Abstract
Brain imaging studies over two decades have delineated the neural circuitry of anxiety and related disorders, particularly regions involved in fear processing and in obsessive-compulsive symptoms. The neural circuitry of fear processing involves the amygdala, anterior cingulate, and insular cortex, while cortico-striatal-thalamic circuitry plays a key role in obsessive-compulsive disorder. More recently, neuroimaging studies have examined how psychotherapy for anxiety and related disorders impacts on these neural circuits. Here we conduct a systematic review of the findings of such work, which yielded 19 functional magnetic resonance imaging studies examining the neural bases of cognitive-behavioral therapy (CBT) in 509 patients with anxiety and related disorders. We conclude that, although each of these related disorders is mediated by somewhat different neural circuitry, CBT may act in a similar way to increase prefrontal control of subcortical structures. These findings are consistent with an emphasis in cognitive-affective neuroscience on the potential therapeutic value of enhancing emotional regulation in various psychiatric conditions.
Los estudios de imaginología cerebral desde hace más de dos décadas ban delineado los circuitos neurales de la ansiedad y los trastornos relacionados, particularmente las regiones involucradas en el procesamiento del miedo y en los síntomas obsesivo compulsivos. El circuito neural del procesamiento del miedo incluye la amígdala y las cortezas cingulada anterior e insular, mientras que el circuito tálamo-estriado-cortical juega un papel clave en el trastorno obsesivo-compulsivo. Más recientemente, los estudios de neuroimágenes han examinado cómo la psicoterapia para la ansiedad y los trastornos relacionados impacta en estos circuitos neurales. En este artículo se realiza una revision sistemática de los hallazgos de estos trabajos, los que comprenden 19 estudios de resonancia magnética functional que examinan las bases neurales de la terapia cognitivo-conductual (TCC) en 509 patientes con ansiedad y trastornos relacionados. Se concluye que aunque la ansiedad y cada uno de los trastornos relationados está mediado por circuitos neurales algo diferentes, la TCC puede actuar de manera similar para aumentar el control prefrontal de las estructuras subcorticales. Estos hallazgos son consistentes con el énfasis de la neurociencia cognitivo-afectiva en el potential valor terapéutico del aumento de la regulación emocional en varias condiciones psiquiátricas.
Ces 20 dernières années, des études d'imagerie cérébrale ont défini les circuits neuronaux de l'anxiété et des troubles apparentés, en particulier les régions impliquées dans les processus de peur et les symptômes obsessionnels-compulsifs. L'amygdale, le cortex insulaire et le cortex cingulaire antérieur sont impliqués dans les circuits neuronaux des processus de peur, tandis que le circuit cortico-striato-thalamique joue un rôle dans les troubles obsessionnels-compulsifs. Plus récemment, des études de neuro-imagerie ont analysé comment la psychothérapie pour l'anxiété et les troubles apparentés influent sur ces circuits neuronaux. Nous menons ici une étude méthodique sur les résultats de ces travaux, qui a permis d'aboutir à 19 études d'imagerie fonctionnelle par résonance magnétique analysant les bases neuronales d'une thérapie cognitivo-comportementale (TCC) chez 509 patients atteints d'anxiété et de troubles apparentés. Bien que l'anxiété et les troubles apparentés soient induits par différents circuits neuronaux, nous concluons que la TCC peut agir de la même façon pour accroître le contrôle préfrontal des structures sous-corticales. Ces résultats sont cohérents avec l'accent mis dans les neurostiences cognitivo-affectives, sur la valeur thérapeutique potentielle de l'amélioration de la régulation émotionnelle dans diverses pathologies psychiatriques.
Introduction
Basic and clinical studies have led to advances in our understanding of the neural circuitry of anxiety and related disorders. Animal studies, for example, have highlighted the role of the amygdala in fear conditioningCitation1 and of the striatum in mediating grooming behaviors.Citation2 Structural and functional brain imaging studies in patients with anxiety and related disorders have shown alterations in analogous regions, identifying a “fear network” incorporating the amygdala, anterior cingulate, and insular cortex,Citation3 and emphasizing the role of cortico-striatal-thalamic-cortical circuitry in obsessive-compulsive and related disorders.Citation4 The cognitive-affective neuroscience of anxiety and related disorders points to the role of discrete types of “false alarm” that can be targeted effectively with psychotherapy.Citation5 More recently, a number of functional magnetic resonance imaging (fMRI) studies have begun to explore the impact of psychotherapy on these neural circuits. Here we review this literature, exploring the question of how cognitive-behavioral therapy (CBT) in particular alters neural circuitry in anxiety and related disorders, after considering animal and human models of anxiety and related disorders.
Animal models of anxiety and related disorders
Animal models have provided valuable insights into the neural circuitry of key cognitive-affective processes relevant to anxiety and related disorders. Basic research on the neurobiology of fear conditioning, for example, has contributed to our understanding of the neural circuitry of the anxiety disorders and of trauma- and stressor-related disorders such as post-traumatic stress disorder (PTSD). Work on the neurobiology of social dominance and submission has arguably been particularly relevant to understanding the neural circuitry of social anxiety disorder (SAD). Animal models of repetitive grooming or other stereotypic behaviors may be useful in providing insights into the neuroanatomy of obsessive-compulsive disorder (OCD). Here we briefly review this work.
