizophrenic patients.Citation97 Microglia activation was found in a small percentage of schizophrenics and is speculated to be a medication effect.Citation98 A type-1 immune activation as an effect of antipsychotic treatment has repeatedly been observed.
Since the type-1 activation predominates in the response of the peripheral immune system in depression, a dominance of microglial activation compared with astrocyte activation should be observed in depression. Glial reductions were consistently found in brain circuits known to be involved in mood disorders, such as in the limbic and prefrontal cortex.Citation99'Citation100 Although several authors did not differentiate between microglial and astrocytic loss, this difference is crucial due to the different effects of the type-l/type-2 immune response. Recent studies, however, show that astrocytes are diminished in patients suffering from depression,Citation101 although the data are not entirely consistent.Citation102 A loss of astrocytes was in particular observed in younger depressed patients: the lack of glial fibrillary acid protein (GFAP)-immunoreactive astrocytes reflects a lowered activity of responsiveness in those cells.Citation101 A loss of astrocytes was found in many cortical layers and in different sections of the dorsolateral prefrontal cortex in depression.Citation103 A reduction of astrocytes has also been observed in the dentate gyrus of an animal model of IFN-α induced depression (Myint et al, personal communication).
Moreover, a loss of astrocytes is associated with an impaired reuptake of glutamate from the extracellular space into astrocytes by high affinity glutamate transporters.Citation104 Impaired glutamate reuptake from the synaptic cleft by astroglia prolongs synaptic activation by glutamate.Citation105 Accordingly, increased glutamatergic activityhas been observed in patients with depression.Citation106
Neuroprotective and neurotoxic metabolites of the tryptophan-kynurenine metabolism in psychiatric disorders
In contrast to microglial cells which produce QUIN, astrocytes play a key role in the production of KYNA in the CNS. Astrocytes are the main source of KYNA.Citation107 The cellular localization of the kynurenine metabolism is primarily in macrophages and microglial cells, but also in astrocytes.Citation108 KMO, a critical enzyme in the kynurenine metabolism, is absent in human astrocytes, however.Citation109 Accordingly, it has been pointed out that astrocytes cannot produce the product 3-hydroxykynurenine (3-HK), but they are able to produce large amounts of early kynurenine metabolites, such as KYN and KYNA.Citation109 This supports the observation that inhibition of KMO leads to an increase in the KYNA production in the CNS.Citation110 The complete metabolism of kynurenine to QUIN is observed mainly in microglial cells, only a small amount of QUIN is produced in astrocytes via a side-arm of the kynurenine metabolism. Therefore, due to the lack of kynurenine-hydroxylase (KYN-OHse),in case of high tryptophan breakdown to KYN, KYNA may accumulate in astrocytes.
A second key player in the metabolization of 3-HK are monocytic cells infiltrating the CNS. They help astrocytes in the further metabolism to QUIN.Citation109 However, the low levels of sICAM-1 (ICAM-1 is the molecule that mainly mediates the penetration of monocytes and lymphocytes into the CNS) in the serum and in the CSF of nonmedicated schizophrenic patients,Citation22 and the increase of adhesion molecules during antipsychotic therapy indicate that the penetration of monocytes may be reduced in nonmedicated schizophrenic patients.Citation57
Quinolinic acid as a depressiogenic and neurotoxic substance
Apart from certain liver cells, only macrophage-derived cells are able to convert tryptophan into quinolinic acidolonic acid.Citation111 Interestingly, in a model of infection, the highest concentrations of QUIN are found in the gray and white matter of the cortex, not in subcortical areas. This finding points out that high levels of QUIN therefore may be associated with cortical dysfunction.Citation112
The strong association between cortical QUIN concentrations and local IDO activity supports the view that the induction of IDO is an important event in initiating the increase of QUIN production.Citation113 In the CNS, invaded macrophages and microglial cells are able to produce QUINCitation111 During a local inflammatory CNS process, the QUIN production in the CNS might increase without changes of the peripheral blood levels of QUIN. The local QUIN production correlates with the level of β2 microglobulin, an inflammatory marker. Local CNS concentrations of QUIN are able to exceed the blood levels by far.Citation112 Peripheral immune stimulation, however, under certain conditions also leads to increased CNS concentration of QUIN.Citation111
A recent study showed that depressive symptoms are related to an high ratio of KYN/KYNA in depression.Citation114 The increase of this ratio reflects that in depressed states KYN may be preferentially metabolized to QUIN, while the KYNA pathway is neglected.
