Abstract
Cross-species affective neuroscience studies confirm that primary-process emotional feelings are organized within primitive subcortical regions of the brain that are anatomically, neurochemically, and functionally homologous in all mammals that have been studied. Emotional feelings (affects) are intrinsic values that inform animals how they are faring in the quest to survive. The various positive affects indicate that animals are returning to “comfort zones” that support survival, and negative affects reflect “discomfort zones” that indicate that animals are in situations that may impair survival. They are ancestral tools for living - evolutionary memories of such importance that they were coded into the genome in rough form (as primary brain processes), which are refined by basic learning mechanisms (secondary processes) as well as by higher-order cognitions/thoughts (tertiary processes). To understand why depression feels horrible, we must fathom the affective infrastructure of the mammalian brain. Advances in our understanding of the nature of primary-process emotional affects can promote the development of better preclinical models of psychiatric disorders and thereby also allow clinicians new and useful ways to understand the foundational aspects of their clients' problems. These networks are of clear importance for understanding psychiatric disorders and advancing psychiatric practice.
Los estudios de las neurociencias afectivas a través de las especies confirman que los sentimientos emocionales como proceso primario están organizados dentro de las regiones subcorticales primitivas del cerebro, las que son anatómica, neuroquímica y funcionalmente homólogas en todos los mamíferos que se han estudiado. Los sentimientos emocionales (afectos) son valores intrínsecos que informan a los animales acerca de cómo se están manejando en la búsqueda de la sobrevivencia. Los diversos afectos positivos indican que los animales están retornando a las “zonas de confort” que permiten la sobrevivencia y los afectos negativos reflejan las “zonas de disconfort” que indican que los animales están en situaciones en que se puede deteriorar la sobrevivencia. Ellos constituyen herramientas ancestrales para vivir, memorias evolutivas de tal importancia que fueron codificadas en el genoma de forma primitiva (como proceso cerebral primario), que han sido refinadas mediante mecanismos de aprendizaje básico (procesos secundarios), como por cogniciones/pensamientos de orden más elevado (procesos terciarios). Para comprender el porqué de los horribles sentimientos depresivos se debe profundizar en la infraestructura afectiva del cerebro de los mamíferos. Los avances en nuestra comprensión acerca de la naturaleza de los afectos emocionales como proceso primario puede favorecer el desarrollo de mejores modelos preclínicos de los trastornos psiquiátricos y por consiguiente también permitir a los clínicos nuevas y útiles vías para comprender los aspectos básicos de los problemas de sus clientes. Estas redes son de gran importancia para la comprensión de los trastornos psiquiátricos y el avance en la práctica psiquiátrica.
Des études de neuroscience affective entre espèces confirment que les principales émotions s'organisent dans les régions sous-corticales primitives du cerveau qui sont anatomiquement, neurochimiquement et fonctionnellement homologues chez tous les mammifères étudiés. Les émotions (affects) sont des données intrinsèques qui informent les animaux sur leurs possibilités de réussite de survie. Les différents affects positifs montrent que les animaux retournent vers les « zones de confort » qui permettent la survie et les affects négatifs témoignent des « zones d'inconfort » qui indiquent que les animaux sont dans des situations qui peuvent menacer leur survie. Ce sont des outils de subsistance ancestraux, souvenirs de l'évolution d'une telle importance qu'ils ont été codés dans le génome sous une forme approximative (comme les processus primaires du cerveau), qui sont affinés par des apprentissages basiques (processus secondaires) ainsi que par des connaissances/pensées d'un ordre supérieur (processus tertiaire). Pour comprendre l'horreur de la dépression, nous devons appréhender l'infrastructure affective du cerveau des mammifères. Des progrès dans notre compréhension de la nature des processus primaires des affects émotionnels peuvent favoriser le développement de modèles précliniques de troubles psychiatriques plus performants et donc aussi offrir aux médecins des voies nouvelles et utiles pour comprendre les aspects fondamentaux des problèmes de leurs patients. Ces réseaux sont très importants pour comprendre les troubles psychiatriques et progresser en psychiatrie.
Psychiatric disorders commonly reflect affective imbalances within the brain. Accordingly, a key question in psychiatric research is the neural nature of emotional feelings. For instance, in depression research, one of the most important unanswered questions is: Why does depression feel so bad? What is the “psychological pain” that leads people to lose their joy of living? Exactly the same affective issues confront us when we study addictions. Here we explore the possibility that chronic affective changes may arise from functional changes in basic emotional systems of the brain. For example, diminished arousability of specific positive affective systems along with elevated activation of distinct negative affective networks may be the fundamental source of depressive affect.
But what systems are they? Here, arguments for the critical importance of brain systems that integrate the distress and despair of separation-distress (overactivity of basic PANIC/GRIEF networks) and the diminished arousal of SEEKING networks that constitute dysphoria will be presented. Excessive arousal of SEEKING urges may contribute substantially to mania and psychostimulant addictions, leading to excessive elation/euphoria, arising from excessive appetitive dopamine SEEKING urges, which can promote unwise life choices.Citation1 (Capitalizations highlight the need for a specialized vocabulary when discussing the evolutionary foundations of the mind. Vernacular terms have excess meanings, and thus will not suffice for clear discourse). Thus, drug addictions share some important affective features with depression; for instance, the dysphoric feelings that accompany both addictive drug withdrawal and depression which reflect diminished SEEKING urges.Citation2 Studies in psychology and neuroscience, as well as in psychiatric syndromes, indicate that there are many distinct emotional feelings within mammalian brains and minds (henceforth BrainMind, a monistic term). We are just beginning to understand the underlying innate, genetically determined, and epigenetically refined aspects of emotional feelings.
They generate characteristic behavioral-instinctual action patterns
They are initially activated by a limited set of unconditional stimuli
The resulting arousals outlast precipitating circumstances
Emotional arousals gate/regulate various sensory inputs into the brain
They control learning and help program higher brain cognitive activities
With maturation, higher brain mechanisms come to regulate emotional arousals.