The neural circuitry of fear conditioning and fear extinction is particularly well conserved across mammalian species. Animal studies of the neural circuitry of fear conditioning have established that stimulation of the amygdala produces physiological and behavioral responses akin to anxiety and panic.Citation6,Citation7 Stimulation of the periaqueductal gray, a brain stem nucleus, also evokes fear responses, including fast muscle contractions, twitching, and blinking.Citation8,Citation9 Moreover, acute stress may diminish the effective regulation of fear, presumably by altering synaptic excitation of glutamate receptors in the prefrontal cortex, as seen in rodent stress.Citation10 Additionally, the role of hippocampal function in contextual fear learning—or Pavlovian conditioning—is well-documented.Citation11 In terms of fear reconsolidation and extinction, medial prefrontal cortex and connections between the amygdala, hippocampus, and entorhinal cortex play a key role.Citation12
Social dominance models have been studied in subordinate primates,Citation13 and implicate mesolimbic regions such as the amygdala, hippocampus, and striatum in the neuropathology of SAD and anxiety disorders. For example, shrinkage of hippocampal volumes can occur in mice exposed to social stress,Citation14 with one hypothesis being that this is mediated by hypothalamic-pituitary-adrenal (HPA) axis Cortisol release.Citation15 Further, hyperactivation of the HPA axis and impaired neurotransmission in the striatum is associated with lower social status and submissiveness in primates.Citation15,Citation16 Conversely, gray matter volume enlargement in the amygdala, hippocampus, striatum, hypothalamus, and raphe nucleus of the brain stem is observed in Macaque monkeys with higher social status.Citation17 Thus, social fear conditioning and stress subordination/social submissiveness appear to involve volume reduction and hyperstimulation of the mesolimbic pathway.Citation13
A range of animal studies have examined the neuroanatomy of grooming and other stereotypies that are relevant to OCD.Citation18 Such stereotypies range from simple motor perseveration to more complex behaviorsCitation19 and there is a growing understanding of the underlying neuroanatomy. In an early study, persistent, stereotyped movements in the aftermath of social deprivation in nonhuman primates were associated with alterations in the cytoarchitecture of the striatum.Citation20 More recent work in mice has found that the dorsal medial striatum and orbitofrontal cortex (OFC) are more engaged, whereas the dorsal lateral striatum appears less engaged when mice lever-press with a goal-directed versus habitual action strategy respectively.Citation21 More recently, optogenetics methods have been used to manipulate neural transmission in rodents, and have demonstrated that chronic hyperstimulation of an excitatory circuit between glutamatergic neurons in the orbitofrontal cortex and the ventromedial striatum triggers compulsive behavior.Citation19,Citation22
Neurocircuitry of anxiety and related disorders
While animal models provide important insights, brain imaging studies using fMRI link uniquely human cognitions and behaviors to the neuropathology of anxiety and related disorders. Functional brain imaging studies typically examine responses to specific anxiety-provoking stimuli, such as angry faces or disturbing scenes, or during cognitive tasks such as response inhibition. These paradigms have highlighted common patterns of neural activation in those with anxiety and related disorders and have led to the development of neural circuitry models. For example, the neural substrates of conditioned fear have emphasized the role of amygdala hypersensitivity; the lateral amygdala for fear acquisition and the central nucleus for fear behaviors.Citation1,Citation23 Furthermore, in OCD hyperactivation of the corticostriatal-thalamic-cortical (CSTC) network, specifically involving the lateral and medial OFC and the dorsal anterior cingulate cortex circuitry, is exacerbated during anxiety-provoking visual stimulus presentation and underlies deficits in fear extinction and behavioral inhibition.Citation24 Hippocampal function mediates appreciation of safe contexts and explicit learning/memory, and deficits in fear extinction and contextual learning in those with anxiety disorders coincide with reduced hippocampal activation to anxiety-provoking stimuli.Citation25
Along with the amygdala and the CTSC networks, activation of the insular cortex, a brain region associated with perceptions of bodily sensations (interoceptive awareness) and emotion regulation also seems to be involved in the experience of anxiety, disgust, and obsessive-compulsive symptoms.Citation26,Citation3,Citation27 For example, altered interoceptive awareness, or an inability to effectively process bodily sensation, is prominent in anxiety states, and could be due to an augmented detection of the difference between observed and expected bodilystates.Citation28 Further, insula function contributes to the interface between basic interoception and self-referential belief states that are driven by prefrontal cortex processes.Citation29 However, insula involvement in heightened anxiety is suggested to be due, not to faulty interoceptive processing per se, but to an amplified self-referential and discrepant prediction about bodily stateCitation29 that may originate in prefrontal cortex and be associated with dysregulated top-down emotion regulation.Citation30,Citation31
While fMRI studies often utilize symptom-provocation paradigms with disorder-specific stimuli, or cognitive tasks, resting-state studies, on the other hand, measure neural activation at rest and further contribute to our understanding of the neural circuitry of anxiety and related disorders.Citation32 For example, a recent metaanalysis examining 28 resting state studies in patients with anxiety disorders demonstrated similarities across PTSD, PD, GAD, SAD, and OCD in broad connectivity alterations between limbic regions—namely, the bilateral amygdalae, insula, and regions associated with the default mode network (DMN—for self-referential thought), central executive network (CEN—for control of emotion) and salience network (SN—for arousal).Citation33 However, resting state connectivity in specific anxietydisorders may be highly variable, and to date only OCD demonstrates consistent functional alterations in the corticostriatal circuitry and the DMN.Citation33
While prefrontal cortex and limbic connectivity is broadly implicated in dysregulation of emotional processing in anxiety disorders in general, discrete functional differences are observed in specific anxiety disorders, and these will now be considered.
PTSD
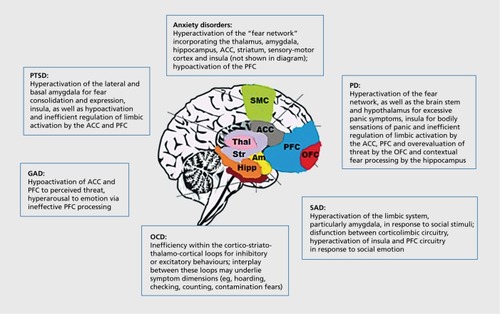
PTSD is a trauma- and stressor-related disorder that affects individuals who have been exposed to a substantial traumatic event (eg, combat, interpersonal violence, natural disaster), and is characterized by intrusive memories of the trauma, avoidance of related cues, and hyperarousal.Citation34 Functional imaging studies over the past two decades have demonstrated that PTSD is accompanied by heightened activation of the amygdala, particularly the lateral and basal amygdala, with aberrant activation between the amygdala and medial prefrontal cortex, hippocampus, and insula.Citation35 Specificaily, excessive activation of the amygdala in those with PTSD is consistently shown by fMRI studies in response to trauma-arousing words.Citation25,Citation36-Citation38 It is suggested by a number of fMRI studies that amygdala hyperactivation is due to ineffective inhibitory control by the medial prefrontal cortex,Citation38 and a recent review confirmed that for PTSD, as opposed to other anxiety disorders, activation of the anterior cingulate cortex (ACC), which forms part of the mPFC inhibitory network, is consistently reduced.Citation3 Additionally, a large meta-analysis of 846 PTSD patients found reduced bilateral hippocampal volume, particularly on the left side.Citation39 The No/Go task, a paradigm where participants are required to withhold their button press response when a stop stimulus is presented, has been used in fMRI studies to demonstrate deficits in response inhibition, and is associated with reduced medial PFC and ACC activation in those with PTSD.Citation40,Citation41 Additionally, hippocampal structure and functional connectivity with PFC is reported to be reduced in those with PTSD, which could contribute to deficits in fear conditioning, fear extinction, and may reflect neurotoxicity associated with significant trauma.Citation42,Citation43
PD
PD is characterized by panic attacks and hyperarousalCitation34 and involves neural dysfunction in the fear network, again involving amygdala hypersensitivity, and dysfunctional emotion regulation in the PFC.Citation44 An early neuroanatomical hypothesis of PD proposed that brain stem and hypothalamus were responsible for the stress and panic responses; the mesolimbic pathway (eg, the amygdala and hippocampus) for fear anticipation, and the prefrontal cortex for phobic reactions and emotion dysregulation.Citation45 More recently this hypothesis has been refined to emphasise “crosstalk” and functional connectivity patterns prevalent in PD, between corticolimbic areas, namely the OFC and ACC for emotion regulation, and the amygdala, hippocampus, and insula for hyperarousal.Citation46 fMRI studies to date support a model of PD that emphasizes cognitive impairment in fear processingCitation46 in that excessive contextual fear learning is processed by the hippocampus, exacerbating amygdala hypersensitivity to threat cues and ineffective emotion regulation via the PFC, which together results in exaggerated pathological fear.
