Abstract
Cellular processes that control transcription of genetic information are critical for cellular function, and are often implicated in psychiatric and neurological disease states. Among the most critical of these processes are epigenetic mechanisms, which serve to link the cellular environment with genomic material. Until recently our understanding of epigenetic mechanisms has been limited by the lack of tools that can selectively manipulate the epigenome with genetic, cellular, and temporal precision, which in turn diminishes the potential impact of epigenetic processes as therapeutic targets. This review highlights an emerging suite of tools that enable robust yet selective interrogation of the epigenome. In addition to allowing site-specific epigenetic editing, these tools can be paired with optogenetic approaches to provide temporal control over epigenetic processes, allowing unparalleled insight into the function of these mechanisms. This improved control promises to revolutionize our understanding of epigenetic modifications in human health and disease states.
Los procesos celulares que controlan la transcripción de la información genética son esenciales para la función celular y con frecuencia se relacionan con enfermedades psiquiátricas y neurológicas. Los mecanismos epigenéticos son uno de los procesos más importantes que permiten relacionar el ambiente celular con el material genético. Hasta hace poco nuestra comprensión de los mecanismos epigenéticos ha estado limitada por la falta de herramientas que puedan manipular selectivamente el epigenoma con precisión genética, celular y temporal, lo que a su vez disminuye el potencial impacto de los procesos epigenéticos como blancos terapéuticos. Esta revisión destaca la aparición de un conjunto de herramientas que facilita un examen consistente y selectivo del epigenoma, Además de permitir la edición epigenética sitio-específica, estas herramientas pueden complementarse con mecanismos optogenéticos para proveer un control temporal sobre los procesos epigenéticos, lo que favorece una mirada novedosa sobre la función de estos mecanismos, Este control perfeccionado promete revolucionar nuestra comprensión de las modificaciones epigenéticas en la salud y en la enfermedad en humanos.
Les processus cellulaires contrôlant la transcription de l'information génétique sont essentiels à la fonction cellulaire et souvent impliqués dans les pathologies psychiatriques et neurologiques. Les mécanismes épigénétiques en sont parmi les plus importants, liant l'environnement cellulaire au matériel génomique. Jusqu'à récemment, le manque d'outils capables de manipuler sélectivement l'épigénome avec une précision génétique, cellulaire et temporelle, a limité notre compréhension des mécanismes épigénétiques et donc diminué l'impact potentiel des processus épigénétiques comme cibles thérapeutiques. Cet article met en lumière une série d'outils nouveaux qui permettent d'interroger l'épigénome de façon à la fois sélective et fiable. Ces outils, en plus de permettre des réarrangements épigénétiques spécifiques du site, peuvent être appariés à des techniques optogénétiques pour contrôler temporellement les processus épigénétiques, donnant un aperçu incomparable du fonctionnement de ces mécanismes. Ce contrôle amélioré va révolutionner notre compréhension des modifications épigénétiques chez l'homme sain et malade.
Introduction
Arising from the interface between DNA and the nuclear environment, so-called “epi”-genetic mechanisms serve to regulate the readout of genetic material and thus serve a critical role in a host of cellular functions. As highlighted throughout this issue of Dialogues in Clinical Neuroscience, epigenetic processes control a wide range of cellular and behavioral phenomena that have relevance to psychiatric disorders. In addition to having demonstrated importance for basic neuronal features such as synaptic potentiation, synaptic scaling, and activity-related signaling, epigenetic mechanisms are dysregulated in diseases or disorders such as addiction, schizophrenia, Alzheimer's disease, and depression. Overall, then, our understanding of epigenetic mechanisms in disease states holds immense relevance for potential therapeutics.
However, the very nature of epigenetic mechanisms poses a number of challenges. For example, pharmacological approaches that target specific neurotransmitter receptor systems are able to utilize the relative exclusivity of a substrate for that receptor, enabling reasonably selective interrogation of the function of that system. By contrast, the epigenetic state at any given gene is established by a multitude of individual epigenetic factors, all of which will also be important for epigenetic regulation at many other genes. This fact implies that current epigenetic tools (including global inhibitors of DNA methyltransferases and histone deacetylases), which target specific enzymes that regulate the epigenome as a whole, can be of only limited use in understanding how epigenetic properties at a specific gene can regulate transcription, neuronal function, and ultimately behavior. Moreover, even where epigenetic patterns at a target gene can be altered (in the presence of other off-target effects), we generally lack the ability to reverse those changes, or to regulate the temporal dynamics of that process. At present, this lack of robust tools to modulate the epigenome limit our ability to generate detailed mechanistic insights, and ultimately serves to delay the next generation of epigenetically targeted therapeutics.
These limitations demonstrate that in order to capitalize on the potential promise of epigenetic therapeutics, we must first understand the basic biology of epigenetic machinery. These efforts are progressing in part due to a rapidly evolving suite of tools that offer the ability to monitor and control the epigenome with extreme precision.Citation1 The intent of this review is to describe emerging approaches that enable controlled epigenetic editing at specific genes and in specific cell types, and to highlight the potential importance of these approaches to specific clinical problems.
Current challenges
The pharmacological toolbox currently available for manipulation of the epigenome includes compounds that, despite clear substrate efficacy, are ultimately suboptimal. DNA methyltransferase (DNMT) and histone deacetylase (HDAC) inhibitors, which are the two most commonly used tools for probing epigenetic function, operate globally at their target enzymes.Citation2 Thus, these drugs can generate significant side effectsCitation3-Citation5that are usually not related to the specific processes under investigation. Nevertheless, these approaches are not without benefits. For example, several of these drugs are small molecule inhibitorsCitation6,Citation7 and therefore readily diffuse across the blood-brain barrier. In addition, in clinical cases that involve broad rearrangement of the epigenome at many genes, it is possible that only a global tool could reverse pathological symptoms.