The increase of QUIN was observed to be associated with several prominent features of depression: decrease in reaction timeCitation115 and cognitive deficits, in particular difficulties in learning.Citation112 In an animal model, an increase of QUIN and 3-hydroxykynurenine was associated with anxiety.Citation116
QUIN was shown to cause an over-release of glutamate in the striatum and in the cortex, presumably by presynaptic mechanisms.Citation117 The QUIN pathway of the kynurenine metabolism - directed by proinflammatorycytokines - might be the key mechanism involved in the increased glutamatergic neurotransmission in MD,Citation106 while it is unclear whether QUIN itself has depressiogenic properties. Thus, an excess of QUIN might be associated with excess glutamatergic activation.
COX-2 inhibition as a therapeutic approach in schizophrenia and depression
COX inhibition provokes differential effects on kynurenine metabolism: while COX-1 inhibition increases the levels of KYNA, COX-2 inhibition decreases them.Citation118 Therefore, psychotic symptoms and cognitive dysfunctions, observed during therapy with COX-1 inhibitors, were assigned to the COX-1 mediated increase of KYNA. The reduction of KYNA levels, by a prostaglandin-mediated mechanism, might be an additional mechanism to the above-described immunological mechanism for therapeutic effects of selective COX-2 inhibitors in schizophrenia.Citation118
Indeed, in a prospective, randomized, double-blind study of therapy with the COX-2 inhibitor celecoxib added on to risperidone in acute exacerbation of schizophrenia, a therapeutic effect of celecoxib was observed.Citation119 Immunologically, an increase of the type-1 immune response was found in the celecoxib treatment group.Citation120 The finding of a clinical advantage of COX-2 inhibition, however, could not be replicated in a second study. Further analysis of the data revealed that the outcome depends on the duration of the disease.Citation121 This observation is in accordance with results from animal studies showing that the effects of COX-2 inhibition on cytokines, hormones, and particularly on behavioral symptoms are dependent on the duration of the preceding changes and the time point of application of the COX-2 inhibitor.Citation122 In subsequent clinical studies following a similar randomized double-blind placebo-controlled add-on design of 400 mg celecoxib to risperidone (in one study risperidone or olanzapine) in partly different patient populations, similar positive results of cyclo-oxygenase inhibition were able to be obtained: in a Chinese population of first-manifestation schizophrenics,Citation123 and in an Iranian sample of chronic schizophrenics.Citation124 In continuously ill schizophrenics, however, no advantage of celecoxib could be found.Citation125 In schizophrenia, COX-2 inhibition showed beneficial effects preferentially in early stages of the disease, the data regarding chronic schizophrenia are controversial, possibly in part due to methodological concerns. The data are still preliminary and further research has to be performed, eg, with other COX-2 inhibitors.
COX-2 inhibition as a possible anti-inflammatory therapeutic approach in depression
Due to the increase of proinflammatory cytokines and PGE2, in depressed patients, anti-inflammatory treatment would be expected to show antidepressant effects also in depressed patients. In particular, COX-2 inhibitors seem to show advantageous results: animal studies show that COX-2 inhibition can lower the increase of the proinflammatory cytokines IL-1β, TNF-α, and of PGE2, but it can also prevent clinical symptoms such as anxiety and cognitive decline, which are associated with this increase of proinflammatory cytokines.Citation122 Moreover, treatment with the COX-2 inhibitor celecoxib - but not with a COX-1 inhibitor - prevented the dysregulation of the IIPA-axis, in particular the increase of Cortisol, one of the biological key features associated with depression.Citation122,Citation126 This effect can be expected because PGE2 stimulates the HPA axis in the CNS,Citation127 and PGE2 is inhibited by COX-2 inhibition. Moreover, the functional effects of IL-1 in the CNS - sickness behavior being one of these effects - were also shown to be antagonized by treatment with a selective COX-2 inhibitor.Citation128
Additionally, COX-2 inhibitors influence the CNS serotonergic system. In a rat model, treatment with rofecoxib was followed by an increase of serotonin in the frontal and the temporoparietal cortex.Citation129 A possible mechanism of the antidepressant action of COX-2 inhibitors is the inhibition of the release of IL-1 and IL-6. Moreover, COX-2 inhibitors also protect the CNS from effects of QUIN, ie, from neurotoxicity.Citation130 In the depression model of the bulbectomized rat, a decrease of cytokine levels in the hypothalamus and a change in behavior have been observed after chronic celecoxib treatment.Citation131 In another animal model of depression, however, the mixed COX-1/COX-2 inhibitor acetylsalicylic acid showed an additional antidepressant effect by accelerating the antidepressant effect of fluoxetine.Citation132
Moreover, we were able to demonstrate a significant therapeutic effect of the COX-2 inhibitor on depressive symptoms in a randomized, double-blind pilot add-on study using the selective COX-2 inhibitor celecoxib in MD.Citation133 Also in a clinical study, the mixed COX-1/COX-2 inhibitor acetylsalicylic acid accelerated the antidepressant effect of fluoxetine and increased the response rate in depressed nonresponders to monotherapy with fluoxetine in a open-label pilot study.Citation134 Currently, a large study with the COX-2 inhibitor cimicoxib is ongoing. For ethical reasons, clinical trials so far have been performed in an add-on design; no monotherapy with a COX-2 inhibitor was studied.
