Abstract
Over the last few years, neuroimaging techniques have contributed greatly to the identification of the structural and functional neuroanatomy of anxiety disorders. The amygdala seems to be a crucial structure for fear and anxiety, and has consistently been found to be activated in anxiety-provoking situations. Apart from the amygdala, the insula and anterior cinguiate cortex seem to be critical, and all three have been referred to as the “fear network.” In the present article, we review the main findings from three major lines of research. First, we examine human models of anxiety disorders, including fear conditioning studies and investigations of experimentally induced panic attacks. Then we turn to research in patients with anxiety disorders and take a dose look at post-traumatic stress disorder and obsessive-compulsive disorder. Finally, we review neuroimaging studies investigating neural correlates of successful treatment of anxiety, focusing on exposure-based therapy and several pharmacological treatment options, as well as combinations of both.
Durante los últimos años las técnicas de neuroimágenes han contribuido de manera importante a la identificación de la neuroanatomía estructural y funcional de los trastornos ansiosos. La amígdala parece ser una estructura crucial para el miedo y la ansiedad, y constantemente se ha encontrado activada en situaciones que provocan ansiedad, Además de la amígdala, la ínsula y la corieza cingulada anterior también parecen ser muy importantes y las tres se han denominado “el circuito del miedo”. En este artículo se revisan los principales hallazgos de tres importantes líneas de investigation. Primero se examinan modelos humanos de los trastornos ansiosos, incluyendo estudios de miedo condicionado e investigaciones de ataques de pánico inducidos experimenialmente. Luego se aborda la investigación en patientes con trastornos ansiosos con especial énfasis en el trastorno por estrés postraumático y el trastorno obsesivo compulsivo. Finalmente se revisan los estudios de neuroimágenes que investigan los correlatos neurales de tratamientos ansiolíticos exitosos, enfocándose en terapias basadas en la exposición y en algunas alternativas psicofarmacológicas, como también en combinaciones de ambas.
Ces dernières années, les techniques de neuro-imagerie ont largement contribué à l'identification de la neuroanatomie structurale et fonctionnelle des troubles anxieux. Les amygdales, structures capitales pour la peur et l'anxiété, sont régulièrement activées dans des situations pourvoyeuses d'anxiété, À côté des amygdales, l'insula et le cortex cingulaire antérieur semblent d'une importance cruciale, ces trois structures ayant été qualifiées de « réseau de la peur ». Dans cet article, nous passons en revue les principaux résultats de trois axes majeurs de recherche. Tout d'abord, nous examinons des modèles humains de troubles anxieux, à l'aide d'études sur le conditionnement de la peur et d'enquêtes sur les attaques de panique induites expérimentalement. Puis nous nous consacrons à la recherche chez les patients atteints de troubles anxieux et nous examinons de près l'état de stress post-traumatique et les troubles obsessionnels compulsifs. Enfin, nous analysons des études de neuroimagerie sur les corrélations neurales du traitement efficace de l'anxiété, en nous concentrant sur un traitement basé sur l'exposition (au stimulus anxiogène) et sur plusieurs traitements pharmacologiques, comme sur l'association des deux.
Introduction
In recent years, the development of neuroimaging techniques such as high-resolution magnetic resonance imaging (MRI), functional magnetic resonance imaging (fMRI), positron emission tomography (PET), or single photon emission tomography (SPECT) has promoted the identification of structural and functional characteristics underlying mental disorders to a great extent. In anxiety disorders, recent neuroimaging techniques have contributed greatly to diagnosis and treatment, and helped to shed light on the neurobiological basis of anxiety in general.Citation1 The number of neuroimaging studies conducted on anxiety disorders has risen constantly since the 1980s.Citation2
According to DSM-IV, anxiety disorders include diagnoses of panic disorder, agoraphobia, post-traumatic stress disorder (PTSD), social anxiety disorder (social phobia), specific phobias, generalized anxiety disorder (GAD), and obsessive-compulsive disorder (OCT)).Citation3 The common feature of the different anxiety disorders is excessive, irrational fear and avoidance of anxiety triggers.Citation3 Numerous studies have been conducted so far to determine structural and functional neural pathways of anxiety disorders and anxiety in general. Furthermore, there have been attempts to disentangle the neurobiological characteristics specific to each disorder.Citation4 However, the number of neuroimaging studies conducted on each anxiety disorder varies greatly. Most of the imaging studies on anxiety disorders published within the last decade focused on PTSD or OCD; less research has been conducted on agoraphobia and generalized anxiety disorder, for example.Citation2 In addition to imaging studies in patients with anxiety disorders, a large body of research has been conducted on anxiety in healthy subjects. For example, fear conditioning studiesCitation5-Citation8 or experimentally induced panic attacks in healthy individualsCitation9 resembled the elevated fear response seen in anxiety disorder patients quite well.