A second group of tools involves the use of traditional genetic knockout/knock-in, transgenic, viral, and/ or RNA interference (RNAi) technology to manipulate specific epigenetic enzymes.Citation8-Citation12 While these approaches typically allow improved substrate specificity, manipulation of specific substrate isoforms or subclasses, and even limited cellular specificity, they still operate universally within a given cell, and therefore lack the ability to modulate the epigenome in specific ways. Based on these issues, it is becoming evident that research progress, and ultimately therapeutic benefit, may hinge on the ability to improve epigenetically targeted manipulations on several fronts. These critical areas are discussed in detail below.
Genetic specificity
Without question, the major shortcoming of current pharmacological and genetic tools is that they lack the specificity to direct epigenetic changes at specific sequences within DNA, or even at specific genes. It is now well understood that behavioral and experience-dependent epigenetic changes can be traced to specific genes and even specific sites within a gene.Citation13-Citation16 The application of genome-scale sequencing tools to the field of neuroepigenetics has confirmed the gene-specific nature of epigenetic changes within the brain,Citation17-Citation19 as well as the gene-specific nature of potential epigenetic treatments.Citation14 With few exceptions, basic research tools that could manipulate the epigenetic landscape at single genes would be vastly preferable to globally active pharmacological agents. As highlighted below, the ability to limit epigenetic remodeling to a single gene would allow the development of new hypotheses regarding epigenetic function, while also drastically reducing undesirable side effects.
Cell type specificity
Behavioral outcomes, including those that require clinical treatment, have physiological correlates that have been traced to specific cell types in defined neuronal circuitry.Citation20-Citation23 In some cases, behavioral patterns can be attributed to incredibly small populations of neurons (eg, interneurons), many of which are embedded in heterogeneous brain regions. In these limited neuronal populations, genetic mutations or epigenetic rearrangements may manifest to generate pathological outcomes. This basic observation implies that the ability to utilize epigenetic treatments to generate therapeutic outcomes will require the capacity to regulate the epigenome in specific cell types. However, this capacity is currently unavailable with current pharmacological tools that affect all cells. With genetic tools, achieving a cell-type specific manipulation is relatively straightforward, in that a cell's own transcriptional properties can be used as a guide for manipulations. For example, Cre-dependent viruses can be used in tandem with transgenic mouse Cre-driver lines to limit epigenetic manipulations to selected cellular populations.Citation21 However, these tools have only been sparsely used in the context of epigenetic manipulation in the intact brain.
Temporal precision
Finally, none of the traditional tools described above have the capacity to modulate the epigenome on timescales commensurate with normal behavioral and neuronal phenomena. Infusion of global inhibitors of the epigenome may require several hours to bind and inhibit their targets and may remain active long after the desired manipulation period, whereas virally expressed constructs can require several weeks to achieve full expression or knockdown of target enzymes. This lag in onset and/or offset may enable the development of compensatory mechanisms that counteract desired effects, thereby diminishing target efficacy. Therefore, a technique that could induce temporally precise epigenetic alterations would be beneficial both for basic understanding of the biochemical nature of these mechanisms, as well as the role of epigenetic changes in behavior and disease states.
Precision epigenetic editing
The recent emergence of approaches that allow tailored editing of the epigenome with the requirements outlined above has been possible in part due to enormous advances in genetic engineering. A common feature of new epigenetic tools is that they employ unique DNA sequences as a molecular homing device for secondary effector proteins that are capable of robust epigenetic reorganization. At the forefront of these approaches are tools built upon the nucleotide sequence recognition capacities native to three different systems: zinc-finger nucleases (ZFNs), transcriptional-activator like effectors (TALEs), and clustered regularly interspaced short palindromic repeats (CRISPR), which interact with Cas9 nucleases. Although these simple biochemical systems evolved for very different purposes, each employ an innate ability to recognize and bind specific DNA sequences, and each can be readily re-engineered to utilize this capacity for interrogation of the epigenome . This review will highlight TALE and CRISPR-based approaches, given their recent emergence, ease of synthesis, and increased efficiency over ZFNs.
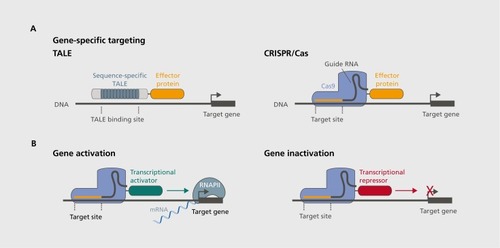
TALE-based strategies utilize a customized version of the site-specific binding system originally found in the bacteria Xanthamonas. The DNA binding motif of TALEs consists of repeating short (33-34aa) protein sequences, which contain variable diresidues that confer binding affinity for a specific nucleotide base within DNA. These repetitive sequences can be strung together in a designated order to create larger engineered repeat arrays that exhibit selective binding preference for known DNA sequences, enabling the ability to use TALEs to bind specific genes or genetic regions (Figure 1) likewise, CRISPR/Cas approaches were also first discovered in bacteria, where they serve as a form of adaptive immune defense against viruses and plasmids.Citation24,Citation25 However, CRISPR tools use engineered “guide” RNA (gRNA), which is a synthetic combination of two separate small RNAs endogenous to the bacterial system.Citation26 These gRNAs have the dual function of binding specific regions of DNA (they can be engineered to bind to almost any site in DNA), and serving as a scaffold to recruit CRISPR associated proteins to DNA (such as the nuclease Cas9). Moreover, Cas9 can be modified such that it has no nuclease activity, but retains its gRNA binding capabilities.Citation27 This general approach, despite its recent emergence, has received much attention due to the relative ease of synthesizing RNA molecules (as opposed to engineered proteins), as well potentially superior selectivity and targeting efficiency.Citation28
In their simplest form, customized TALE repeat arrays or synthetic CRISPR gRNAs are used to direct cleavage of specific sequences of DNA, which is highly useful for deletion of genetic material in genome engineering.Citation29-Citation32 However, almost simultaneously with the emergence of these techniques, many groups realized that the basic DNA binding capabilities of these tools could also be used to target fused effector proteins to DNA (Figure 1). Citation33-Citation35 Thus, beyond the relativelysimple ability to cut or nick double-stranded DNA, TALE and CRISPR approaches can ferry other cargo to DNA, including transcription factors, generic transcriptional activators, and transcriptional repressors (Figure 1B).Citation1,Citation21,Citation33,Citation35 These tools therefore enable relatively straightforward yet highly robust interrogation of the functional roles of specific genes and gene products. However, more germane to the focus of this review, these tools can localize enzymes that regulate specific epigenetic modifications directly to genes of interest (or even promoter, intragenic, or enhancer sites in DNA). The potential use of these components in tandem with enzymes that modify the epigenome is addressed below, with special focus on the two most well understood epigenetic mechanisms: DNA methylation and histone modifications.