Conclusion
A large number of findings point out that inflammation plays a pivotal role in the pathogenesis of major psychiatric disorders, in particular in MD and in schizophrenia. The differential influence of cytokines and proinflammatory mediators, which are altered in schizophrenia and MD, on the enzyme IDO and the tryptophan/kynurenine metabolism result in alterations of the serotonergic, glutamatergic, and dopaminergic neurotransmissions; these alterations are typically found in schizophrenia and MD. The tryptophan/kynurenine metabolism, however, generates neurotoxic and neuroprotective metabolites, an imbalance in this metabolism contributes to the production of either the neurotoxic metabolite QUIN or the neuroprotective metabolite KYNA, both exhibiting different effects on the glutamatergic neurotransmission. Additionally, a direct influence of cytokines on neurotransmitters has been noted. Moreover, cytokines can also act in a neurotoxic and neuroprotective manner. Anti-inflammatory drugs, however, are candidates for antidepressants and antipsychotics, which might be more related to the pathophysiology of these disorders compared with the neurotransmitter disturbances. The neurotransmitter disturbances might be a final common pathway of different pathological pathways in schizophrenia and depression, the immunological pathway might be true for a subgroup of patients suffering from these disoders. COX-2 inhibitors - most studies have been performed with celecoxib - have been shown in invitro experiments, animal studies, and clinical trials by several groups of researchers to exhibit antidepressant and antipsychotic properties. Other anti-inflammatory therapeutic approaches will be of interest in the future, and possibly support the hypothesis that inflammation is an important pathogenetic factor in depression and schizophrenia.
Selected abbreviations and acronyms
| COX | = | cyclo-oxygenase |
| IDO | = | indoleamine 2,3-dioxygenase |
| IL | = | interleukin |
| KYN | = | kynurenine |
| KYNA | = | kynurenic acid |
| MD | = | major depression |
| OUIN | = | quinolinic acid |
| TDO | = | tryptophan 2,3-dioxygenase |
| TNF | = | tumor necrosis factor |
REFERENCES
- Machado-VieiraR.SalvadoreG.IbrahimLA.az-GranadosN.ZarateCA.Jr.Targeting glutamatergic signaling for the development of novel therapeutics for mood disorders.Curr Pharm Des.2009151595161119442176
- SwerdlowNR.van BergeijkDP.BergsmaF.WeberE.TalledoJ.The effects of memantine on prepulse inhibition.Neuropsychopharmacology.2009341854186419242406
- MyintAM.KimYK.VerkerkR.ScharpeS.SteinbuschH.LeonardB.Kynurenine pathway in major depression: evidence of impaired neuroprotection.J Affect Disord.20079814315116952400
- MüllerN.SchwarzMJ.The immunological basis of glutamatergic disturbance in schizophrenia: towards an integrated view.J Neurotransmission.2007(suppl)269280
- MillierN.SchwarzMJ.The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression.Mol Psychiatry.200712988100017457312
- MillsCD.KincaidK.AltJM.HeilmanMJ.HillAM.M-1/M-2 macrophages and the Th1/Th2 paradigm.J Immunol.20001646166617310843666
- BrownAS.BeggMD.GravensteinS.et al.Serologic evidence of prenatal influenza in the etiology of schizophrenia.Arch Gen Psychiatry.20046177478015289276
- BukaSL.GoldsteinJM.SeidmanLJ.TsuangMT.Maternal recall of pregnancy history: accuracy and bias in schizophrenia research.Schizophr Bull.20002633535010885635
- ZuckermanL.WeinerI.Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring.J Psychiatr Res.20053931132315725430
- GattazWF.AbrahaoAL.FoccaciaR.Childhood meningitis, brain maturation and the risk of psychosis.Eur Arch Psychiatry Clin Neurosci.2004254232614991375
- KoponenH.RantakallioP.VeijolaJ.JonesP.JokelainenJ.IsohanniM.Childhood central nervous system infections and risk for schizophrenia.Eur Arch Psychiatry Clin Neurosci.200425491314991373
- BrownAS.The risk for schizophrenia from childhood and adult infections.Am J Psychiatry.200816571018178749