The present review attempts to create a global overview of the current findings of structural MRI, fMRI, and PET studies in the field of anxiety disorders. In the following, we first discuss research on models of anxiety in healthy subjects, then turn to clinical studies in anxiety patients, and conclude with an outlook on the possibility of visualizing the effects of pharmacological and psychotherapeutic treatment of anxiety disorders using neuroimaging techniques.
Modeling anxiety in healthy individuals
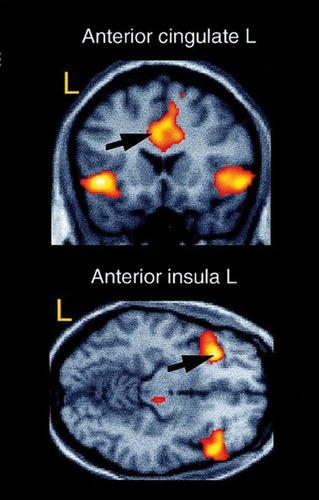
Classical fear conditioning was one of the first experimental paradigms employed to study the functional neuroanatomy of anxiety in healthy humans.Citation10 In fear conditioning studies, a previously neutral stimulus is repeatedly paired with an aversive stimulus which by itself elicits an autonomic fear response. After several paired presentations, the previously neutral stimulus becomes “conditioned” and elicits the autonomic fear response alone. In a well-known study by Büchel et al,Citation5 neutral faces were conditioned with an unpleasantly loud tone. After conditioning, presentation of the conditioned stimulus evoked brain activity in the anterior cingulate cortex, the anterior insula, and the amygdala (). Interestingly, amygdala activation decreased over time, indicating a rapid habituation of this structure.Citation5,Citation10 The finding that the amygdala, the insula, and the anterior cingulate cortex are part of an aversive conditioning network has been replicated many times within the last years.Citation8 Furthermore, the results of the early fear conditioning studies already pointed to the “fear network” commonly found to be activated in imaging studies in anxiety disorders during symptom provocation. Not only the acquisition of anxiety but also mechanisms of extinction can be modeled by fear conditioning paradigms.Citation8 During extinction of a conditioned fear response, the previously neutral stimulus is repeatedly presented without the aversive stimulus and the conditioned fear response is gradually eliminated. Neuroimaging of fear extinction revealed that most of the regions involved in fear conditioning are active during the extinction process as well.Citation8 Again, and most consistently, activation in the fear network, including the amygdala,Citation11 the insula,Citation12 and the anterior cingulate cortex,Citation11 was found during extinction. Moreover, there is evidence for activation in prefrontal regions during fear extinctionCitation13 that might reflect a regulating effect of prefrontal structures on the amygdalar fear reaction, in that the expression of fear as a reaction to a fearful stimulus is inhibited.Citation14,Citation15 Extinction of fear is a process which is important for the treatment of anxiety disorders, particularly for exposure -based psychotherapeutic approaches, and changes in functional neuroanatomy seen during extinction resemble the functional changes after successful treatment of anxiety disorders quite well (see below).
