Abstract
Affective neuroimaging has contributed to our knowledge of generalized anxiety disorder (GAD) through measurement of blood oxygenation level-dependent (BOLD) responses, which facilitate inference on neural responses to emotional stimuli during task-based functional magnetic resonance imaging (fMRI). In this article, the authors provide an integrated review of the task-based affective fMRI literature in GAD. Studies provide evidence for variable presence and directionality of BOLD abnormalities in limbic and prefrontal regions during reactivity to, regulation of, and learning from emotional cues. We conclude that understanding the sources of this variability is key to accelerating progress in this area. We propose that the cardinal symptom of GAD—worry—predominantly reflects stimulus-independent mental processes that impose abnormal, inflexible functional brain configurations, ie, the overall pattern of information transfer among behaviorally relevant neural circuits at a given point in time. These configurations that are inflexible to change from the incoming flux of environmental stimuli may underlie inconsistent task-based findings.
Las neuroimágenes de las emociones han contribuido al conocimiento del trastorno de ansiedad generalizada (TAG) a través de la medición de las respuestas de la señal BOLD, la cual refleja el nivel de oxigenación de la sangre del cerebro; esto facilita la inferencia de respuestas neuronales a los estímulos emocionales durante la imaginería funcional por resonancia magnética (RNMf) con una tarea determinada. En este artículo, los autores revisan de manera integradora la literatura sobre la RNMf emocional en base a una tarea en el TAG. Los resultados de estudios aportan evidencia sobre la variabilidad de la presencia y dirección de las anormalidades de la señal BOLD en las regiones límbicas y prefrontales durante la reactividad a claves emocionales, en la regulación y en el aprendizaje que se hace de éstas. Se concluye que la comprensión de los orígenes de esta variabilidad es clave para acelerar el progreso en esta área. Se propone que el síntoma cardinal del TAG -la preocupación excesiva- es reflejo principalmente de los procesos mentales independientes de estímulos que imponen configuraciones cerebrales funcionales anormales y rígidas; como por ejemplo, el esquema global de transferencia de información entre los circuitos neurales conductualmente relevantes en un momento dado. Estas configuraciones que no se modifican por el flujo de entrada de los estímulos ambientales, pueden estar a la base de resultados contradictorios luego de una tarea determinada.
La neuro-imagerie des émotions a enrichi notre connaissance de l'anxiété généralisée (AG) en mesurant les réponses par le signal BOLD qui reflète le taux d'oxygénation du sang dans le cerveau, ce qui facilite l'inférence des réponses neuronales aux stimuli émotionnels pendant l'imagerie fonctionnelle par résonance magnétique (IRMf) lors d'une tâche donnée. Dans cet article, les auteurs analysent de façon globale la littérature sur l'IRMf émotionnelle lors d'une tâche donnée dans l'AG. Des études ont montré une présence et une direction variables des anomalies du signal BOLD dans les régions limbiques et préfrontales lors de la réactivité à certains indices émotionnels, lors de leur régulation et de l'apprentissage qui en est fait. Nous en concluons que la compréhension des origines de cette variabilité est un élément clé d'une progression accélérée dans ce domaine. Selon nous, le symptôme cardinal de l'AG, l'inquiétude excessive ou l'appréhension, reflète de façon prédominante les processus mentaux indépendants du stimulus qui imposent des configurations anormales et rigides du cerveau fonctionnel, par exemple, le schéma global du transfert de l'information au sein des circuits neuronaux comportementalement pertinents à un instant donné. Ces configurations, qui ne se modifient pas lors du flux entrant des stimuli environnementaux, peuvent être à la base de résultats contradictoires lors d'une tâche donnée.
Introduction
Generalized anxiety disorder (GAD) is a prevalent and debilitating anxiety disorder that is associated with significant distress, functional impairment, and human and economic burden.Citation1,Citation2 The cardinal symptom feature of GAD is uncontrollable, pervasive worry and anxiety occurring more days than not for at least 6 months, occurring alongside somatic and emotional symptoms, such as restlessness, feeling keyed up or on edge, being easily fatigued, difficulty concentrating, irritability, muscle tension, and sleep disturbance.
In recent years, significant resources have been devoted to understanding the neurobiological mechanisms of the disorder. These efforts have profoundly shaped scientific understanding through noninvasive imaging methods, such as functional magnetic resonance imaging (fMRI), which utilize intensity changes in a type of magnetic resonance (MR) signal to track hemodynamic changes in the brain. These hemodynamic changes (known as the blood oxygenation-level dependent or BOLD response) can be utilized as proxy measures for neuronal function, allowing researchers to infer what brain regions in GAD are activated during a given behavior, the degree to which these activations may be abnormal, and how the synchrony of brain function across two or more regions may be perturbed. In aggregate, these tools have provided much-needed insight regarding the functional brain abnormalities observed in the GAD phenotype. In this paper, the authors provide an integrated review of the emotional task-based fMRI literature in GAD. Resting state, cognitive studies, and pre/post treatment comparisons have been excluded from the scope of this review due to space constraints. The goal of this effort is threefold: (i) to synthesize and integrate the body of literature for the purposes of clearly disseminating key patterns of findings to the interested reader; (ii) to highlight relevant trends over time in the design, conduction, and findings of GAD imaging studies; and (iii) to provide future directions and general guidelines for continued research in this area.
