Abstract
We review critical trends in imaging genetics as applied to schizophrenia research, and then discuss some future directions of the field. A plethora of imaging genetics studies have investigated the impact of genetic variation on brain function, since the paradigm of a neuroimaging intermediate phenotype for schizophrenia first emerged. It was initially posited that the effects of schizophrenia susceptibility genes would be more penetrant at the level of biologically based neuroimaging intermediate phenotypes than at the level of a complex and phenotypically heterogeneous psychiatric syndrome. The results of many studies support this assumption, most of which show single genetic variants to be associated with changes in activity of localized brain regions, as determined by select cognitive controlled tasks. From these basic studies, functional neuroimaging analysis of intermediate phenotypes has progressed to more complex and realistic models of brain dysfunction, incorporating models of functional and effective connectivity, including the modalities of psycho-physiological interaction, dynamic causal modeling, and graph theory metrics. The genetic association approaches applied to imaging genetics have also progressed to more sophisticated multivariate effects, including incorporation of two-way and three-way epistatic interactions, and most recently polygenic risk models. Imaging genetics is a unique and powerful strategy for understanding the neural mechanisms of genetic risk for complex CNS disorders at the human brain level.
Se revisan las tendencias más importantes en imágenes y genética aplicadas a la investigación en esquizofrenia y se discuten algunas perspectivas a futuro en este campo. Gran cantidad de estudios de imágenes y genética han investigado el impacto de la variación genética en la función cerebral desde que apareció el paradigma de un fenotipo intermedio de neuroimágenes para la esquizofrenia. Inicialmente se postuló que los efectos de los genes susceptibles para la esquizofrenia tendrían una mayor penetración a nivel de los fenotipos intermedios de neuroimágenes con base biológica que a nivel de un síndrome psiquiátrico complejo y fenotípicamente heterogéneo. Los resultados de muchos estudios apoyan esta hipótesis y la mayoría de ellos muestra variantes genéticas únicas que se asocian con cambios en la actividad de regiones cerebrales localizadas, como se puede determinar a través de la selección de tareas cognitivas controladas. A partir de estos estudios básicos, el análisis de neuroimágenes funcionales de los fenotipos intermedios ha progresado hacia modelos de disfunciones cerebrales más complejos y realistas, incorporando modelos de conectividad funcional y efectiva, que incluyen las modalidades de interacción psicófisiológíca, el modelo causal dinámico y las mediciones de la teoría de losgrafos. Los enfoques de asociación genética aplicados a las imágenes y genética también han progresado hacia efectos multivariados más sofisticados, incluyendo la incorporación de interacciones epistáticas de dos o tres vías, y más recientemente modelos de riesgo poligénico. Las imágenes y genética constituyen una estrategia única y poderosa para la comprensión de los mecanismos neurales de riesgo genético de trastornos complejos del sistema nervioso central a nivel del cerebro humano.
Nous examinons l'évolution déterminante de la neuro-imagerie génétique appliquée a la recherche sur la schizophrénie, puis nous analysons les futures possibilités de ce domaine. Une pléthore d'études associant la neuro-imagerie et la génétique a recherché l'influence de la variation génétique sur la fonction cérébrale, depuis l'émergence initiale d'un paradigme de phénotype intermédiaire de schizophrénie en neuro-imagerie. II a d'abord été postulé que les effets des gènes de susceptibilité a la schizophrénie seraient plus pénétrants au niveau des phénotypes intermédiaires de neuro-imagerie basés sur la biologie, qu'au niveau d'un syndrome psychiatrique complexe et phénotypiquement hétérogène. Les résultats de plusieurs études soutiennent cette hypothèse, la plupart mettant en évidence des variants génétiques uniques associés à des changements de l'activité de régions cérébrales localisées, déterminés par des tâches cognitives contrôlées définies. À partir de ces études fondamentales, l'analyse fonctionnelle de la neuro-imagerie des phénotypes intermédiaires a évolue vers des modèles plus complexes et réels de dysfonction cérébrale, comportant des modèles de connectivite effective et fonctionnelle, comme les modalités d'interaction psychophysiologique, la modélisation causale dynamique et les méthodes théoriques graphiques. Les méthodes d'association génétique appliquées a la neuro-imagerie génétique ont aussi progressé vers des effets multivariés plus sophistiqués, englobant des interactions épistatiques à 2 et 3 voies et plus récemment des modèles de risque polygénique. La neuro-imagerie génétique est une méthode puissante et originale de compréhension des mécanismes neuronaux du risque génétique pour les troubles complexes du SNC chez l'être humain.