- DalmanC.AllebeckP.GunnellD.et al.Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects.Am J Psychiatry.2008165596518056223
- KörschenhausenDA.HampelHJ.AckenheilM.PenningR.MüllerN.Fibrin degradation products in post mortem brain tissue of schizophrenics: a possible marker for underlying inflammatory processes.Schizophr Res.1996191031098789908
- BechterK.Mild encephalitis underlying psychiatric disorders - A reconsideration and hypothesis exemplified on Borna disease.Neurol Psychiatry Brain Res.200195570
- ReichenbergA.KrausT.HaackM.SchuldA.PollmacherT.YirmiyaR.Endotoxin-induced changes in food consumption in healthy volunteers are associated with TNF-alpha and IL-6 secretion.Psychoneuroendocrinology.20022794595612383455
- ReichenbergA.YirmiyaR.SchuldA.et al.Cytokine-associated emotional and cognitive disturbances in humans.Arch Gen Psychiatry.20015844545211343523
- WilkeI.AroltV.RothermundtM.WeitzschC.HornbergM.KirchnerH.Investigations of cytokine production in whole blood cultures of paranoid and residual schizophrenic patients.Eur Arch Psychiatry Clin Neurosci.19962462792848863007
- MüllerN.RiedelM.AckenheilM.SchwarzMJ.Cellular and humoral immune system in schizophrenia: a conceptual re-evaluation.World J Biol Psychiatry.2000117317912607212
- Sperner-UnterwegerB.MillerC.HolznerB.WidnerB.FleischhackerWW.FuchsD.Measurement of neopterin, kynurenine and tryptophan in sera of schizophrenic patients. In: Müller N, ed;Psychiatry, Psychoiinrnunology. and Viruses. Vienna, Austria; New York, NY: Springer;1999115119
- MüllerN.AckenheilM.HofschusterE.MempelW.EcksteinR.Cellular immunity in schizophrenic patients before and during neuroleptic treatment.Psychiatry Res.1991371471601678892
- SchwarzMJ.RiedelM.AckenheilM.MüllerN.Decreased levels of soluble intercellular adhesion molecule-1 (slCAM-1) in unmedicated and medicated schizophrenic patients.Biol Psychiatry.200047293310650446
- HaackM.Hinze-SelchD.FenzelT.et al.Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis.J Psychiatr Res.19993340741810504009
- MolholmHB.Hyposensitivity to foreign protein in schizophrenic patients.Psychiatr Quarterly.194216565571
- RiedelM.SpellmannI.SchwarzMJ.StrassnigM.SikorskiC.MôllerHJ.MüllerN.Decreased T cellular immune response in schizophrenic patients.J Psychiatr Res.2006413716434055
- BreseeC.RapaportMH.Persistently increased serum soluble interleukin-2 receptors in continuously ill patients with schizophrenia.Int J Neuropsychopharmacol.20091286186519366488
- PotvinS.StipE.SepehryAA.GendronA.BahR.KouassiE.Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review.Biol Psychiatry.20086380180818005941
- CazzulloCL.ScaroneS.GrassiB.et al.Cytokines production in chronic schizophrenia patients with or without paranoid behaviour.Prog Neuropsychopharmacol Biol Psychiatry.1998229479579789879
- LinA.KenisG.BignottiS.et al.The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6.Schizophr Res.1998329159690329
- Sperner-UnterwegerB.WhitworthA.KemmlerG.et al.T-cell subsets in schizophrenia: a comparison between drug-naive first episode patients and chronic schizophrenic patients.Schizophr Res.199938617010427611
- SchwarzMJ.ChiangS.MüllerN.AckenheilM.T-helper-1 and T-helper2 responses in psychiatric disorders.Brain Behavlmmun.200115340370
- van KammenDP.McAllister-SistilliCG.KelleyME.Relationship between immune and behavioral measures in schizophrenia. In: Wieselmann G, ed.Current Update in Psychoirnrnunohgy. Vienna, Austria; New York, NY: Springer;19975155
- MittlemanBB.CastellanosFX.JacobsenLK.RapoportJL.SwedoSE.ShearerGM.Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease.J Immunol.1997159299429999300724
- MüllerN.HofschusterE.AckenheilM.MempelW.EcksteinR.Investigations of the cellular immunity during depression and the free interval: evidence for an immune activation in affective psychosis.Prog Neuropsychopharmacol Biol Psychiatry.1993177137308255983
- MaesM.MeltzerHY.BuckleyP.BosniansE.Plasma-soluble interleukin-2 and transferrin receptor in schizophrenia and major depression.Eur Arch Psychiatry Clin Neurosci.19952443253297772617