Another experimental model of human anxiety is the induction of panic attacks with panicogenic substances, like the synthetic neuropeptide cholecystokinintetrapeptide (CCK-4). A panic attack is a period of intense fear and anxiety along with numerous physical symptoms, eg, sweating, trembling, chest pain, and discomfort; the recurrence of unexpected, sudden panic attacks characterizes panic disorder.Citation3 CCK-4-induced panic attacks closely resemble spontaneously occurring panic attacks experienced by panic disorder patients,Citation16,Citation17 and CCK-4 is assumed to be an ideal and valid agent for the experimental induction of panic attacks.Citation18 CCK-4induced panic can therefore serve as a useful model to study the pathophysiology and neurobiological basis of panic disorder.Citation19 In studies investigating the functional neuroanatomy of CCK-4-induced panic, CCK-4 and placebo injections are delivered during PET or fMRI scanning and brain activity is recorded meanwhile.Citation9,Citation20-Citation21 Contrasting brain activity during CCK-4, placebo, and periods of anticipatory anxiety with baseline activity then reveals what brain regions might be involved in the generation of panic attacks. Eser et alCitation9 found large responses to CCK-4 injection in the ventral anterior cingulate cortex (ACC), middle and superior frontal gyrus, precuneus, middle and superior temporal gyrus, occipital lobe, sublobar areas, cerebellum, and brain stem. Moreover, amygdala activation during CCK-4 was significantly higher than during placebo, especially in individuals that experienced more severe symptoms of fear. This finding indicates that the experience of CCK-4 induced fear might be related to the extent of amygdala activation and emphasizes its role in fear and anxiety.Citation9 Furthermore, CCK-4 models of panic disorder not only serve to uncover the functional neuroanatomy of panic attacks but can also point to putative genomic risk factors for anxiety,Citation22 the influence of personality factors on proneness to anxiety,Citation23,Citation24 or the effect of drugs on brain activity and symptoms of fear.Citation25-Citation26
To summarize, human models of anxiety in healthy individuals can help to reveal neural processes underlying the development of anxiety disorders, the expression of fear during symptom provocation, and the extinction of fear during treatment of anxiety. Brain structures found to be involved in fear conditioning in healthy humans (ie, the fear networkCitation10) have been shown to underlie clinically relevant anxiety disorders as well.
Neuroimaging of anxiety disorders
The majority of functional neuroimaging studies investigating anxiety disorders employed a symptom provocation paradigm. They contrasted a negative emotional condition (eg, pictures of feared objects or situations) with a neutral or positive condition to elicit anxiety-specific brain activity, and then compared activity in anxiety disorder patients with healthy controls.Citation4 For example, individuals with a social anxiety disorder were confronted with pictures of angry faces,Citation7 PTSD patients were exposed to pictures of trauma-related scenes and sounds,Citation27 and spider phobic individuals saw pictures of spiders.Citation28 One of the most consistent findings of these studies is a hyperactivity of the amygdala during symptom provocation that is related to the experienced symptoms of fear.Citation2,Citation29-Citation31 The amygdala is a group of nuclei located in the medial temporal lobe. It is involved in several fear and emotion related processes like fear conditioning,Citation10 the regulation of stress effects on memory,Citation32 reward learning,Citation33 and the processing of emotionally and socially relevant information.Citation34-Citation35 Recently, more general approaches assume that the amygdala codes salience or relevanceCitation35 or valueCitation33 and is therefore a crucial structure for a larger number of processes. Apart from the amygdala, further brain regions like the anterior cingulate cortex and the insula were shown to be involved in the development and maintenance of anxiety disorders as well. They have previously been referred to as “the fear network.”Citation8,Citation10 The insula is a central structure for emotion processing,Citation36 for subjective feelings and interoceptive awareness,Citation37,Citation38 and the anterior cingulate cortex plays an important role in approach and avoidance and fear learning.Citation39,Citation40 In general, all of the fear network regions seem to be involved in “the processing of emotions as they relate to the self”Citation1 and thus play a role in fear and anxiety as well. Imaging studies in almost all of the anxiety disorders have consistently demonstrated enhanced activation in the fear network during symptom provocation.