Methods
In line with the scope of the review, we focused on identifying studies that investigated emotional task-based BOLD fMRI responses in individuals with the diagnosis of GAD. The following criteria were instituted: (i) primary publication in English; (ii) primary comparison of a diagnostically defined GAD sample (or subsample from larger studies investigating anxiety transdiagnostically) against at least one healthy comparison group and/or other patient group; (iii) use of BOLD fMRI during an affective task as a primary outcome measure; and (iv) sample size within each cell of at least eight participants (case studies were excluded). To identify studies, the authors conducted a literature search in PubMed using broadly defined search terms: [“generalized anxiety” OR “generalised anxiety” OR “GAD”] AND [“imaging” OR “fMRI” OR “neuroimaging”]. The scope of articles was further shortened through review of search results and assessment of whether each study met the above criteria.
Results
Using the search terms listed above, a total of 608 articles were initially identified. Individual review of each narrowed this pool down to 30 studies (Table I) Citation3-Citation32 We review the results below, subdivided by functional domain assessed.
TABLE I. Table I. Affective neuroimaging studies of generalized anxiety disorder.Citation3-Citation32
Facial affect processing
Numerous BOLD imaging studies in GAD have focused on the assessment of brain responses to facial expressions of emotion, particularly fear. Fear faces robustly activate limbic circuitry, such as the insula and amygdala,Citation33 which are implicated as being hyperactive in various anxiety manifestations.Citation26,Citation34 The amygdala is crucial for the induction of a fear response to an external stimulusCitation35 and is critically involved in fear learningCitation36 and emotion perception.Citation37 The insula is implicated in representing the physiological state of the body, a process known as interoception,Citation38 which provides information regarding changes in internal body signals upon which subjective emotional states are based.Citation39 Evidence for hyperactive amygdala and insula function in anxiety disorders, such as social anxiety and specific phobia are robust,Citation34,Citation40 but results in GAD have been mixed. In adults with GAD, some studies have observed hyperactive amygdala responses to processing fearful vs happy facesCitation26 and processingCitation10 of and adaptation to face-word emotional conflict,Citation12 whereas others have found decreased amygdala activation to gender identification of fearful vs neutral facesCitation6 and viewing of negative pictures,Citation15 and yet others have found no differences in amygdala activation during fearful face processing,Citation5 facial affect processing of angry, fearful, or neutral faces,Citation30 or viewing of aversive pictures.Citation8,Citation29 At least one study observed a hyperactive anterior insula response to threatening vs neutral picture viewing in the absence of amygdala abnormalities.Citation29 Findings in adolescent and child samples have also been mixed, with amygdala hyperactivity detected in an adolescent GAD sample only when they were attending to their own subjective feelings of fear in response to a fearful face but not when attending to the affect of the face itself.Citation4
Other studies have used a dot-probe paradigm with angry and neutral faces to probe amygdala reactivity. The dot-probe utilizes a rapid presentation of a pair of stimuli (usually an emotional and neutral face) followed by a probe in the location of one of the preceding stimuli. The participant signals the location of the probe as quickly as possible, which facilitates inference on attentional bias, ie, the extent to which the participant's attention was drawn to the location of the emotional cue and the level of difficulty disengaging from the emotional cue. These studies observed hyperactivity of amygdala responses in youth with GAD only when the angry faces were masked from conscious awareness through rapid replacement of the emotional probe with a neutral one.Citation7 When faces were consciously processed, the amygdala response was normal, but the ventrolateral prefrontal cortex (vlPFC) was hyperactive.Citation3 Anxiety symptoms were inversely correlated with degree of vlPFC activation, suggesting a potential compensatory function. This finding converges with a study in adult GAD that observed a hyperactive lateral prefrontal response to consciously processed angry faces without any amygdalar differences.Citation6 Although the dot-probe has not yet been examined in adult GAD with imaging, these studies preliminarily suggest that amygdala abnormalities may be modulated by the focus or level of attention given to an emotional cue during the task.