- IrwinM.Immune correlates of depression.Adv Exp Med Biol.199946112410442164
- MüllerN.SchwarzMJ.Immunology in anxiety and depression. In: Kasper S, den Boer JA, Sitsen JMA, eds.Handbook of Depression and Anxiety,2nd ed. New York, NY: Marcel Dekker;2002267288
- MikovaO.YakimovaR.BosniansE.KenisG.MaesM.Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis.Eur Neuropsychopharmacol.20011120320811418279
- SluzewskaA.RybakowskiJ.BosmansE.et al.Indicators of immune activation in major depression.Psychiatry Res.1996641611678944394
- RothermundtM.AroltV.FenkerJ.GutbrodtH.PetersM.KirchnerH.Different immune patterns in melancholic and non-melancholic major depression.Eur Arch Psychiatry Clin Neurosci.2001251909711407444
- BonaccorsoS.LinAH.VerkerkR.et al.Immune markers in fibromyalgia: comparison with major depressed patients and normal volunteers.J Affect Disord.19984875829495605
- MaesM.ScharpeS.MeltzerHY.et al.Increased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: further evidence for an immune response.Psychiatry Res.1994541431607761549
- MaesM.ScharpeS.MeltzerHY.et al.Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamicpituitary-adrenal axis in severe depression.Psychiatry Res.19934911277511248
- SchiepersOJ.WichersMC.MaesM.Cytokines and major depression.Prog Neuropsychopharmacol Biol Psychiatry.20052920121715694227
- MyintAM.LeonardBE.SteinbuschHW.KimYK.Th1, Th2, and Th3 cytokine alterations in major depression.J Affect Disord.20058816717316126278
- JunTY.PaeCU.HoonH.ChaeJH.BahkWM.KimKS.SerrettiA.Possible association between - G308A tumour necrosis factor-alpha gene polymorphism and major depressive disorder in the Korean population.Psychiatr Genet.20031317918112960751
- RieckmannP.NunkeK.BurchhardtM.et al.Soluble intercellular adhesion molecule-1 in cerebrospinal fluid: an indicator for the inflammatory impairment of the blood-cerebrospinal fluid barrier.J Neuroimmunol.1993471331408103775
- SchäferM.HornM.SchmidtF.et al.Correlation between slCAM-1 and depressive symptoms during adjuvant treatment of melanoma with interferon-alpha.Brain Behav lmmun.200418555562
- ThomasAJ.FerrierIN.KalariaRN.et al.Elevation in late-life depression of intercellular adhesion molecule-1 expression in the dorsolateral prefrontal cortex.Am J Psychiatry.20001571682168411007725
- DimopoulosN.PiperiC.SaloniciotiA.et al.Elevation of plasma concentration of adhesion molecules in late-life depression.Int J Geriatr Psychiatry.20062196597116927406
- MendlovicS.MozesE.EilatE.et al.Immune activation in non-treated suicidal major depression.Immunol Lett.19996710510810232390
- PenttinenJ.Hypothesis: low serum cholesterol, suicide, and interleukin2. Am J Epidemiol.19951417167187709913
- NassbergerL.Traskman-BendzL.Increased soluble interleukin-2 receptor concentrations in suicide attempters.Acta Psychiatr Scand.19938848528372695
- BaronDA.HardieT.BaronSH.Possible association of interleukin-2 treatment with depression and suicide.J Am Osteopath Assoc.1993937998008365929
- MüllerN.RiedelM.SchwarzMJ.et al.Immunomodulatory effects of neuroleptics to the cytokine system and the cellular immune system in schizophrenia. In Wieselmann G, ed.Current Update in Psychoimmunology. Vienna, Austria; New York, NY: Springer;19975767
- MüllerN.EmplM.RiedelM.SchwarzM.AckenheilM.Neuroleptic treatment increases soluble IL-2 receptors and decreases soluble IL-6 receptors in schizophrenia.Eur Arch Psychiatry Clin Neurosci.19972473083139477010
- MüllerN.RiedelM.HadjamuM.SchwarzMJ.AckenheilM.GruberR.Increase in expression of adhesion molecule receptors on T helper cells during antipsychotic treatment and relationship to blood-brain barrier permeability in schizophrenia.Am J Psychiatry.199915663463610200747
- PollmächerT.SchuldA.KrausT.HaackM.Hinze-SelchD.[On the clinical relevance of clozapine-triggered release of cytokines and soluble cytokine-receptors].Fortschr Neurol Psychiatr.200169(suppl 2)S65S7411533853
- OzekM.ToreciK.AkkokI.GuvenerZ.[Influence of therapy on antibody-formation],Psychopharmacologia.1971214014125098938