Emotional nomenclature can be confusing. Here primary-process (ie, basic or primordial) emotional networks are defined in terms of neural and behavioral criteria. Basic emotional networks can be defined by six criteria:
Affects are the subjectively experienced aspects of emotions, commonly called feelings. Critical evidence now indicates that primary-process emotional affects are mammalian/human birthrights that arise directly from genetically encoded emotional action circuits that anticipate key survival needs. They mediate what philosophers have called “intentions-in-action” (Table I).
Table I. Levels of control in brain emotion-affective processing
Primary-process emotional feelings within mammalian brains - namely the experienced aspects of the unconditioned emotional brain systems (ie, “instinctual” integrative BrainMind systems) in action. From a philosophical point of view, they control “intentions-in-action.”
Secondary emotional processes that arise from simple emotional learning, such as classical and operant conditioning that has been well studied in animal models, especially FEAR conditioning.
Tertiary-process emotions are the intrapsychic ruminations and thoughts about one's lot in life. Such higherorder affective-cognitions that promote “intentions-toact” and are elaborated by medial-frontal regions, which can only be well studied in humans (Table I).
Until we understand the neurobiological nature of basic emotional feelings within the human BrainMind, our understanding of psychiatric disorders will remain woefully incomplete. Because of striking cross-species homologies in mammalian primary-process emotional systems, animal models may provide optimal guidance for deciphering brain affective mechanisms that also operate in our species. This review will delve into various levels of emotional control, especially the first:
It is among the inherited subcortical primary-process instinctual tools for living that the foundations of human emotional lives reside, and neurochemical imbalances there can lead to persistent affective imbalances of psychiatric significance.Citation3 Also, it is reasonable to currently postulate that the secondary and tertiary emotional levels of organization remain critically linked to the dynamics of primary processes, which serve as a foundation for diverse higher psychological functions.
The mammalian brain is clearly an organ where evolutionary layering remains evident at both the anatomical and chemical levels, and striking cross-species homologies exist in the more ancient primary-process neural regions.Citation4 In contrast, higher brain functions, which are much harder to study in preclinical models, are more distinct across species. Such neuroevolutionary facts allow us to envision primary emotional processes in humans that are homologous across mammals, permitting animal models to effectively illuminate how primordial emotional feelings - ancestral states of consciousness - emerge from human brain activities.Citation5 In addition, advances in understanding subcortical emotional brain organization, especially its evolutionary roots, can illuminate certain higher tertiary-process BrainMind functions, permitted by massive encephalization in primates. Here, some of the cross-species primary -process emotional systems that help us decipher the foundations of emotions in normal human mental life, as well as psychiatric conditions, will be described.Citation6 However, first it should be noted that there are historical forces at work that are delaying such integration.
Many still believe in James-Lange's 125-year-old conjecture that emotional feelings reflect neocortical “readout” of bodily autonomic arousals. For a sampling of such opinions from prominent investigators see the video of Charlie Rose's 8th Brain Series on May 26, 2010.Citation7 Regrettably, this time-honored theoretical vision has essentially no consistent support. However, evidence that affective feelings arise directly from medial subcortical networks is consistent and substantial.Citation8 The primary-process networks for emotional instincts run from midbrain periaqueductal gray (PAG) regions to medial diencephalon to various basal ganglia nuclei (amygdala, bed nucleus of the stria terminalis, nucleus accumbens, etc) that interact with paleocortical brain functions (eg, cingulate, insular, as well as medial- and orbitofrontal cortices) and more indirectly with certain neocortical regions to provide integration with higher cognitive activities. The subcortical locus of affect generation strongly suggests that the foundational principles of human emotions can be understood by studying these brain structures and functions in other animals.Citation9
Historical perspectives and the role of animal models in biological psychiatry
Twentieth-century thinking about psychiatric issues can be divided into two phases: the first half of the century focused heavily on emotional and related psychological complexities, especially through Freud-inspired psychoanalytic theory. Because of the immaturity of neuroscience, this eventually led to the study of the mind without a brain - a top-down speculative perspective with little scientific basis. The second half of the century, after the discovery of several highly effective psychiatric medications, was framed more in a Krapelinian context - psychiatric diagnostic categories were linked to diverse brain mechanisms, which were studied objectively. This has now led to abundant ruthless reductionism, where mental (experienced) aspects of brain functions are inadequately considered in the genesis of psychiatric disorders, especially when preclinical models are used to clarify underlying principles. This has led to the increasing study of living brains without feelings - without a mind. This is ontologically unsatisfactory.
The above traditions can now be blended, illuminating how our ancestral affective BrainMind contributes to and often causes psychiatric problems. But the absence of a general solution to how emotional feelings are created in the brain continues to impede development of neuroscientifically coherent psychiatric nosologies (reflected in the current discussions regarding DSM-5 definitions). Detailed understanding of primary emotional systems in animal models may yield psychologically relevant endophenotypes for psychiatry.Citation10
“The difficulty of characterizing the circuitry and mechanisms that underlie higher brain functions.” Regrettably Hyman largely neglected the emotional difficulties that arise from imbalanced lower emotionalaffective brain functions that can be studied in animals.
The “complexity of the genetic and developmental underpinnings of normal and abnormal behavioral variation” that prevents integration between diagnostic labels and brain pathophysiology. This is surely so, but many current emotion-free genetic-psychiatric linkage studies are providing few insights. Perhaps more the-oretically focused studies that include affective issues can lead to faster progress.Citation12
The “unsatisfactory nature of current animal models of mental disorders.” The key problem here may be our relative unwillingness to discuss the nature of affective experience in animals, which prevents development of preclinical brain emotional-network models that could better clarify primary-affective issues.
However, preclinical models pose major problems, as emphasized by the past director of NIMH, Steve Hyman, Citation11who highlighted three dilemmas of current research in facilitating more coherent future nosologies (eg, DSM-5). They were (my commentary in italics):
The rest of this article will highlight: (i) how emotional states can be understood neuroscientifically through animal models; and (ii) how such knowledge can impact clinical practice in biological psychiatry, with a focus on depression.
