Abstract
Trauma-related disorders, such as posttraumatic stress disorder (PTSD) are remarkably common and debilitating, and are often characterized by dysregulated threat responses. Across numerous epidemiological studies, females have been found to have an approximately twofold increased risk for PTSD and other stress-related disorders. Understanding the biological mechanisms of this differential risk is of critical importance. Recent data suggest that the pituitary adenylate cyclase-activating polypeptide (PACAP) pathway is a critical regulator of the stress response across species. Moreover, increasing evidence suggests that this pathway is regulated by both stress and estrogen modulation and may provide an important window into understanding mechanisms of sex differences in the stress response. We have recently shown that PACAP and its receptor (PAC1R) are critical mediators of abnormal processes after psychological trauma. Notably, in heavily traumatized human subjects, there appears to be a robust sex-specific association of PACAP blood levels and PAC1R gene variants with fear physiology, PTSD diagnosis, and symptoms, specifically in females. The sex-specific association occurs within a single-nucleotide polymorphism (rs2267735) that resides in a putative estrogen response element involved in PAC1R gene regulation. Complementing these human data, the PAC1R messenger RNA is induced with fear conditioning or estrogen replacement in rodent models. These data suggest that perturbations in the PACAP-PAC1R pathway are regulated by estrogen and are involved in abnormal fear responses underlying PTSD.
Los trastornos relacionados con el trauma, como el trastorno por estrés postraumático (TEPT) en humanos, son extraordinariamente comunes y desgastadores, y a menudo están caracterizados por respuestas desreguladas a la amenaza. En numerosos estudios epidemiológicos se ha encontrado que las mujeres tienen un riesgo aumentado al doble para el TEPT y otros trastornos relacionados con el estrés. La comprensión de los mecanismos biológicos de este riesgo diferencial es de gran importancia. Hay datos recientes que sugieren que, a través de las especies, la vía del polipéptido activador de la adenilato ciclasa hipofisiaria (PAACH) tiene una regulación central en la respuesta de estrés. Sin embargo, hay evidencia creciente que sugiere que esta vía está regulada por el estrés y la modulación estrogénica y puede aportar una ventana importante para la comprensión de los mecanismos de las diferencias por sexo en la respuesta de estrés. Nosotros hemos mostrado recientemente que el PAACH y su receptor (R1PAC) son importantes mediadores de los procesos anormales después del trauma psicológico. Es notable que, específicamente en mujeres víctimas de grandes traumas, al parecer hay una potente asociación específica entre los niveles sanguíneos de PAACH y variantes del gen R1PAC con la fisiología del miedo, y síntomas y diagnóstico de TEPT. La asociación específica con el sexo ocurre con el polimorfismo del nucleótido único (rs2267735) que se encuentra en un elemento de la respuesta putativa de estrógeno involucrada en la regulación del gen R1PAC. Como complemento a estos datos humanos, en modelos de roedores el ARN mensajero del R1PAC está inducido por el condicionamiento al miedo o por el reemplazo de estrógenos. Estos datos sugieren que las perturbaciones en la vía del PACCH-R1PAC están reguladas por estrógenos y participan en las respuestas anormales al miedo del TEPT.
Les troubles liés aux traumatismes, comme les troubles du stress post-traumatique (TSPT) chez les humains, sont extrêmement courants et invalidants et souvent caractérisés par une dérégulation des réponses à la menace. De nombreuses études épidémiologiques indiquent que les femmes ont environ deux fois plus de risque de TSPT et d'autres troubles liés au stress. La compréhension des mécanismes biologiques de ce risque différentiel est d'une importance essentielle. Selon des données récentes, la voie du PACAP (pituitary adenylate cyclase-activating polypeptide) est un régulateur capital de la réponse au stress commun à l'ensemble des espèces. De plus, il existe des preuves croissantes de la régulation de ces voies par la modulation à la fois du stress et des estrogènes et de leur apport d'une ouverture importante dans la compréhension du mécanisme des différences selon le sexe dans la réponse au stress. Nous avons récemment montré que la PACAP et son récepteur (PAC1R) sont des médiateurs essentiels des processus anormaux après un traumatisme psychologique. Il semble y avoir, en particulier chez les humains fortement traumatisés, une importante association des concentrations sanguines de PACAP et des variants du gène PAS1R spécifiques du sexe avec la physiologie de la peur, le diagnostic de TSPT et des symptômes, en particulier chez les femmes. L'association spécifique au sexe est liée à un polymorphisme nucléotidique (rs2267735) localisé dans une séquence d'ADN supposée être un élément de réponse à l'estrogène impliqué dans la régulation du gène PAC1R. Pour compléter ces données humaines, l'ARN messager du PAC1R est induit avec le conditionnement à la peur ou le remplacement des estrogènes dans des modèles murins. Ces données suggèrent que les perturbations dans la voie du PACAP-PAC1R sont régulées par les estrogènes et impliquées dans les réponses anormales à la peur sous-tendant le TSPT.