GAD
GAD is characterized by debilitating, chronic, excessive, and uncontrollable worry about a variety of topics.Citation34 In contrast to the other anxiety disorders, fMRI studies of GAD have reported hypofunction of prefrontal and anterior cingulate regions, as documented in a recent review.Citation47 The review included 14 fMRI studies and examined the neural correlates of the contemporary models of GAD, namely: 10 studies adhering to the emotional dysregulation theory.Citation48 The other studies included in the review examined other related models, namely; the conditioned fear overgeneralization model and the intolerance of uncertainty- theory.Citation47 The emotion dysregulation theory asserts that patients with GAD experience emotional hyperarousal,Citation49 contributing to maladaptive emotion regulation and unsuccessful attempts to either minimize or over-control emotions. Emotional dysregulation in GAD across 10 fMRI studies to date has been linked to a reduced PFC and ACC activation during emotion regulation tasks (for review, see refs 47,49).
SAD
SAD is defined as an excessive fear of social situations; such that avoidance or significant distress ensues to disrupt normal functioning.Citation34 Functional neuroimaging studies of SAD have found increased limbic activation especially in the amygdala and particularly in response to emotional stimuli of a social and self -critical nature.Citation50 Additionally, dysfunctional interplay between the limbic system and prefrontal cortex regions in SAD (eg, medial prefrontal cortex, dorsal raphe, striatum, locus coeruleus, insular cortex, and anterior cingulate cortex), involving γ-aminobutyric acid (GABA), dopaminergic, and oxytocin neurotransmitter systems may underlie symptoms and volumetric changes.Citation50-Citation52 In a recent meta-analysis of brain function in 17 studies of SAD patients, increased limbic processing in response to emotional faces was significant, specifically hyperactivation of the amygdala, the parahippocampal gyrus, and the globus pallidus in comparison with healthy controls.Citation53 Additionally, hyperactivation of the insula, putamen, the superior temporal gyrus, medial frontal regions and the cuneus was observed in the SAD cases compared with Williams-Beuren Syndrome—a disorder on the opposite end of the social anxiety spectrum.Citation53 Thus, increased limbic activation to socially emotive stimuli appears to be present in those with SAD, and it is possible that, consistent with other anxiety disorders, inefficient top-down regulation by prefrontal cortex networks of excessive limbic responses may contribute to social anxiety symptoms.
OCD
OCD is characterized by recurring thoughts and beliefs (obsessions), as well as physical or mental acts performed repeatedly that are difficult to resist (compulsions).Citation34 Detailed modeling of the neural circuitry of OCD has emerged from various imaging studies. Early workCitation54 proposed that the behaviorally disinhibiting direct basal ganglia pathway and the behaviorally inhibiting or moderating indirect basal ganglia system alters “neural tone” and may mediate the expression of OCD symptoms. More recent studies of the neuroanatomy and function of OCD have continued to implicate CSTC circuits in the pathophysiology of OCD.Citation19 The CSTC circuitry is comprised of parallel loops, which appear to support inhibitory (eg, freezing, motor repetition) or excitatory (eg, compulsivity, fear conditioning) behaviors and cognitions, and interplay between different areas of these loops may explain the different OCD symptom dimensions (eg, hoarding, checking, cleaning, counting).Citation55 Such models are supported by a recent multicenter mega-analysis,Citation56 which found reduction in ACC, dorsomedial prefrontal cortex, and inferior frontal gyrus volumes in OCD, with group-by-age interactions in striatum, OFC, and insula. In line with functional deficits in prefrontal cortex circuitry in those with OCD, a recent meta-analysis of structural differences reported reduction in prefrontal cortex volumes, but also increased striatal volumes.Citation4 Aberrant activation of CSTC circuitry may also explain executive dysfunction seen in OCD.Citation57 For example, a recent meta-analysis of 110 previous studies revealed that individuals with OCD are impaired on tasks measuring most aspects of executive function and that these impairments are not influenced by motor slowness or depression.Citation58 Executive dysfunction may be associated with excessive modulatory top-down prefrontal cortical activation of excessive basal ganglia network activation.Citation59
A systematic review of the neural correlates of CBT for anxiety and related disorders
Animal models and functional brain imaging studies provide an important foundation for the investigation of how CBT alters the neurocircuitry of anxiety and related disorders. CBT remains the most widely recommended first-line psychotherapy for anxiety and related disordersCitation60 and aims to alter affect, behavior, and cognitions (A-B-C) that contribute to misperceptions of threat and behavior, and to strengthen self-awareness of emotional responses to anxiety-related stimuli. A key outcome of CBT is better emotion regulation of anxiety responses.Citation61 From this perspective, we now systematically review functional MRI studies that have examined the neural correlates of CBT for each of the main anxiety and related disorders in turn. The neural correlates of anxiety disorders are summarized in .
Literature search
We searched PubMed, Medline, and Google Scholar, and conducted a manual search of the publication reference lists from 1995 until May 2015 using the following search criteria: FMRI + CBT + ANXIETY; FMRI + CBT + POSTTRAUMATIC STRESS DISORDER; FMRI + CBT + PANIC DISORDER; FMRI + CBT + GENERALISED ANXIETY DISORDER: FMRI + CBT + SOCIAL ANXIETY DISORDER; FMRI + CBT + OBSESSIVE COMPULSIVE DISORDER. We also substituted FMRI for MRI and NEURAL, and CBT for PSYCHOTHERAPY as additional searches. Publications were included if they were: (i) written in English; (ii) used CBT and not other methods (eg, other psychological therapies or pharmacotherapy); (iii) used fMRI and not other imaging modalities (which are outside the scope of the aims of this article); (iv) examined patient populations other than anxiety and related disorders. This systematic search yielded 19 fMRI studies for inclusion in this review. We now describe the findings in relation to the separate anxiety and related disorders. See Table I for details of included studies.
Table I FMRI studies of the neural correlates of cognitive behavioral therapy (CBT) in anxiety and related disorders. RCT, randomized controlled trial; PTSD, post-traumatic stress disorder; GAD, generalized anxiaety disorder; OFC, orbitofrontal cortex; VLPFC, ventrolateral prefrontal cortex
PTSD
Two fMRI studies have identified neural correlates of CBT for PTSD, which has the neural characteristics of hyperactivation of the amygdala and reduced PFC and ACC activation. In the first fMRI study, 14 PTSD patients' neural responses to masked fearful faces were measured before and after 8 weeks of CBT that involved education, imagined and in vivo exposure, and cognitive therapy.Citation62 The authors found that 7 patients only were treatment responders (eg, at least 50% improvement on symptom scores), and that poor treatment response was associated with pretreatment increases in bilateral amygdala and ventral ACC. The authors suggest that excessive pretreatment neural responses in these regions may signal deficits in emotion regulation during treatment and may hinder CBT efficacy. A second fMRI study used the No/Go task to examine inhibitory neural function following 8 weeks' CBT treatment.Citation63 It was found that after CBT there was greater left dorsal striatal and frontal network activation during inhibitory control, which was associated with lower PTSD symptom severity, indicating that improvements to inhibitory control contribute to better treatment response.