The emergence of imaging genetics to investigate the impact of individual genetic variation on brain function was presaged by the methodological application of functional magnetic resonance imaging (fMRI) to schizophrenia research. In 1996, it was first proposed that functional magnetic resonance imaging, because of its technical advantages over nuclear imaging techniques related to enhanced spatial and temporal resolution and noninvasiveness, would enable individual brain phenotype characterization for genetic association studies.Citation1 Further, two seminal reports of gene variation associated with altered brain activity served as an initial proof-of-principle and heralded the onset of imaging genetics. In 2000, variation in the ApoE genotype was reported to be associated with altered activity in brain regions affected by Alzheimer's disease during a memory task (in hippocampus, parietal, and prefrontal regions)Citation2 and in 2001, a functional variation in the catechol-O-methyltransferase (COMT) genotype was reported to be associated with altered prefrontal activity during a working memory task, setting the stage for subsequent investigations of the impact of individual genetic variation on brain activity, as detectable by fMRI.Citation3
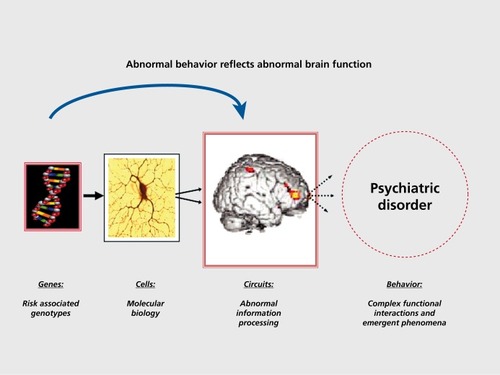
Concurrent with the methodological advances of imaging genetics was the conceptual advance of appreciating the neuroimaging intermediate phenotype as a manifestation of biological risk for a psychiatric syndrome. It was reasoned that susceptibility genes for schizophrenia do not directly encode for the clinical syndrome with which they are associated, that is, they do not directly encode for psychopathology, such as first-rank symptoms of hallucinations or delusions; rather susceptibility genes for schizophrenia are affecting basic biology — the development of brain cells and neural systems that underlie the expression of the clinical symptoms of the syndrome. It was posited that the effects of susceptibility genes would be more penetrant, ie, “closer to the gene,” at the level of biologically based neuroimaging intermediate phenotypes rather than at the level of a complex and phenotypically heterogeneous psychiatric syndrome.Citation4 Neuroimaging intermediate phenotypes, akin to cognitive or electrophysiological intermediate phenotypes, could therefore be used to enhance the potential to link genetic variation to a complex psychiatric disorder, such as schizophrenia.
Hundreds of published articles have ensued, describing studies to investigate the association of genetic variation with brain activity as pertinent to schizophrenia and other CNS disorders. We currently review several critical trends in the evolution of the field of imaging genetics as applied to schizophrenia research. We then discuss where we are poised to go next: innovations in imaging analysis and genetics analysis, effective connectivity modeling, and polygenic risk models are on the peak of the next wave of imaging genetics.
The neuroimaging intermediate phenotype
The neuroimaging intermediate phenotype is conceptually analogous to an intermediate phenotype for common complex medical disorders. It is logical to assume that genes would show stronger associations with the biological substrates contributing to risk of a disorder, with measurable quantitative traits along a pathophysiologic causal pathway, intermediate to the end complex syndrome. Intermediate phenotypes in other realms of medicine include lipid level as an intermediate phenotype for heart disease, sodium homeostasis as an intermediate phenotype for hypertension, and body mass index as an intermediate phenotype for diabetes.Citation5,Citation6 We favor the term “intermediate phenotype” over the more popular term “endophenotype,” though the two terms are essentially interchangeable. The term “endophenotype” (which was introduced into psychiatric genetics in the 1970sCitation7) initially referred to a trait that is “internal” that may be discoverable by a “biochemical test or microscopic examination,” but is not external or overtlymanifest. Also, the term “endophenotype” does not emphasize the concept of intermediacy in pathogenicity. Criteria for the establishment of a neuroimaging-based intermediate phenotype for schizophrenia, as in other fields of medicine, are that the intermediate phenotype: (i) is heritable; (ii) is found with increased frequency in healthy relatives of ill probands; (iii) exists temporally before the onset of the clinical illness in the pathophysiological pathway to the emergence of the clinical syndrome. As expounded in a review by Tan et al, evidence for each of these criteria has accumulated for the syndrome of schizophrenia, with cognitive dysfunction often integral and assayable at the brain level by task-based neuroimaging intermediate phenotypes.Citation8 Each of these criteria are consistent with the assumption that the intermediate phenotype is genetically and biologically less complex than the clinical syndrome, and that genes showing association at the syndromal level will show greater effect sizes (penetrance) on variation in intermediate phenotypes.
According to the neuroimaging genetics paradigm, to simply demonstrate that a susceptibility gene for schizophrenia impacts brain function is a necessary but not sufficient biological proof of a mechanism of susceptibility. This is because many, if not most, genes expressed in the brain, are apt to have a brain effect of some sort. A sine qua non of this proof is to show that the physiological intermediate phenotype associated with a susceptibilitygene for schizophrenia is itself linked to illness risk. To make this link, it is necessary to demonstrate that the physiological intermediate phenotype is a characteristic of individuals who are at increased genetic risk but do not manifest the clinical syndrome. The ideal samples in which to demonstrate this are unaffected relatives, eg, cotwins, siblings. This has been done for a number of brain-associated intermediate phenotypes related to increased risk for schizophrenia, including cognitive dysfunctions and neuroimaging phenotypes.Citation9-Citation14 Thus, the study of healthy relatives as a target population is critical for establishing the link between genetic association with clinical risk, and genetic association with biological risk. Having identified a neuroimaging phenotype related to increased genetic risk for illness, investigators can ask the question of whether genetic variation in a gene of interest maps onto the specific phenotype, as an indication of its putative neural mechanism of risk. The question arises of which population to choose to conduct this test. Neuroimaging studies of only affected subjects is confounded by illness-associated epiphenomena that are difficult to control, including smoking history, medical comorbidities, chronic illness burden, or prolonged neuroleptic exposure. This makes results in patient samples difficult to interpret, as the associations may reflect an interaction of the gene with any of these epiphenomena. Instead, the imaging genetics paradigm to test a specific gene-association hypothesis, le, the association of variation in a putative susceptibility gene and brain function linked to increased genetic risk, is best performed in healthy subjects. Healthy individuals possess common at-risk genotypes, but are not themselves symptomatic or clinically ill, thereby reducing the effect of confounding variables. This approach isolates the simple biologic effect of the genetic variation on brain function().