Emotion theory - old beliefs and new realities
Primary-process emotion approaches to the BrainMind are not well represented in modern psychology, psychiatry, or even neuroscience. The most widely acknowledged theory of emotional feelings remains the JamesLange conjecture (see above) that advanced the counterintuitive idea of life-challenging situations (ie, when inadvertently confronted by a grizzly bear in the woods) resulting first in various bodily symptoms of autonomic arousal, and emotional experiences following only after bodily arousals are “read out” by higher cognitive processes. This has promoted the misleading belief that emotions are just a subset of cognitive process. If one defines cognitive processes as neural handling of incoming sensory stimuli, a disciplined distinction can be made between cognitive and primaryprocess emotional processes, with the former consisting of externally sourced information processing and the latter being internal state-control processes, as done here. When one moves to higher levels of processing, secondary (learning), and tertiary processes (thought) levels of analysis, cognitive and emotional issues do get more conflated.
Another bias impeding progress is the fact that many psychologists believe that emotions arise not from brain evolution but from social-developmental learning based on primal gradients (dimensions) of arousal and valence.Citation13 This “experimental convenience” - namely a convenient conceptual way to study human emotions verbally - goes back to the 19th-century work of Wilhelm Wundt, but it has never been firmly connected to neuroscientific facts. Such dimensional approaches effectively focus on the diverse languages of emotion (ie, tertiary processes) with no compelling strategy for unraveling primary-process emotional networks. To this day, abundant “battles” are waged between psychologists who espouse “basic emotion” views in human research and those who prefer dimensional views. The “basic emotion” approaches posit a variety of distinct, inherited brain emotional systems; the “dimensional” views envision distinct emotions simply to reflect verbal labeling of locations in some type of continuous affective space that is defined by two continuous axes: generalized forms of: (i) low and high arousal; and (ii) positive and negative valence.
The study of primary-process brain mechanisms of emotions, best pursued in animal models, provides a bridge that can help settle such debates. A primaryprocess/basic emotion view may prevail in many subcortical regions, and constructivist/dimensional approaches may effectively parse higher emotional concepts as processed by the neocortex (Table I). In other words, such debates may simply reflect investigators working at different levels of control.
The Affective Neuroscience Citation3 strategy relies on preclinical evidence for the existence of a variety of primaryprocess emotional networks in mammalian brains. These networks are identified by distinct emotional behaviors evoked with highly localized electrical stimulation of the brain (ESB) sites which exist almost exclusively in subcortical regions. Such instinct-generating sites also generate emotional feelings, as monitored by “reward” and “punishment” attributes. In other words, animals care whether such emotional states are evoked. The likelihood that there are just singular types of “good” and “bad” feelings (positive and negative valence) among the subcortical affective networks is unlikely; humans report a variety of emotional feelings that generally correspond to the types of emotional actions evoked in animals.Citation14 Also, a single primordial dimension of arousal must be questioned: the psychological feeling of emotional intensity is regulated by many systems - eg, acetylcholine, dopamine, glutamate, histamine, norepinephrine, serotonin, and various neuropeptides - leaving open the possibility of distinct types of arousal in lower regions of the brain. Perhaps at a tertiary-process conceptual (neocortical) level, we do conflate feelings into positive and negative - “good” and “bad” - categories, but that is a heuristic simplification (a Wittgensteinian “word game”) promoted by our thinking processes. But can the neocortex generate emotional feelings on its own?
The emotional-behavioral coherence of organisms is fully formed in subneocortical regions of the brain - eg, just consider that physical PLAY, the most complex basic social emotion, persists after neodecortication.Citation16
Both the emotional-behavioral and affective (reward and punishment) aspects of ESB are most readily obtained, with the lowest current levels, from the most ancient midbrain regions (PAG or central gray) rather than from higher emotional regions (eg, amygdala, cingulate, and frontal cortices).Citation17
Cognitive working-memory fields concentrated in dorsolateral frontal cortical regions have a “seesaw” relationship with subcortical emotional-affective systems, so that their activities are commonly reciprocally related.Citation18
- Human brain imaging of intense emotional experiences (anger, fear, sadness, and joy) “light up” subcortical brain regions, homologous in all mammals.Citation19
No scientist who has worked on primary-process brain emotional systems has ever subscribed to the JamesLange conjecture that affective feelings are only experienced when unconscious sensory information about bodily arousals reaches the neocortex. Beside Walter Cannon's seminal critique,Citation15 abundant modern findings contradict that view:
The second point above is critical. There is a remarkable correspondence between ESB sites yielding emotional action patterns (the various distinct instinctual-behavioral profiles, described below for each of seven primary emotional processes) and their capacity to sustain “reinforced” learning in animals and intense emotional feelings in humans. Accordingly, we can use a dual-aspect monism strategy to study emotional feelings - ie, ESB evoked RAGE behaviors reflect angry-type feelings (animals turn off such ESBCitation20), while evoked PLAY behaviors reflect joyful-type feelings - ESB evoking play-vocalizations sustain self-stimulation reward,Citation21 etc. (In physics, a related “dual-aspect” strategy - concurrent acceptance of “wave” and “particle” descriptions of electromagnetic radiation - is needed to make sense of available data). In the present view, the affective states generated by primordial brain emotional networks may have been among the first experiences that existed in brain evolution. Without them, higher consciousness (frontal neocortical executive functions) may not have evolved.Citation22 In evolutionary terms, all primal emotional systems are rooted in yet deeper and more ancient processes. For example, the psychological pain of separation-distress/GRIEF may have arisen from earlier physical pain systems of the brain.Citation23
The primary-process emotional-affective networks of mammalian brains
Brain research supports the existence of at least seven primary-process (basic) emotional systems - SEEKING, RAGE, FEAR, LUST, CARE, GRIEF (formerly PANIC), and PLAY - concentrated in ancient subcortical regions of all mammalian brains.