Introduction
Of the trauma-and stress-related disorders, posttraumatic stress disorder (PTSD) is the most prevalent and well-understood. It is triggered by exposure to acute or reccurring traumatic events and characterized by four distinct symptom clusters: intrusive re-experiencing, avoidance, hyperarousal, as well as negative cognitions and mood (Diagnostic and Statistical Manual of Mental Disorders, 5th edition [DSM-5]). Accordingly, the major features of PTSD reflect deficits in stress-and fear memory-related mechanisms. In comparison with other trauma-related disorders, the epidemiological patterns of PTSD are fascinating—60% to 90% of Americans are exposed to PTSD risk factors, yet PTSD only manifests in 8% to 12% of the general population.Citation1 Given the low disease penetrance, despite frequent exposure to environmental risk factors, it is both surprising and alarming that adult females exhibit a twofold higher lifetime prevalence of PTSD and greater symptom severity than males and prepubescent females.Citation2-Citation5 This incongruity within PTSD's broader disease profile highlights the following: interindividual variability significantly negotiates PTSD risk, and one such variability, sex, differentially influences PTSD susceptibility, potentially via sex-dependent risk factors.Citation6,Citation7
Many studies suggest that complex interactions between biological and environmental risk factors generate changes within neural substrates that regulate biological responsivity to trauma exposure.Citation6 These intermediate phenotypes, in turn, promote maladaptive behavioral changes that manifest as PTSD-associated symptom clusters. Until recently, an overreliance on male cohorts in preclinical and clinical investigations has limited the field's understanding of how biological and environmental risk factors shape PTSD vulnerability in a sex-dependent manner. However, a recent convergence of studies implicates the pituitary adenylate cyclase-activating polypeptide (PACAP)ergic system, a fear- and stress-regulating biological system, in shaping sex-dependent PTSD risk. These studies have provided insights into sex-specific biological and environmental sculptors of PTSD susceptibility, as well as predictive PTSD biomarkers that may be potentially used to more effectively diagnose and treat female PTSD patients. Below, we will examine the intersection between sex-specific PTSD risk factors and the PACAPergic system to determine how the two collaborate to differentially shape PTSD susceptibility in females. In doing so, we will demonstrate the interconnectivity of biological and environmental risk factors and how this intersection influences sex-specific PTSD etiology and symptomatology.Citation8
Introduction to the PACAPergic system
The PACAPergic system is a highly conserved biological system comprised of several polypeptides of varying sizes, owing to posttranslational proteolytic processing of the PACAP precursor protein that is encoded by the adenylate cyclase-activating polypeptide 1 gene (ADCYAP1). Citation9,Citation10 Two polypeptides, a 27- and a 38-aminoacid peptide (PACAP27 and PACAP38, respectively), are the most abundantly expressed and biologically active in central nervous system and peripheral tissues, with PACAP38 being tenfold to 100-fold more abundant than PACAP27.Citation11 Subsequent to the isolation of PACAP, its three cognate receptors were identified and found to be highly expressed in the periphery and the brain.Citation12 Of the three receptors, only the PACAP type 1 receptor (PAC1R) selectively binds PACAP27 and PACAP38 with high affinity.Citation13
The PAC1R is a G-protein coupled receptor which, upon PACAP binding, recruits either Gαs and/or Gαq - coupled second-messenger pathways.Citation10,Citation14 Alternative splice events in the N-terminal and third intracellular loop of the PAC1R transcript diversify PAC1R properties, such as ligand affinity and coupling to second-messenger pathways in the central nervous system.Citation15,Citation16 These diverse second-messenger pathways engage intracellular downstream effectors with pleiotropic consequences, such as activity-dependent structural and functional plasticity within neuroendocrine circuits that control stress and fear processes.Citation14,Citation17-Citation19 Supportive of this are early examinations of rodent PACAP38 and PAC1R transcript and protein expression patterns which show both being highly expressed within brain regions known to subserve stress and fear processes.Citation11
Estrogenic regulation of the PACAPergic system
It is also important to note that ADCYAP1R1 (the PAC1R gene) contains several predicted estrogen response elements (EREs).Citation20 EREs are consensus sequences within the promoter region of target genes to which activated estrogen receptors directly bind to regulate gene transcription.Citation21-Citation23 This is important to note because levels of estradiol, one of the predominant gonadal hormones in adult females, vacillate across the female reproductive cycle. Thus, in sexually mature females, PAC1R expression and PAC1R-associated functions are potentially regulated in an estrogen-dependent manner. Indeed, long-term estradiol treatment of ovariectomized female rats, via continuous release estrogen pellets, has been shown to increase ADCYAP1R1 and ADCYAP1 transcript expression within the bed nucleus of the stria terminalis (BNST).Citation20 In part, these results parallel earlier studies showing that brain ADCYAP1 transcript levels vary across the rat estrous cycle with the greatest change occurring during proestrusCitation24—a period characterized by peak gonadal hormones, including estradiol.Citation25 Overall, these data suggest that the expression of PACAP-PAC1Rs is dynamically regulated by cycling levels of estradiol, and this dependency may confer sex-specific PACAP-PAC1R functional consequences, particularly within neural circuits of fear and stress.
PACAP-PAC1R and the stress response
Collectively, rodent studies indicate that the PACAP-ergic system is a master regulator of both central and peripheral stress responses after threat exposure.Citation26 Anatomically, PACAP and PAC1R immunoreactive cells are present within stress-regulating brain regions.Citation27-Citation29 Furthermore, the functional consequences of this expression pattern is notable after chronic stress exposure, which results in elevated PACAP and PAC1R transcript expression in these brain regions.Citation30 Similarly, other studies demonstrate that increasing central PACAP levels, via intracerebroventricular injections in behaviorally naive rats, enhance physiological hallmarks of stress, such as increased glucocorticoid releaseCitation31 and sympathetic activation.Citation32,Citation33 Parallel to these results, global loss of the PACAP or PAC1Rs attenuates normal molecular, physiological, and behavioral consequences of environmental stressors,Citation34 whereas manipulations of PACAP levels or PAC1R activity, specifically within stress-responsive brain regions, are accompanied by enhancement of stress-related behavioral phenotypes, such as startle responsesCitation27,Citation30 and anxiety-like behaviors.Citation27,Citation33,Citation35,Citation36 These data not only support the role of the PACAPergic system in stress-response mechanisms, but also suggest that PACAP-PAC1R interactions are upstream of many well-known stress mediators. Altogether, these experiments demonstrate that PACAP-PAC1R functions, particularly within stress-regulating neural substrates, are principal facilitators of molecular, physiological, and behavioral consequences of stress exposure.Citation36,Citation37
Sex-dependency of PACAP-PAC1R regulation of stress responsivity
There are few studies that have examined whether PACAP-PAC1R regulation of the stress response exhibits sex dependency. Of those, there appears to be no influence of the primary gonadal hormone in females, estradiol, on PACAP recruitment of corticosterone. However, these experiments were carried out in ovariectomized ratsCitation38; therefore, additional studies, particularly in naturally cycling rodents, may offer greater translational insight into the relationship between sex and PACAP-PAC1R regulation of stress responsivity. The data discussed in the previous paragraph strongly argue that PACAP-PAC1R functions within stress-regulating brain regions—such as the amygdala, hippocampus, prefrontal cortex, and BNSTCitation39-Citation41—play an important role in stress responsivity to environmental threats. Notably, these brain regions are sexually dimorphic, abnormal in PTSD cohorts, and under significant gonadal hormone control,Citation42,Citation43 implying that within the sex-dependent constraints of these stress-regulating circuits, PACAP-PAC1R actions, or lack thereof, may confer differential sex-dependent vulnerabilities to stress-related pathologies such as PTSD, as discussed further below.