Some neuroimaging caveats
In contrast to functional neuroimaging, structural imaging has not yielded robust intermediate phenotypes for schizophrenia, although a myriad of structural MRI studies have revealed whole brain and region-specific volume and other differences between patients with schizophrenia and controls.Citation15-Citation17 As discussed elsewhere,Citation18 variation in structural MRI measurements mav be attributable to artifacts other than the disease itself, physiological alterations in brain tissue (eg, tissue perfusion, body fat, or water content),Citation19 or differences in image acquisition and analysis techniques. Alterations in body weight,Citation20 alcohol intake,Citation21 steroid administration and hormonal status,Citation22 can also change brain volume. Of critical importance in evaluating patients with chronic schizophrenia, medication can alter brain volume, sometimes rapidly; lithium carbonate reportedlyincreased cortical gray matter volume by 3% in patients with bipolar affective disorder after 4 weeks of treatment,Citation23 and multiple studies have reported basal ganglia volume change with neuroleptics over a duration of time intervals, including longitudinal studies of first-episode patients.Citation24,Citation25 Furthermore, there has been a lack of reliability in reports of the heritability of brain structures, with volume reductions in unaffected siblings inconsistent across brain regions and different studies, and not consistently overlapping with genetic liability to schizophrenia, decreasing the utility of structural volumetric indices as intermediate phenotypes.Citation26,Citation77 Increasingly, a growing number of research groups have used resting-state fMRI to map brain networks as well as diffusion tensor imaging (DTI) to investigate white matter abnormalities in patients with schizophrenia compared with controls. These modalities and investigations, however, are beyond the current focus of imaging genetics in the present review, but have been reviewed in depth elsewhere.Citation28-Citation32 We would emphasize two caveats with respect to these approaches: (i) the analysis of resting fMRI patterns is very sensitive to variation in head motion and to the mental state of the subject, making problematic comparisons between ill and well samplesCitation33; and (ii) DTI is based on highly derived data which are highly susceptible to many artifacts and biological events unrelated to white matter structure, rendering interpretation of the results also highly problematic.Citation34
In considering activation-based fMRI as applied to schizophrenia research there are methodological assumptions and limitations to recognize — the same limitations of fMRI in general — in its application to elucidating other CNS disorders or any cognitive function.Citation35 Firstly, brain mapping is predicated on the assumption of a modular organization of the brain, that there is a functional segregation with specialized and spatially separated modules, with interconnection of the entities and functional integration by distributed systems. Only according to this assumption can fMRI be then employed to reveal hierarchical decompositions of brain functional units. Another assumption is that a brain structure can be conceptualized as an information processing entity, with an input, a local processing capacity, and an output. Yet, the traditional cortical input-elaboration-output scheme, a correlate of the perception-cognition-action tripartite model, may be an oversimplification. In contributing to neuronal output, a change in balance between excitation and inhibition may predominate over a more straightforward hierarchical connectivity feedforward and feedback model.Citation36,Citation37 Further, as for all hemodynamic-based modalities, fMRI measures a surrogate signal of brain function, but then justifies the assignment of a functional role to an ”active“ area, presuming the change in signal results from the change of activity of a neuronal population. Also, the blood oxygen level-dependent (BOLD) signal reflects neuronal mass activity that may limit its neurobiological inferences, even while being advantageous in some global instances. According to one estimate there are about 90 to 100 000 neurons under 1 mm2 of cortical surface,Citation38 or as another metric, an unfiltered fMRI voxel contains 5.5 million neurons, and 2.2-5. 5x1010 synapses, in contrast to traditional microelectrode recordings. Lastly, for task-based fMRI neuroimaging studies, using a block design, a subtraction method is required to compare a task state with an investigator-designed control state, requiring a detailed task analysis to determine subtraction components and their interactions. Despite the above select critiques, fMRI remains the best tool at present for gaining insight into brain function, as many of these considerations are rendered relatively nonsys tematic because of the principle of every subject serving as his/her own control and the comparison of signals from one state to another. Thus, fMRI activation approaches allow for the ability to test discrete hypotheses about definable brain functions, especially the impact of genetic variation on these functions.
Identifying gene associations with regions of brain activation and neural circuits
Early studies mapped single genes selected based on a measure of candidacy, to an area of brain activation. Examples in addition to COMT mentioned above, include the association of a GRM3 variant with inefficient prefrontal function during a working memory task,Citation39 or the association of the short (S) variant In a variable repeat sequence in the promoter of the serotonin transporter gene, 5-HTTLPR, with altered amygdala activity during an emotionally evocative task.Citation40
Illustrative of genetic vulnerability maps of brain function based on imaging genetics approaches, Rasetti et al reviewed neuroimaging intermediate phenotypes of schizophrenia and the gene variants associated with them, catalogued by cognitive task.Citation41 For example, working memory tasks have revealed abnormal engagement of: dorsolateral prefrontal cortex associated with CACNA1C, COMT, GAD1, GRM3, DRD2, KCNH2, MTHFR, and RGS4; ventrolateral prefrontal cortex associated with COMT, PRODH, and RGS4; thalamus and hippocampus associated with NRG1, DAOA/G72, and DISCI; and parietal lobe associated with PRODH. Cognitive control tasks (designed to challenge executive function of goal-directed behavior in the presence of conflict) have identified abnormal engagement of the anterior cingulate cortex associated with COMT,DRD2, and MAOA; of the dorsolateral prefrontal cortex (DLPFC) associated with DTNBPl,DRD2,MAOA, COMT: and of the parietal cortex associated with DRD2, and MAOA.Citation42 Memory encoding tasks recently identified abnormal engagement of hippocampus parahlppocampus regionCitation43 and association of the hippocampus with BDNF, COMT, DISCI, GRM3, and KCNH2. It is worthwhile to note that most association studies of brain function have used single gene variants and risk haplotypes emerging from linkage studies and more recently genome wide association studies, with differing levels of genetic evidence for each candidate gene, though there has been no systematic approach to date to selecting genes for imaging genetics studies.