In sum, affective neuroscientific analysis of basic emotions is based on several highly replicable facts: (i) Coherent emotional-instinctual behaviors can be aroused by electrically stimulating very specific subcortical regions of the brain; (ii) Wherever one evokes emotional action patterns with ESB, there are accompanying affective experiences. Again, the gold standard for this assertion is the fact that the brain stimulations can serve as “rewards” when positive-emotions are aroused - eg, SEEKING, LUST, CARE, and aspects of PLAY. When negative emotions are aroused - RAGE, FEAR, GRIEF - animals escape the stimulation; (iii) The above behavioral and affective changes are rarely, if ever, evoked from higher prefrontal neocortical regions, suggesting that higher brain areas may not have the appropriate circuitry to generate affective experiences, although the neocortex can clearly regulate (eg, inhibit) emotional arousals and, no doubt, prompt emotional feelings by dwelling on life problems.
The emotional primes are summarized in several monographs, with another appearing soon.Citation24 Thumbnail descriptions are provided below, with one key reference for each.
The SEEKING/desire system
This extensive network confluent with the medial forebrain bundle (MFB) is traditionally called the “brain reward system.” In fact, this is a general-purpose appetitive motivational system that is essential for animals to acquire all resource needs for survival, and it probably helps most other emotional systems to operate effectively. It is a major source of life “energy”, sometimes called “libido.” In pure form, it provokes intense and enthusiastic exploration and appetitive anticipatory excitement/learning. When fully aroused, SEEKINGCitation25 fills the mind with interest and motivates organisms to effortlessly search for the things they need, crave, and desire. In humans, this system generates and sustains curiosity from the mundane to our highest intellectual pursuits. This system becomes underactive during addictive drug withdrawal, chronic stress, and sickness, and with accompanying feelings of depression. Overactivity of this system can promote excessive and impulsive behaviors, along with psychotic delusions and manic thoughts. All antipsychotics reduce arousability of this “reality-creating” mechanism of the brain. The term “reality-creating” is used to highlight the fact that this system appears to generate causal convictions about the nature of the world from the perception of correlated events (for a full discussion see Chapter 8 of Affective Neuroscience Citation3).
Neuroanatomically, SEEKING circuitry corresponds to the extensive medial forebrain bundle and major dopamine-driven, self-stimulation “reward” circuitry coursing from ventral midbrain to nucleus accumbens and medial frontal cortex, where it can promote frontal cortical functions related to planning and foresight. Rather than being a “pleasure or reinforcement system,” SEEKING coaxes animals to acquire resources needed for survival. It promotes learning by mediating anticipatory eagerness, partly by coding predictive relationships between events. It promotes a sense of engaged purpose in both humans and animals, and is diminished in depression and the dysphoria of withdrawal from addictive drugs. This is further highlighted by the simple fact that bilateral lesions of the system produce profound amotivational states in animals (all appetitive behaviors are diminished) and the elevated threshold for self-stimulation reward probably reflects the dysphoria state.
The RAGE/anger system
When SEEKING is thwarted, RAGECitation26 is aroused. Anger is provoked by curtailing animals' freedom of action. RAGE is a reliably provoked ESB of a neural network extending from the medial amygdala and hypothalamus to the dorsal PAG. RAGE lies close to and interacts with trans-diencephalic FEAR systems, highlighting the implicit source of classic “fight-flight” terminology. It invigorates aggressive behaviors when animals are irritated or restrained, and also helps animals defend themselves by arousing FEAR in their opponents. Human anger may get much of its psychic energy from the arousal of this brain system; ESB of the above brain regions can evoke sudden, intense anger attacks, with no external provocation. Key chemistries which arouse this system are the neuropeptide Substance P and glutamate, while endogenous opioids and y-aminobutyric acid (GABA) inhibit the system. A prediction is that glutamate and Substance P receptor antagonists (eg, aprepitant) may help control human anger. Additional medicines to control RAGE could presumably be developed through further detailed understanding of RAGE circuitry.
The FEAR/anxiety system
The evolved FEARCitation27 circuit helps to unconditionally protect animals from pain and destruction. FEAR-ESB leads animals to flee, whereas much weaker stimulation elicits a freezing response. Humans stimulated in these same brain regions report being engulfed by an intense free-floating anxiety that appears to have no environmental cause. Key chemistries that regulate this system are Neuropeptide Y and corticotrophin releasing factor (CRF); anti-anxiety agents such as the benzodiazepines inhibit this system by facilitating GABA transmission.
The LUST/sexual systems
Sexual LUST,Citation28 mediated by specific brain circuits and chemistries, distinct for males and females, is aroused by male and female sex hormones, which control many brain chemistries including two “social neuropeptides” - oxytocin transmission is promoted by estrogen in females and vasopressin transmission by testosterone in males. These brain chemistries help create gender-specific sexual tendencies. Oxytocin promotes sexual readiness in females, as well as trust and confidence, and vasopressin promotes assertiveness, and perhaps jealous behaviors, in males. Distinct male and female sexual tendencies are promoted by these steroid hormones early in life, with sexual activation by gonadal hormones at puberty. Because brain and bodily sex characteristics are independently organized, it is possible for animals that are externally male to have female-typical sexual urges and, others with female external characteristics to have maletypical sexual urges. The dopamine-driven SEEKING system participates in the search for sexual rewards just as for all other types of rewards, including those relevant for the other social-emotional systems described below.
The CARE/maternal nurturance system
Brain evolution has provided safeguards to assure that parents (usually the mother) take care of offspring. Some of the chemistries of sexuality, for instance oxytocin, have been evolutionarily redeployed to mediate maternal care - nurturance and social bonding - suggesting there is an intimate evolutionary relationship between female sexual rewards and maternal motivations.Citation29 The shifting hormonal tides at the end of pregnancy (declining progesterone, and increasing estrogen, prolactin, and oxytocin) invigorate maternal urges days before the young are born. This collection of hormonal and associated neurochemical changes also help assure strong maternal bonds with offspring.