PD
Four fMRI studies to date have examined the neural correlates of CBT for PD. PD is associated with amygdala hypersensitivity and dysregulation in the PFC and CBT has been shown to effectively alter these neural patterns. The first study compared 42 PD patients with healthy controls and found that a 12-week course of manualized CBT altered neural responses to fear-conditioned stimuli.Citation64 Specifically, reduced left inferior frontal gyrus (IFG) was observed post-CBT treatment in the PD group, as well as increased functional connectivity between the IFG and regions of the fear network, namely the amygdalae, insulae, and anterior cingulate cortex. In another study, 49 medication-free PD patients, who received manualized CBT treatment and who were categorized as either respond ers or nonresponders based on at least 50% symptom reduction, were measured for neural responses during fear conditioning.Citation65 The authors reported that successful treatment was associated with an increase in right hippocampal activation when using cognitive strategies in response to fear-based stimuli. Furthermore, significant symptom reduction was associated with an inhibitory functional coupling between the anterior cingulate cortex and the amygdala that did not change over time. The authors suggest that neuroplasticity within frontal-amygdala networks may mediate improvements in safety signaling. In another study, CBT treatment efficacy in 23 PD patients was best predicted, during maintenance of emotion by activation in the insula/anterior cingulate cortex, and during reappraisal of emotion by activation in the occipital and supramarginal gyrus.Citation66 In the largest multicenter study of the effects of CBT on neural correlates of symptom reduction in patients with PD,Citation67 49 patients who completed at least 4 weeks of CBT treatment were examined using a fear conditioning task. The authors reported that neural activation to fear conditioning and extinction predicted whether patients became responders or nonresponders after treatment, with 70% accuracy. Fear conditioning was associated with increased neural responses in the precentral gyrus, occipital cortex, and OFC. Fear extinction by contrast was associated with increased activation in the putamen, paracingulate cortex, and occipital and frontal cortices.
GAD
Four studies have examined the effects of CBT on neural activation in patients with GAD, which is characterized by hypoactivation in the mPFC and ACC, perhaps due to excessive limbic responsivity to perceived threat stimuli. In the first fMRI study with a facial emotion recognition task, 7 pediatric patients with a predominant GAD diagnosis were examined after an 8-week course of CBT that focused on exposure and skills training (versus 7 patients who chose SSRI treatment).Citation68 It was found that left amygdala hyperactivation prior to treatment predicted a reduction in clinical severity scores following CBT. In a similar fMRI study using an emotional probe task, 7 adolescents with GAD showed increased right vlPFC response following 8 weeks of CBT treatment.Citation69 The authors conclude that increased vlPFC response is likely indicative of improvements to modulation of anxiety responses originating in the amygdala. In a third study of 25 adults being treated with CBT for GAD, hippocampal activation during maintenance of response to emotional stimuli, and anterior insula, superior temporal, supramarginal, and superior frontal gyrus activation during reappraisal of emotion were among the best predictors of better treatment response.Citation66 In the final study to date, of 21 adults with GAD who undertook CBT treatment compared with healthy controls, at pretreatment those with GAD showed reduced responses in the amygdala, insula, and anterior cingulate to pictures of happy faces, and greater amygdala-insula connectivity.Citation70 After 10 sessions of CBT there was attenuated amygdalar and subgenual anterior cingulate activation to fear/angry faces and heightened insular responses to happy faces with no effects on connectivity. The authors suggest a dual process effect of CBT on those with GAD, in that neural responses to positive stimuli are heightened and neural responses to fearful stimuli attenuated. This may perhaps be due to strengthened PFC functioning that is better able to regulate the experience of positive and negative emotions.
SAD
The neural correlates of SAD are an excessive limbic response to socially emotive stimuli. Four studies have so far examined the effects of CBT on patients with SAD. The first study examined neural responses to emotional faces/scenes in 39 patients with SAD being treated with CBTCitation71 The authors report increased neural activation in temporal occipital regions to emotional stimuli before treatment predicts lower clinical severity after CBT. Similarly, in a study examining the neural correlates of a 12-week program of CBT in 14 patients with SAD, successful intervention predicts enhanced pretreatment activation to threatening faces in superior and middle temporal gyrus (for visual processing) and dorsal anterior cingulate cortex, dorsomedial prefrontal cortex (for cognitive regulation of emotion).Citation72 Furthermore, this study revealed that greater threat processing of fearful faces prior to treatment corresponds with greater prefrontal cortex and insula activation in the SAD group. Another study examined the effects of cognitive reappraisal after 16 individual sessions of CBT in 75 patients with SAD.Citation73 More positive responses and reappraisals of negative self-belief statements at the end of treatment were associated with greater prefrontal cortex activation and greater connectivity between prefrontal cortex and amygdala that coincided with reductions in negative emotion ratings. A similar later study by the same group, this time examining the neural correlates of emotional responsivity to social evaluation after the same type of CBT was conducted in a subset of 59 patients with SAD.Citation74 CBT altered emotional reactivity in terms of increased right superior frontal gyrus (SFG), inferior parietal lobule (IPL) and middle occipital gyrus (MOG) activation during social praise. In contrast, CBT was followed by increases in right SFG and IPL and decreases in left posterior superior temporal gyrus (pSTG) during social criticism. Finally, CBT increased brain responses in right SFG and MOG, and decreased left pSTG activation during reappraisal of emotion.
OCD
The neural correlates of OCD involve cortico-striato-thalamo-cortical (CSTC) networks, for example, excessive PFC modulation of excessive limbic responsivity to emotional stimuli, although the various symptom dimensions of OCD likely reflect different neural patterns within the CSTC loops. Five fMRI studies so far have examined the effects of CBT in pediatric and adult samples. The first fMRI study used the Stroop task that evokes conflicting responses to stimulus targets that included symptom provocation in 10 outpatients with OCD.Citation75 The authors reported that after 12 weeks' of CBT there was a reduction in PFC activation to conflicting responses, and an increase in the parietal cortex and cerebellum to anxiety-provoking stimuli. A later reverse-learning (RL) fMRI study measured the effects of CBT using exposure and response prevention on frontostriatal circuitry in 10 patients with OCD.Citation76 It was found that before CBT, RL was associated with reduced OFC and striatal activation (caudate), and that after CBT there was increased striatal activation (putamen) during RL. A later fMRI study used the Flanker task to probe conflict processing in a pediatric sample of 25 with OCD who completed 8 weeks of CBT.Citation77 The authors concluded that the OCD group had increased ACC and bilateral insula activation during error/high conflict tasks that was only partially altered by CBT. In a more recent fMRI study examining the effects of 3 months' CBT on neural function during a personalized image exposure probe task in 35 OCD patients, anterior cingulate and orbitofrontal cortex activation was higher during exposure to personalized obsession-inducing images.Citation78 Further, activation to the anxiety-provoking images, in the anterior cingulate and left orbitofrontal decreased with symptom improvement after CBT. In another fMRI study examining the neural correlates of CBT for the most common OCD symptom dimension, namely contamination and washing obsessions, heightened activation of the amygdala and decreased dorsolateral prefrontal cortex activation (associated with cognitive control) was indicative of significant reduction in anxiety symptoms after 12 weeks of CBTCitation79 The authors concluded that in OCD successful recruitment of limbic structures during fear exposure may be beneficial for successful treatment outcome, whereas excessive top-down control via recruitment of the prefrontal cortex during CBT may hinder the effects of treatment.