Imaging genetics approaches have progressed to associating gene variants with multiple regions of activation, with disease-relevant risk circuits and putative distributed functional networks, rather than isolated, single regions. After all, brain information processing does not occur as discrete activation “blobs,” but as activity across distributed neural systems and circuits. Thus, circuit-based phenotypes would be expected to have greater fidelity in showing genetic association at the level of brain function, since in principle, the more realistic the phenotype, the stronger the genetic association. As schizophrenia is an emergent property of neural system function, not isolatable to a singular brain region or localized regional defect, but likely attributable to network-based neurointegrative deficits, neuroimaging and intermediate phenotyplng strategies have progressed to better understand distributed networks associated with Increased genetic risk. To identify a functional network or interregional coupling, functional connectivity between spatially remote regions is inferred based on temporal coherence, by identifying regions of coactivation.Citation44 Statistical analyses used for functional connectivity include mapping based on seed voxel correlations, principal component analysis, independent component analysis, and partial least squares methods.
The functional connectivity literature within schizophrenia research has largely focused on PFC connectivity, especially the DLPFC and anterior cingulate, and DLPFC interaction with the medial temporal lobe, specifically the hippocampal formation (HF), and interaction with the DLPFC-thalamus.Citation45 For the DLPFC, abnormal connectivity has been identified in multiple studies in patients with schizophrenia and in high-risk subjectsCitation46-Citation49 and various genetic associations have been established with this putative circuit, during working memory tasks. For example during the n-back working memory task, COMT-associated activation changes mapped onto a prefrontal-parietal-striatal circuit, involving the DLPFC, ventrolateral prefrontal cortex (VLPFC), the posterior parietal regions, and the striatum.Citation50 A PRODH schizophrenia risk haplotype was associated with increased striatal-frontal functional connectivity, while the protective haplotype was associated with decreased striatal-frontal functional connectivity.Citation51 A 7 single-nucleotide polymorphism (SNP) haplotype of PPPIRIB (encoding DARPP-32) was associated with functional coupling and increased activation of the striatum and prefrontal cortex.Citation52 An RGS4 variant was found to impact frontoparietal and frontotemporal coupling.Citation53 A CACNAIC risk SNP was associated with decreased prefrontal-hippocampal connectivity.Citation54 While some functional connectivity studies have employed a classic intermediate phenotype strategy, testing for regions of correlation in affected subjects as well as unaffected relatives, many studies appear to query the association of risk susceptibility gene variants with correlated regions in healthy subjects or affected patients alone, with the putative neural circuit then pending validation as an intermediate phenotype.
Psycho-physiological interaction (PPI) analysis is an alternative approach to estimating connectivity, and measures a regionally specific response in terms of an interaction between a cognitive (or sensorimotor) process and activity in another part of the brain. The supposition is that the remote region is either the source of afferents that confer functional specificity on the target region or is activated by efferents that are specifically active during the task. PPI, therefore, allows for the exploration of the effects of an independent variable (eg, genotype) on taskrelated differences in interregional connectivity.Citation55 As a specific example, combining information about activity in the parietal region, mediating attention to a particular stimulus, and information about the stimulus, PPI aims to identify regions that respond to that stimulus when, and only when, activity in the parietal region is high. If such an interaction exists, then one might infer that the parietal area is modulating responses to the stimulus for which the area is selective. While this approach offers a deeper functional probe of network activity than simple time series correlation analyses, it is still based on a correlation of activity and not on a directional model of activity in one region influencing activity in another.
In one study, during the N-back task, DLPFC-HF coupling was identified in both patients with schizophrenia and their unaffected relatives, and associated with a ZNF804A risk allele, using seeded connectivity as well as PPI approaches.Citation56 PPI analysis showed a reduction in task load-related modulation of coupling between the right DLPFC and bilateral HF, in patients and siblings, compared with controls. Further, subjects homozygous for the risk-associated allele of ZNF804A showed a disruption in task-related modulation of right DLPFC-left HF coupling in the PPI analysis. The seeded connectivity analysis showed similar results to the PPI analysis in DLPFC-HF coupling.
Overall then, functional connectivity analysis offers some insight into correlation between different brain regions, but is limited in that it does not account for directionality, influence, or causality between putatively interacting regions; it makes no assumptions about the nature of underlying pathways, their structure, nor anatomical connectivity. So while correlative methods provide a way to characterize neural functional networks by temporal coherence of Inter-regional activation patterns, it yields neither an understanding of driving neural origins nor of the directionality of the observed network.
The next wave of imaging genetics: effective connectivity modeling
In contrast to functional connectivity approaches, effective connectivity analyses promise extended insight, referring explicitly to the influence that one neuronal system exerts over another, and may be used to better explain integration within a distributed neural system. Models employed in analyzing imaging data to uncover effective connectivity are based on regression models, or structural equation models, and these models may be linear or nonlinear. Dynamic causal modeling (DCM) is a type of effective connectivity analysis that yields directional, pathway information and allows for a quantification of the influence of a given neural region over another.Citation57,Citation58 DCM analysis, introduced in 2003 for fMRI data, is a Bayesian framework for inferring hidden neuronal states from measurements of brain activity; it is a hypothesisdriven approach, requiring an a priori definition of a set of interconnected neural areas that mediate a given function of interest.Citation59 DCMs are generative models of brain responses, which provide estimates of neurobiologically interpretable quantities including strength of synaptic connections among neuronal populations and their context-dependent modulation.Citation60 Causality in DCM is based on control theory, ie, causal interactions among hidden state variables that are expressed by differential equations that describe how the present state of one neuronal population causes dynamics in another via synaptic connection, and how these interactions change under the influence of external perturbations (eg, experimental manipulations) or brain activity. DCM tests hypotheses about neuronal mechanisms, allowing one to specify a generative model of measured brain data, which is a probabilistic mapping from experimentally controlled manipulations to observed data, via neuronal dynamics.