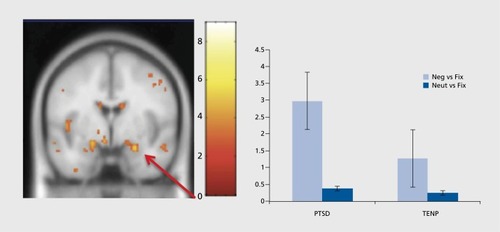
The most extensive research in the field of neuroimaging in anxiety disorders has been conducted on PTSD.Citation2 PTSD is an anxiety disorder that is caused by the experience of an extremely stressful event that involved actual or threatened death, serious injury, or a threat to the physical integrity of self or others. PTSD is characterized by re-experiencing this traumatic event, avoidance of the stimuli associated with the event, and a persistently increased arousal.Citation3 Functional neuroimaging studies have recurrently demonstrated amygdalar hyperactivity in PTSDCitation41-Citation43 () and hypoactivity in the medial prefrontal cortex and anterior cingulate cortex.Citation44 There is evidence for reduced hippocampal activity as well.Citation45 In current models of PTSD, amygdalar hyperactivity reflects the persistently elevated fear response, and hypoactivity in frontal regions suggests a reduced potential for top-down regulation of fearCitation46 and fear extinction.Citation44,Citation47 The hippocampus provides information about the context of a situation and the attenuated hippocampal response might be attributable to difficulties in identifying safe contexts.Citation46 In addition to the functional abnormalities described above, structural changes in several brain regions, including the hippocampus, amygdala, and medial prefrontal cortex, have been demonstrated in PTSD patients as well.Citation44 Interestingly, not all people exposed to a traumatic event develop PTSD as a consequence. Hence, this raises the question of whether the structural and functional abnormalities predispose to or follow the development of PTSD, and there seem to be mixed results in the literature.Citation48 However, studies conducted so far point to a two-way relationship. They indicate that some of the observed abnormalities, like reduced hippocampal volume,Citation49 can be a predisposing factor for the development of PTSD on the one hand, but also be a consequence of the disorder and show a further decrease over time.Citation50
Another anxiety disorder that has attracted much attention in neuroimaging research within the last few years is OCD.Citation2 OCD is characterized by the presence of recurrent and persistently disturbing thoughts and images (obsessions), mostly followed by repetitive behaviors (compulsions) to reduce anxiety. Compulsions typically include washing, ordering, or checking.Citation3 According to a widely accepted model, the cortico-striatal model of OCD, the primary pathology of OCD lies within the striatum, specifically the caudate nucleus.Citation46 A striatal dysfunction leads via direct and indirect pathways to inefficient thalamic gating, which in turn results in hyperactivity within the orbitofrontal and anterior cingulate cortex.Citation46 Orbitofrontal hyperactivity is associated with the occurrence of intrusive thoughts, while hyperactivity within the anterior cingulate cortex is considered to be reflected in unspecific anxiety arising from these thoughts. Within this model, compulsions are assumed to be performed to compensatory activate the striatum, achieve thalamic gating, and thus neutralize intrusive thoughts and anxiety.Citation46 The cortico-striatal model is consistent with neuroimaging studies demonstrating abnormal functional connectivityCitation51 and increased brain activity in orbitofrontal and ACC regions during restCitation52 and during presentation of OCD-related stimuli.Citation53-Citation55 Consistent with findings from functional imaging studies, structural abnormalities in OCD patients have been found in key regions of the fronto-striatal circuit, like the orbitofrontal cortex, the anterior cingulate cortex, the basal ganglia, and the thalamus.Citation56
Although OCD is considered an anxiety disorder, there is limited evidence for a prominent role of the amygdala in the pathophysiology of this disorder,Citation53-Citation57 and anxiety symptoms have rather been linked to hyperactivity in the anterior cingulate cortex.Citation46 Simon et alCitation55 addressed this issue and investigated brain activation during individually tailored symptom provocation. As expected, they demonstrated increased activation of fronto-striatal areas in OCD-patients compared with healthy controls in response to OCD-related stimuli, contrasted with neutral and generally aversive but symptom-unrelated stimuli. However, amygdala hyperactivation in patients was found during OCD-related symptom provocation and during presentation of unrelated aversive stimuli.Citation55 Thus, the authors argue that amygdala hyperactivation in OCD patients might reflect general emotional hyperarousal rather than OCD-related anxiety.
In summary, studies in patients with anxiety disorders rather consistently demonstrated activity of the “fear network” during symptom provocation. Symptoms of anxiety are considered to be due to a pathologically hyperactivated amygdala and insufficient top-down regulation by frontal brain regions. However, at least in OCD, there seems to be a network of regions distinctlyactivated in this disorder. Further research will probably identify more specific regions involved in the development and maintenance of each anxiety disorder.