Manipulating DNA methylation
DNA methylation processes in neurons have been shown to regulate multiple types of memory formation and long-term behavioral change, and these behavioral phenomena have been linked to changes in DNA methylation profiles at numerous plasticity-related genes.Citation9-Citation36-Citation43 Furthermore, alterations in DNA methylation status at specific genes are frequently implicated in psychiatric and neurological disease states.Citation9,Citation44-Citation51 Recent landmark studies have revealed that certain processes related to DNA methylation are highly enriched within adult neurons, suggesting the potential importance of this modification for a host of neuronal functions. For example, although it was previously believed that DNA methylation occurred only at cytosine-guanine dinucleotides within DNA, recent genome-wide sequencing approaches using enriched neuronal populations have uncovered widespread methylation of cytosine nucleotides in other sequence contexts.Citation17 Intriguingly, neuronal methylation in these contexts emerges during brain development and is correlated with synaptogenesis, while remaining nearly absent in glial cells.Citation17 Furthermore, it is now clear that activity- and experience-dependent processes within neurons can regulate active demethylation of DNA,Citation52-Citation54 via hydroxylation of methylcytosine by the Tet family of methylcytosine dioxygenasesCitation55-Citation58 and subsequent base excision repair by thymine DNA glycosylases.Citation59-Citation61
However, despite the dual observations that DNA methylation is altered in a gene-specific manner in the brain, and that DNA methylation is required for many behavioral and neuronal properties, the relationship between these observations remains unclear due to the inability to selectively control these changes in a systemic fashion. Contributing to this confusion is evidence suggesting that neuronal DNA methylation can both promote and repress gene expression, depending on the context and the genes in question.Citation39,Citation62-Citation64 Additionally, the precise transcriptional role of DNA hydroxymethylation also remains an open question, although the presence of this modification is correlated with actively transcribed genes in the nervous system.Citation64
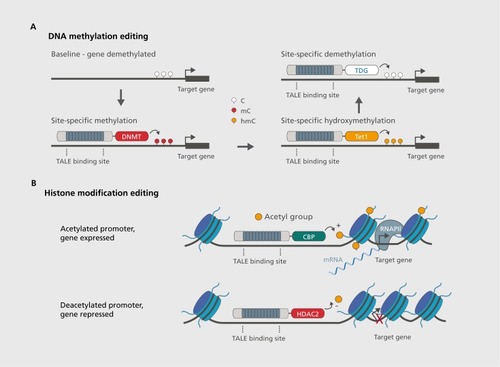
Thus, targeting of DNA methylation enzymes to specific DNA sequences with TALE or CRISPR-based tools has the potential to revolutionize our understanding of the functional consequences of DNA methylation and demethylation.Citation65 A general proof-of-concept for this approach has already been demonstrated using several targeting strategies.Citation66-Citation69 For example, targeting of the mammalian DNA methyltransferases Dnrnt3a directly to the MASPIN or SOX2 genes in breast cancer cell lines led to stable increases in DNA methylation at these genes, which were heritable across cell division and associated with robust gene repression.Citation67 Likewise, demethylation of specific nucleotides in human cells has been accomplished by fusing the catalytic domain of the Tetl enzyme to a custom TALE array targeting several genes individually.Citation68 Finally, targeted DNA demethylation has also been accomplished byfusing thymine deglycosylase (TDG) to the DNA binding domain of a transcription factor.Citation70
As these studies indicate, it is clear that both methylation and demethylation of specific DNA sequences can be accomplished with these engineered approaches. Indeed, it would seem possible to modulate almost any aspect of DNA methylation status using this general template . with the only limitations being the ability to accurately monitor the potential changes in question, and the ability to choose the correct location to modulate transcriptional activity. However, both of these limitations have been diminished by overlapping advances in whole-genome sequencing tools that have been developed to track DNA methylation status in a comprehensive and relatively unbiased fashion.Citation17,Citation71-Citation73 Future studies should employ these sequencing tools to select the most robust and effective strategies for manipulation of epigenetic material. For example, the promoter regions of active genes tend to be demethylated, meaning that tools directing demethylation machinery to these sites would be ineffective, whereas direction of DNMTs to these endogenous loci would likely repress transcription and reduce gene expression.
Histone modifications
As with DNA methylation, there is ample evidence that histone modifications are important, regulators of behavioral and synaptic plasticity, trans-generational epigenetic inheritance, and neurological or psychiatric disease states.Citation11,Citation14,Citation36,Citation43,Citation74-Citation81 Occurring at specific amino acid residues on protruding histone tails, these modifications (which include histone acetylation, methylation, and phosphorylation and are reviewed in great detail elsewhereCitation13,Citation82), have a generally well-understood relationship with transcriptional activity. However, the number of combinatorial possibilities at any given histone,Citation83,Citation84 together with the overall complexity of the chromatin environment, has limited our ability to understand the functional direction of this relationship.