The GRIEF/separation distress system
system was initially called the PANIC system, but few understood the intent of that primary-process terminology, so we shifted to the more comprehensible tertiary-process term of GRIEFCitation30 (highlighting once more terminological problems in emotion research: what are the differences between the tertiary-level emotions of bereavement, grief, and mourning, for instance?). In any event, young socially dependent animals have powerful emotional systems to solicit nurturance. They exhibit intense crying when lost, alerting caretakers to attend to their offspring. ESB mapping of this separation-distress system has highlighted circuitry running from dorsal PAG to anterior cingulate, and it is aroused by glutamate and CRF and inhibited by endogenous opioids, oxytocin, and prolactin - the major social-attachment, socialbonding chemistries of the mammalian brain. These neurochemicals are foundational for the secure attachments that are so essential for future mental health and happiness. It is still worth considering that panic attacks may reflect sudden endogenous spontaneous loss of feelings of security (acute separation-distress) rather than sudden FEAR. We predict that these circuits are tonically aroused during human grief and sadness, feelings that accompany low brain opioid activity.
The PLAY/rough-and-tumble, physical socialengagement system
Young animals have strong urges for physical play - running, chasing, pouncing, and wrestling. These “aggressive” - assertive actions are consistently accompanied by positive affect - an intense social joy - signaled in rats by making abundant high frequency (~50 kHz) chirping sounds, resembling laughter. One key function of social play is to learn social rules and refine social interactions. Subcortically concentrated PLAYCitation31 urges may promote the epigenetic construction of higher social brain functions, including empathy. Further studies of this system may lead to the discovery of positive affect promoting neurochemistries that may be useful in treating depression.Citation32
These seven emotional networks provide psychiatric research with various endophenotypes important for advancing psychiatric understanding of affective order and disorder. For preclinical modeling, these emotional systems provide a variety of affectively important BrainMind networks to guide not only psychiatrically relevant research, but as already highlighted, the development of more specifically acting psychiatric medicines. To highlight one concrete possibility, there will follow a brief focus on how such systems may help us understand the genesis and better treatment of depression.
Emotional networks and depression
A key research question for affective disorders is why depression feels so bad. Specifically, which negative affect generating networks within mammalian brains helps generate depressive pain that leads to chronic despair?
Although all the affective networks of the mammalian brain can be influenced by depression - from diminished CARE and PLAY to elevated FEAR and RAGE - the “painfulness” of depressive affect may be engendered most persistently (i) by sustained overactivity of GRIEF, which promotes a downward cascade toward chronic despair, following a theoretical view originally formulated by John Bowlby.Citation33 This promotes (ii) the sustained dysphoria of depression which may be due largely to abnormally low activity of the reward-SEEKING system. For an extensive discussion, along with expert commentaries, see ref 34.
This vision allows investigators to focus on specific network analyses as opposed to the nonspecific stress models most commonly employed. Many stressors are used to evoke depressive phenotypes in animals - ranging from physical restraint and various punishments to intense psychological losses such as enforced maternal or social isolation and social defeat in adult aggressive encounters.Citation35 Few models specifically modify or monitor activities of specific emotional networks such as GRIEF and SEEKING. Rather, they typically use very general outcome measures - timidity during exploration (eg, center crosses in open fields), various diminished pleasure responses (eg, diminished sexuality and consumption of sweets) and varieties of learned helplessness (eg, diminished struggling when placed into water). For extensive summaries of such models, see the whole issue of Neuroscience & Biobehavioral Reviews devoted to this topic (2006, vol 29).
As a result, existing research typically focuses on general brain consequences of stress - from changing brain norepinephrine and serotonin dynamics to many other brain changes.Citation36 However, such general brain chemical changes may not specifically clarify the morbid mood of depression. The amines regulate rather general brain functions that influence all emotions and related cognitive processes. We now need strategies that aim to study the more specific affective changes that characterize depression. This requires a specific emotional network approach.
Primary-process emotional-systems analyses provide preclinical models where specific types of affective change can be manipulated and studied, and new treatments can be developed based on the neurochemical characteristics of the relevant circuits. For instance, the separation-distress/GRIEF “protest” gateway to depression may engender “psychological pain” that can cascade toward “despair” and sustained clinical depression.Citation30,Citation34 The entry to despair may reflect diminished SEEKING urges, promoting lack of initiative and lethargy, thereby further amplifying dysphoria. Thus, primary-process affective neuroscience is beginning to highlight distinct emotional networks that may specifically help explain why depression feels bad. This suggests potential benefits of relatively safe mu-opioid agonists, such as the mixed agonist-antagonists buprenorphine, and kappa antagonists for treating depression (see below).
An affective neuroscientific perspective on why depression feels so bad
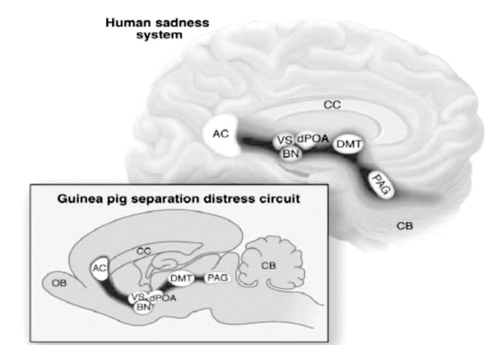
As noted already, John Bowlby first emphasized that depressive affects are related to the experiences of social attachments and social loss. This is, epidemiologically, now a well-supported conclusion.Citation37 Bowlby's insight about the crucial role of separation distress - the acute “protest” or “panic” responses to social loss, especially in young animals - allows neuroscience to clarify the “painfulness” of social loss. The GRIEF system of several species has been mapped to similar brain regions (), and this may be a key to the acute psychological pain of social loss. Indeed, the higher reaches of this system in the anterior cingulate are targeted in recent deep brain stimulation (DBS) initiatives for treatment-resistant depressions.Citation38 A focus on the neurochemical controls in this system provides other options for medicinal development. Likewise, facilitation of SEEKING urges should further facilitate recovery, whether by joyful life activities, pharmacological stimulation of SEEKING reserves, or even DBS of the nucleus accumbens and MFB.Citation39
Opioids that activate mu receptors are especially effective in reducing arousal of GRIEF/separation distress in animals.Citation42 Each of the above neurochemical controls (eg, opioids and oxytocin) provides novel options to reduce the psychic pain of depression in ways that are currently not clinically used. Indeed, reasonably safe opioids, such as ultra-low-dose buprenorphine, are very effective antidepressants for individuals who have obtained no relief from standard antidepressants.Citation43 Similarly, drugs that inhibit CRF and glutamate, the key neurochemistries that promote separation calls (vocalizations made when young animals are separated from mothers or siblings, ie, GRIEF), have yielded promising antidepressant effects.Citation44,Citation45
In sum, GRIEF circuitry evolved from general pain mechanisms, well over a hundred million years ago (birds possess a homologous system). This emotional system forges social bonds and dependencies between infants and caretakers, and probably regulates adult social relationships and solidarity. The affective consequences of severed attachment bonds make adults suffer in a distinct way, commonly called grief, but this is not yet clinical depression.