DCM has begun to be applied to imaging genetics. Using a DCM approach, distributed circuits that putatively underlie working memory — prefrontal-parietal and prefrontal-striatal circuits — were identified in healthy, normal subjects, and COMT, DRD2, and AKT1 functional variants were associated with the circuits.Citation61 The goal of the study was to engage a hypothesis-driven strategy to study component dopamine signaling processes, both D2-mediated and non-D2-mediated aspects of dopamine signaling. The investigators developed a working memory task that allowed dissociation of working memory into sub-processes, specifically maintenance of information and manipulation of information. In accordance with the DCM approach, models of prefrontal-subcortical-parietal networks were generated (each model's nodes, connections, and inputs were generated) during working memory maintenance and manipulation events, and the optimal model with the highest group Bayes factor was determined. The best DCMs for maintenance were primarily prefrontal-parietal connections, while for manipulation, the circuit that best fit the data was a prefrontalstriatal network. These results fit remarkably well with data from nonhuman primates about subprocesses in working memory and the principal networks engaged. The cortical network engaged during maintenance is presumed to be a non-D2 dominated network, and indeed, only COMT showed association with activity in this network. In contrast, the cortical-striatal network is expected to be D2-dominated, and all three genes showed effects on this network. This study illustrates the greater fidelity of genetic association based on more realistic models of brain information processing.
In a study using nonlinear DCM, subjects at high familial risk of schizophrenia performed a sentence completion task, and the connection strength of the mediodorsal (MD) thalamus and inferior frontal gyrus (IFG) was investigated, revealing lower connection strength in the at-risk subjects.Citation62 Bayesian Model Selection was used to compare the optimal bilinear and nonlinear models, and Bayesian Model Averaging was used to assess the connection strengths with the gating from the MD thalamus and the IFG, with nonlinear models providing better explanation of the data. In another study, dynamic causal models were applied to fMRI data to investigate how brain connectivity during an associative emotional learning task is affected by different PPPIRIB variants (DARPP32-encoding), in healthy subjects.Citation63 A PPPIRIB variant was associated with increased connectivity between the inferior frontal gyrus (IFG), amygdala and parahippocampal gyrus (PHG), with directionality of the connectivity determined to be from the IFG to the PHG. In addition to emerging effective connectivity analyses by DCM, connectivity is being explored from a more systems-level, hierarchical perspective, using graph theory metrics to describe the structural and functional composition of neural circuits. In graph theory, the correlated activity across multiple, distributed preselected brain regions can be expressed in terms of a graph, having various quantitative parameters, such as nodes, hubs, edges, pathway length, and connectivity strength.Citation64,Citation65 The “hubs” of these networks correspond to the most highly interconnected neural regions, which typically map to the association cortices of the human brain. The strength of each node is defined as its average connectivity with all other nodes, and the graph's size is defined by the number of nodes in the largest connected component; a larger graph size indicates fewer disconnected nodes.Citation66,Citation67
Accumulating evidence suggests that the small-world topological properties of brain functional networks are altered in patients with schizophrenia. In one study, in 31 patients with schizophrenia compared with 31 healthy controls, functional connectivity between 90 cortical and subcortical regions was estimated by partial correlation analysis and thresholded to construct a set of unidirected graphs.Citation68 The healthy subjects demonstrated efficient small-world properties, whereas topological parameters of brain networks — strength and degree of connectivity — were decreased in patients with schizophrenia, especially in the prefrontal, parietal, and temporal lobes, consistent with a hypothesis of dysfunctional integration. In another study, in a sample of 203 patients with schizophrenia, compared with 259 healthy controls, multimodal network organization was noted to be abnormal, as measured by topological and distance metrics of anatomical network organization, abstracted from fMRI data.Citation69 Patients with schizophrenia, compared with controls, demonstrated reduced hierarchy throughout the small-world regime, and increased connection distance in the multimodal cortical network. The loss of frontal hubs and the emergence of nonfrontal hubs was also noted, supporting the hypothesis of schizophrenia as a dysconnectivity syndrome, impacting the efficiency of a frontally dominated hierarchical network of multimodal cortical connections. Though the impact of genetic variation on network topology based on graph analyses has not yet been reported, moderate levels of heritability have been found for brain graph topology measured in a twin study using EEG, suggesting that genetic variation may Impact small-world organization and brain graph metrics.Citation70
The next wave of imaging genetics: polygenic risk