To bridge this gap in knowledge, several recent papers have employed TALE-based strategies to target histone methyltransferases, histone demethylases, histone acetyltransferases, and histone deacetylases directly to endogenous DNA sequences.Citation1,Citation85 These efforts have been largely successful for a broad range of histone-modifying enzymes. For example, Konermann, et alCitation1 fused either histone methyltransferases or histone deacetylases to a TALE repeat array targeting the Grm2 and Neurog2 gene promoters in neuronal cultures. In general, these manipulations resulted in significant increases in histone methylation and decreases in histone acetylation, each of which led to 1.5-3 fold decrease in mRNA levels. Likewise, another study fused the histone demethylase LSD1 to TALE repeat domains that targeted non-genic “enhancer” sites that, transcribe small noncoding RNAs that, are hypothesized to regulate the transcription of nearby genes.Citation85,Citation86This study found that histone demethylation at these sites resulted in reduced enhancer RNA and decreased transcription of nearby-gene targets, and was thus critical for revealing the actual function of these sites.Citation85 Both of these studies are in line with several others that targeted histone-modifying enzymes to DNA in less specific ways.Citation69
However, an interesting observation noted in the first report is that certain histone modifying enzymes were effective at silencing one gene, but ineffective at silencing another.Citation1 While this outcome may simply reflect differences between the TALE target sites selected for these genes, it may also highlight the possibility that endogenous epigenetic states lead to differing interpretations of these new marks, or even protect against the new marks completely. Additionally, it is also possible that certain cofactors must also be present in order to produce the desired epigenetic effects. In either case, this result was not completely predictable given the previous state of knowledge. Together, these studies have been among the first to demonstrate a clear functional outcome of altering epigenetic status at a specific gene or genetic locus, and have unveiled key features of functional genomic and epigenetic elements that were previously not understood.
These studies provide clear support for the concept that artificially induced histone modifications can be very useful tools for dissecting epigenetic processes in the central nervous system (Figure 2B). A simple example of how this may work is the robust decrease in hippocampal Brain-derived neurotrophic factor (Bdnf) mRNA in mouse models of depression (social defeat stress).Citation87,Citation88 This deficit in Bdnf levels is associated with a repressive histone modification (dimethylation at lysine 27 on histone H3) at specific Bdnf isoform promoters, which are also an epigenetic target of antidepressants that reverse depression-like behavior in this model.Citation88 A prediction that becomes testable with sequence-specific epigenetic editing is that selective demethylation of this histone mark would similarly reverse depression like symptoms. Likewise, the direction of histone deacetylases to these promoters should enhance depressionlike behaviors, whereas direction of histone acetyltransferases (such as Creb-binding protein, or CBP) to these promoter sites should reverse and possibly even prevent depression -like behaviors. As is evident, with this simple example, targeted epigenetic editing will enable researchers to conduct experiments that, determine the causal relationship between epigenetic changes and behavioral phenomena, without the potential confounds that, emerge using globally active epigenetic pharmaceuticals.
Optoepigenetics
Given the ability to selectively modulate epigenetic states at. any gene, it is easy to recognize the potential strengths of combining this capability with the temporal precision and tunability of optogenetics,Citation89 which would enable temporally specific modulation of epigenetic states on a timescale that is similar to many behavioral and neuronal phenomena. However, unlike traditional optogenetic approaches, which utilize membrane-bound ion channels or G-protein coupled receptors to manipulate the physiology of neurons, the ability to se lectively alter the epigenome requires proteins that are active in the nuclear environment but remain responsive to light stimulation.
A potential solution for this challenge has recently been explored using the light-sensitive protein Cryptochrome 2 (Cry 2) originally found in Arabidopsis, where it mediates phototropism.Citation90 Intriguingly, upon photostimulation with a blue light source, Cry2 alters its protein conformation, which recruits a binding partner called CIB1. This basic capability has been repurposed for use in neurons, where these distinct components can be shuttled to the nucleus with the attachment of a nuclear localization signal found in other nuclear proteins.Citation1 The utility of this system was first demonstrated by fusing CIB1 to a generic transcriptional activator, and fusing Cry2 to a TALE that targeted a selected DNA locus (see Figure 3A for details). Upon blue light, stimulation, the induction of Cry2-CIB1 binding localized the transcriptional activator to the gene of interest, generating robust. (~20-fold) increases in mRNA levels within hours. Further, after cessation of light stimulation, gene expression levels returned to baseline by 12 h after stimulation, indicating remarkable temporal control of transcriptional properties. Furthermore, this study also generated the first, optoepigenetic demonstration in neuronal systems by fusing the HDAC complex Sin3a to CIB1, allowing localization of this repressive histone modification to the target, gene upon light stimulation. Indeed, blue light resulted in substantial decreases in histone acetylation at this target gene, as well as significant, decreases in mRNA levels.Citation1
As the components of this approach are not, endogenous to the mammalian nervous system, TALE -mediated epigenetic and optoepigenetic tools require transfection into neuronal cultures, or viral expression in the intact brain. While this poses certain challenges (viral packaging constraints, efficiency of viral expression), it. also presents an opportunity to employ readily available viral targeting strategies adapted from optogeneticsCitation89,Citation91,Citation92 that can enable cell- or even projection-specific epigenetic modulation (Figure 3B-D). For example, the ventral tegmental area (VTA) and its projection targets are often implicated in psychiatric diseases,Citation93,Citation94 and epigenetic modulation in this brain region is critical for reward-related learning.Citation39However, in addition to the well-studied dopaminergic projection originating from this region and terminating in the nucleus accumbens (NAc), the VTA also contains multiple non-dopaminergic neuronal subtypes and sends projections to functionally diverse brain regions.Citation95 As detailed in , a combination of viral and transgenic tools could be highly useful for targeting the epigenome of these specific neuronal populations. For example, the use of viruses that depend on Cre recombinase in tandem with transgenic mice that only express Cre recombinase in dopamine neurons would allow cell-type specific targeting of the epigenome with optical fibers localized to the VTA (Figure 3C). This powerful approach would enable not only the first optically driven epigenetic reorganization in this brain region, but would also provide the first detailed studies of cell-type specific epigenetic processes. As such, this approach has the potential to fundamentally alter the way in which epigenetics manipulations are conducted.