Fear regulation as a biomarker of PTSD in humans
Patients with PTSD, as compared with trauma-exposed controls, also exhibit abnormally high conditioned fear responses,Citation44-Citation46 as well as deficits in fear inhibitionCitation47-Citation49 and extinction.Citation50 These pathological fear mechanisms are hypothesized to result from either one or a combination of the following dysregulated fear-memory processes: (i) an inability to habituate to aversive environmental stimuli; (ii) impaired capacity to inhibit or extinguish fear memory; and/or (iii) an overconsolidation of the initial fear memory. To examine these dysregulated fear mechanisms, Pavlovian fear conditioning and extinction or fear inhibition behavioral paradigms extremely valuable in dissecting the pathophysiology of PTSD in preclinical and clinical have been shown to be studies.Citation51-Citation53 More specifically, these paradigms permit the examination of species-specific indices of fear acquisition and inhibition long after the aversive stimuli is removed from the experimental environment.Citation54 Altogether, these data argue in favor of experimental models of fear learning, extinction, and inhibition as useful models for understanding PTSD pathophysiology.
Fear-memory processes and the PACAPergic system
Consistent with PACAP-PAC1R expression within neural substrates of fear-memory, functional and behavioral analyses show that PACAP-PAC1R actions also play a role in fear-memory mechanisms. Whereas global PAC1R loss, in rodent models, does not lead to deficits in declarative memory or cued-fear memory retrieval, significant deficits in contextual fear memory are observed,Citation55 thereby demonstrating that PAC1R signaling plays a critical role in hippocampal-dependent fear-memory circuits. Moreover, this deficiency in context-related associative learning is also accompanied by impaired long-term synaptic plasticity in the hippocampusCitation55 —a relatively long-lasting functional change in synaptic strength that is thought to represent the cellular basis for fear-memory consolidation.Citation56 Similarly, hippocampal- and amygdala-specific PAC1R pharmacological inhibition attenuate contextual fear-memory recall, albeit cued-fear-memory consolidation was not assessed in this study.Citation57 These experimental results suggest that PACAP-PAC1R actions are enlisted, within neural substrates of the fear-memory circuit, to promote contextual fear-learning mechanisms. Supportive of this conclusion are classical fear-conditioning studies, where a previously neutral tone paired with a series of foot shocks increased both PACAP and PAC1R transcripts in the amygdala, BNST, and prefrontal cortex of mice.Citation20 Furthermore, a positive correlation found between prefrontal cortex and amygdala PAC1R transcript levels and the degree of fear learning was also observed.Citation20 Altogether, these data argue that PACAP-PAC1R actions are engaged within fear-learning neural circuits to promote fear-memory consolidation and retrieval. Given the evidence of PACAP-PAC1R function as central to fear and stress processes and the deterioration of these processes in PTSD symptomatology,Citation58 the PACAP-PAC1R system has emerged as an attractive candidate in the examination of PTSD susceptibility.
Peripheral PACAP levels as a biomarker for sex-specific PTSD risk
As alluded to earlier, the hallmark features of PTSD symptom clusters are maladaptive stress responsivity and fear-memory processes after trauma exposureCitation58 (DSM-5). It is, therefore, unsurprising that many stress-and fear-regulating neurobiological systems, such as the PACAPergic system, are dysregulated in PTSD patients. Interestingly, dysfunctions in the PACAP-PAC1R system appear to uniquely facilitate PTSD vulnerability in sexually mature females rather than males. Relatively recently, our lab found that peripheral PACAP38 levels were positively correlated with total PTSD symptoms, specifically in adult female PTSD cohorts, with no association between peripheral PACAP38 and PTSD symptoms found in male PTSD counterparts despite males also exhibiting similarly high blood PACAP38 levels.Citation20 Furthermore, peripheral PACAP38 levels were also predictive of PTSD diagnosis specifically for female, but not male, PTSD cohorts. Although differently characterized in the DSM-IV and the DSM-5, the three primary symptom clusters of PTSD, ie, intrusive re-experiencing, avoidance, and hyperarousal, were also positively correlated with peripheral PACAP38 levels in females, with higher levels of PACAP38 predictive of these subscale symptoms specifically in female, but not male, PTSD cohorts.Citation20 These associations were replicated in a similar sample of highly traumatized adult females and remained statistically significant even when controlling for age, total trauma exposure, and other disorders often comorbid with PTSD, such as depression and substance abuse.Citation20 Furthermore, the association between peripheral PACAP38 levels and inhibition of conditioned fear was also examined. Females with elevated PACAP38 levels demonstrated impaired inhibition of conditioned fear, as exhibited by an inability to discriminate between the conditioned stimulus (danger cue) and unconditioned stimulus (safety cue).Citation20 Overall, these data suggest that blood PACAP38 levels are associated with PTSD symptoms, specifically in female cohorts.
Sex-specific genetic risk for PTSD
The significant influence of genetic factors in PTSD susceptibility is evident in twin studies, which compellingly demonstrate that genetic factors account for 30% to 70% of PTSD risk.Citation59 Intriguingly, this heritability of PTSD is higher in females than malesCitation6—evidence that indicates a higher contribution of heritability to PTSD vulnerability in females. Molecular studies have identified over 20 genetic polymorphisms that are more frequent in PTSD cohorts than controls (reviewed in Norrholm and ResslerCitation60). However, until recently, none of the 20 PTSD-associated genetic polymorphisms were associated with female-specific PTSD risk, despite females exhibiting greater PTSD heritability. This has made it difficult to examine how genetic variability interacts with environmental risk factors to modulate sex-specific PTSD risk.