Separation distress is only the gateway to depression
The acute GRIEF response may need to be supplemented by other neuroaffective changes before individuals cascade into sustained depressive lassitude and despair. Cytokines that promote sickness feelings (eg, Interleukin 1) and endogenous inflammatory cascades have been proposed as possible causal vectors; both may operate, in part, by diminishing SEEKING arousals.Citation46 A sustained depressive phenotype may arise when diminished SEEKING urges allow the behavioral manifestations of GRIEF (the “protest” phase of separation distress) to diminish. This need not mean that the intrapsychic pain of GRIEF also disappears. Indeed, if the psychic pain is sustained, the dysphoria of diminished SEEKING could further elevate negative affect. Thus, depressive affect may start with psychological pain (GRIEF, with concurrent SEEKING arousal) followed by “giving up” (consisting of sustained psychic pain, accompanied by the lethargic anhedonia of diminished SEEKING).
Diminished brain reward in preclinical models of depressive states is well established,Citation47 but it is not yet clear how this happens. A promising candidate is elevated dynorphin activity along SEEKING circuitry. Indeed, dynorphin mediates the negative affect arising from loss in competitive social encounters.Citation48 Again, this suggest that severe depression may be optimally counteracted by medicines that reduce both social-loss induced psychic pain and depleted SEEKING resources; low-dose buprenorphine can counteract both through its mu-opioid agonist and kappa-receptor antagonism effects. Addictive tendencies are markedly reduced since higher doses block mu receptors which blunt opioid tolerance and escalating addictive dosing.
Thus, although negative affective changes in the opioidand oxytocin-driven attachment and affectional systems may be the pivotal precipitants of psychological pain that is the entry point for a depressive cascade, it may be diminished SEEKING that pushes the system into a sustained clinically significant dysphoria. This scenario does not exclude the potential contribution of other biogenic amine imbalances in depression - changes in overall brain arousal can reinforce the above affective changes. Because of the affective complexity and diversity of depression, many variants on these basic themes can be envisioned, yielding many subtypes of depression. It would be premature to try to relate the emotional primes to the various subtypes - anxious, agitated, etc - but to simply indicate that FEAR overactivity may contribute to anxious forms, while the GRIEF separation-distress system might contribute more to melancholic forms, while selectively diminished SEEKING may contribute to those forms where agitation is not prevalent.
The critical point is that detailed clarification of dedicated emotional-affective circuits in mammalian brain should allow us eventually to invest in more direct affective strategies to understand and treat depression as well as other psychiatric disorders accompanied by imbalanced affective states.Citation10 This may be a substantial advance over generalized stress models, for it is easier to envision how to focus on changes in specific brain emotional circuits rather than more global stress-induced brain changes. Affective circuit perspectives also coax us to consider the potential benefits of strengthening various positive emotional systems to promote affective homeostasis. For instance, therapeutic approaches that promote the positive hedonics of social CARE and PLAY systems may increase treatment options that could yield better outcomes than existing therapies.
To develop this last theme a little further, when we develop antidepressants that can rapidly and specifically promote desired affective rebalancing, we might consider developing complementary psychotherapeutic approaches where clinicians explicitly seek to utilize the power of positive affective systems of clients' brains. For instance, the “power of PLAY” in adult psychotherapy remains largely unused, although preclinical benefits for childhood problems such as excessive impulsivity have been documented.Citation49 Considering that PLAY can promote the expression of various neurotrophins like brain-derived neurotrophic factor,Citation50 and insulin-like growth factor 1,Citation32 it is to be expected that playful interactions, just like exercise, may have antidepressant effects, and the resulting neuroplasticities may reinforce better and longer-lasting psychotherapeutic benefits. Affective neuroscientific thinking suggests many other new avenues for medicinal developments since all primary-process emotional systems seem to have unique neuropeptidergic controls.Citation51
Summary: the promise of new therapeutic approaches
In the above context, it would not only be of interest to explore novel psychotherapeutic approaches that might specifically influence endogenous neurochemical controls of the other affective networks of mammalian brains, but clinicians may seek to estimate the primary-process emotional strengths and weaknesses of clients so as to better envision the major emotional forces that may have become imbalanced in major forms of emotional distress. Of course, primary processes in humans can only be estimated through tertiary-process verbal reports. Although there are shortcomings in such approaches, we have developed the Affective Neuroscience Personality Scales to provide a tool whereby clinicians may better estimate the primary-process emotional traits in normal as well as psychiatric patients.Citation52
A better understanding of the emotional endophenotypes discussed here may help guide clinicians to deal more strategically with the raw and troublesome feelings of their clients, and give them clearer explanations of the sources of their distress. This may be beneficial for many patients. The approach also provides new avenues, yet to be developed, that better recruit the personal affective resources of clients to promote healing. Therapists who can work effectively with the basic emotions - reframing and recontextualizing hurtful memories so they can be reconsolidated in the context of positive feelings - may be able to promote more lasting therapeutic change than those that seek to remain more strictly at cognitive levels of interaction. This is not to minimize the ability of cognitive processes to reframe stressful life events and to regulate negative emotionality through the analysis of life options, but to suggest that more direct work with the nature of affects is a perspective that remains underdeveloped.