Conflicting results may also be accounted for by abnormal amygdala responses in GAD to facial stimuli other than fearful faces, which are often used as baseline or comparator conditions in affective imaging tasks. For example, one study in adult GAD observed diminished amygdala and insula responses to a happy face-processing comparator condition that was contrasted with fearful and angry face processing, resulting in an exaggerated contrast magnitude with differences driven by the baseline condition only.Citation23 Another study observed similarly increased magnitude of amygdala responses to neutral faces, but not angry faces, in adults with GAD.Citation21
Similarly, amygdala interactions with other brain structures may influence variability, often investigated through context-dependent functional connectivity, ie, the degree of synchrony of BOLD responses in two regions in interaction with task conditions. A higher degree of synchrony is thought to indicate that two regions show greater connectivity during one task condition relative to another. One study observed elevated amygdala-insula connectivity in adults with GAD during processing of fearful and angry vs happy facial expressions,Citation23 consistent with the role of these regions in emotion processingCitation41 and in anxietyCitation34 more generally. Findings for abnormal amygdala-prefrontal connectivity in GAD are also abundant. A recent study observed increased connectivity between the amygdala and dorsomedial prefrontal cortex (dmPFC) and dorsal anterior cingulate (dACC) during the processing of fearful vs happy faces in a transdiagnostic anxiety sample composed of individuals with GAD and social anxiety (this finding was also present in the GAD sample alone), and higher levels of anxiety symptoms were related to greater connectivity of the amygdala with the dACC/ dmPFC.Citation24 Similarly, another study observed increased connectivity during the processing of threatening vs neutral pictures between the amygdala and the dorsal mid-cingulate cortex, which is anatomically and functionally related to the dACC,Citation42 as well as increased connectivity between the anterior insula and dorsal mid-cingulate.Citation29
In contrast, other findings demonstrate deficient amygdala-ventral anterior cingulate (vACC) connectivity in GAD, which supports a specific type of implicit emotional regulatory activity—emotional conflict adaptation. In the first of two studies,Citation10 GAD participants displayed a reduced dmPFC response to face-word emotional conflict (participants identified fearful or happy facial expressions overlaid with either the congruent or incongruent emotional word), as well as deficient vACC connectivity with the amygdala, which displayed a hyperactive response across all trial types. In healthy individuals, greater vACC-amygdala connectivity was related to the ability to adapt (ie, reduce reaction time slowdown) to the interference arising from an incongruent face-word emotional conflict trial when that trial was preceded by another incongruent trial vs when that trial was preceded by a congruent trial. Deficient dmPFC activation and exaggerated amygdala reactivity to emotional conflict trials is consistent with a study in late-life GAD that observed deficient dlPFC recruitment and exaggerated amygdala reactivity during an emotional Stroop task,Citation14 a conceptually related paradigm in which participants identify font color during presentation of negative and neutral words. The second study using the face-word emotional conflict paradigm in GAD observed deficient vACC activation, exaggerated amygdala reactivity, and deficient vACC-amygdala connectivity during emotional conflict adaptation, which was common across both GAD and major depression.Citation12 Thus, GAD is associated with selectively diminished connectivity between the amygdala and the vACC/ventromedial prefrontal cortex (vmPFC) during the implicit regulation of emotional reactivity, but increased amygdala connectivity with the dACC/dmPFC during reactivity itself. This dorsal/ventral distinction in amygdala connectivity abnormalities in GAD parallels research that implicates the vACC/vmPFC in inhibition of fear and fear extinction, whereas the dACC/dmPFC and dorsal-mid-cingulate have been implicated in fear expression/generationCitation43 and aversive amplification.Citation24
The PFC has also demonstrated abnormalities during emotional reactivity paradigms in GAD, such as hyperactive dACC/dmPFC and vlPFC responses in youth with GAD during assessment of their own emotional response to fear faces,Citation4 viewing of emotional vs neutral images during a continuous processing task,Citation17 and hyperactive vlPFC responses during a dot-probe paradigm with angry and neutral faces.Citation3 In adults with GAD, one study observed attenuated medial and lateral prefrontal responses to fearful, angry, sad, and happy faces (vs neutral),Citation13 but others observed exaggerated lateral prefrontal responses to angry facesCitation6 and exaggerated dorsal mid-cingulate and dorsolateral prefrontal (dlPFC) activation to threatening vs neutral pictures, which was specific to GAD relative to healthy individuals and participants with social anxiety (SAD) or panic disorder (PD).Citation29
In summary, GAD demonstrates a variable pattern of prefrontal and limbic activation and connectivity abnormalities during the processing of facial affect, which is in stark contrast to other anxiety disorders that demonstrate more consistent patterns of hyperactivity in regions such as the amygdala and insula.Citation34,Citation40 The source of this variability is currently unclear, but it may be consequent to factors varying across studies, such as depth of processing and attentional engagement necessitated by the task, as well as age-related changes in amygdala-frontal dynamics during emotion perception.Citation44 Secondarily, the varying directionality and presence of abnormalities during facial affect processing may reflect shifting functional configurations of brain regions involved in emotional reactivity and emotion regulation due to variation in internal/affective contexts, such as degree of worry or physiological arousal, which may moderate effective inhibition of limbic activation.