PAC1R genetic variability as biomarker for female-specific PTSD risk
Taking into consideration the greater degree of heritability in PTSD vulnerability and the correlation between peripheral PACAP38 levels and PTSD symptomatology specifically in female PTSD cohorts, genetic polymorphisms in the PACAP and PAC1R loci (ADCYAP1 and ADCYAP1R1, respectively) were assessed to determine whether PACAP-PAC1R genetic variability is associated with PTSD diagnosis and symptoms, as well as physiological and psychological intermediate phenotypes in highly traumatized civilians. Using a tag-single-nucleotide-polymorphism (tag-SNP) approach, investigators examined 44 SNPs in ADCYAP1 and ADCYAP1R1. However, only one SNR rs2267735 in ADCYAP1R1 was found to be significantly associated with PTSD diagnosis specifically in female, but not male, PTSD subjects, including an additional cohort that served as replication source.Citation20 Although genetic variations in and around ADCYAP1R1 are implicated in other mental disorders, such as major depression and schizophrenia,Citation61,Citation62 this rs2267735 polymorphism was not associated with these and other severe psychiatric disorders, such as bipolar disorder and Alzheimer disease,Citation20 implying that rs2267735 may be specifically predictive of PTSD diagnosis in adult females.
We also explored whether the ADCYAP1R1 risk genotype was also predictive of PTSD symptomatology in a sex-specific manner. Moreover, analyses of the relationship between the ADCYAP1R1 risk allele and PTSD symptoms revealed a replicable, dose-dependent relationship between the risk allele and total PTSD symptoms, with female “CC” carriers demonstrating greater PTSD symptoms than “CG” or “GG” carriers.Citation20 Additional analyses revealed that physiological measures of stress and fear are also differentially associated with the ADCYAP1R1 SNR Even after controlling for trauma, age, and race, the ADCYAP1R “CC” genotype was strongly associated with higher levels of hyperarousal symptoms than those observed in “CG” or “GG” female carriers.Citation20 Furthermore, when we assessed physiological measures of fear, using fear-potentiated startle and dark-enhanced startle, female carriers of the “CC” genotype had impaired startle discrimination and enhanced fear responsivity, respectively, as compared with “CG” or “GG” female carriers. Intriguingly, both measures of fear responsivity are consistently impaired in PTSD cohorts.Citation63,Citation64
In further support of its contributions to sex-specific PTSD risk, rs2267735 was also located within a predicted ERE of the ADCYAP1R1 locus. Given the estrogen-dependency of ADCYAP1R1 transcript expressionCitation20 and evidence of estradiol-ERE interactions in gene-expression regulation,Citation21,Citation22,Citation65 we utilized a previously analyzed data set, with combined brain messenger RNA (mRNA) expression and genome-wide association data, to examine the functional implications of ADCY AP1R1 rs2267735 on central PAC1R transcript expression. Overall, these data show that females carrying the ADCYAP1R1 rs2267735 risk genotype (“CC”) expressed significantly less cortical ADCYAP1R1 mRNA than male “CC” carriers or females that did not carry the risk allele.Citation20 Although additional studies are warranted, these results suggest that the “CC” risk allele potentially impairs estrogen-dependent ADCYAP1R1 transcript expression, thus resulting in lower AD CYAP1R1 mRNA levels. Intriguingly, the sex-dependency of the ADCY-AP1R1 risk genotype does not manifest until puberty, consistent with a role for cycling estrogen in its regulation.Citation66 Indeed, the fearCitation67 stress-regulatingCitation68 and anxiolyticCitation69,Citation70 roles of testosterone may, in part, explain why the “CC” risk allele, though associated with pathological threat responsivity in both prepubescent males and females, is no longer predictive of PTSD diagnosis and physiological fear responses in postpubescent males.Citation20 Though additional studies are needed, these collective data suggest that impaired estradiol-ERE interactions, as a result of the “CC” risk allele, may act as one of the mechanisms through which sexually mature females gain greater PTSD vulnerability.
Neuroimaging profiles as intermediate phenotypes of sex-specific PTSD risk
As alluded to earlier, PTSD-implicated stress- and fear-neural circuits are also sexually dimorphic. For example, healthy males and females exhibit a number of sex differences in region-specific sizes and differential neural activation patterns during fear-learning tasksCitation53,Citation71,Citation72 and after exposure to stressors.Citation73 Interestingly, sex differences in PTSD diagnosis, symptom duration, symptom severity, and functional impairment emerge after adolescence, a period characterized by striking changes in cycling levels of gonadal hormones.Citation74,Citation75 The coincidental emergence of differential PTSD vulnerability alongside the onset of gonadal hormone cyclicityCitation52,Citation72 suggests that activational effects of gonadal hormones, within the confines of sexually dimorphic stress- and fear-neural circuits, lead to sex-dependent differences in trauma responsivity.
In light of this, it is also important to investigate intermediate phenotypes related to the ADCYAP1R1 SNP to further understand why this risk allele, in females, is predictive of exaggerated arousal responses characteristic of PTSD psychophysiology. To do this, we used functional magnetic resonance imaging (fMRI) analyses in a sample of adult females who carried or lacked the risk allele and who have experienced a moderate to high level of civilian traumaCitation76 in order to assess the consequence of the ADCYAP1R1 risk allele on amygdala and hippocampal activation, as well as the functional connectivity between the two. These brain regions were chosen as they are often implicated in PTSD pathophysiologyCitation77; furthermore, it is also known that PACAP and PAC1Rs exhibit high densityCitation12,Citation28 and facilitate synaptic plasticity,Citation78,Citation79 as well as additional biological functions within these brain regions.Citation80
This study found that in response to unconditioned fearful-face stimuli, females with “CC” risk alleles, as compared with females with the “GC” or “GG” nonrisk alleles, demonstrate increased activation of both the amygdala and hippocampus, as well as reduced functional connectivity between the two (). Citation76 These data are consistent with previous studies that show greater fear responsivity (ie, dark-enhanced startle) in females with the ADCYAP1R1 “CC” genotype than in “GC” or “CC” carriers.Citation20 Furthermore, these activational changes within fear-regulating neural circuits are characteristic of neurobiological profiles of PTSD,Citation81,Citation82 suggesting that the ADCYAP1R1 “CC” risk allele increases the PTSD vulnerability profile of female carriers by dysregulating neural circuits that subserve unconditioned fear responsivity.