Just as imaging genetics will continue to incorporate increasingly sophisticated analytic methodologies, so too will imaging genetics evolve to incorporate increasingly sophisticated models of genetic risk, reflective of the increasingly apparent polygenic complexity of psychiatric syndromes. Genome-wide association studies (GWAS) have indicated a highly significant polygenic component of schizophrenia risk, possibly involving up to thousands of common alleles of very small effect, at the population level.Citation71
While early imaging genetics used intermediate phenotypes to assess the impact of single gene variants, recent studies have increasingly tended towards epistatic models of gene interaction. In 2007, Tan et al reported a two-way risk variant epistasis: the COMT-Val risk allele, associated with reduced prefrontal dopamine, and the GRM3 risk allele, related to suboptimal glutamatergic function, interacted to give disproportionately inefficient DLPFC activation in a working memory task.Citation72 Further, the inefficiency was associated with reduced frontoparietal functional connectivity. Nicodemus et al reported the first 3-way interaction using neuroimaging genetics to assess the risk susceptibility of the NRGI molecular pathway, finding epistasis between NRGI, and its tyrosine kinase receptor ERBB4, in a 3-way interaction with a variant of AKT1.Citation73 The statistical interaction was biologically validated by fMRI, in which healthy individuals carrying all three at-risk genotypes for NRGI, ERBB4, and AKT1 were disproportionately less efficient in DLPFC processing than any other combinations of one or two at-risk genotypes. Of note, lower-level interactions were not observed between NRGI, ERBB4, and AKTI, suggesting that the interaction, and the NRGI pathway, was necessary for the observed fMRI effect of inefficiency. Other reports of epistasis in neuroimaging genetics include association of variants of with altered DLPFC activation, during working memory tasks including DISCI-CIT-NDELI, MTHFR-COMT, and COMTRGS4.Citation74-Citation76
Imaging genetics is further evolving towards modeling increasing genetic complexity, by utilizing a polygenic risk score or propensity score of genetic risk for schizophrenia in fMRI studies. A range of options for constructing a polygenic score may be considered, selection of markers according to their P-values in association studies, and different methods for weighting markers in the score.Citation77 Only a handful of studies utilizing a polygenic risk score have been reported to date, using both functional and structural neuroimaging, and for multiple psychiatric syndromes. Walton et al calculated a genetic risk score for schizophrenia, the additive effect of 41 SNPS from 34 putative risk genes, and found a positive relationship between the genetic risk score and left DLPFC inefficiency during a working memory task.Citation78 Holmes et al reported a structural anatomic association with polygenic risk for Major Depressive Disorder (MDD) In a sample of 1050 healthy young adults with no history of psychiatric illness. Using risk scores derived from large MDD GWAS analyses, an MDD polygenic score was found to be associated with reduced cortical thickness in the left medial prefrontal cortex, a structural variation that is believed to influence vulnerability to MDD.Citation79 In a third study, increasing polygenic risk allele load for bipolar affective disorder (BPAD) was associated with increased activation in limbic regions previously implicated in BPAD, including the anterior cingulate cortex and amygdala during a verbal fluency task.Citation80 So, while a few early imaging genetics studies have employed the polygenic risk score, use of the polygenic score approach remains to be assessed and validated in larger-scale, more robust studies, with an explicit focus on schizophrenia and with various models of the risk score calculation possible.
Conclusion
As highlighted in this review, initial methodological and conceptual advances in functional neuroimaging launched the epoch of imaging genetics. Since then, development of genetic vulnerability maps of the brain, identifying neuroimaging intermediate phenotypes of schizophrenia and the risk variants associated with them, have become a major research industry. While imaging genetics to date has led to an increased understanding of schizophrenia pathophysiology and potential sites of pharmacologic intervention, a new wave of imaging genetics is fueled by even further methodological and conceptual advances. Effective connectivity-modeling promises to offer causal and directional insight into brain networks and circuitry, and polygenic risk modeling promises to incorporate genetic models reflective of the polygenic complexity of the schizophrenia syndrome.
REFERENCES
- WeinbergerDR.MattayV.CallicottJ.et alfMRI applications in schizophrenia research.Neuroimage.19964S118S1269345536
- BookheimerSY.StrojwasMH.CohenMS.et alPatterns of brain activation in people at risk for Alzheimer's disease.N Engl J Med.200034345045610944562
- EganMR.GoldbergTE.KolachanaBS.et alEffect of COMT Val108/1 58 Met genotype on frontal lobe function and risk for schizophrenia.Proc Natl Acad Sci U S A.2001986917692211381111
- EganMF.WeinbergerDR.Neurobiology of schizophrenia.Curr Opin Neurobiol.199777017079384552
- CastelliWP.AndersonK.WilsonPW.LevyD.Lipids and risk of coronary heart disease. The Framingham Study.Ann Epidemiol.1992223281342260
- O'ConnorDT.InselPA.ZieglerMG.et alHeredity and the autonomic nervous system in human hypertension.Curr Hypertens Rep.20002162210982526