Imaging neural correlates of treatment in anxiety disorders
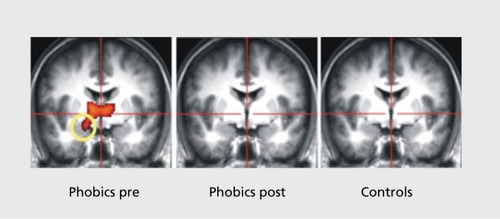
Among psychotherapeutic interventions, cognitive-behavioral therapy (CBT), particularly exposure therapy, has been shown to be highly effective in the treatment of anxiety disorders.Citation58 During exposure therapy, patients are systematically and repeatedly exposed to the anxiety-provoking stimulus or situation until their fear subsides. The exact neural mechanisms of this potent intervention remain to be determined. However, exposure therapy appears to be quite similar to the process of fear extinction and thus might recruit similar brain structures as well. In line with this assumption, several studies have been conducted within the last few years that demonstrated changes in brain structure and function after successful anxiety treatment with exposure therapy. Goossens et alCitation59 demonstrated altered patterns of neural functioning after successful treatment of specific phobia. People suffering from specific phobia show an elevated fear response cued by the presence or anticipation of a specific object or situation.Citation3 Common phobic stimuli are animals, heights, flying, receiving an injection, and seeing blood. On the neuronal level, confrontation with or anticipation of the phobic stimulus usually produces an elevated response in the fear network, in patients with specific phobia.Citation4 In a sample of spider phobic individuals, amygdala activity decreased after successful exposure therapy, compared with pretreatment activity (). Furthermore, a normalization of insular and anterior cingulate cortex activity was found.Citation59 In OCD, changes in patterns of brain activity were seen after CBT comprising exposure and response prevention strategies.Citation60,Citation61 Dickie et alCitation62 investigated the neural correlates of recovery from PTSD and found activity in the hippocampus and the subgenual anterior cingulate cortex to correlate with improvement in PTSD symptoms. Activity in the amygdala and ventral-medial prefrontal cortex was associated with current symptom severity.Citation62,Citation63
A novel line of research investigated the application of D-cycloserine, a partial N-methyl-D-aspartate (NMDA) receptor agonist, in combination with exposure-based therapy in the treatment of anxiety disorders. D-cycloserine facilitates the effectiveness of exposure therapy, in that it speeds up fear extinction processes.Citation64 Neuroimaging in spider phobic patients suggests that during symptom provocation D-cycloserine enhances activation in regions involved in cognitive control and interoceptive integration, like the prefrontal cortex, the anterior cingulate cortex, and the insula.Citation65 On the behavioral level, this neural modulation might become evident in enhanced extinction of fear.
In addition to exposure-based therapies, there is also evidence for neural changes associated with other psychotherapeutic concepts. For example, behavioral changes in patients with social anxiety disorder after mindfulness-based stress reduction (MBSR) therapy seem to be reflected by distinct patterns of neural activity.Citation66
With regard to psychotherapy research, neuroimaging techniques offer the opportunity to monitor structural and functional neuronal changes as a result of psychotherapy that occur along with changes in patients' perception and behavior and might help to further refine and optimize psychotherapeutic strategies.
Psychopharmacological first-line treatments of anxietydisorders include antidepressant treatment with selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs).Citation67 Positive effects of antidepressant medication can be demonstrated using neuroimaging techniques, too. Citalopram, for example, attenuated amygdala response to aversive facesCitation68 and reduced activity in prefrontal regions, the striatum, the insula, and paralimbic regions during listening to worry sentences in GAD.Citation69 Thus, SSRI treatment in anxiety disorders seems to alter abnormal neural processes that were found to be key characteristics of fear and anxiety. The anticonvulsant drug pregabalin has an anxiolytic potential, too, and is approved for the use in GAD. In a recent study in healthy individuals, pregabalin attenuated amygdalar and insular activity during anticipation of and during emotional processing.Citation70
The neuropeptide oxytocin has stress-reducing and attachment enhancing effects and facilitates social encounters.Citation71,Citation72 Thus, it might also have positive effects on emotion regulation in patients suffering from abnormally elevated fear of social situations. In patients with social anxiety disorder, oxytocin attenuated the heightened amygdala activation in response to fearful faces.Citation73 Hence, it appears to modulate the exaggerated amygdala activity during confrontation with social stimuli in pathological social anxiety. These lines of research suggest that neuroimaging techniques could potentially identify common neural pathways of anxiety treatment, and therefore help us to understand how new pharmacological treatment options for anxiety disorders might work.
Furthermore, there is evidence that pretreatment patterns of functional neuronal activity might predict whether a patient responds to a particular intervention or not.Citation74 Structural neuroanatomical characteristics were shown to predict response to psychotherapy as well. Bryant et alCitation75 demonstrated in PTSD patients that a smaller volume of the rostral anterior cingulate cortex predicted nonresponse to CBT. The authors assume that exposure-based CBT is, similarly to the extinction of conditioned fear, a process that requires anterior cingulate cortical structures.Citation11 Thus, larger volumes of the anterior cingulate cortex would lead to better control over fear responses during exposure therapy and enhanced extinction, and consequently result in better responding to CBTCitation75 Therefore, pretreatment characteristics in structural and functional neuroanatomy might become important predictors for the kind of treatment that suits best for a particular patient.