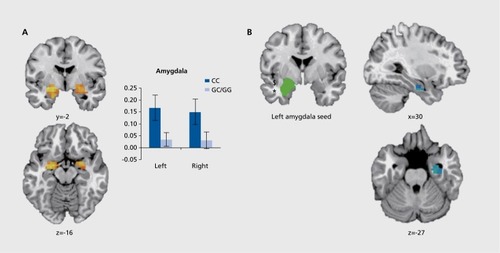
In comparison with unconditioned fear responses, fMRI studies revealed that contextual, but not cued, fear conditioning resulted in a gene-dose-dependent decrease in hippocampal activation in female carriers of the “C” PTSD risk allele during the late acquisition of contextual fear, with no differences found during habituation, early acquisition, or extinction.Citation83 Interestingly, these data parallel deficits in contextual, but not cued, fear learning found in mice with global PAC1R lossCitation84 and suggest that decreased ADCYAP1R1 mRNA expression, associated with the female-specific risk genotype,Citation20 attenuates hippocampal activation and, by extension, impairs contextual processing during fear learning. Indeed, PTSD cohorts exhibit impaired hippocampaldependent contextual fear conditioning.Citation85 Collectively, this work demonstrates genetic penetrance of the ADCYAP1R1 risk allele at the level of PTSD-implicated neural circuits which, in turn, manifest as intermediate phenotypes of the ADCYAP1R1 risk allele during unconditioned and conditioned fear.
Interactions between genetic and environmental risk factors in sex-specific PTSD risk
Twin studies provide compelling evidence that although genetic factors account for 30% to 70% of PTSD risk, the remaining variance in PTSD vulnerability is accounted for by individual-specific environmental exposure.Citation86 This level of specificity implies that trauma characteristics play an important role in shaping individual PTSD risk.Citation7 Experience of trauma, defined by the DSM-5 as having “experienced, witnessed, or [been] confronted with an event or events that involved actual or threatened death or serious injury, or a threat to the physical integrity of the self or others,” can be segregated into two broad categories. The first is systemic/neurogenic, which represents a homeostatic challenge or a physical stimulus that is recognized by somatic, visceral, or circumventricular sensory pathways and requires an immediate “systemic” reaction that is triggered by reflexive mechanisms.Citation87 The second category is processive, which is of psychological origin and which recruits brain regions involved in higher-order processing/decision making and represents responses mounted in anticipation of, rather than in reaction to, a homeostatic threat. This type of trauma is influenced by previous experiences (eg, associative learning) or species-specific predispositions (eg, the aversion to snakes in humans) and allows the organism to detect novel stimuli that predict sources of harm.Citation88 When activated by environmental threat, psychological trauma engages mediators in the corticolimbic circuitry that subserve decision making, learning, and memory, as well as emotionality. However, it is important to note that though both types of trauma theoretically recruit distinct neural circuits, at times, trauma types are not entirely separable, as physical stressors may, to varying degrees, have psychosocial facets and vice versa.Citation89 Nonetheless, these distinct differences in trauma features may confer differential interactions between environmental and genetic risk factors and, as a result, confer differential PTSD vulnerabilities.
Though the effects of trauma characteristics on sex-specific PTSD risk are not as clear, differences in PTSD vulnerability may, partly, be due to differences in specific trauma types to which each sex is exposed.Citation74,Citation90 Indeed, females experience higher rates of sexual traumaCitation91 than males, who in general experience higher rates of trauma,Citation92 as well as higher rates of nonsexual assaults and physical violence.Citation93 Thus, it is possible that males and females engage different trauma-related neural processes more frequently.
PACAP signaling is required for biological responsivity to psychological trauma
Given that females have a higher exposure rate to psychological trauma and are predisposed to PACAP-PAC1R PTSD risk factors, it is also interesting to note that, in rodent models, the trauma type (psychological vs physical) also appears to differentially recruit the PACAPergic system. This is partly demonstrated in transgenic mice that globally lack PACAP protein (PACAP-knockout mice). PACAP-knockout mice, as compared with controls, demonstrate blunted hypothalamic-pituitary-adrenal (HPA) axis activation and impaired stress-coupled molecular activity after psychological stressors, such as restraint stress,Citation34,Citation94 light exposure after constant dark,Citation95 or open field exposure.Citation94 Contrarily, global loss of PACAP does not impair HPA activation, as measured by plasma corticosterone levels, after systemic/neurogenic stressors, such as immune, metabolic, or cold-exposure challenges.Citation34,Citation36,Citation94 These data suggest that, unlike physical stressors, psychological stressors depend on PACAP signaling to recruit molecular and neuroendocrine processes. Though additional studies are needed, these data collectively indicate that the predominance of psychological stress exposure in female cohorts may lead to a greater dependence on PACAP-mediated biological responsivity in females than in males, after environmental trauma exposure. Thus, impairment of the PACAPergic system and, in turn, an inadequate stress response to environmental threats—a risk factor for PTSDCitation96,Citation97—will have greater pathological consequences for females.
There also appears to be a within-trauma-type effect of trauma persistence on PTSD vulnerability. In mice, the PACAP-dependence of HPA axis activation after psychological stressors is consistently greater for prolonged trauma than for acute psychological trauma.Citation34 In support of this, associations between the ADCYAP1R1 rs2267735 risk allele and childhood maltreatment—one of the most robust environmental risk factors for PTSD among trauma-exposed adultsCitation98,Citation99—were moderated by the number of exposures to childhood maltreatment (CM) in female PTSD, but not depression, cohorts.Citation100 Moreover, CM-exposed females carrying one or more of the ADCYAP1R1 “C” alleles showed increased risk for PTSD, as well as greater PTSD severity.Citation100 These data suggest a within-trauma-type effect of the PACAPergic system after psychological trauma.