![Figure 3. Optical tools enable temporally precise modulation of epigenetic state. (A) Basic targeting strategy using the blue light sensitive protein cryptochrome 2 (Cry2) and its binding partner CIB1. Dual viral expression of tandem constructs (transcriptional-activator like effector [TALE] machinery + Cry2; CIB1 + epigenetic effector protein) enables site-specific localization of Cry2 to DNA. In the presence of blue light (delivered here via an optical fiber), Cry2 alters its conformation and binds CIB1, recruiting the epigenetic effector directly to the target gene. In the absence of blue light, the epigenetic effector is not targeted to DNA. (B-C) The use of conventional viral tools and transgenic animal models enables robust region, cell type, and pathway-specific epigenetic modification. For example, the ventral tegmental area (VTA) contains a heterogeneous neuronal population, including dopamine (DA) neurons that project to target structures such as the nucleus accumbens (NAc) and prefrontal cortex, as well as glutamatergic (Glu) and γ-aminobutyric acid (GABA)ergic projections. Viruses expressing TALE+Cry2 and CIB1+effector proteins could be infused directly into the ventrotegmental area (VTA), where localized blue light delivery would induce gene-specific epigenetic alterations in all coexpressing neurons (B). Application of a virus dependent on Cre-recombinase and a transgenic mouse line that expresses Cre only in tyrosine hydroxylase (Th)-containing dopamine neurons could further limit epigenetic alterations only to dopamine neurons (C). Finally, packaging of Cre-dependent CIB1 -effector constructs into a transsynaptic virus could be used to isolate specific projection targets (for example, the NAc), and combination with transgenic Th-Cre mouse lines could limit epigenetic alterations specifically to dopamine neurons that project from the VTA to the NAc (D).](/cms/asset/8d4e660f-e789-4f50-acde-bb637944ce66/tdcn_a_12130972_f0003_oc.jpg)
The eccentric biology of the epigenome: insights for therapeutics
Given the plethora of excellent reviews focusing on the mechanistic details of epigenetic processes,Citation13,Citation57,Citation96-Citation98 this review has not addressed the precise biochemical nature of these modifications. However, it is worth mentioning that, several epigenetic mechanisms have biochemical properties that make them favorable candidates for supporting long-lasting behavioral changes that arise from environmental experiences. For example, histones are relatively long-lasting proteins, with turnover rates often an order of magnitude or more lower than other proteins.Citation99,Citation100 Histone variants with different functional properties can become incorporated into chromatin in an experience-dependent manner, engaging new regulatory templates and gene expression patterns.Citation101 Together, these attributes make histone variants and histone modifications attractive candidates for longterm information storage. Likewise, DNA methylation can be self-perpetuating, making it. a remarkably stable epigenetic modification that can last the lifetime of a cell, and even perpetuate during cell division.Citation102 In fact, these basic mechanisms that are critical players in establishing and maintaining cellular phenotype, even in classically non-dividing cells such as neurons. It, is this fundamental capacity that makes therapeutics that, can selectively target the epigenome such an attractive and promising idea.
However, even apart from the vast contribution of the epigenome to neuronal function, approaches that involve epigenetic manipulation have several distinct practical advantages over other approaches. The first advantage is that in comparison to tools that seek to manipulate neuronal function by interfering with RNA translation, enzymatic activity, or receptor signaling, the unit of operation at the epigenetic scale is comparatively small. For example, within a single neuron, there may at any given time be thousands of neurotransmitter receptors of a particular type, hundreds or thousands of mRNA copies that will be translated into that receptor, and an indefinite number of signaling molecules that are operating to control the function of that receptor. However, even in the most complex neurons, there are at most two copies of the genetic material that codes for that, receptor. Therefore, manipulations that alter the epigenome at this level are potentially capable of much stronger functional regulation than RNAi or receptorbased approaches.
A second advantage is that, unlike other manipulations wherein cessation of treatment is generally accompanied by declining therapeutic benefit, epigenetic manipulations may capitalize on the unique biochemistryoutlined above, and therefore enable continued target manipulation even in a drug-free state. While this trait could certainly be a double-edged sword (extending potential benefits as well as side effects), it is nevertheless a potentially favorable option. For example, this form of “hit-and-run” therapeutic could be useful in situations where continued drug dosing can be toxic, or where compliance with scheduled prescriptions in an issue.
Finally, new epigenetically targeted approaches could be extremely useful in tandem with available pharmacological and behavioral therapies. These traditional approaches are acting on top of long-lasting epigenetic marks (some of which may have been inherited), and so it is possible that this biochemical inertia could profoundly influence the success or failure of these approaches. Indeed, the success of a treatment might require a favorable epigenetic milieu in a patient, and drugs that fail to permanently reverse clinical symptoms may possess this limitation due to an inability to act on stable epigenetic landscapes. Treatments that operate directly at the level of the epigenome will bypass this challenge. Thus, a third strength of epigenetic tools is their potential combination with traditional approaches in order to create a favorable condition for pharmacological efficacy.