- GottesmanII.GouldTD.The endophenotype concept in psychiatry: etymology and strategic intentions.Am J Psychiatry.200316063664512668349
- TanHY.CallicottJH.WeinbergerDR.Intermediate phenotypes in schizophrenia genetics redux: is it a no brainer?Mol Psychiatry.20081323323818285755
- CannonTD.ZorrillaLE.ShtaselD.et alNeuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers.Arch Gen Psychiatry.1994516516618042914
- GoldbergTE.TorreyEF.GoldJM.et alGenetic risk of neuropsychological impairment in schizophrenia: a study of monozygotic twins discordant and concordant for the disorder.Schizophr Res.19951777848541253
- ToulopoulouT.PicchioniM.RijsdijkF.et alSubstantial genetic overlap between neurocognition and schizophrenia: genetic modeling in twin samples.Arch Gen Psychiatry.2007641348135518056542
- EganMF.GoldbergTE.GscheidleT.et alRelative risk for cognitive impairments in siblings of patients with schizophrenia.Biol Psychiatry.2001509810711527000
- CallicottJH.EganMF.MattayVS.et alAbnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. AmJ Psychiatry.2003160709719
- RasettiR.WeinbergerDR.Intermediate phenotypes in psychiatric disorders.CurrOpin Genet Dev.201121340348
- HoneaR.CrowTJ.PassinghamD.MackayCE.Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies.Am J Psychiatry.20051622233224516330585
- ShentonME.DickeyCC.FruminM.McCarleyRW.A review of MRI findings in schizophrenia.Schizophr Res.20014915211343862
- WrightIC.Rabe-HeskethS.WoodruffPW.DavidAS.MurrayRM.BullmoreET.Meta-analysis of regional brain volumes in schizophrenia.Am J Psychiatry.2000157162510618008
- WeinbergerDR.McClureRK.Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry: what is happening in the schizophrenic brain?Arch Gen Psychiatry.20025955355812044198
- HajnalJV.SaeedN.OatridgeA.et alDetection of subtle brain changes using subvoxel registration and subtraction of serial MR images.J Comput Assist Tomogr.1995196776917560311
- SwayzeVW 2nd.AndersenA.ArndtS.et alReversibility of brain tissue loss in anorexia nervosa assessed with a computerized Talairach 3-D proportional grid.Psychol Med.1996263813908685294
- SchrothG.NaegeleT.KloseU.MannK.PetersenD.Reversible brain shrinkage in abstinent alcoholics, measured by MRI.Neuroradiology.1988303853893211313
- DentonER.HoldenM.ChristE.et alThe identification of cerebral volume changes in treated growth hormone-deficient adults using serial 3D MR image processing.J Comput Assist Tomogr.20002413914510667673
- MooreGJ.BebchukJM.WildsIB.ChenG.ManjiHK.Lithium-induced increase in human brain grey matter.Lancet.20003561241124211072948
- ScheepersFE.de WiedCC.HulshoffPol HE.et alThe effect of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics.Neuropsychopharmacology.200124475411106875
- CorsonPW.NopoulosP.MillerDD.ArndtS.AndreasenNC.Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics.Am J Psychiatry.19991561200120410450260
- HoneaRA.Meyer-LindenbergA.HobbsKB.et alIs gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings.Biol Psychiatry.20086346547417689500
- OwensSF.PicchioniMM.EttingerU.et alPrefrontal deviations in function but not volume are putative endophenotypes for schizophrenia.Brain.20121352231224422693145
- GreiciusMD.KrasnowB.ReissAL.MenonV.Functional connectivity in the resting brain: a network analysis of the default mode hypothesis.Proc Natl Acad Sci USA.200310025325812506194
- BroydSJ.DemanueleC.DebenerS.HelpsSK.JamesCJ.Sonuga-BarkeEJ.Default-mode brain dysfunction in mental disorders: a systematic review.Neurosci Biobehav Rev.20093327929618824195
- van den HeuvelMP.Hulshoff PolHE.Exploring the brain network: a review on resting-state fMRI functional connectivity.Eur Neuropsychopharmacol.20102051953420471808
- SamartzisL.DimaD.Fusar-PoliP.KyriakopoulosM.White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies.J Neuroimag. In press.2013doi 10.1 111/J.15526569
- WhitfordTJ.KubickiM.ShentonME.Diffusion tensor imaging, structural connectivity, and schizophrenia.Schizophr Res Treatment. In press.2011doi 10.1155/2011/709523
- BucknerRL.KrienenFM.YeoBT.Opportunities and limitations of intrinsic functional connectivity MRI.Nat Neurosci.20131683283723799476
- JonesDK.KnoscheTR.TurnerR.White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI.Neuroimage.20137323925422846632
- LogothetisNK.What we can do and what we cannot do with fMRI.Nature.200845386987818548064
- DouglasRJ.MartinKA.Neuronal circuits of the neocortex.Annu Rev Neurosci.20042741945115217339
- BruneiN.WangXJ.Effects of neuromodulation in a cortical network model of object working memory dominated by recurrent inhibition.J Comput Neurosci.200111638511524578
- RockelAJ.HiornsRW.PowellTP.The basic uniformity in structure of the neocortex.Brain.19801032212446772266
- EganMF.StraubRE.GoldbergTE.et alVariation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia.Proc Natl Acad Sci U S A.2004101126041260915310849
- HaririAR.MattayVS.TessitoreA.et alSerotonin transporter genetic variation and the response of the human amygdala.Science.200229740040312130784
- RasettiR.WeinbergerDR.Intermediate phenotypes in psychiatric disorders.Curr Opin Genet Dev.201121348340
- SambataroF.MattayVS.ThurinK.et alAltered cerebral response during cognitive control: a potential indicator of genetic liability for schizophrenia.Neuropsychopharmacology.20133884685323299932
- Di GiorgioA.GelaoB.CaforioG.et alEvidence that hippocampalparahippocampal dysfunction is related to genetic risk for schizophrenia.Psychol Med.2012111
- FristonKJ.Functional and effective connectivity in neuroimaging: a synthesis. Hum.Brain Mapp.199425678