Conclusions
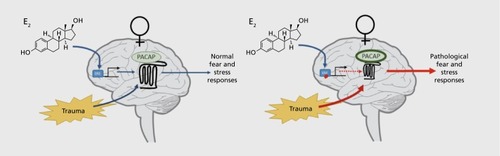
The PACAP and PAC1R systems serve as a system-wide mediator of stress responses. Convergent animal and human data suggest a robust role for this system within neural nodes that regulate stress and fear processes elicited by trauma exposures. Furthermore, data from multiple sources now suggest a role for dysregulated PACAP-PAC1R in PTSD. Notably, in humans, this effect seems most robust in females and increasing data suggest that this is due to estrogenic regulation of the ADCYAP1R1 gene in combination with the sex-specific dominance of trauma type recruiting stress and fear processes (). There are also compelling data for significant interactions between the ADCYAP1R1 risk allele and persistence of childhood maltreatment in female PTSD cohorts. These data indicate that trauma amount differentially interacts with PAC1R genetic risk factors and may reflect a greater impact of PAC1R dysfunction under conditions where its recruitment is crucial for effective physiological and psychological responses to trauma. Much is yet to be learned about the role of sex biology, estrogen regulation, and PACAP-PAC1R functions, but these may make up one of the more robust “sex x environment interactions” models in understanding brain function related to sex, trauma, and PTSD.
Research in the author's laboratories is supported by funds from R01 MH096764 and R01 MH108665. The authors have no conflicts of interest to disclose.
REFERENCES
- BangasserDA.ValentinoRJ.Sex differences in stress-related psychiatric disorders: neurobiological perspectives.Front Neuroendocrinol.201435330331924726661
- BreslauN.DavisGC.PetersonEL.SchultzL.Psychiatric sequelae of posttraumatic stress disorder in women.Arch Gen Psychiatry.199754181879006404
- AlimTN.GravesE.MellmanTA.et alTrauma exposure, posttraumatic stress disorder and depression in an African- American primary care population.J Natl Med Assoc.200698101630163617052054
- ResnickHS.KilpatrickDG.DanskyBS.SaundersBE.BestCL.Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women.J Consult Clin Psychol.19936169849918113499
- PynoosRS.FrederickC.NaderK.et alLife threat and posttraumatic stress in school-age children.Arch Gen Psychiatry.19874412105710633689093
- SartorCE.McCutcheonW.PommerNE.et alCommon genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women.Psychol Med.20114171497150521054919
- SteinMB.JangKL.TaylorS.VernonPA.LivesleyWJ.Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study.Am J Psychiatry.2002159101675168112359672
- DiasBG.ResslerKJ.PACAP and the PAC1 receptor in post-traumatic stress disorder.Neuropsychopharmacology2G13381245246
- VaudryD.Falluel-MorelA.BourgaultS.et alPituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery.Pharmacol Rev.200961328335719805477
- MiyataA.JiangL.DahlRD.et alIsolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38).Biochem Biophys Res Commun.199017026436482383262
- ArimuraA.Somogyvari-VighA.MiyataA.MizunoK.CoyDH.KitadaC.Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes.Endocrinology.19911295278727891935809
- JooKM.ChungYH.KimMK.et alDistribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PACT receptor) in the rat brain.J Comp Neurol.2004476438841315282712
- ShiversBD.GoresTJ.GottschallPE.ArimuraA.Two high affinity binding sites for pituitary adenylate cyclase-activating polypeptide have different tissue distributions.Endocrinology.19911286305530652036976
- SpenglerD.WaeberC.PantaloniC.et alDifferential signal transduction by five splice variants of the PACAP receptor.Nature.199336564421701758396727
- AjpruS.McArthurAJ.PigginsHD.SugdenD.Identification of PAC1 receptor isoform mRNAs by real-time PCR in rat suprachiasmatic nucleus.Brain Res Mol Brain Res.20021051-2293712399105
- PilzerI.GozesI.VIP provides cellular protection through a specific splice variant of the PACAP receptor: a new neuroprotection target.Peptides.200627112867287616905223
- KondoT.TominagaT.IchikawaM.lijimaT.Differential alteration of hippocampal synaptic strength induced by pituitary adenylate cyclase activating polypeptide-38 (PACAP-38).Neurosci Lett.19972212-31891929121696
- TyeKM.DeisserothK.Optogenetic investigation of neural circuits underlying brain disease in animal models.Nat Rev Neurosci.201213425126622430017
- TyeKM.PrakashR.KimSY.et alAmygdala circuitry mediating reversible and bidirectional control of anxiety.Nature.2011471738835836221389985
- ResslerKJ.MercerKB.BradleyB.et alPost-traumatic stress disorder is associated with PACAP and the PAC1 receptor.Nature.2011470733549249721350482
- DriscollMD.SathyaG.MuyanM.KlingeCM.HilfR.BambaraRA.Sequence requirements for estrogen receptor binding to estrogen response elements.J Biol Chem.19982734529321293309792632
- HeldringN.PikeA.AnderssonS.et alEstrogen receptors: how do they signal and what are their targets.Physiol Rev.200787390593117615392
- KlingeCM.Estrogen receptor interaction with estrogen response elements.Nucleic Acids Res.200129142905291911452016
- MooreJP Jr.BurgerLL.DalkinAC.WintersSJ.Pituitary adenylate cyclase activating polypeptide messenger RNA in the paraventricular nucleus and anterior pituitary during the rat estrous cycle.Biol Reprod.200573349149915917345
- FataJE.ChaudharyV.KhokhaR.Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17β-estradiol during the estrous cycle.Biol Reproduction.2001653680688
- HammackSE.MayV.Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies.Biol Psychiatry.201578316717725636177
- MissigG.RomanCW.VizzardMA.BraasKM.HammackSE.MayV.Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain.Neuropharmacology.201486384824998751
- CondroMC.MatyniaA.FosterNN.et alHigh-resolution characterization of a PACAP-EGFP transgenic mouse model for mapping PACAPexpressing neurons.J Comp Neurol. 2016 May 14. Epub ahead of print. doi:10.1002/cne.24035.