Discussion
We have summarized contemporary models of anxiety and related disorders (eg, PTSD, PD, GAD, SAD, OCD) from the perspectives of animal studies and human magnetic resonance imaging (fMRI), in order to provide a foundation upon which to consider how CBT may alter the neurocircuitry of these conditions. We conclude that although each of the anxiety and related disorders is mediated by somewhat different neural circuitry, CBT may act in a similar way to increase prefrontal control of subcortical structures. In summary, anxiety disorders and PTSD are associated with exaggerated fear network activation to anxiety-provoking stimuli, involving limbic structures such as the amygdala, hippocampus, striatum, ACC, and insula.Citation1,Citation3 Dysregulation in corticostriatal circuitry, on the other hand, is thought to underlie the neuropathology of obsessive-compulsive and related disorders.Citation24 Such differences across circuitry may help explain symptom-specific traits for the various anxiety and related disorders, and may be considered targets for effective psychotherapy.Citation30 Discrete differences across these circuits may explain symptom-specific traits for the various anxiety and related disorders, and must be considered for effective psychotherapy. For example, fMRI studies of PTSD and PD report heightened activation of the fear network, particularly involving the amygdala and in response to anxiety-provoking stimuli,Citation35,Citation44 while those with SAD show similar neural responses in the fear circuitry, but rather in relation to social stimuli.Citation50 Conversely, GAD has been linked to hypofunction in the PFC and ACC that is associated with emotion regulation,Citation47 and those with OCD show altered cortico-striatal-thalamic-cortical (CSTC) circuitry, and hyperactivation of both PFC and limbic networks.Citation19
How CBT may affect neural function in the anxiety disorders
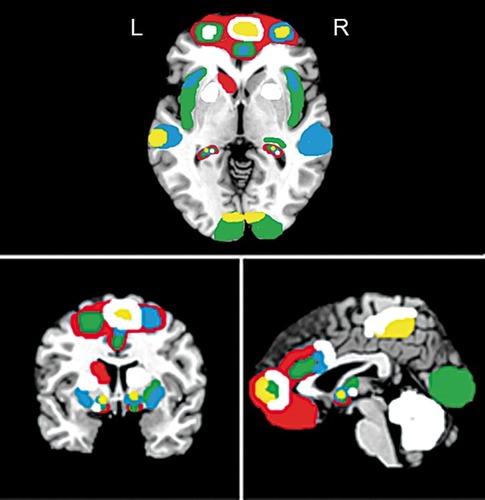
CBT is currently the most widely used and effective treatment for anxiety and related disorders,Citation60 although there are other effective treatments that will be mentioned below. An important outcome of CBT is improved emotional regulation.Citation61 Indeed, the data reviewed here suggest that neural circuitry underlying emotion processing and regulation (prefrontal cortex and limbic system) appears most sensitive to change following psychotherapy of anxiety and related disorders.Citation91 That said, in terms of neural outcomes of CBT treatment for the various anxiety and related disorders, while there are broad similarities, there are also discrete differences. For example, CBT for PTSD is associated with greater left dorsal striatal and frontal network activation during inhibitory control, which may indicate that improvements to the inhibitory control neural network contribute to better treatment response.Citation63 CBT for PD correlates with increased activation between the PFC and regions of the fear network, such as the amygdala and hippocampus, which is related to improved cognitive appraisal of emotional responses.Citation64,Citation65,Citation92 In those with GAD, CBT appears to increase PFC activation, which may in turn be more effective at modulating pretreatment amygdala, hippocampal and insula activation, to enable better processing of positive and negative emotional stimuli.Citation49,Citation68-Citation70 For those with SAD, CBT appears to increase PFC and occipito-temporal activation that coincides with alterations in limbic circuitry and improvements in cognitive appraisal and emotionality.Citation71-Citation74 Finally, in patients with OCD, reduced responses in limbic and other areas (including amygdala, striatum and ACC) appear to be most consistently observed following CBT, with some indication that PFC networks are strengthened.Citation54,Citation76-Citation79 However, it has also been suggested that excessive top-down control at the start of CBT may hinder the effects of treatment for OCD.Citation79 Some of these studies also suggest that pretreatment neural responses to disorder-specific stimuli maypredict the effectiveness of CBT, and these findings are briefly discussed below. Regional brain functional differences associated with changes following CBT in anxiety disorders are shown in .
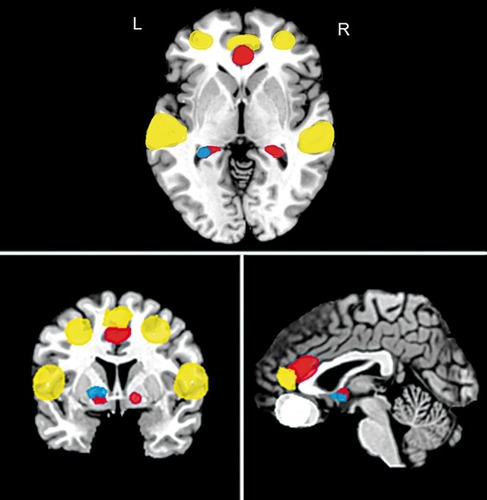
Pre-CBT neural predictors of outcome
In a study of the effects of CBT on PTSDCitation80 only half the sample (n=7) were treatment responders (eg, at least 50% improvement on symptom severity scores). Poor treatment response was associated with pretreatment increases in bilateral amygdala and ventral ACC. The authors suggest that this could be indicative of significant dysregulation of the limbic system due to dysfunction of the PFC circuitry, which may adversely affect treatment success. Conversely, in patients with GAD, left amygdala hyperactivation prior to treatment predicted a reduction in clinical severity scores following CBTCitation68 It was suggested that in those with GAD at least, a high level of emotional responsivity to disorder-salient stimuli at the start of treatment may help to drive the development of new cognitive strategies for the effective regulation of emotion after a course of CBT. In an fMRI study of SAD patients, increased pretreatment response to threatening faces in the superior and middle temporal gyrus, dorsal anterior cingulate cortex and dorsomedial prefrontal cortex predicted symptom reduction following CBT, and this may be indicative of an intact cognitive regulation PFC network that responds to treatment.Citation72 Neural activation during a reverse learning task in patients with OCD has also been associated with reduced OFC and striatal activation (caudate) prior to treatment.Citation76 PreCBT functional differences that may predict the effectiveness of treatment are illustrated in .