- TanakaKF.ShintaniF.FujiiY.YagiG.AsaiM.Serum interleukin-18 levels are elevated in schizophrenia.Psychiatry Res.200096758010980328
- MaesM.BosmansE.De JonghR.KenisG.VandoolaegheE.NeelsH.Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression.Cytokine.199798538589367546
- SongC.LeonardBE.An acute phase protein response in the olfactory bulbectomised rat: effect of sertraline treatment.Med Sci Res.199422313314
- ZhuJ.BengtssonBO.MixE.ThorellLH.OlssonT.LinkH.Effect of monoamine reuptake inhibiting antidepressants on major histocompatibility complex expression on macrophages in normal rats and rats with experimental allergic neuritis (EAN).Immunopharmacology.1994272252448071062
- MaesM.SongC.LinAH.BonaccorsoS.et al.Negative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretion.Neuropsychopharmacology.19992037037910088138
- SeidelA.AroltV.HunstigerM.RinkL.BehnischA.KirchnerH.Cytokine production and serum proteins in depression.Scand J Immunol.1995415345387539545
- LanquillonS.KriegJC.Bening-Abu-ShachU.VedderH.Cytokine production and treatment response in major depressive disorder.Neuropsychopharmacology.20002237037910700656
- KenisG.MaesM.Effects of antidepressants on the production of cytokines.Int J Neuropsychopharmacol.2002540141212466038
- O'SullivanJB.RyanKM.CurtinNM.HarkinA.ConnorTJ.Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration.Int J Neuropsychopharmacol.20091268769919046481
- VollmarP.NesslerS.KalluriSR.HartungHP.HemmerB.The antidepressant venlafaxine ameliorates murine experimental autoimmune encephalomyelitis by suppression of pro-inflammatory cytokines.Int J Neuropsychopharmacol.20091252553618922202
- SluzewskaA.RybakowskiJK.LaciakM.et al.lnterleukin-6 serum levels in depressed patients before and after treatment with fluoxetine.Ann N Y Acad Sci.19957624744767668562
- FrommbergerUH.BauerJ.HaselbauerP.FraulinA.RiemannD.BergerM.lnterleukln-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission.Eur Arch Psychiatry Clin Neurosci.19972472282339332905
- MaesM.MeltzerHY.BosmansE.BergmansR.VandoolaegheE.RanjanR.DesnyderR.Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression.J Affect Disord.1995343013098550956
- PollakY.YirmiyaR.Cytokine-induced changes in mood and behaviour: implications for 'depression due to a general medical condition', immunotherapy and antidepressive treatment.Int J Neuropsychopharmacol.2002538939912466037
- MtabajiJP.MankuMS.HorrobinDF.Actions of the tricyclic antidepressant clomipramine on responses to pressor agents. Interactions with prostaglandin E2.Prostaglandins.197714125132897208
- YaronI.ShiraziI.JudovichR.LevartovskyD.CaspiD.YaronM.Fluoxetine and amitriptyline inhibit nitric oxide, prostaglandin E2, and hyaluronic acid production in human synovial cells and synovial tissue cultures.Arthritis Rheum.1999422561256810616001
- HestadKA.TonsethS.StoenCD.UelandT.AukrustP.Raised plasma levels of tumor necrosis factor alpha in patients with depression: normalization during electroconvulsive therapy.J ECT.20031918318814657769
- DimitrovS.LangeT.TiekenS.FehmHL.BornJ.Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans.Brain Behav Immun.20041834134815157951
- SchwarczR.PellicciariR.Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities.J Pharmacol Exp Ther.200230311012235226
- HilmasC.PereiraEF.AlkondonM.RassoulpourA.SchwarczR.AlbuquerqueEX.The brain metabolite kynurenic acid inhibits alpha? nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications.J Neurosci.2001217463747311567036
- GrohmannU.FallarinoF.PuccettiP.Tolerance, DCs and tryptophan: much ado about IDO.Trends Immunol.20032424224812738417
- TakikawaO.YoshidaR.KidoR.HayaishiO.Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase.J Biol Chem.1986261364836532419335
- MillerCL.LlenosIC.DulayJR.BarilloMM.YolkenRH.WeisS.Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia.Neurobiol Dis.20041561862915056470
- AloisiF.RiaF.AdoriniL.Regulation of T-cell responses by CNS antigenpresenting cells: different roles for microglia and astrocytes.Immunol Today.20002114114710689302