- GoosensKA.Hippocampal regulation of aversive memories.Curr Opin Neurobiol.201121346046621546244
- HammackSE.CheungJ.RhodesKM.et alChronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior.Psychoneuroendocrinology.200934683384319181454
- TanidaM.ShintaniN.MoritaY.et alRegulation of autonomic nerve activities by central pituitary adenylate cyclase-activating polypeptide.Regul Pept.20101611-3738020171991
- AgarwalA.HalvorsonLM.LegradiG.Pituitary adenylate cyclaseactivating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene.Brain Res Mol Brain Res.20051381455715882914
- DoreR.lemoloA.SmithKL.WangX.CottoneP.SabinoV.CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP.Neuropsychopharmacology.201338112160216923657440
- StrothN.LiuY.AguileraG.EidenLE.Pituitary adenylate cyclase-activating polypeptide controls stimulus-transcription coupling in the hypothalamic-pituitary-adrenal axis to mediate sustained hormone secretion during stress.J Neuroendocrinol.2011231094495521824204
- RomanCW.LezakKR.HartsockMJ.et alPAC1 receptor antagonism in the bed nucleus of the stria terminalis (BNST) attenuates the endocrine and behavioral consequences of chronic stress.Psychoneuroendocrinology20144715116525001965
- LehmannML.MustafaT.EidenAM.HerkenhamM.EidenLE.PACAP-deficient mice show attenuated corticosterone secretion and fail to develop depressive behavior during chronic social defeat stress.Psychoneuroendocrinology201338570271523062748
- MustafaT.JiangSZ.EidenAM.WeiheE.ThistlethwaiteI.EidenLE.Impact of PACAP and PAC1 receptor deficiency on the neurochemical and behavioral effects of acute and chronic restraint stress in male C57BL/6 mice.Stress.201518440841825853791
- LezakKR.RoelkeE.HarrisOM.et alPituitary adenylate cyclase-activating polypeptide (PACAP) in the bed nucleus of the stria terminalis (BNST) increases corticosterone in male and female rats.Psychoneuroendocrinology.201445112024845172
- SabatinelliD.FortuneEE.LiQ.et alEmotional perception: meta-analyses of face and natural scene processing.Neuroimage.20115432524253320951215
- PhanKL.WagerTD.TaylorSF.LiberzonI.Functional neuroimaging studies of human emotions.CNS Spectr.20049425826615048050
- PhanKL.TaylorSF.WelshRC.HoSH.BrittonJC.LiberzonI.Neural correlates of individual ratings of emotional salience: a trial-related fMRI study.Neuroimage.200421276878014980580
- AdmonR.LubinG.RosenblattJD.et alImbalanced neural responsivity to risk and reward indicates stress vulnerability in humans.Cereb Cortex.2013231283522291028
- ShvilE.SullivanGM.SchaferS.et alSex differences in extinction recall in posttraumatic stress disorder: a pilot fMRI study.Neurobiol Learn Mem.201411310110824560771
- MoreyLC.BensonKT.An investigation of adherence to diagnostic criteria, revisited: clinical diagnosis of the DSM-IV/DSM-5 section II personality disorders.J Pers Disord.201630113014425905732
- MoreyM.Fernandez-MarmiesseA.CastineirasD.FragaJM.CouceML.CochoJA.Corrigendum to “A glimpse into past, present, and future DNA sequencing” [Mol. Genet. Metab. 110 (2013) 3-24].Mol Genet Metab.2015114348425877474
- LissekS.PowersAS.McClureEB.et alClassical fear conditioning in the anxiety disorders: a meta-analysis.Behav Res Ther.200543111391142415885654
- JovanovicT.ElyT.FaniN.et alReduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample.Cortex.20134971884189123020899
- JovanovicT.NorrholmSD.BlandingNQ.et alImpaired fear inhibition is a biomarker of PTSD but not depression.Depress Anxiety.201027324425120143428
- JovanovicT.NorrholmSD.FennellJE.et alPosttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity.Psychiatry Res.20091671-215116019345420
- NorrholmSD.JovanovicT.OlinIW.et alFear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity.Biol Psychiatry.201169655656321035787
- PitmanRK.RasmussonAM.KoenenKC.et alBiological studies of post-traumatic stress disorder.Nat Rev Neurosci.2012131176978723047775
- GrahamBM.MiladMR.Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women.Biol Psychiatry.201373437137823158459
- GloverEM.JovanovicT.MercerKB.et alEstrogen levels are associated with extinction deficits in women with posttraumatic stress disorder.Biol Psychiatry.2012721192422502987
- RasmussonAM.CharneyDS.Animal models of relevance to PTSD.Ann N Y Acad Sci.19978213323519238215
- OttoC.KovalchukY.WolferDP.et alImpairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice.J Neurosci.200121155520552711466423
- ChoJH.DeisserothK.BolshakovVY.Synaptic encoding of fear extinction in mPFC-amygdala circuits.Neuron.20138061491150724290204
- SchmidtSD.MyskiwJC.FuriniCR.SchmidtBE.CavalcanteLE.IzquierdoI.PACAP modulates the consolidation and extinction of the contextual fear conditioning through NMDA receptors.Neurobiol Learn Mem.201511812012425490058
- MeewisseML.ReitsmaJB.de VriesGJ.GersonsBP.OlffM.Cortisol and post-traumatic stress disorder in adults: systematic review and metaanalysis.Br J Psychiatry.200719138739217978317
- SteinMB.JangKL.LivesleyWJ.Heritability of social anxiety-related concerns and personality characteristics: a twin study.J Nerv Ment Dis.2002190421922411960082
- NorrholmSD.ResslerKJ.Genetics of anxiety and trauma-related disorders.Neuroscience.2009164127228719540311
- HashimotoR.HashimotoH.ShintaniN.et alPituitary adenylate cyclase-activating polypeptide is associated with schizophrenia.Mol Psychiatry.200712111026103217387318
- AragarnN.WangKS.PanY.Genome-wide association analysis of gender differences in major depressive disorder in the Netherlands NESDA and NTR population-based samplesJ Affect Disord.2011133351652121621269
- JovanovicT.NorrholmSD.BlandingNQ.et alFear potentiation is associated with hypothalamic-pituitary-adrenal axis function in PTSD.Psychoneuroendocrinology201035684685720036466