Other treatments and the effects on neural processing
According to a recent meta-analysis of treatment studies for anxiety disorders,Citation60 a range of effective pharmacotherapies and psychotherapies exist. These include mindfulness therapies, which aim to focus cognitions on the breath, self and body sensations; relaxation, individual CBT, group CBT, psychodynamic therapy that brings nonconscious thoughts and feelings to the fore; therapies without face-to-face contact (eg, Internet therapies), eye movement desensitization reprocessing (EMDR) that uses eye movement to mimic sleep rhythms to reset emotional responses, and interpersonal therapy.
In terms of other psychotherapies, 8 weeks' mindfulness-based stress reduction in GAD patients appears to reduce hyperactivity in the amygdala, and increase VLPFC activation, which may suggest that mindfulness promotes regulation of emotion, or reduction of emotional reactivity, as well as strengthening cognitive control of emotion in patients with GAD.Citation95 Mindfulnessbased therapy in patients with SAD is associated with improved self-esteem, more positive self- appraisal, lowered anxiety and increased prefrontal cortex activation to emotional stimuli.Citation96 Internet-based CBT for patients with SAD is associated with a reduction in amygdala reactivity to emotional faces after treatment, as well as increased connectivity between the prefrontal cortex, ACC, and amygdala that corresponds to reductions in self-reported clinical symptoms and lack of relapse 1 year later.Citation97,Citation98
The relationship between genetic variants and neurocircuitry of CBT treatment
The effects of psychotherapy for anxiety and related disorders are likely to be influenced by the relationship between gene variants and neural circuitry pertaining to emotion regulation, however, more research needs to be conducted in this area. In one study, long alleles of the monoamine oxidase A (MAOA) gene in the uVNTR promoter polymorphism region were linked to PD symptoms.Citation99 Those patients with the protective short alleles of the MAOA polymorphism had increased anterior cingulate cortex activation during fear conditioning in the presence of fearful stimuli at the beginning of CBT, and greater inferior parietal cortex activation after CBT In another study of PD patients, inhibitory ACC-amygdala coupling during fear conditioning was characteristic of treatment response and was associated with presence of the L/L genotype of the serotonin transporter gene SLC6A4(5-HTTLPR)Citation64,Citation100. Another study of PD patients showed inhibitory ACC-amygdala coupling during fear conditioning that had previously been shown to characterize treatment response in this sample was driven by CBT responders with the L/L genotype of the serotonin transporter gene.Citation101 Thus, there is growing evidence that genetic variation may be associated with specific alterations of neural responses during CBT of anxiety and related disorders.
Limitations and future directions
While there has been progress in knowledge of how psychotherapy for anxiety and related disorders impacts neural function, particularly in networks pertaining to cognitive regulation of emotion, there is still wide heterogeneity in fMRI studies, which prevents the drawing of firm conclusions. For example, fMRI studies typically utilize different paradigms, including fear conditioning, reverse learning, reappraisal, fear extinction, symptom provocation paradigms with anxiety-relevant stimuli and cognitive tasks (eg, response inhibition and reverse learning tasks such as Go/No Go and Flanker Tasks). On the one hand, such an approach is consistent with an emphasis on endophenotypes that are relevant across the anxiety and related disorders, and on the other hand there is also a need to clearly relate imaging work to the specific range of symptoms seen in the anxiety and related disorders. Although the field has progressed, additional studies are clearly needed. While it is possible that brain-imaging technologiesCitation102 will eventually generate single patient level predictions to guide clinical decision-making,Citation66 we are currently far from that point. In the interim, work on the neurocircuitry of psychotherapy has made an important contribution to the consolidation of both neurobiological models of anxiety and related disorders, as well as models of the mechanisms of treatment intervention.
DJS supported by the Medical Research Council (MRC) South Africa.
REFERENCES
- MorrisonFG.ResslerKJ.From the neurobiology of extinction to improved clinical treatments.Depress Anxiety.201431427929024254958
- GraybielAM.RauchSL.Toward a neurobiology of obsessive-compulsive disorder.Neuron.200028234334711144344
- EtkinA.WagerTD.Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia.Am J Psychiatry.2007164101476148817898336
- RaduaJ.van den HeuvelOA.SurguladzeS.Mataix-ColsD.Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders.Arch Gen Psychiatry.201067770171120603451
- SteinDJ.Advances in the understanding the anxiety disorders: The cognitive-affective neuroscience of 'False Alarms'Ann Clin Psychiatry200618373182
- LeDouxJ.Fear and the brain: where have we been, and where are we going?Biol Psychiatry199644122912389861466
- LeDouxJ.The amygdala.Curr Biol.20071720R868R87417956742
- JenckF.MoreauJ-L.MartinJR.Dorsal periaductal gray-induced aversion as a stimulation of panic anxiety: elements of face and predictive validity.Psychiatry Res.1995571811917480384
- ViannaDML.Landeira-FernandezJ.BrandaoML.Dorsolateral and ventral regions of the periaqueductal gray matter are involved in distinct types of fear.Neurosci Biobehav Rev.20012571171911801296
- MusazziL.TreccaniG.PopoliM.Functional and structural remodeling of glutamate synapses in prefrontal and frontal cortex induced by behavioral stress.Front Psychiatry.20156:6011025653621
- NeesF.PohlackST.Functional MRI studies of the hippocampus.Front Neurol Neurosci.201434859424777133
- BaldiE.BucherelliC.Brain sites involved in fear memory reconsolidation and extinction of rodents.Neurosci Biobehav Rev.20155316019025887284
- ReusGZ.Dos SantosMA.AbelairaHM.QuevedoJ.Animal models of social anxiety disorder and their validity criteria.Life Sci.201411411325132362
- TseYC.MontoyaI.WongAS.et alA longitudinal study of stress-induced hippocampal volume changes in mice that are susceptible or resilient to chronic social defeat.Hippocampus.20142491120112824753271
- ShivelyCA.Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys.Biol Psychiatry.19984498828919807643
- SteinDJ.WesternbergHG.LiebowitzMR.Social anxiety disorder and generalized anxiety disorder: serotonergic and dopaminergic neurocircuitry,J Clin Psychiatry200263suppl121912027115
- NoonanMP.SalletJ.MarsRB.et alA neural circuit covarying with social hierarchy in macaques.PLoS Biol.2014129e100194025180883
- Camilla d'AngeloLS.EagleDM.GrantJE.FinebergNA.RobbinsTW.ChamberlainSR.Animal models of obsessive-compulsive spectrum disorders.CNS Spectr.2014191284924093759