- Meyer-LindbergA.BullmoreE.Functional brain imaging in schizophrenia. In:Schizophrenia.Third Ed. Oxford, UK: Blackwell Publishing Ltd2011;334353
- HoneyGD.BullmoreET.SharmaT.De-coupling of cognitive performance and cerebral functional response during working memory in schizophrenia.Schizophr Res.200253455611728837
- AnticevicA.RepovsG.CrystalJH.BarchDM.A broken filter: prefrontal functional connectivity abnormalities in schizophrenia during working memory interference.Schizophr Res.201214181422863548
- WolfDH.GurRC.ValdezJN.et alAlterations of fronto-temporal connectivity during word encoding in schizophrenia.Psychiatry Res.200715422123217360163
- WolfRC.VasicN.SambataroF.et alTemporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia.Prog Neuropsychopharmacol Biol Psychiatry.2009331464147319666074
- TanHY.ChenQ.GoldbergTE.et alCatechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory.J Neurosci.200727133931340118057197
- KempfL.NlcodemusKK.KolachanaB.et alFunctional polymorphisms in PRODH are associated with risk and protection for schizophrenia and fronto-striatal structure and function.PLoS Genet.20084e100025218989458
- Meyer-LindenbergA.StraubRE.LipskaBK.et alGenetic evidence implicating DARPP-32 in human frontostriatal structure, function, and cognition.J Clin Invest.200711767268217290303
- BuckholtzJW.Meyer-LindenbergA.HoneaRA.et alAllelic variation in RGS4 impacts functional and structural connectivity in the human brain.J Neurosci.2007271584159317301167
- PaulusFM.BedenbenderJ.KrachS.et alAssociation of rs1006737 in CACIMA1C with alterations in prefrontal activation and fronto-hippocampal connectivity.Hum Brain Mapp.20133430431322042765
- FristonKJ.BuechelC.FinkGR.MorrisJ.RollsE.DolanRJ.Psychophysiological and modulatory interactions in neuroimaging.Neurolmage.19976218229
- RasettiR.SambataroF.ChenQ.CallicottJH.MattayVS.WeinbergerDR.Altered cortical network dynamics: a potential intermediate phenotype for schizophrenia and association with ZNF804A.Arch Gen Psychiatry.2011681207121721810628
- KJFriston.BLi.JDaunizeau.KEStephan.Network discovery with DCM.Neurolmage.20115612021221
- TostH.BilekE.Meyer-LindenbergA.Brain connectivity in psychiatric imaging genetics.Neuroimage.2012622250226022100419
- FristonKJ.HarrisonL.PennyW.Dynamic causal modelling.Neuroimage.2003191273130212948688
- StephanKE.PennyWD.MoranRJ.den OudenHE.DaunizeauJ.FristonKJ.Ten simple rules for dynamic causal modeling.Neuroimage.2010493099310919914382
- TanHY.ChenAG.KolachanaB.et alEffective connectivity of AKT1mediated dopaminergic working memory networks and pharmacogenetics of anti-dopaminergic treatment.Brain.20121351436144522525159
- DauvermannMR.WhalleyHC.RomaniukL.et alThe application of nonlinear Dynamic Causal Modelling for fMRI in subjects at high genetic risk of schizophrenia.Neuroimage.201373162923384525
- Curcic-BlakeB.SwartM.Ter HorstGJ.LangersDR.KemaIP.AlemanA.Variation of the gene coding for DARPP-32 (PPP1R1B) and brain connectivity during associative emotional learning.Neuroimage.2012591540155021878394
- AchardS.SalvadorR.WhitcherB.SucklingJ.BullmoreE.A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs.J Neurosci.200626637216399673
- BullmoreE.BassettD.Brain graphs: graphical models of the human brain connectome.Annu Rev Clin Psychol.2011711314021128784
- BassettDS.NelsonBG.MuellerBA.CamchongJ.LimKO.Altered resting state complexity in schizophrenia.Neuroimage.2012592196220722008374
- BullmoreET.BassettDS.Brain graphs: graphical models of the human brain connectome.Annu Rev Clin Psychol.2011711314021128784
- LiuY.LiangM.ZhouY.et alDisrupted small-world networks in schizophrenia.Brain.2008131(Pt 4)94596118299296
- BassettDS.BullmoreE.VerchinskiBA.et alHierarchical organization of human cortical networks in health and schizophrenia.J Neurosci.2008289239924818784304
- SmitDJ.StamCJ.PosthumaD.BoomsmaDl.de GeusEJ.Heritability of “small-world” networks in the brain: a graph theoretical analysis of resting-state EEG functional connectivity.Hum Brain Mapp.2008291368137818064590
- International Schizophrenia Consortium: Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder.Nature.200946074875219571811
- TanHY.ChenQ.SustS.et alEpistasis between catechol-O-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function.Proc Natl Acad Sci US A.20071041253612541
- NicodemusKK.LawAJ.RadulescuE.et alBiological validation of increased schizophrenia risk with NRG1, ERBB4, and AKT1 epistasis via functional neuroimaging in healthy controls.Arch Gen Psychiatry.201067991100120921115
- NicodemusKK.CallicottJH.HigierRG.et alEvidence of statistical epistasis between DISCI, CITand NDEL1 impacting risk for schizophrenia: biological validation with functional neuroimaging.Hum Genet.201012744145220084519
- RoffmanJL.WeissAP.DeckersbachT.et alInteractive effects of COMT Val108/158Met and MTHFR C677T on executive function in schizophrenia.Am J Med Genet B Neuropsychiatr Genet.2008147B99099518186041
- BuckholtzJW.SustS.TanHY.et alfMRI evidence for functional epistasis between COMT and RGS4.Mol Psychiatry.200712893895,88517895922
- DudbridgeF.Power and predictive accuracy of polygenic risk scores.PLoS Genet.20139e100334823555274
- WaltonE.TurnerJ.GollubRL.et alCumulative genetic risk and prefrontal activity in patients with schizophrenia.Schizophr Bull.20133970371122267534
- HolmesAJ.LeePH.HollinsheadMO.et alIndividual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk.J Neurosci.201232180871810023238724
- WhalleyHC.PapmeyerM.SprootenE.et alThe influence of polygenic risk for bipolar disorder on neural activation assessed using fMRI.Transl Psychiatry.20122e13022760554