Affective learning and regulation
Beyond emotional reactivity, GAD also manifests abnormalities in downstream processes, such as learning to associate cues with affective outcomes,Citation45 and in deliberately regulating emotional responses to such cues.Citation46,Citation47 Two studies have observed abnormal prefrontal responses during the explicit downregulation of emotion in response to affective images. During cognitive reappraisal of aversive images, individuals with GAD displayed deficient activation in dlPFC and dmPFC regionsCitation19 that are crucial for supporting this emotion regulation technique,Citation48 a phenotype that was also observed in individuals with PD. Another study similarly observed reduced dACC recruitment across individuals with GAD, SAD, or comorbid GAD/SAD during completion of a cognitive reappraisal paradigm, as well as a second paradigm assessing top-down attentional control.Citation15 Thus, these findings are consistent with an emotional dysregulatory perspective on GAD,Citation47 providing evidence for deficient recruitment of brain regions during explicit emotion regulation in GAD that are consistently implicated in healthy individuals as supporting this psychological process.Citation48 This may also be a characteristic shared across different anxiety manifestations.Citation15,Citation19
Other studies have investigated affective learning in individuals with GAD, abnormalities of which may underlie processes thought to be involved in the etiology of the disorder, eg, threat generalization and avoidance learning.Citation45 One study in women observed that GAD was associated with a reduced capacity for vmPFC discrimination of a fear-conditioned stimulus from stimuli of similar perceptual characteristics, such that those with GAD displayed a flatter slope of vmPFC activation change as a function of stimulus similarity, ie, vmPFC activation was not as high for stimuli most different from the fear-conditioned stimulus.Citation20 This is consistent with the role of the vmPFC in fear inhibition and safety learningCitation49 and suggests that GAD is associated with a deficient ability of the brain to signal safety in contexts that resemble those associated with threat but that have no threat potential.
This same female GAD sample also displayed an abnormally heightened response of the ventral tegmental area (VTA) to stimuli that resembled the fear conditioned stimulus but were never actually paired with shock.Citation22 This latter finding is quite novel and implicates dysfunction of a key node of the mesolimbic dopaminergic system in GAD, which has classically been associated with reward and approach behavior but is also increasingly recognized as being involved in anticipation and response to aversive stimuli.Citation50 This system, of which the VTA is a crucial part, is critically implicated in learning stimulus-outcome associations and modifying the predictive value of a stimulus for a particular outcome.Citation51 This process has been increasingly examined using computational models of operant reinforcement learning in probabilistic decision-making tasks combined with imaging, known as model-based fMRI.Citation52 With this method, researchers are able to derive from behavioral data individual computational model parameters of expected value (the degree to which a stimulus signals a potential future positive outcome) and prediction error (an adjustment signal that is used to update the expected value of a stimulus via trial and error learning) in paradigms that invoke decision making to obtain rewards or avoid punishments. These individual parameters are then convolved with the hemodynamic response function on a trial-by-trial basis to identify areas of the brain displaying BOLD signal dynamics that conform to this pattern of information processing. A recent study in adults observed that GAD was associated with deficient prediction error signaling in key regions implicated in decision making and affective learning regardless of trial outcome.Citation32 These regions included the vmPFC, the dACC/dmPFC, anterior insula, posterior cingulate, and ventral striatum. Moreover, when trial outcomes were punishing, the GAD group displayed deficient modulation by punishment prediction errors in the bilateral putamen, globus pallidus, and caudate. This valence-specific striatal abnormality during an incentivized learning task in GAD is partially consistent with a study in pediatric anxiety that observed a valence-specific putamen abnormality in children with GAD during anticipation of increasing magnitudes of monetary gains, but not losses,Citation16 suggesting some continuity of dysfunctional incentive learning circuitry across development.