In conclusion, affective neuroscience also has implications for the future development of animal models of psychiatric disorders. Currently preclinical models are rather deficient, as highlighted by Steven Hyman (see above).Citation11 What has been lacking so far is a more direct focus on manipulating specific emotional processes to simulate psychiatric disorders and to also have outcome measures that are not so general (eg, gross locomotor activity, swimming, and other stress-provoked changes that cannot be easily linked to specific brain affective circuits). By using an affective neuroscience approach, we can now monitor affective states by the ethological-emotional patterns of animals, especially diverse emotional vocalizations that can be used as direct “self-reports” of changes in affective states.Citation53,Citation54 Also, even though preclinical models can tell us a great deal about brain emotional and stress-induced changes that cannot be harvested in other ways, we must recognize that such approaches cannot penetrate the tertiary-process cognitive complexities that make human emotional life so rich and full of conflicts and devilishly complex vicissitudes. However, what a cross-species affective neuroscience strategy does provide is a better and more precise focus on the diverse forms of affective distress and euphoria that can arise from the basic emotional circuits of all mammalian brains, leading to concrete hypotheses of how each system may contribute to higher mental processes. For such a discussion of RAGE circuitry, see ref 55 and the relations of GRIEF and SEEKING systems for further understanding of addictions,Citation54,Citation56,Citation57 and depression.Citation34,Citation58-Citation60 Such issues are central for many psychiatric concerns.
A final issue that deserves attention is how such viewpoints may relate to psychiatric disorder susceptibility issues. One general principle might be that better evaluation of basic emotional personality traits may provide a tool for analyzing such relationships.Citation52 Although it is premature to reach any conclusions, we hypothesize that heightened constitutional sensitivity of GRIEF systems and endogenous underactivity of SEEKING urges would facilitate the emergence of depression in response to stressors. To evaluate this, we have generated genetic lines of animals that exhibit high and low positive affect based on heritability of emotional vocalizations.Citation61 Preliminary work suggests that the high positive affect animals may be resistant to depression while low ones may be more susceptible to depression.Citation62 Related work has been pursued at the genetic level by others.Citation63
Once we have a clear scientific understanding of the primary emotional processes of mammalian brains, we may be able to employ the concept of endophenotypes more effectively than it is currently used.Citation10 Such foundational knowledge may serve as a useful roadmap for gathering knowledge useful for the next generation of progress in biological psychiatry.
REFERENCES
- KasselJD.edSubstance Abuse and Emotion. Washington, DC: American Psychological Association2010see in particular137168
- KhantzianEJ.Understanding addictive vulnerability: an evolving psychodynamic perspective. (with commentaries by Johnson B, Koob GF, Morrison V, Panksepp J, Yorke C, and response by Khantzian EJ). Neuropsychoanalysis.2003 5556
- PankseppJ.Affective Neuroscience: the Foundations of Human and Animal Emotion. New York, NY: Oxford University Press1998
- MacLeanP.The Triune Brain in Evolution. New York, NY: Plenum Press1990
- PankseppJ.The affective brain and core-consciousness: how does neural activity generate emotional feelings? In Lewis M, Haviland JM, Barrett LF, eds. Handbook of Emotions. New York, NY: Guilford20084767
- PankseppJ.At the interface between the affective, behavioral and cognitive neurosciences: decoding the emotional feelings of the brain. Brain Cogn. 20035241412812799
- The Anxious Brain. Available at: http://www.charlierose.com/view/interview/11028?sponsor_id=1. Accessed October 2010
- LiottiM.PankseppJ.On the neural nature of human emotions and implications for biological psychiatry. In Panksepp J, ed. Textbook of Biological Psychiatry. Hoboken, NJ: Wiley20043374
- PankseppJ.Affective consciousness: core emotional feelings in animals and humans. Conscious Cogn. 2005141969
- PankseppJ.Emotional endophenotypes in evolutionary psychiatry. Progr Neuro-Psychopharmacol BioPsychiatry. 200630774784
- HymanSE.Can neuroscience be integrated into the DSM-V? Nat Rev Neurosci. 2007872573217704814
- MeaneyMJ.Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Ann Rev Neurosci. 2001241611192
- RussellJA.Core affect and the psychological construction of emotion. Psychol Rev. 200311014517212529060
- PankseppJ.Mood changes: In Vinken PJ, Bruyn GW, Klawans HL, eds. Handbook of Clinical Neurology (Revised series). Vol 1(45). Clinical Neuropsychology. Amsterdam, the Netherlands: Elsevier Science Publishers 1985271285
- CannonWB.The James-Lange theory of emotions: a critical examination and an alternative theory. Am J Psychol. 192739106124
- PankseppJ.NormansellLA.CoxJF.SiviyS.Effects of neonatal decortication on the social play of juvenile rats. Physiol Behav. 1994564294437972392
- PankseppJ.The periconscious substrates of consciousness: Affective states and the evolutionary origins of the SELF. J Conscious Stud. 19985566582
- NorthoffG.Psychopathology and pathophysiology of the self in depression - neuropsychiatric hypothesis. J Affect Disord. 200710411417379318
- DamasioAR.GrabowskiTJ.BecharaA.et alSub-neocortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 200031049105611017179
- PankseppJ.Aggression elicited by electrical stimulation of the hypothalamus in albino rats. Physiol Behav. 197163113165169915
- BurgdorfJ.WoodPL.KroesRA.MoskalJR.PankseppJ.Neurobiology of 50-kHz ultrasonic vocalizations in rats: Electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 200718227428317449117
- PankseppJ.On the embodied neural nature of core emotional affects. J Conscious Stud. 200512158184