- KimYK.MyintAM.VerkerkR.ScharpeS.SteinbuschH.LeonardB.Cytokine changes and tryptophan metabolites in medication-naive and medication-free schizophrenic patients.Neuropsychobiology.20095912312919390223
- MellorAL.MunnDH.Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation?Immunol Today.19992046947310500295
- MunnDH.ShafizadehE.AttwoodJT.BondarevI.PashineA.MellorAL.Inhibition of T cell proliferation by macrophage tryptophan catabolism.J Exp Med.19991891363137210224276
- WeissG.MurrC.ZollerH.et al.Modulation of neopterin formation and tryptophan degradation by Th1- and Th2-derived cytokines in human monocytic cells.Clin Exp Immunol.199911643544010361231
- AlberatiGD.RicciardiCP.KohlerC.CesuraAM.Regulation of the kynurenine metabolic pathway by interferon-gamma in murine cloned macrophages and microglial cells.J Neurochem.19966699610048769859
- BraunD.LongmanRS.AlbertML.A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation.Blood.20051062375238115947091
- MyintAM.KimYK.Cytokine-serotonin interaction through IDO: a neurodegeneration hypothesis of depression.Med Hypotheses.20036151952514592780
- ChiarugiA.CalvaniM.MeliE.TraggiaiE.MoroniF.Synthesis and release of neurotoxic kynurenine metabolites by human monocyte-derived macrophages.J Neuroimmunol.200112019019811694334
- LidbergL.BelfrageH.BertilssonL.EvendenMM.AsbergM.Suicide attempts and impulse control disorder are related to low cerebrospinal fluid 5-HIAA in mentally disordered violent offenders.Acta Psychiatr Scand.200010139540210823300
- MannJJ.MaloneKM.Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients.Biol Psychiatry.1997411621719018386
- BonaccorsoS.MarinoV.PuzellaA.et al.Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system.J Clin Psychopharmacol.200222869011799348
- CapuronL.NeurauterG.MusselmanDL.et al.Interferon-alphainduced changes in tryptophan metabolism, relationship to depression and paroxetine treatment.Biol Psychiatry.20035490691414573318
- AloisiF.PennaG.CeraseJ.MenendezIB.AdoriniL.IL-12 production by central nervous system microglia is inhibited by astrocytes.J Immunol.1997159160416129257819
- RothermundtM.FalkaiP.PonathG.et al.Glial cell dysfunction in schizophrenia indicated by increased S100B in the CSF.Mol Psychiatry.2004989789915241436
- BayerTA.BusleiR.HavasL.FalkaiP.Evidence for activation of microglia in patients with psychiatric illnesses.Neurosci Lett.199927112612810477118
- CotterD.ParianteC.RajkowskaG.Glial pathology in major psychiatric disorders. In Agam G, Belmaker RH, Everall I, eds.The Post-Mortern Brain in Psychiatric Research. Boston, MA: Kluwer Acad Pub;2002291324
- RajkowskaG.Depression: what we can learn from postmortem studies.Neuroscientist.2003927328412934710
- Miguel-HidalgoJJ.BaucomC.DilleyG.et al.Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder.Biol Psychiatry.20004886187311063981
- DavisS.ThomasA.PerryR.OakleyA.KalariaRN.O'BrienJT.Glial fibrillary acidic protein in late life major depressive disorder: an immunocytochemical study.J Neurol Neurosurg Psychiatry.20027355656012397151
- RajkowskaG.Astroglia in the cortex of schizophrenics: histopathology finding.World J Biol Psychiatry.2005674
- ChoudaryPV.MolnarM.EvansSJ.et al.Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression.Proc Natl Acad Sci USA.2005102156531565816230605
- DanboltNC.Glutamate uptake.Prog Neurobiol.200165110511369436
- SanacoraG.GueorguievaR.EppersonCN.et al.Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression.Arch Gen Psychiatry.20046170571315237082
- HeyesMP.ChenCY.MajorEO.SaitoK.Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types.Biochem J.19973263513569291104
- KissC.Ceresoli-BorroniG.GuidettiP.ZielkeCL.ZielkeHR.SchwarczR.Kynurenate production by cultured human astrocytes.J Neural Transm.200311011412541009
- GuilleminGJ.KerrSJ.SmytheGA.et al.Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection.J Neurochem.20017884285311520905
- ChiarugiA.CarpenedoR.MoroniF.Kynurenine disposition in blood and brain of mice: effects of selective inhibitors of kynurenine hydroxylase and of kynureninase.J Neurochem.1996676926988764597