In aggregate, these findings point toward abnormal neural and behavioral processes supporting not only reactivity to emotional cues, but also the downstream processes that underlie the regulation of emotional responses to these salient stimuli and the learning and behaviors that follow. Notably, these abnormalities encompass regions of the dorsal and ventral prefrontal cortex and basal ganglia, which is consistent with the role of these circuits in supporting goal-directed behavior,Citation53 associative learning,Citation54 and in regulating emotional state implicitlyCitation10 and explicitly.Citation48 Thus, the GAD neurophenotype manifests a disturbance of behaviorally relevant neural circuits across contexts, rather than a more circumscribed and focal abnormality of structures underlying the fear response and anxiety per se.Citation34
Worry and perseverative cognition
As the cardinal symptom feature of GADCitation55 and the center point of its clinical conceptual models,Citation56,Citation57 early imaging studies focused on assessing worry and its neural correlates. An imaging study using worry-inducing and neutral spoken sentences demonstrated similar levels of activation in the dACC and dmPFC across both individuals with GAD and healthy comparison subjects, but only in those with GAD did this elevated regional activity persist into a post worry-induction resting state scan.Citation9 Furthermore, higher elevations in dACC/dmPFC post-worry resting state activity were correlated with higher self-reported levels of worry. Similarly, another study in elderly GAD participants observed that worry induction elicited robust activation in the amygdala and insula across those with GAD and healthy comparison subjects, but the pattern of activation was more prominently frontal in those with GAD.Citation28 A third study in elderly GAD participants during worry induction and reappraisal observed reduced connectivity between the dmPFC and the dlPFC during worry reappraisal, suggesting an inability of the dmPFC to couple with lateral prefrontal regions for regulation of worry engagement.Citation25 These findings highlight a pattern of frontal predominance and inflexibility in GAD during states of worry, which is broadly consistent with recent findings that have investigated patterns of connectivity before and after induction of perseverative cognition, ie, worry and rumination. One study investigating amygdala connectivity at rest before and after induction of perseverative cognition observed that individuals with GAD displayed decreased connectivity between the amygdala and the dACC/dmPFC before induction of perseverative cognition that was as prominent an abnormality as the increased connectivity observed between these regions after the induction.Citation27 This latter finding converges with other resting state studies that have observed reduced connectivity between the amygdala and dACC/dmPFC in adults with GAD.Citation58 Furthermore, another study in a largely overlapping sample of these participants also observed that before perseverative cognition induction, individuals with GAD displayed less task-induced deactivation of the posterior cingulate and precuneus, the posterior nodes of the resting state default-mode network that display frequent deactivation in response to task demands and elevated activity at rest.Citation59 In aggregate, these findings support the contention that the brain in GAD is characterized by a striking level of inflexibility in response to changing environmental/behavioral demands,Citation60 which is consistent with the clinical observation that persistent worry and negative beliefs are generally unamenable to change from contradictory evidence in the environment.Citation61
Discussion
These findings highlight limbic and prefontal abnormalities in GAD across behavioral processes and developmental stages. Perhaps more so than any other anxiety disorder, the presence and directionality of these abnormalities varies greatly across studies. Given the significant heterogeneity of findings observed, what can be gleaned from this body of work that will inform future studies and lead to a deeper and more nuanced understanding of the GAD neurophenotype?
We suggest that understanding the sources of this variability is key to progress in this area. Of the anxiety disorders, GAD is unique in that the cardinal, disorder-defining feature is by its very nature potentially independent of environmental input—that is, worry is persistent, inflexible, future-oriented, and does not necessitate any perceptual stimulus or cue in order to arise in the individual's subjective experience. We do not mean that worry does not arise as a consequence of a cue or stimulus, but rather that it has a larger potential for stimulus independence relative to other anxiety symptoms such as avoidance, emotional reactivity, and other cued fear states. Thus, the stimulus-independent potential for worry to arise and persist suggests two critical points: (i) the neurocircuitry orchestrating the worry cascade is probably heavily dependent on top-down medial prefrontal systems implicated in stimulus-independent mental activity, ie, the anterior default mode network, consistent with the prominent GAD mPFC abnormalities reviewed in this paper, as well as imaging studies of worry in healthy individualsCitation62; and (ii) predominant stimulus-independent brain states in GAD that form the foundation for worry are more resistant to change from the perpetual flux of environmental input and more likely to persist in a chronic, inflexible manner. This deduction is consistent with recent clinical theoretical developments that posit worry is not a way to avoid negative emotion in GADCitation56 but actually serves to induce a mild, persistent state of negative affect that facilitates an attenuated magnitude of affective shifts arising from stimuli in the environment (known as the contrast avoidance theoryCitation63). The idea of a stimulus-independent, maladaptive medial prefrontal “hyperorganizalion” of the internal milieu is consistent with findings for a reduced capacity of the brain in GAD to discriminate among stimuli signaling safety vs threat,Citation20 reduced modulation of brain activation by environmental feedback,Citation32 and a decreased ability to shift brain and physiological states in response to environmental demands.Citation9,Citation27,Citation31
If accurate, the wide variability in task-based activation and connectivity abnormalities in GAD would therefore be expected. Specifically, task-based manipulations are less likely to influence regional brain configurations in a reliable way in GAD, and these configurations are more likely to be dictated by stimulus-independent factors that are resistant to change by experimental input. Thus, depending on the stimulus-independent factors influencing the functional configurations of limbic and prefrontal regions in a given GAD sample, abnormalities may be assessed as increased activation, decreased activation, or null activation differences. We illustrate this potential dynamic in (), wherein the range of potential brain states in GAD is proposed to be broader than that of healthy individuals and individuals with other anxiety disorders, but the shifts among these various states are proposed to be less frequent and more invariant to experimental manipulations.