- AhmariSE.DoughertyDD.Dissecting OCD circuits: from animal models to targeted treatments.Depress Anxiety.201532855056225952989
- MartinLJ.SpicerDM.LewisMH.GluckJP.CorkLC.Social deprivation of infant rhesus monkeys alters the chemoarchitecture of the brain: I. Subcortical regions.J Neurosci.19911111334433581682426
- GremelCM.CostaRM.Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions.Nat Commun.20134226423921250
- BurguièreE.MonteiroP.FengG.GraybielAM.Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors.Science.201334061371243124623744950
- AhmariSE.SpellmanT.DouglassNL.et alRepeated corticostriatal stimulation generates persistent OCD-like behavior.Science.20133401234123923744948
- MiladMR.RauchSL.Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways.Trends Cogn Sci.2012161435122138231
- RauchSL.ShinLM.PhelpsEA.Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research-past, present, and future.Biol Psychiatry.200660437638216919525
- SteinDJ.AryaM.PietriniP.RapoportJL.SwedoSE.Neurocircuitry of disgust and anxiety in obsessive-compulsive disorder: a positron emission tomography study.Metab Brain Dis.2006212-3255265
- GasquoinePG.Contributions of the insula to cognition and emotion.Neuropsychol Rev.2014242778724442602
- PaulusMP.SteinMB.An insular view of anxiety.Biol Psychiatry200660438338716780813
- PaulusMP.SteinMB.Interoception in anxiety and depression.Brain Struct Funct.20102145-645146320490545
- SteinDJ.Emotional regulation: implications for the psychobiology of psychotherapy.CNS Spectr.200813319519818323752
- HolzschneiderK.MulertC.Neuroimaging in anxiety disorders.Dialogues Clin Neurosci.201113445346122275850
- WaitesAB.StanislavskyA.AbbottDF.et alEffect of prior cognitive state on resting state networks measured with functional connectivity.Hum Brain Mapp.2005241596815382248
- PetersonA.ThomeJ.FrewenP.LaniusRA.Resting-state neuroimaging studies: a new way of identifying differences and similarities among the anxiety disorders?Can J Psych.2014596294300
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders.5th ed. Washington, DC: American Psychiatric Association Press2013
- MichopoulosV.NorrholmSD.JovanovicT.Diagnostic biomarkers for posttraumatic stress disorder: promising horizons from translational neuroscience research.Biol Psychiatry.201578534435325727177
- ProtopopescuX.PanH.TuescherO.et alDifferential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects.Biol Psychiatry.200557546447315737660
- ShinLM.WrightCI.CannistraroPA.et alA functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder.Arch Gen Psychiatry.200562327328115753240
- StevensJS.JovanovicT.FaniN.et alDisrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder.J Psychiatr Res.201347101469147823827769
- O'DohertyDC.ChittyKM.SaddiquiS.BennettMR.LagopoulosJ.A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder.Psychiatry Res.2015232113325735885
- CarrionVG.GarrettA.MenonV.WeemsCF.ReissAL.Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth.Depress Anxiety.200825651452617598145
- JovanovicT.ElyT.FaniN.et alReduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample.Cortex.20134971884189123020899
- BremnerJD.ElzingaB.SchmahlC.VermettenE.Structural and functional plasticity of the human brain in posttraumatic stress disorder.Prog Brain Res.200816717118618037014
- AdmonR.MiladMR.HendlerT.A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities.Trends Cogn Sci.201317733734723768722
- GeigerMJ.NeufangS.SteinDJ.DomschkeK.Arousal and the attentional network in panic disorder.Hum Psychopharmacol.2014 659960325311787
- GormanJM.HirschfeldRM.NinanPT.New developments in the neurobiological basis of anxiety disorders.Psychopharmacol Bull.200236suppl 2496712490823
- SantosM.D'AmicoD.DierssenM.From neural to genetic substrates of panic disorder: Insights from human and mouse studies.Eur J Pharmacol.201575912714125818748
- MochcovitchMD.da Rocha FreireRC.GarciaRF.NardiAE.A systematic review of fMRI studies in generalized anxiety disorder: evaluating its neural and cognitive basis.J Affect Disord.201416733634225020268
- BeharE.DiMarcoID.HeklerEB.MohlmanJ.StaplesAM.Current theoretical models of generalized anxiety disorder (GAD): conceptual review and treatment implications.J Anxiety Disord.20092381011102319700258
- BallTM.SteinMB.PaulusMP.Toward the application of functional neuroimaging to individualized treatment for anxiety and depression.Depress Anxiety.2013311192093325407582
- Freitas-FerrariMC.HallakJE.TrzesniakC.et alNeuroimaging in social anxiety disorder: a systematic review of the literature.Prog Neuropsychopharmacol Biol Psychiatry.201034456558020206659
- HattinghCJ.IpserJ.TrampSA.et alFunctional magnetic resonance imaging during emotion recognition in social anxiety disorder: an activation likelihood meta-analysis.Front Hum Neurosci.2013634723335892
- MarazzitiD.AbelliM.BaroniS.CarpitaB.RamacciottiCE.Dell'OssoL.Neurobiological correlates of social anxiety disorder: an update.CNS Spectr.201520210011124571962
- BinelliC.SubiràS.BatallaA.et alCommon and distinct neural correlates of facial emotion processing in social anxiety disorder and Williams syndrome: A systematic review and voxel-based meta-analysis of functional resonance imaging studies.Neuropsychologia.201464C20521725194208
- Jr BaxterLR.SaxenaS.BrodyAL.et alBrain mediation of obsessive-compulsive disorder symptoms: evidence from functional brain imaging studies in the human and nonhuman primate.Semin Clin Neuropsychiatry.199611324710229782
- PennartzCM.BerkeJD.GraybielAM.et alCorticostriatal interactions during learning, memory processing, and decision making.J Neurosci.200929128311283819828796
- de WitSJ.AlonsoP.SchwerenL.et alMulticenter voxel based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder.Am J Psychiatry201417134034924220667
- PaulsDL.AbramovitchA.RauchSL.GellerDA.Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective.Nat Rev Neurosci.201415641042424840803
- SnyderHR.KaiserRH.WarrenSL.HellerW.Obsessive-compulsive disorder is associated with broad impairments in executive function: A meta-analysis.Clin Psychol Sci.20153230133025755918
- NakaoT.OkadaK.KanbaS.Neurobiological model of obsessive-compulsive disorder: evidence from recent neuropsychological and neuroimaging findings.Psychiatry Clin Neurosci.201468858760524762196
- BandelowB.ReittM.RöverC.MichaelisS.GörlichY.WedekindD.Efficacy of treatments for anxiety disorders: a meta-analysis.Int Clin Psychopharmacol.201513018319225932596
- MessinaI.SambinM.PalmieriA.VivianiR.Neural correlates of psychotherapy in anxiety and depression: a meta-analysis.PLoS One.201389e7465724040309
- BryantRA.FelminghamK.KempA.et alAmygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder.Psychol Med.200838455556118005496
- FalconerE.AllenA.FelminghamKL.WilliamsLM.BryantRA.Inhibitory neural activity predicts response to cognitive-behavioral therapy for posttraumatic stress disorder.J Clin Psychiatry.201374989590124107763