In summary, in the future, neuroimaging techniques might enable therapists and researchers to continuously monitor treatment success. Furthermore, structural and functional neuroimaging studies seem to be a promising tool to reveal the neural mechanisms underlying anxiety disorders and thus may lead to the development of more effective therapeutic options. They might also help to specifically assign patients to treatments that promise to be most effective for a certain individual.
Conclusion
In conclusion, in the present article we have provided an overview of the results of current neuroimaging studies in fear and anxiety. Studies in human models of anxiety, as well as investigations in anxiety disorder patients consistently implicated the crucial role of the “fear network,” comprising the amygdala, insula, and anterior cingulate cortex, for the development and maintenance of anxietydisorders. Effective psychotherapeutic and pharmacological treatments of anxiety seem to specifically alter patterns of brain activation in these structures.
REFERENCES
- PaulusMP.The role of neuroimaging for the diagnosis and treatment of anxiety disorders.Depress Anxiety.20082534835618412061
- DamsaC.KoselM.MoussallyJ.Current status of brain imaging in anxiety disorders.Curr Opin Psychiatry.2009229611019122541
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Text Revision. Washington, DC: American Psychiatric Association;2000
- EtkinA.WagerTD.Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia.Am J Psychiatry.20071641476148817898336
- BuchelC.MorrisJ.DolanRJ.FristonKJ.Brain systems mediating aversive conditioning: an event-related fMRI study.Neuron.1998209479579620699
- GrillonC.Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology.Biol Psychiatry.20025295897512437937
- KluckenT.KagererS.SchweckendiekJ.TabbertK.VaitlD.StarkR.Neural, electrodermal and behavioral response patterns in contingency aware and unaware subjects during a picture-picture conditioning paradigm.Neuroscience.200915872173118976695
- SehlmeyerC.SchoningS.ZwitserloodP.et al.Human fear conditioning and extinction in neuroimaging: a systematic review.PLoS One.20094e586519517024
- EserD.LeichtG.LutzJ.et al.Functional neuroanatomy of CCK-4-induced panic attacks in healthy volunteers.Hum Brain Mapp.20093051152218095276
- BuchelC.DolanRJ.Classical fear conditioning in functional neuroimaging.Curr Opin Neurobiol.20001021922310753800
- PhelpsEA.DelgadoMR.NearingKl.LeDouxJE.Extinction learning in humans: role of the amygdala and vmPFC.Neuron.20044389790515363399
- GottfriedJA.DolanRJ.Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value.Nat Neurosci.200471144115215361879
- YaguezL.CoenS.GregoryLJ.et al.Brain response to visceral aversive conditioning: a functional magnetic resonance imaging study.Gastroenterology.20051281819182915940617
- QuirkGJ.LikhtikE.PelletierJG.PareD.Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons.J Neurosci.2003238800880714507980
- Sotres-BayonF.CainCK.LeDouxJE.Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex.Biol Psychiatry.20066032933616412988
- BradwejnJ.KoszyckiD.MeterissianG.Cholecystokinin-tetrapeptide induces panic attacks in patients with panic disorder.Can J Psychiatry.19903583852180549
- BradwejnJ.KoszyckiD.ShriquiC.Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder. Clinical and behavioral findings.Arch Gen Psychiatry.1991486036102069490
- BradwejnJ.KoszyckiD.Cholecystokinin and panic disorder: past and future clinical research strategies.Scand J Clin Lab Invest Suppl.2001234192711713976
- EserD.SchuleC.BaghaiT.et al.Evaluation of the CCK-4 model as a challenge paradigm in a population of healthy volunteers within a proofof-concept study.Psychopharmacology (Berl).200719247948717318504
- SchunckT.ErbG.MathisA.et al.Functional magnetic resonance imaging characterization of CCK-4-induced panic attack and subsequent anticipatory anxiety.Neuroimage.2006311197120816600640