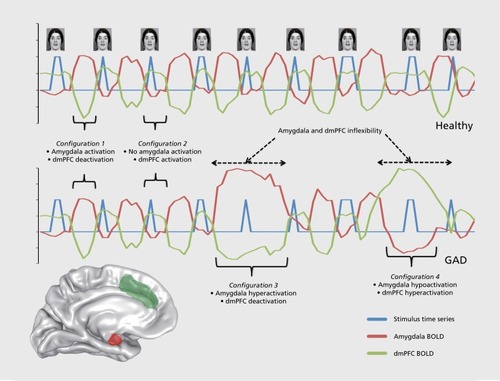
If variability is the norm rather than the exception, how can task-based imaging be used to better understand the brain dynamics in GAD? First, it will be crucially important to bring other convergent measures to bear on imaging findings in order to better contextualize their significance. The field has already begun to move in this direction with the recent incorporation of physiological measures such as heart rate variabilityCitation27,Citation31 as well as computational methods that allow for inference concerning the information processed by dysfunctional substrates.Citation32 Second, the variability in activation values across the task itself may in fact be extremely informative to explore in regards to GAD. Characterization of the amplitudes of low frequency fluctuations in GAD at rest have already yielded potentially valuable insights in this area,Citation64,Citation65 but to the authors' knowledge no such investigations have been undertaken in a task context. Of particular importance would be to understand how variability in stimulus-cued activation changes over the course of time and is influenced by other stimulus-independent factors such as mood, arousal level, predominance of worry or rumination, and physiological state. Third, a careful consideration and assessment of the baseline comparator condition in imaging paradigms will be crucial, as existing findings already indicate abnormal responses to stimuli often used as the subtractive condition for a contrast.Citation21,Citation23 Thus, multiple baseline or comparator conditions might be warranted, as well as careful theoretical considerations of which stimuli are most suitable for this purpose. Furthermore, understanding how spontaneous fluctuations in BOLD signal might contribute differently to variations in task-evoked activationCitation66 in GAD might be an important area for focus, particularly given the prominent resting state abnormalities observed in the disorderCitation58,Citation64 and the variability in task-based findings. Finally, consistent with the recent explosion of “big data” analytic techniques and their application to neuroscience,Citation67 the time is ripe for the application of these principles to the study of GAD. More specifically, the overwhelming majority of imaging studies in this area have relied exclusively on univariate statistical techniques to characterize brain dynamics, which are well-characterized methods with an illustrious history of application to brain mapping.Citation68 However, multivariate statistical methods such as multivariate pattern analysis, representational similarity analysis, and machine learning algorithms are being increasingly applied to the study of mental disordersCitation69 and neuroscience more broadly.Citation70 Such multivariate tools could be eminently useful for characterizing the functional neuroanatomy of GAD, in which a wide degree of variability in observed abnormalities may derive, at least in part, from the confluence of multiple classifiable abnormal brain states across subjects in a sample, or even within the same subject at different points in time. Moreover, the ability to understand how similar observed levels of activation in a given region, eg, the amygdala, may signify very different information-processing states depending upon the concomitant activation level in other relevant brain structures will be paramount to better understanding the significance of regional brain configurations and how differential access to and assumption of these configurations may reveal unique information regarding worry and GAD.
Selected abbreviations and acronyms
| BOLD | = | blood oxygenation-level dependent |
| dmPFC | = | dorsomedial prefrontal cortex |
| fMRI | = | functional magnetic resonance imaging |
| GAD | = | generalized anxiety disorder |
| vACC | = | ventral anterior cingulate cortex |
| vmPFC | = | ventromedial prefrontal cortex |
Gregory A. Fonzo was partially supported by a grant from the National Institute of Mental Health T32 MH019938. All authors report no conflicts of interest.
REFERENCES
- WittchenHU.Generalized anxiety disorder: prevalence, burden, and cost to society.Depress Anxiety.200216416217112497648
- HoffmanDL.DukesEM.WittchenHU.Human and economic burden of generalized anxiety disorder.Depress Anxiety.2008251729017146763
- MonkCS.NelsonEE.McClureEB.et alVentrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder.Am J Psychiatry.200616361091109716741211
- McClureEB.MonkCS.NelsonEE.et alAbnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder.Arch Gen Psychiatry.20076419710617199059
- WhalenPJ.JohnstoneT.SomervilleLH.et alA functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder.Biol Psychiatry.200863985886317964548
- BlairK.ShaywitzJ.SmithBW.et alResponse to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders.Am J Psychiatry.200816591193120218483136
- MonkCS.TelzerEH.MoggK.et alAmygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder.Arch Gen Psychiatry.200865556857618458208