Conclusions and future directions
At present, the tools described in this review are generally only compatible with basic research uses in animal models or in vitro assays, where they will likely prove critical for unraveling the epigenetic mechanisms of neuronal plasticity, memory formation, and transgenerational epigenetic inheritance. Nevertheless, the ability to bidirectionally modulate the readout of genetic material will be useful across health-related research fields, potentially enabling rapid screening and verification of new targets arising from high-throughput screening approaches. Additionally, the relatively rapid emergence of these tools has created excitement for their potential application to human conditions.Citation32,Citation103,Citation104 In the future, modified versions of these tools may be packaged into nanoplatforms for delivery into the brain,Citation105 or targeted to specific brain areas with using phage librariesCitation106 or antibody-coated nanoparticle approaches.Citation107
The further advance of this general class of tools could also allow the development of robust new treatment options for psychiatric and neurological disease states, especially for clinical cases that are resistant to current approaches.Citation108 Epigenetic alterations could be targeted to the specific cell types that generate deleterious behavioral patterns, and even done so in a temporally specific manner in order to coordinate with effective behavioral treatments for anxiety disorders, post-traumatic stress disorder, addiction, or depression. Such approaches will allow unprecedented control over the molecular and circuit, mechanisms underlying these phenomena, ushering in the new age of epigenetic therapeutics.
JJD is supported by the National Institute on Drug Abuse (DA034681), startup funds from UAB, and the Evelyn F. McKnight Brain Research Foundation. Many thanks to Andrew Kennedy, Garrett Kaas, Garret Stuber, David Sweatt, and Feng Zhang for helpful discussions on the subject matter of this review.
REFERENCES
- KonermannS.BrighamMD.TrevinoAE.et alOptical control of mammalian endogenous transcription and epigenetic states.Nature.201350047247623877069
- SzyfM.Epigenetics, DNA methylation, and chromatin modifying drugs.Annu Rev Pharmacol Toxicol.20094924326318851683
- ShakespearMR.HaliliMA.IrvineKM.FairlieDP.SweetMJ.Histone deacetylases as regulators of inflammation and immunity.Trends Immunol.20113233534321570914
- LykoF.BrownR.DNA methyltransferase inhibitors and the development of epigenetic cancer therapies.J Natl Cancer Inst.2005971498150616234563
- BruecknerB.LykoF.DNA methyltransferase inhibitors: old and new drugs for an epigenetic cancer therapy.Trends Pharmacol Sci.20042555155415491775
- BruecknerB.Garcia BoyR.SiedleckiP.et alEpigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases.Cancer Res.2005656305631116024632
- ZhangY.LiW.LaurentT.DingS.Small molecules, big roles — the chemical manipulation of stem cell fate and somatic cell reprogramming.J Cell Sci.2012125(Pt 23)5609562023420199
- FengJ.ZhouY.CampbellSL.et alDnmtl and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons.Nat Neurosci.20101342343020228804
- LaPlantQ.VialouV.CovingtonHE. 3rdet alDnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens.Nat Neurosci.2010131137114320729844
- SultanFA.WangJ.TrontJ.LiebermannDA.SweattJD.Genetic deletion of Gadd45b, a regulator of active DNA demethylation, enhances long-term memory and synaptic plasticity.J Neurosci.201232170591706623197699
- GuanJS.HaggartySJ.GiacomettiE.et alHDAC2 negatively regulates memory formation and synaptic plasticity.Nature.2009459556019424149
- OliveiraAM.HemstedtTJ.BadingH.Rescue of aging-associated decline in Dnmt3a2 expression restores cognitive abilities.Nat Neurosci.2012151111111322751036
- GraffJ.TsaiLH.Histone acetylation: molecular mnemonics on the chromatin.Nat Rev Neurosci.2013149711123324667
- GraffJ.JosephNF.HornME.SamieiA.MengJ.SeoJ.et alEpigenetic priming of memory updating during reconsolidation to attenuate remote fear memories.Cell.201415626127624439381
- DayJJ.SweattJD.Epigenetic treatments for cognitive impairments.Neuropsychopharmacology ;201237247260
- JiangY.LangleyB.LubinFD.et alEpigenetics in the nervous system.J Neurosci.200828117531175919005036
- ListerR.MukamelEA.NeryJR.et alGlobal epigenomic reconfiguration during mammalian brain development.Science.2013341123790523828890
- GuoJU.MaDK.MoH.et alNeuronal activity modifies the DNA methylation landscape in the adult brain.Nat Neurosci.2011141345135121874013
- GuoJU.SuY.ShinJH.et alDistribution, recognition and regulation of non-CpG methylation in the adult mammalian brain.Nat Neurosci.20141721522224362762
- KvitsianiD.RanadeS.HangyaB.TaniguchiH.HuangJZ.KepecsA.Distinct behavioural and network correlates of two interneuron types in prefrontal cortex.Nature.201349836336623708967
- JenningsJH.SpartaDR.StamatakisAM.et alDistinct extended amygdala circuits for divergent motivational states.Nature.201349622422823515155
- SpartaDR.JenningsJH.UngRL.StuberGD.Optogenetic strategies to investigate neural circuitry engaged by stress.Behav Brain Res.2013255192523684554
- KimSY.AdhikariA.LeeSY.et alDiverging neural pathways assemble a behavioural state from separable features in anxiety.Nature.201349621922323515158
- BarrangouR.FremauxC.DeveauH.et alCRISPR provides acquired resistance against viruses in prokaryotes.Science.20073151709171217379808
- GasiunasG.BarrangouR.HorvathP.SiksnysV.Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria.Proc Natl Acad Sci U S A.2012109E2579E258622949671