- KamkwalalaA.NorrholmSD.PooleJM.et alDark-enhanced startle responses and heart rate variability in a traumatized civilian sample: putative sex-specific correlates of posttraumatic stress disorder.Psychosom Med.201274215315922286850
- KlingeCM.Estrogen receptor interaction with co-activators and corepressors.Steroids.200065522725110751636
- JovanovicT.NorrholmSD.DavisJ.et alPAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children.Mol Psychiatry.201318774274322776899
- GrahamBM.MiladMR.Inhibition of estradiol synthesis impairs fear extinction in male rats.Learn Mem.201421734735024939838
- HandaRJ.NunleyKM.LorensSA.LouieJP.McGivernRF.BollnowMR.Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors.Physiol Behav.19945511171248140154
- McDermottCM.LiuD.SchraderLA.Role of gonadal hormones in anxiety and fear memory formation and inhibition in male mice.Physiol Behav.201210551168117422226989
- HodosyJ.ZelmanovaD.MajzunovaM.et alThe anxiolytic effect of testosterone in the rat is mediated via the androgen receptor.Pharmacol Biochem Behav.2012102219119522546276
- CahillL.UncapherM.KilpatrickL.AlkireMT.TurnerJ.Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an fMRI investigation.Learn Mem.200411326126615169855
- ZeidanMA.IgoeSA.LinnmanC.et alEstradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats.Biol Psychiatry.2011701092092721762880
- GoldsteinJM.JerramM.AbbsB.Whitfield-GabrieliS.MakrisN.Sex differences in stress response circuitry activation dependent on female hormonal cycle.J Neurosci.201030243143820071507
- BreslauN.DavisGC.AndreskiP.PetersonEL.SchultzLR.Sex differences in posttraumatic stress disorder.Arch Gen Psychiatry.19975411104410489366662
- SeemanMV.Psychopathology in women and men: focus on female hormones.Am J Psychiatry.199715412164116479396940
- StevensJS.AlinliLM.FaniN.et alPACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus.ProcNatl Acad Sci USA.2014111831583163
- DannlowskiU.KugelH.HuberF.et alChildhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala.Hum Brain Mapp.201334112899290922696400
- ChoJH.ZushidaK.ShumyatskyGP.CarlezonWA Jr.MeloniEG.BolshakovVY.Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit.J Neurosci.20123241141651417723055486
- YangK.LeiG.JacksonMF.MacdonaldJF.The involvement of PACAP/ VIP system in the synaptic transmission in the hippocampus.J Mol Neurosci.201042331932620414742
- LindholmD.SkoglosaY.TakeiN.Developmental regulation of pituitary adenylate cyclase activating polypeptide (PACAP) and its receptor 1 in rat brain: function of PACAP as a neurotrophic factor.Ann N Y Acad Sci.19988651891969928012
- StevensJS.JovanovicT.FaniN.et alDisrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder.J Psychiatr Res.201347101469147823827769
- RauchSL.WhalenPJ.ShinLM.et alExaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study.Biol Psychiatry.200047976977610812035
- PohlackST.NeesF.RuttorfM.et alNeural mechanism of a sex-specific risk variant for posttraumatic stress disorder in the type i receptor of the pituitary adenylate cyclase activating polypeptide.Biol Psychiatry.2015781284084725680674
- PanthongA.KanjanapothiD.TaesotikulT.PhankummoonA.PanthongK.ReutrakulV.Anti-inflammatory activity of methanolic extracts from Ventilago harmandiana Pierre.J Ethnopharmacol.2004912-323724215120445
- BrewinCR.The nature and significance of memory disturbance in posttraumatic stress disorder.Annu Rev Clin Psychol.2011720322721219190
- LyonsMJ.GoldbergJ.EisenSA.et alDo genes influence exposure to trauma? A twin study of combat.Am J Med Genet.199348122278357033
- HermanJP.Neural control of chronic stress adaptation.Front Behav Neurosci.201376123964212
- OhmanA.MinekaS.Fears, phobias, and preparedness: toward an evolved module of fear and fear learning.Psychol Rev.2001108348352211488376
- JoelsM.BaramTZ.The neuro-symphony of stress.Nat Rev Neurosci.200910645946619339973
- UrsanoRJ.FullertonCS.EpsteinRS.et alAcute and chronic posttraumatic stress disorder in motor vehicle accident victims.Am J Psychiatry.1999156458959510200739
- WalkerJL.CareyPD.MohrN.SteinDJ.SeedatS.Gender differences in the prevalence of childhood sexual abuse and in the development of pediatric PTSD.Arch Womens Ment Health.20047211112115083346
- OlffM.LangelandW.DraijerN.GersonsBP.Gender differences in posttraumatic stress disorder.Psychol Bull.2007133218320417338596
- MarmarCR.WeissDS.SchlengerWE.et alPeritraumatic dissociation and posttraumatic stress in male Vietnam theater veterans.Am J Psychiatry.199415169029078185001
- TsukiyamaN.SaidaY.KakudaM.et alPACAP centrally mediates emotional stress-induced corticosterone responses in mice.Stress.201114436837521438773
- HatanakaM.TanidaM.ShintaniN.et alLack of light-induced elevation of renal sympathetic nerve activity and plasma corticosterone levels in PACAP-deficient mice.Neurosci Lett.2008444215315618722505
- YoungEA.BreslauN.Saliva Cortisol in posttraumatic stress disorder: a community epidemiologic study.Biol Psychiatry.200456320520915271590
- YehudaR.SouthwickSM.KrystalJH.BremnerD.CharneyDS.MasonJW.Enhanced suppression of Cortisol following dexamethasone administration in posttraumatic stress disorder.Am J Psychiatry.1993150183868417586
- BrewinCR.AndrewsB.ValentineJD.Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults.J Consult Clinical Psychol.200068574876611068961
- OzerEJ.BestSR.LipseyTL.WeissDS.Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis.Psychol Bull.20031291527312555794
- UddinM.ChangSC.ZhangC.et al ADCYAP1R1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment.Depress Anxiety.201330325125823280952