- JavanmardM.ShlikJ.KennedySH.VaccarinoFJ.HouleS.BradwejnJ.Neuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time points.Biol Psychiatry.19994587288210202575
- EserD.UhrM.LeichtG.et al.Glyoxalase-I mRNA expression and CCK-4 induced panic attacks.J Psychiatr Res.201145606320542521
- EserD.WenningerS.BaghaiT.SchuleC.RupprechtR.Impact of state and trait anxiety on the panic response to CCK-4.J Neural Transm.200811591792018414777
- ToruI.AluojaA.VohmaU.et al.Associations between personality traits and CCK-4-induced panic attacks in healthy volunteers.Psychiatry Res.201017834234720471107
- SchunckT.MathisA.ErbG.et al.One milligram of lorazepam does not decrease anxiety induced by CCK-4 in healthy volunteers: investigation of neural correlates with BOLD MRI.J Psychopharmacol.201125525920498136
- ZwanzgerP.EserD.NothdurfterC.et al.Effects of the GABA-reuptake inhibitor tiagabine on panic and anxiety in patients with panic disorder.Pharmacopsychiatry.20094226626919924586
- BremnerJD.StaibLH.KaloupekD.SouthwickSM.SouferR.CharneyDS.Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study.Biol Psychiatry.19994580681610202567
- SchweckendiekJ.KluckenT.MerzCJ.et al.Weaving the (neuronal) web: fear learning in spider phobia.Neuroimage.20115468168820673801
- BremnerJD.Brain imaging in anxiety disorders.Exp Rev Neurother.20044275284
- RauchSL.ShinLM.WrightCI.Neuroimaging studies of amygdala function in anxiety disorders.Ann NY Acad Sci.200398538941012724173
- KentJM.RauchSL.Neurocircuitry of anxiety disorders.Curr Psychiatry Rep.2003526627312857529
- RoozendaalB.McEwenBS.ChattarjiS.Stress, memory and the amygdala.Nat Rev Neurosci.20091042343319469026
- MorrisonSE.SalzrnanCD.Re-valuing the amygdala.Curr Opin Neurobiol.20102022123020299204
- SabatinelliD.FortuneEE.LiQ.et al.Emotional perception: meta-analyses of face and natural scene processing.Neuroimage.2011542524253320951215
- AdolphsR.What does the amygdala contribute to social cognition?Ann NY Acad Sci.20101191426120392275
- PhanKL.WagerT.TaylorSF.LiberzonI.Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI.Neuroimage.20021633134812030820
- CraigAD.How do you feel? Interoception: the sense of the physiological condition of the body.Nat Rev Neurosci.2002365566612154366
- CritchleyHD.WiensS.RotshteinP.OhmanA.DolanRJ.Neural systems supporting interoceptive awareness.Nat Neurosci.2004718919514730305
- FreemanJH.JrCuppernellC.FlanneryK.GabrielM.Limbic thalamic, cingulate cortical and hippocampal neuronal correlates of discriminative approach learning in rabbits.Behav Brain Res.1996801231368905135
- BuchananSL.PowellDA.Cingulate cortex: its role in Pavlovian conditioning.J Comp Physiol Psychol.1982967557747142487
- ShinLM.OrrSP.CarsonMA.et al.Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD.Arch Gen Psychiatry.20046116817614757593
- VermettenE.SchmahlC.SouthwickSM.BremnerJD.Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder.Psychopharmacol Bull.20074083017285093
- BrohawnKH.OffringaR.PfaffDL.HughesKC.ShinLM.The neural correlates of emotional memory in posttraumatic stress disorder.Biol Psychiatry.2010681023103020855060
- ShinLM.RauchSL.PitmanRK.Amygdala, medial prefrontal cortex, and hippocampal function in PTSD.Ann NY Acad Sci.20061071677916891563
- HayesJP.LabarKS.McCarthyG.et al.Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combatrelated PTSD.J Psychiatr Res.20114566066921047644
- DeckersbachT.DoughertyDD.RauchSL.Functional imaging of mood and anxiety disorders.J Neuroimaging.20061611016483270
- BremnerJD.ElzingaB.SchmahlC.VermettenE.Structural and functional plasticity of the human brain in posttraumatic stress disorder.Prog Brain Res.200816717118618037014
- RobinsonBL.ShergillSS.Imaging in posttraumatic stress disorder.Curr Opin Psychiatry.201124293321088585
- GilbertsonMW.ShentonME.CiszewskiA.et al.Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma.Nat Neurosci.200251242124712379862
- FelminghamK.WilliamsLM.WhitfordTJ.et al.Duration of posttraumatic stress disorder predicts hippocampal grey matter loss.Neuroreport.2009201402140619794316