- PankseppJ.Brain opioids: a neurochemical substrate for narcotic and social dependence. In: Cooper S, ed. Progress in Theory in Psychopharmacology. London, UK: Academic Press 1981149175
- PankseppJ.BivenL.Archaeology of Mind. New York, NY: Norton 2011
- AlcaroA.HuberR.PankseppJ.Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev. 20075628332117905440
- SiegelA.The Neurobiology of Aggression and Rage. Boca Raton, FL; CRC Press 2005
- PankseppJ.FuchsT.IacbucciP.The basic neuroscience of emotional experiences in mammals: the case of subcortical FEAR circuitry and implications for clinical anxiety. Applied Animal Behav Sci. 2010. In press
- PfaffDW.Drive: Neurobiological and Molecular Mechanisms of Sexual Behavior. Cambridge, MA: MIT Press 1999
- NumanM.InselTR.The Neurobiology of Maternal Behavior. New York: Springer 2003
- PankseppJ.The neurobiology of social loss in animals: some keys to the puzzle of psychic pain in humans. In. Jensen-Campbell LA, MacDonald G, eds. Social Pain: Neuropsychological and Health Implications of Loss and Exclusion. American Psychological Association, Washington, DC. 20101152
- PellisS.PellisV.The Playful Brain: Venturing to the Limits of Neuroscience. Oxford, UK: One World 2009
- BurgdorfJ.KroesRA.BeinfeldMC.PankseppJ.MoskalJR.Uncovering the molecular basis of positive affect using rough-and-tumble play in rats: a role for insulin-like growth factor I. Neuroscience. 201016876977720350589
- BowlbyJ.Attachment and Loss, Vol. 3. Loss: Sadness and Depression. London, UK: Hogarth Press 1980
- WattDF.PankseppJ.Depression: an evolutionarily conserved mechanism to terminate separation-distress? A review of aminergic, peptidergic, and neural network perspectives. (with commentaries)Neuropsychoanalysis. 2009115104
- McEwenBS.Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 20078787390417615391
- HarroJ.OrelandL.Depression as a spreading adjustment disorder of monoaminergic neurons: A case for primary implications of the locus coeruleus. Brain Res Rev. 2001387912811750928
- HeimC.NemeroffCB.The impact of early adverse experiences on brain systems involved in the patho-physiology of anxiety and affective disorders. Biol Psychiatry. 199946 1509152210599479
- MaybergHS.LozanoAM.VoonV.et alDeep brain stimulation for treatment-resistant depression. Neuron. 20054565166015748841
- SchlaepferTE.CohenMX.FrickC.etal.Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 20083336837717429407
- HermanBH.PankseppJ.Ascending endorphinergic inhibition of distress vocalization. Science. 1981211106010627466377
- PankseppJ.Feeling the pain of social loss. Science. 200330223723914551424
- PankseppJ.HermanB.ConnerR.BishopP.ScottJP.The biology of social attachments: opiates alleviate separation distress. Biol Psychiatry. 19781360761883167
- BodkinJA.ZornbergGL.LukasSE.ColeJO.Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 19951549577714228
- HolsboerF.The role of peptides in treatment of psychiatric disorders. J Neural Transm. 200364(suppl)1734
- ZarateCA.SinghJB.CarlsonPJ.et alA randomized trial of an Nmethyl-D-aspartate antagonist in treatment- resistant major depression. Arch Gen Psychiatry. 20066385686416894061
- AnismanH.MeraliZ.HayleyS.Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: Comorbidity between depression and neurodegenerative disorders. Progr Neurobiol. 200885174
- NestlerEJ.CarlezonWA Jr.The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006591151115916566899
- McLaughlinJP.Marton-PopoviciM.ChavkinC.Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003235674568312843270
- PankseppJ.Can PLAY diminish ADHD and facilitate the construction of the social brain. J Can Acad Child Adolesc Psychiatry. 200710576618392153
- GordonNS.BurkeS.AkilH.WatsonJ.PankseppJ.Socially induced brain fertilization: play promotes brain derived neurotrophic factor expression. Neurosci Lett. 2003341172012676333
- PankseppJ.HarroJ.The future of neuropeptides in biological psychiatry and emotional psychopharmacology: goals and strategies. In. Panksepp J, ed. Textbook of Biological Psychiatry. Hoboken, NJ: Wiley 2004627660
- DavisKL.PankseppJ.NormansellL.The affective neuroscience personality scales: Normative data and implications. Neuropsychoanalysis. 200352129
- KnutsonB.BurgdorfJ.PankseppJ.Ultrasonic vocalizations as indices of affective states in rat. Psychol Bull. 200212896197712405139
- PankseppJ.KnutsonB.BurgdorfJ.The role of emotional brain systems in addictions: a neuro-evolutionary perspective. Addiction. 20029745946911964061
- PankseppJ.ZellnerM.Towards a neurobiologically based unified theory of aggression. Rev Int Psychol Sociale/Int Rev Soc Psychol. 2004173761
- PankseppJ.Evolutionary substrates of addiction: the neurochemistries of pleasure seeking and social bonding in the mammalian brain. In. Kassel JD, ed. Substance Abuse and Emotion. Washington, DC: American Psychological Association 2010137168
- PankseppJ.NocjarC.BurgdorfJ.PankseppJB.HuberR.The role of emotional systems in addiction: a neuroethological perspective. In: Bevins RA, Bardo MT, eds. 50th Nebraska Symposium on Motivation: Motivational Factors in the Etiology of Drug Abuse. Lincoln: Nebraska 200485126
- PankseppJ.WattDW.Why does depression hurt? Ancestral primaryprocess separation-distress (PANIC/GRIEF) and diminished brain reward (SEEKING) processes in the genesis of depressive affect. Psych Interpers Biol Proc. 2011. In press
- Schoene-BakeJ-C.ParpaleyY.WeberB.PankseppJ.HurwitzTA.CoenenVA.Tractographic analysis of historical lesion-surgery for depression. Neuropsychopharmacology. 2010352553256320736994
- ZellnerM.SolmsM.WattDW.PankseppJ.Affective neuroscientific and neuropsychoana lytic approaches to two intractable psychiatric problems: why depression feels so bad and what addicts really want. Neurosci Biobehav Rev. 2011. In press
- BurgdorfJ.PankseppJ.BrudzynskiSM.MoskalJR.Breeding for 50-kHz positive affective vocalizations in rats. Behavior Genetics. 200535677215674533
- BurgdorfJ.PankseppJ.BrudzynskiSM.et alThe effects of selective breeding for differential rates of 50-kHz ultrasonic vocalizations on emotional behavior in rats. Dev Psychobiol. 200851344618819097
- KrishnanV.HanMH.GrahamDL.et alMolecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007131691700