- JinekM.ChylinskiK.FonfaraI.HauerM.DoudnaJA.CharpentierE.A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity.Science.201233781682122745249
- QiLS.LarsonMH.GilbertLA.et alRepurposing CRISPR as an RNAguided platform for sequence-specific control of gene expression.Cell.20131521173118323452860
- ShenB.ZhangW.ZhangJ.et alEfficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. WatMethods.201411399402
- CongL.RanFA.CoxD.et alMultiplex genome engineering using CRISPR/Cas systems.Science.201333981982323287718
- RanFA.HsuPD.LinCY.et alDouble nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity.Cell.20131541380138923992846
- RanFA.HsuPD.WrightJ.AgarwalaV.ScottDA.ZhangF.Genome engineering using the CRISPR-Cas9 system.Nat Protocols.2013822812308
- MaliP.YangL.EsveltKM.AachJ.GuellM.DiCarloJE.et alRNA-guided human genome engineering via Cas9.Science.201333982382623287722
- CongL.ZhouR.KuoYC.CunniffM.ZhangF.Comprehensive interrogation of natural TALE DNA-binding modules and transcriptional repressor domains.Nat Commun.2012396822828628
- GilbertLA.LarsonMH.MorsutL.et alCRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes.Cell.201315444245123849981
- Perez-PineraP.KocakDD.VockleyCM.et alRNA-guided gene activation by CRISPR-Cas9-based transcription factors.Nat Methods.20131097397623892895
- MillerCA.CampbellSL.SweattJD.DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity.Neurobiol Learn Mem.20088959960317881251
- MillerCA.GavinCF.WhiteJA.et alCortical DNA methylation maintains remote memory.Nat Neurosci.20101366466620495557
- MillerCA.SweattJD.Covalent modification of DNA regulates memory formation.Neuron.20075385786917359920
- DayJJ.ChildsD.Guzman-KarlssonMC.et alDNA methylation regulates associative reward learning.Nat Neurosci.2013161445145223974711
- RothTL.LubinFD.FunkAJ.SweattJD.Lasting epigenetic influence of early-life adversity on the BDNF gene.Biol Psychiatry.20096576076919150054
- GuptaS.KimSY.ArtisS.et alHistone methylation regulates memory formation.J Neurosci.2010303589359920219993
- WeaverIC.CervoniN.ChampagneFA.et alEpigenetic programming by maternal behavior.Nat Neurosci.2004784785415220929
- LubinFD.RothTL.SweattJD.Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory.J Neurosci.200828105761058618923034
- GraysonDR.JiaX.ChenY.et alReelin promoter hypermethylation in schizophrenia.Proc Natl Acad Sci U S A.20051029341934615961543
- NohJS.SharmaRP.VeldicM.et alDNA methyltransferase 1 regulates reelin mRNA expression in mouse primary cortical cultures.Proc Natl Acad Sci U S A.20051021749175415671176
- HuangHS.AkbarianS.GAD1 mRNA expression and DNA methylation in prefrontal cortex of subjects with schizophrenia.PloS One.20072e80917726539
- KundakovicM.ChenY.GuidottiA.GraysonDR.The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes.Mol Pharmacol.20097534235419029285
- AllanAM.LiangX.LuoY.et alThe loss of methyl-CpG binding protein 1 leads to autism-like behavioral deficits.Hum Mol Genet.2008172047205718385101
- CoppietersN.DieriksBV.LillC.FaullRL.CurtisMA.DragunowM.Global changes in DNA methylation and hydroxymethylation in Alzheimer's disease human brain.Neurobiol Aging.2013351334134424387984
- Sanchez-MutJV.AsoE.PanayotisN.et alDNA methylation map of mouse and human brain identifies target genes in Alzheimer's disease.Brain.2013136(Pt 10)3018302724030951
- SabunciyanS.AryeeMJ.IrizarryRA.et alGenome-wide DNA methylation scan in major depressive disorder.PloS One.20127e3445122511943
- KaasGA.ZhongC.EasonDE.et alTET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation.Neuron.2013791086109324050399
- RudenkoA.DawlatyMM.SeoJ.et alTet1 is critical for neuronal activity-regulated gene expression and memory extinction.Neuron.2013791109112224050401
- SzulwachKE.LiX.LiY.et al5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging.NatNeurosci.20111416071616
- TahilianiM.KohKP.ShenY.et alConversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1.Science.200932493093519372391
- GuoJU.SuY.ZhongC.MingGL.SongH.Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain.Cell.201114542343421496894
- GuoJU.SuY.ZhongC.MingGL.SongH.Emerging roles of TET proteins and 5-hydroxymethylcytosines in active DNA demethylation and beyond.Cell Cycle.2011102662266821811096
- KriaucionisS.HeintzN.The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain.Science.200932492993019372393
- CortellinoS.XuJ.SannaiM.et alThymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair.Cell.2011146677921722948
- KohliRM.ZhangY.TET enzymes, TDG and the dynamics of DNA demethylation.Nature.201350247247924153300
- ShenL.WuH.DiepD.et alGenome-wide analysis reveals TET- and TDG-dependent 5-methylcytosine oxidation dynamics.Cell.201315369270623602152
- WuH.CoskunV.TaoJ.et alDnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes.Science.201032944444820651149
- ChahrourM.JungSY.ShawC.et alMeCP2, a key contributor to neurological disease, activates and represses transcription.Science.20083201224122918511691
- MellenM.AyataP.DewellS.KriaucionisS.HeintzN.MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system.Cell.20121511417143023260135
- JurkowskaRZ.JeltschA.Silencing of gene expression by targeted DNA methylation: concepts and approaches.Methods Mol Biol.201064914916120680833
- MinczukM.PapworthMA.KolasinskaP.MurphyMP.KlugA.Sequence-specific modification of mitochondrial DNA using a chimeric zinc finger methylase.Proc Natl Acad Sci U S A.2006103196891969417170133