- JangJH.KimJH.JungWH.et al.Functional connectivity in fronto-subcortical circuitry during the resting state in obsessive-compulsive disorder.Neurosci Lett.201047415816220302914
- KangDH.KwonJS.KimJJ.et al.Brain glucose metabolic changes associated with neuropsychological improvements after 4 months of treatment in patients with obsessive-compulsive disorder.Acta Psychiatr Scand.200310729129712662252
- BreiterHC.RauchSL.KwongKK.et al.Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder.Arch Gen Psychiatry.1996535956068660126
- SchienleA.SchaferA.StarkR.WalterB.VaitlD.Neural responses of OCD patients towards disorder-relevant, generally disgust-inducing and fear-inducing pictures.Int J Psychophysiol.200557697715935263
- SimonD.KaufmannC.MuschK.KischkelE.KathmannN.Fronto-striato-limbic hyperactivation in obsessive-compulsive disorder during individually tailored symptom provocation.Psychophysiology.20104772873820158678
- KwonJS.JangJH.ChoiJS.KangDH.Neuroimaging in obsessive-compulsive disorder.Expert Rev Neurother.2009925526919210199
- van den HeuvelOA.VeltmanDJ.GroenewegenHJ.et al.Amygdala activity in obsessive-compulsive disorder with contamination fear: a study with oxygen-15 water positron emission tomography.Psychiatry Res.200413222523715664794
- OlatunjiBO.CislerJM.DeaconBJ.Efficacy of cognitive behavioral therapy for anxiety disorders: a review of meta-analytic findings.Psychiatr Clin North Am.20103355757720599133
- GoossensL.SunaertS.PeelersR.GriezEJ.SchruersKR.Amygdala hyperfunction in phobic fear normalizes after exposure.Biol Psychiatry.2007621119112517706612
- NakataniE.NakgawaA.OharaY.et al.Effects of behavior therapy on regional cerebral blood flow in obsessive-compulsive disorder.Psychiatry Res.200312411312014561429
- SaxenaS.GorbisE.O'NeillJ.et al.Rapid effects of brief intensive cognitive-behavioral therapy on brain glucose metabolism in obsessive-compulsive disorder.Mol Psychiatry.20091419720518180761
- DickieEW.BrunetA.AkeribV.ArmonyJL.Neural correlates of recovery from post-traumatic stress disorder: a longitudinal fMRI investigation of memory encoding.Neuropsychologic.20114917711778
- DickieEW.BrunetA.AkeribV.ArmonyJL.An fMRI investigation of memory encoding in PTSD: influence of symptom severity.Neuropsychologia.2008461522153118321537
- GrillonC.D-cycloserine facilitation of fear extinction and exposurebased therapy might rely on lower-level, automatic mechanisms.Biol Psychiatry.20096663664119520359
- AupperleRL.HaleLR.ChambersRJ.et al.An fMRI study examining effects of acute D-cycloserine during symptom provocation in spider phobia.CNS Spectr.20091455657120095368
- GoldinPR.GrossJJ.Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder.Emotion.201010839120141305
- RavindranLN.SteinMB.The pharmacologic treatment of anxiety disorders: a review of progress.J Clin Psychiatry.20107183985420667290
- Del-BenCM.DeakinJF.McKieS.et al.The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study.Neuropsychopharmacology.2005301724173415827569
- Hoehn-SaricR.SchlundMW.WongSH.Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder.Psychiatry Res.2004131112115246451
- AupperleRL.RavindranL.TankersleyD.et al.Pregabalin influences insula and amygdala activation during anticipation of emotional images.Neuropsychopharmacology.2011361466147721430645
- CarterCS.GrippoAJ.Pournajafi-NazarlooH.RuscioMG.PorgesSW.Oxytocin, vasopressin and sociality.Prog Brain Res.200817033133618655893
- DonaldsonZR.YoungLJ.Oxytocin, vasopressin, and the neurogenetics of sociality.Science.200832290090418988842
- LabuschagneI.PhanKL.WoodA.et al.Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder.Neuropsychopharmacology.2010352403241320720535
- McClureEB.AdlerA.MonkCS.et al.fMRI predictors of treatment outcome in pediatric anxiety disorders.Psychopharmacology (Bed).200719197105
- BryantRA.FelmingharnK.WhitfordTJ.et al.Rostral anterior cingulate volume predicts treatment response to cognitive-behavioural therapy for posttraumatic stress disorder.J Psychiatry Neurosci.20083314214618330460