- NitschkeJB.SarinopoulosI.OathesDJ.et alAnticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response.Am J Psychiatry.2009166330231019122007
- PaulesuE.SambugaroE.TortiT.et alNeural correlates of worry in generalized anxiety disorder and in normal controls: a functional MRI study.Psychol Med.201040111712419419593
- EtkinA.PraterKE.HoeftF.MenonV.SchatzbergAF.Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder.Am J Psychiatry.2010167554555420123913
- MaslowskyJ.MoggK.BradleyBP.et alA preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder.J Child Adolesc Psychopharmacol.201020210511120415605
- EtkinA.SchatzbergAF.Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders.Am J Psychiatry.2011168996897821632648
- PalmME.ElliottR.McKieS.DeakinJF.AndersonIM.Attenuated responses to emotional expressions in women with generalized anxiety disorder.Psychol Med.20114151009101820716396
- PriceRB.EldrethDA.MohlmanJ.Deficient prefrontal attentional control in late-life generalized anxiety disorder: an fMRI investigation.Transl Psychiatry.20111e4622833192
- BlairKS.GeraciM.SmithBW.et alReduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder.Biol Psychiatry.201272647648222592057
- GuyerAE.ChoateVR.DetloffA.et alStriatal functional alteration during incentive anticipation in pediatric anxiety disorders.Am J Psychiatry.2012169220521222423352
- StrawnJR.BitterSM.WeberWA.et alNeurocircuitry of generalized anxiety disorder in adolescents: a pilot functional neuroimaging and functional connectivity study.Depress Anxiety.2012291193994722628125
- YassaMA.HazlettRL.StarkCE.Hoehn-SaricR.Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder.J Psychiatr Res.20124681045105222575329
- BallTM.RamsawhHJ.Campbell-SillsL.PaulusMP.SteinMB.Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders.Psychol Med.20134371475148623111120
- GreenbergT.CarlsonJM.ChaJ.HajcakG.Mujica-ParodiLR.Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization.Depress Anxiety.201330324225023139148
- HolzelBK.HogeEA.GreveDN.et alNeural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training.Neuroimage Clin.2013244845824179799
- ChaJ.CarlsonJM.DedoraDJ.GreenbergT.ProudfitGH.Mujica-ParodiLR.Hyper-reactive human ventral tegmental area and aberrant mesocorticolimbic connectivity in overgeneralization of fear in generalized anxiety disorder.J Neurosci.201434175855586024760845
- FonzoGA.RamsawhHJ.FlaganTM.et alCognitive-behavioral therapy for generalized anxiety disorder is associated with attenuation of limbic activation to threat-related facial emotions.J Affect Disord.2014169768525171782
- RobinsonOJ.KrimskyM.LiebermanL.AllenP.VytalK.GrillonC.Towards a mechanistic understanding of pathological anxiety: the dorsal medial prefrontal-amygdala 'aversive amplification' circuit in un-medicated generalized and social anxiety disorders.Lancet Psychiatry.20141429430225722962
- AndreescuC.SheuLK.TudorascuD.et alEmotion reactivity and regulation in late-life generalized anxiety disorder: functional connectivity at baseline and post-treatment.Am J Geriatr Psychiatry.201523220021424996397
- FonzoGA.RamsawhHJ.FlaganTM.et alCommon and disorder-specific neural responses to emotional faces in generalised anxiety, social anxiety and panic disorders.Br J Psychiatry.2015206320621525573399
- MakovacE.MeetenF.WatsonDR.et alAlterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in generalized anxiety disorder.Biol Psychiatry.2016801078679526682467
- MohlmanJ.EldrethDA.PriceRB.StaplesAM.HansonC.Prefrontal-limbic connectivity during worry in older adults with generalized anxiety disorder.Aging Ment Health.201721442643826566020
- BuffC.BrinkmannL.NeumeisterP.et alSpecifically altered brain responses to threat in generalized anxiety disorder relative to social anxiety disorder and panic disorder.Neuroimage Clin.20161269870627761400
- KarimH.TudorascuDL.AizensteinH.WalkerS.GoodR.AndreescuC.Emotion reactivity and cerebrovascular burden in late-life GAD: a neuroimaging study.Am J Geriatr Psychiatry.2016211110401050
- OttavianiC.WatsonDR.MeetenF.MakovacE.GarfinkelSN.CritchleyHD.Neurobiological substrates of cognitive rigidity and autonomic inflexibility in generalized anxiety disorder.Biol Psychol.2016119314127345596
- WhiteSF.GeraciM.LewisE.et alPrediction error representation in individuals with generalized anxiety disorder during passive avoidance.Am J Psychiatry.2016174211011727631963
- Fusar-PoliP.PlacentinoA.CarlettiF.et alFunctional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies.J Psychiatry Neurosci.200934641843219949718
- EtkinA.WagerTD.Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia.Am J Psychiatry.2007164101476148817898336
- FeinsteinJS.AdolphsR.DamasioA.TranelD.The human amygdala and the induction and experience of fear.Curr Biol.2011211343821167712
- MiladMR.RosenbaumBL.SimonNM.Neuroscience of fear extinction: implications for assessment and treatment of fear-based and anxiety related disorders.Behav Res Ther.201462172325204715
- AndersonAK.PhelpsEA.Lesions of the human amygdala impair enhanced perception of emotionally salient events.Nature.2001411683530530911357132