- RivenbarkAG.StolzenburgS.BeltranAS.et alEpigenetic reprogramming of cancer cells via targeted DNA methylation.Epigenetics.2012735036022419067
- MaederML.AngstmanJF.RichardsonME.et alTargeted DNA demethylation and activation of endogenous genes using programmable TALE-TET1 fusion proteins.Nat Biotechnol.2013311137114224108092
- de GrooteML.VerschurePJ.RotsMG.Epigenetic editing: targeted rewriting of epigenetic marks to modulate expression of selected target genes.Nucleic Acids Res.201240105961061323002135
- GregoryDJ.MikhaylovaL.FedulovAV.Selective DNA demethylation by fusion of TDG with a sequence-specific DNA-binding domain.Epigenetics.2012734434922419066
- YuM.HonGC.SzulwachKE.et alTet-assisted bisulfite sequencing of 5-hydroxymethylcytosine.Nat Protoc.201272159217023196972
- ListerR.EckerJR.Finding the fifth base: genome-wide sequencing of cytosine methylation.Genome Res.20091995996619273618
- ListerR.GregoryBD.EckerJR.Next is now: new technologies for sequencing of genomes, transcriptomes, and beyond.Curr Opin Plant Biol.20091210711819157957
- Sadri-VakiliG.KumaresanV.SchmidtHD.et alCocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine.J Neurosci.201030117351174420810894
- VassolerFM.WhiteSL.SchmidtHD.Sadri-VakiliG.PierceRC.Epigenetic inheritance of a cocaine-resistance phenotype.Nat Neurosci.201316424723242310
- KumarA.ChoiKH.RenthalW.TsankovaNM.et alChromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum.Neuron.20054830331416242410
- MazeI.CovingtonHE 3rdDietzDM.et alEssential role of the histone methyltransferase G9a in cocaine-induced plasticity.Science.201032721321620056891
- FischerA.SananbenesiF.WangX.DobbinM.TsaiLH.Recovery of learning and memory is associated with chromatin remodelling.Nature.200744717818217468743
- MalvaezM.Sanchis-SeguraC.VoD.LattalKM.WoodMA.Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference.Biol Psychiatry.201067364319765687
- GraffJ.MansuyIM.Epigenetic dysregulation in cognitive disorders.Eur J Neurosci.2009301819508697
- KoshibuK.GraffJ.BeullensM.et alProtein phosphatase 1 regulates the histone code for long-term memory.J Neurosci.200929130791308919828821
- DayJJ.SweattJD.Epigenetic mechanisms in cognition.Neuron.20117081382921658577
- JenuweinT.AllisCD.Translating the histone code.Science.20012931074108011498575
- LeeJS.SmithE.ShilatifardA.The language of histone crosstalk.Cell.201014268268520813257
- MendenhallEM.WilliamsonKE.ReyonD.et alLocus-specific editing of histone modifications at endogenous enhancers.Nat Biotechnol.2013311133113624013198
- KimTK.HembergM.GrayJM.et alWidespread transcription at neuronal activity-regulated enhancers.Nature.201046518218720393465
- TsankovaN.RenthalW.KumarA.NestlerEJ.Epigenetic regulation in psychiatric disorders.Nat Rev Neurosci.2007835536717453016
- TsankovaNM.BertonO.RenthalW.KumarA.NeveRL.NestlerEJ.Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action.Nat Neurosci.2006951952516501568
- TyeKM.DeisserothK.Optogenetic investigation of neural circuits underlying brain disease in animal models.Nat Rev Neurosci.20121325126622430017
- AhmadM.JarilloJA.SmirnovaO.CashmoreAR.Cryptochrome bluelight photoreceptors of Arabidopsis implicated in phototropism.Nature.19983927207239565033
- JenningsJH.RizziG.StamatakisAM.UngRL.StuberGD.The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding.Science.20133411517152124072922
- JenningsJH.StuberGD.Tools for resolving functional activity and connectivity within intact neural circuits.Curr Biol.201424R41R5024405680
- RenthalW.NestlerEJ.Epigenetic mechanisms in drug addiction.Trends Mol Med.20081434135018635399
- KrishnanV.NestlerEJ.The molecular neurobiology of depression.Nature.200845589490218923511
- FieldsHL.HjelmstadGO.MargolisEB.NicolaSM.Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement.Annu Rev Neurosci.20073028931617376009
- KloseRJ.BirdAP.Genomic DNA methylation: the mark and its mediators.Trends Biochem Sci.200631899716403636
- DulacC.Brain function and chromatin plasticity.Nature.201046572873520535202
- KouzaridesT.Chromatin modifications and their function.Cell.200712869370517320507
- ToyamaBH.SavasJN.ParkSK.et alIdentification of long-lived proteins reveals exceptional stability of essential cellular structures.Cell.201315497198223993091
- CommerfordSL.CarstenAL.CronkiteEP.Histone turnover within nonproliferating cells.Proc Natl Acad Sci U S A.198279116311656951165
- BonischC.HakeSB.Histone H2A variants in nucleosomes and chromatin: more or less stable?Nucleic Acids Res.201240107 1941
- DayJJ.SweattJD.DNA methylation and memory formation.NatNeurosci.20101313191323
- GersbachCA.Perez-PineraP.Activating human genes with zinc finger proteins, transcription activator-like effectors and CRISPR/Cas9 for gene therapy and regenerative medicine.Expert Opin Ther Targets. 201415
- ZhouY.ZhuS.CaiC.et alHigh-throughput screening of a CRISPR/ Cas9 library for functional genomics in human cells.Nature.201450948749124717434
- ParkJH.GuL.von MaltzahnG.RuoslahtiE.BhatiaSN.SailorMJ.Biodegradable luminescent porous silicon nanoparticles for in vivo applications.Nat Mater.2009833133619234444
- PasqualiniR.RuoslahtiE.Organ targeting in vivo using phage display peptide libraries.Nature.19963803643668598934
- KolharP.AnselmoAC.GuptaV.et alUsing shape effects to target antibody-coated nanoparticles to lung and brain endothelium.Proc Natl Acad Sci US A.20131101075310758
- MaybergHS.LozanoAM.VoonV.McNeelyHE.SeminowiczD.HamaniC.et alDeep brain stimulation for treatment-resistant depression.Neuron.20054565166015748841