- KircherT.AroltV.JansenA.et alEffect of cognitive-behavioral therapy on neural correlates of fear conditioning in panic disorder.Biol Psychiatry.20137319310122921454
- LuekenU.StraubeB.KonradC.et alNeural substrates of treatment response to cognitive-behavioral therapy in panic disorder with agoraphobia.Am J Psychiatry.2013170111345135523982225
- BallTM.SteinMB.RamsawhHJ.Campbell-SillsL.PaulusMP.Single-subject anxiety treatment outcome prediction using functional neuroimaging.Neuropsychopharmacology.20143951254126124270731
- HaanED.WoltersLH.Behandeling van dedwangstoomis bij kinderen en adolescenten, Bedwing jedwang.Houten, the Netherlands: Bohn Stafleu van Loghum 2009
- McClureEB.AdlerA.MonkCS.et alfMRI predictors of treatment outcome in pediatric anxiety disorders.Psychopharmacology (Berl).200719119710516972100
- MaslowskyJ.MoggK.BradleyBP.et alA preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder.J Child Adolesc Psychopharmacol.201020210511120415605
- FonzoGA.RamsawhHJ.FlaganTM.et alCognitive-behavioral therapy for generalized anxiety disorder is associated with attenuation of limbic activation to threat-related facial emotions.J Affect Disord.2014169768525171782
- DoehrmannO.GhoshSS.PolliFE.et alPredicting treatment response in social anxiety disorder from functional magnetic resonance imaging.JAMA Psychiatry.2013701879722945462
- KlumppH1.FitzgeraldDA.PhanKL.Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder.Prog Neuropsychopharmacol Biol Psychiatry.201345839123665375
- GoldinPR.ZivM.JazaieriH.HahnK.HeimbergR.GrossJJ.Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial.JAMA Psychiatry.201370101048105623945981
- GoldinPR.ZivM.JazaieriH.WeeksJ.HeimbergRG.GrossJJ.Impact of cognitive-behavioral therapy for social anxiety disorder on the neural bases of emotional reactivity to and regulation of social evaluation.Behav Res Ther.2014629710625193002
- NakaoT.NakagawaA.YoshiuraT.et alBrain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study.Biol Psychiatry.200557890191015820711
- FreyerT.KlöppelS.TüscherO.et alFrontostriatal activation in patients with obsessive-compulsive disorder before and after cognitive behavioral therapy.Psychol Med.201141120721620236568
- HuyserC.VeltmanDJ.WoltersLH.de HaanE.BoerF.Developmental aspects of error and high-conflict-related brain activity in pediatric obsessive-compulsive disorder: a fMRI study with a Flanker task before and after CBT.J Child Psychol Psychiatry.201152121251126021793825
- MorgièveM.N'DiayeK.HaynesWl.et alDynamics of psychotherapyrelated cerebral haemodynamic changes in obsessive compulsive disorder using a personalized exposure task in functional magnetic resonance imaging.Psychol Med.20144471461147324001313
- OlatunjiBO.Ferreira-GarciaR.CaserasX.et alPredicting response to cognitive behavioral therapy in contamination-based obsessive-compulsive disorder from functional magnetic resonance imaging.Psychol Med.201312113
- BryantRA.MouldsML.GuthrieRM.DangST.NixonRDV.Imaginal exposure alone and imaginal exposure with cognitive restructuring in treatment of posttraumatic stress disorder.J Consult Clin Psychol.20037170671212924676
- GlosterAT.WittchenHU.EinsleF.et alMechanism of action in CBT (MAC): methods of a multi-center randomized controlled trial in 369 patients with panic disorder and agoraphobia.Eur Arch Psychiatry Clin Neurosci.2009259155166
- GlosterAT.WittchenHU.EinsleF.et alPsychological treatment for panic disorder with agoraphobia: a randomized controlled trial to examine the role of therapist-guided exposure in situ in CBT.J Consult Clin Psychol20117940642021534651
- CraskeMG.RoseRD.LangA.et alComputer-assisted delivery of cognitive behavioral therapy for anxiety disorders in primary-care settings.Depress Anxiety.20092623524219212970
- BeidelDC.TurnerSM.MorrisTL.Behavioral treatment of childhood social phobia.J Consult Clin Psychol.20006861072108011142541
- KendallPC.HedtkeKA.Cognitive-Behavioral Therapy for Anxious Children: Therapist Manual. 3rd ed. Ardmore, PA: Workbook Publishing, Inc 2006
- HopeDA.HeimbergRG.TurkCL.Managing Social Anxiety: a Cognitive-Behavioral Therapy Approach. New York, NY: Oxford University Press. 2006
- HohagenF.WinkelmannG.Rasche-RuchleH.et alCombination of behaviour therapy with fluvoxamine in comparison with behaviour therapy and placebo. Results of a multicentre study.Br J Psychiatry1998 suppl 357178
- BouvardM.Obsessive Compulsive Disorder Principles, Therapies, Applications. Paris, France: Masson: 2006 [In French]
- KoranLM.HannaGL.HollanderE.NestadtG.SimpsonHB.American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder.Am J Psychiatry200716455317849776
- SalkovskisPM.Understanding and treating obsessive-compulsive disorder.Behav Res Ther.199937S29S5210402695
- FrewenPA.DozoisDJ.LaniusRA.Neuroimaging studies of psychological interventions for mood and anxiety disorders: Empirical and methodological review.Clin Psychol Rev.20082822824617602811
- HahnT.KircherT.StraubeB.et alPredicting treatment response to cognitive behavioral therapy in panic disorder with agoraphobia by integrating local neural information.JAMA Psychiatry.2015721687425409415
- BurghardtNS.BauerEP.Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: implications for underlying fear circuits.Neuroscience.201324725327223732229
- ShinDJ.JungWH.HeY.et alThe effects of pharmacological treatment on functional brain connectome in obsessive-compulsive disorder.Biol Psychiatry.201475860661424099506
- HölzelBK.HogeEA.GreveDN.et alNeural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training.Neuroimage Clin.2013244845824179799
- GoldinP.ZivM.JazaieriH.HahnK.GrossJJ.MBSR vs aerobic exercise in social anxiety: fMRI of emotion regulation of negative self-beliefs.Soc Cogn Affect Neurosci.201381657222586252
- MånssonKN.CarlbringP.FrickA.et alAltered neural correlates of affective processing after internet-delivered cognitive behavior therapy for social anxiety disorder.Psychiatry Res.2013214322923724064198
- MånssonKN.FrickA.BoraxbekkCJ.et alPredicting long-term outcome of Internet-delivered cognitive behavior therapy for social anxiety disorder using fMRI and support vector machine learning.Transl Psychiatry.2015175:e530
- ReifA.RichterJ.StraubeB.et alMAOA and mechanisms of panic disorder revisited: from bench to molecular psychotherapy.Mol Psychiatry.201419112212823319006
- de CarvalhoMR.DiasGP.CosciF.et alCurrent findings of fMRI in panic disorder: contributions for the fear neurocircuitry and CBT effects.Expert Rev Neurother.201010229130320136384
- LuekenU.StraubeB.WittchenHU.et alTherapy genetics: anterior cingulate cortex-amygdala coupling is associated with 5-HTTLPR and treatment response in panic disorder with agoraphobia.J Neural Transm.2015122113514425223844
- EtkinA.PittengerC.PolanHJ.KandelER.Toward a neurobiology of psychotherapy: Basic science and clinical applications.J Neuropsych Clin Neurosci.2005172145158