- SaitoK.CrowleyJS.MarkeySP.HeyesMP.A mechanism for increased quinolinic acid formation following acute systemic immune stimulation.J Biol Chem.199326815496155038340378
- HeyesMP.SaitoK.LacknerA.WileyCA.AchimCL.MarkeySP.Sources of the neurotoxin quinolinic acid in the brain of HIV-1-infected patients and retrovirus-infected macaques.FASEB J.1998128818969657528
- HeyesMP.SaitoK.CrowleyJS.Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease.Brain.1992115124912731422788
- WichersMC.KoekGH.RobaeysG.VerkerkR.ScharpeS.MaesM.IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity.Mol Psychiatry.20051053854415494706
- HeyesMP.BrewBJ.MartinA.et al.Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status.Ann Neurol.1991292022091826418
- LapinIP.Neurokynurenines (NEKY) as common neurochemical links of stress and anxiety.Adv Exp Med Biol.200352712112515206724
- ChenQ.SurmeierDJ.ReinerA.NMDA and non-NMDA receptor-mediated excitotoxicity are potentiated in cultured striatal neurons by prior chronic depolarization.Exp Neurol.199915928329610486197
- SchwielerL.ErhardtS.ErhardtC.EngbergG.Prostaglandin-mediated control of rat brain kynurenic acid synthesis-opposite actions by COX-1 and COX-2 isoforms.J Neural Transm.200511286387215517427
- MüllerN.RiedelM.ScheppachC.et al.Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia.Am J Psychiatry.20021591029103412042193
- MüllerN.UlmschneiderM.ScheppachC.et al.COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy.Eur Arch Psychiatiy Clin Neurosci.20042541422
- MüllerN.RiedelM.DehningS.et al.Is the therapeutic effect of celecoxib in schizophrenia depending from duration of disease?Neuropsychopharmacology.200429176
- CasoliniP.CatalaniA.ZuenaAR.AngelucciL.Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat.J Neurosci Res.20026833734312111864
- ZhangY.Chun ChenD.Long TanY.ZhouDF.A double-blind, placebo-controlled trial of celecoxib addes to risperidone in first-episode and drugnaive patients with schizophrenia [abstract].Eur Arch Psychiatry Clin Neurosci.2006256(suppl 2) II/50
- AkhondzadehS.TabatabaeeM.AminiH.Ahmadi AbhariSA.AbbasiSH.BehnamB.Celecoxib as adjunctive therapy in schizophrenia: a doubleblind, randomized and placebo-controlled trial.Schizophr Res.20079017918517208413
- RapaportMH.DelrahimKK.BreseeCJ.MadduxRE.AhmadpourO.DolnakD.Celecoxib augmentation of continuously ill patients with schizophrenia.Biol Psychiatry.2005571594159615953498
- HuF.WangX.PaceTW.WuH.MillerAH.Inhibition of COX-2 by celecoxib enhances glucocorticoid receptor function.Moi Psychiatry.20051042642815700047
- SongC.LeonardBE.Fundamentals of Psychoneuroimmunology.Chichester, NY: J Wiley and Sons;2000
- CaoC.MatsumuraK.OzakiM.WatanabeY.Lipopolysaccharide injected into the cerebral ventricle evokes fever through induction of cyclooxygenase-2 in brain endothelial cells.J Neurosci.1999197167259880592
- SandriniM.VitaleG.PiniLA.Effect of rofecoxib on nociception and the serotonin system in the rat brain,inflamm Res.20025115415912005206
- Salzberg-BrenhouseHC.ChenEY.EmerichDF.et al.Inhibitors of cyclooxygenase-2, but not cyclooxygenase-1 provide structural and functional protection against quinolinic acid-induced neurodegeneration.J Pharmacol Exp Ther.200330621822812676885
- MyintAM.SteinbuschHW.GoegheganL.LuchtmanD.KimYK.LeonardBE.Effect of the COX-2 inhibitor celecoxib on behavioural and immune changes in an olfactory bulbectomised rat model of depression.Neuroimmunomoduiation.2007146571
- BrunelloN.AlboniS.CaponeG.et al.Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression.Int Clin Psychopharmacol.20062121922516687993
- MüllerN.SchwarzMJ.DehningS.et al.The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a doubleblind, randomized, placebo controlled, add-on pilot study to reboxetine.Mol Psychiatry.20061168068416491133
- MendlewiczJ.KriwinP.OswaldP.SoueryD.AlboniS.BrunelloN.Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study.Int Clin Psychopharmacol.20062122723116687994