- CraigAD.Interoception: the sense of the physiological condition of the body.Curr Opin Neurobiol.200313450050512965300
- CraigAD.How do you feel - now? The anterior insula and human awareness.Nat Rev Neurosci.2009101597019096369
- IpserJC.SinghL.SteinDJ.Meta-analysis of functional brain imaging in specific phobia.Psychiatry Clin Neurosci.201367531132223711114
- KoberH.BarrettLF.JosephJ.Bliss-MoreauE.LindquistK.WagerTD.Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies.Neuroimage.2008422998103118579414
- MarguliesDS.KellyAM.UddinLQ.BiswalBB.CastellanosFX.MilhamMP.Mapping the functional connectivity of anterior cingulate cortex.Neuroimage.200737257958817604651
- EtkinA.EgnerT.KalischR.Emotional processing in anterior cingulate and medial prefrontal cortex.Trends Cogn Sci.2011152859321167765
- MatherM.The affective neuroscience of aging.Annu Rev Psychol.20166721323826436717
- GrupeDW.NitschkeJB.Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective.Nat Rev Neurosci.201314748850123783199
- DeckerML.TurkCL.HessB.MurrayCE.Emotion regulation among individuals classified with and without generalized anxiety disorder.J Anxiety Disord.200822348549417512697
- MenninDS.HeimbergRG.TurkCL.FrescoDM.Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder.Behav Res Ther.200543101281131016086981
- BuhleJT.SilversJA.WagerTD.et alCognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies.Cereb Cortex.201424112981299023765157
- SchillerD.LevyI.NivY.LeDouxJE.PhelpsEA.From fear to safety and back: reversal of fear in the human brain.J Neurosci.20082845115171152518987188
- LammelS.LimBK.MalenkaRC.Reward and aversion in a heterogeneous midbrain dopamine system.Neuropharmacology.201476pt B35135923578393
- KeiflinR.JanakPH.Dopamine prediction errors in reward learning and addiction: from theory to neural circuitry.Neuron.201588224726326494275
- O'DohertyJP.HamptonA.KimH.Model-based fMRI and its application to reward learning and decision making.Ann N Y Acad Sci.20071104355317416921
- HaberSN.BehrensTE.The neural network underlying incentivebased learning: implications for interpreting circuit disruptions in psychiatric disorders.Neuron.20148351019103925189208
- AbrahamAD.NeveKA.LattalKM.Dopamine and extinction: a convergence of theory with fear and reward circuitry.Neurobiol Learn Mem.2014108657724269353
- WittchenHU.HoyerJ.Generalized anxiety disorder: nature and course.J Clin Psychiatry. 2001 62 (suppl 11): 15-19; discussion 20-11.
- BorkovecTD.AlcaineO.BeharE.Avoidance theory of worry and generalized anxiety disorder. In: Heimberg RG, Turk CL, Mennin DS, eds.Generalized Anxiety Disorder: Advances in Research and Practice. New York, NY: Guilford Press.200477108
- DugasMJ.GagnonF.LadouceurR.FreestonMH.Generalized anxiety disorder: a preliminary test of a conceptual model.Behav Res Ther.19983622152269613027
- EtkinA.PraterKE.SchatzbergAF.MenonV.GreiciusMD.Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder.Arch Gen Psychiatry.200966121361137219996041
- GreiciusMD.KrasnowB.ReissAL.MenonV.Functional connectivity in the resting brain: a network analysis of the default mode hypothesis.Proc Natl Acad Sci U S A.2003100125325812506194
- FonzoGA.EtkinA.Brain connectivity reflects mental and physical states in generalized anxiety disorder.Biol Psychiatry.2016801073373527765158
- RuscioAM.SeitchikAE.GentesEL.JonesJD.HallionLS.Perseverative thought: a robust predictor of response to emotional challenge in generalized anxiety disorder and major depressive disorder.Behav Res Ther.2011491286787422030295
- SteinfurthEC.AliusMG.WendtJ.HammAO.Physiological and neural correlates of worry and rumination: support for the contrast avoidance model of worry.Psychophysiology.201754216117127766641
- NewmanMG.LleraSJ.A novel theory of experiential avoidance in generalized anxiety disorder: a review and synthesis of research supporting a contrast avoidance model of worry.Clin Psychol Rev.201131337138221334285
- OathesDJ.PatenaudeB.SchatzbergAF.EtkinA.Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging.Biol Psychiatry.201577438539325444162
- WangW.HouJ.QianS.et alAberrant regional neural fluctuations and functional connectivity in generalized anxiety disorder revealed by resting-state functional magnetic resonance imaging.Neurosci Lett.2016624788427163197
- FoxMD.SnyderAZ.ZacksJM.RaichleME.Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses.Nat Neurosci.200691232516341210
- FengS.HolmesP.Will big data yield new mathematics? An evolving synergy with neuroscience.IMA J Applied Math.201681343245627516705
- XuJ.PotenzaMN.CalhounVD.et alLarge-scale functional network overlap is a general property of brain functional organization: reconciling inconsistent fMRI findings from general-linear-model-based analyses.Neurosci Biobehav Rev.2016718310027592153
- WangXJ.KrystalJH.Computational psychiatry.Neuron.201484363865425442941
- MarblestoneAH.WayneG.KordingKP.Toward an integration of deep learning and neuroscience.Front Comput Neurosci.2016109427683554
