Abstract
In recent years, animal models in psychiatric research have been criticized for their limited translational value to the clinical situation. Failures in clinical trials have thus often been attributed to the lack of predictive power of preclinical animal models. Here, I argue that animal models of voluntary drug intake—under nonoperant and operant conditions—and addiction models based on the Diagnostic and Statistical Manual of Mental Disorders are crucial and informative tools for the identification of pathological mechanisms, target identification, and drug development. These models provide excellent face validity, and it is assumed that the neurochemical and neuroanatomical substrates involved in drug-intake behavior are similar in laboratory rodents and humans. Consequently, animal models of drug consumption and addiction provide predictive validity. This predictive power is best illustrated in alcohol research, in which three approved medications—acamprosate, naltrexone, and nalmefene—were developed by means of animal models and then successfully translated into the clinical situation.
Los modelos animales en investigación psiquiátrica han sido criticados en los últimos años por su limitado valor para ser trasladados a la situación clínica. Las fallas en los ensayos clínicos a menudo han sido atribuidas a la falta de poder predictivo de los modelos animales preclínicos. En este artículo se argumenta que los modelos animales de ingesta voluntaria de drogas—bajo condiciones operantes y no operantes—y los modelos de adicciones basados en el Manual Diagnóstico y Estadístico de los Trastornos Mentales constituyen herramientas cruciales e informativas para la identificación de mecanismos patológicos, identificación de blancos y desarrollo de fármacos. Estos modelos aportan una excelente validez aparente y se asume que los sustratos neuroquímicos y neuroanatómicos involucrados en la conducta de ingesta de drogas son similares en los roedores de laboratorio y en los humanos. En consecuencia, los modelos animales de consumo de drogas y adicciones aportan validez predictiva. Este poder predictivo está mejor ilustrado en la investigación con alcohol, en la cual tres medicamentos aprobados (acamprosate, naltrexona y nalmefene) fueron desarrollados a partir de modelos animales y luego trasladados exitosamente a las situaciones clínicas.
Ces dernières années, les modèles animaux en recherche psychiatrique ont été critiqués pour leur valeur translationnelle limitée en situation clinique. Les échecs des études cliniques ont donc souvent été attribués au manque de puissance prédictive des modèles précliniques animaux. Pour ma part j'estime que les modèles animaux de prise volontaire de substances —sous conditions opératoires et non opératoires— et les modèles d'addiction basés sur le DSM (Diagnostic and Statistical Manual of Mental Disorders) sont des outils essentiels et informatifs pour identifier les mécanismes pathologiques et les cibles thérapeutiques et pour développer les médicaments. La validité apparente de ces modèles est excellente et on considère que les substrats neuro-chimiques et neuro-anatomiques impliqués dans le comportement de prise de substance sont similaires chez les rongeurs de laboratoire et les humains. Les modèles animaux de consommation de substances et d'addiction ont donc une validité prédictive. C'est dans la recherche sur l'alcool que cette puissance prédictive s'illustre le mieux, trois médicaments autorisés (acamprosate, nal-trexone et nalméfène) ayant été développés grâce aux modèles animaux et transférés ensuite avec succès en situation clinique.
Introduction
Over the past two decades, major advances have been made in the neurobiological understanding of brain functions and psychiatric disorders. Despite this huge knowledge gain, new and clinically useful treatment developments remain limited. The fact that many preclinically validated mechanisms fail during clinical development, which has led to the general opinion that animal models in psychiatric research do not provide good predictive validity. Consequently, the strategic decision made by most pharmaceutical companies has been to stop drug development programs in the field of psychiatry.Citation1 However, failed trials for psychiatric disorders do not necessarily invalidate preclinical animal models and identified drug targets.Citation2
In the position paper by Bespalov et al,Citation2 the authors argue that the rigor with which preclinical data is obtained—and the resulting robustness of the data—is quite low. Thus, few preclinical studies report randomization, blinding, or sample-size calculations—factors that are critical for designing clinical trials. Furthermore, the generalizability of preclinical data is often not considered. This is not only an issue pertaining to laboratory conditions and animal strains, age, and sex, but also to long-term drug administration and tolerance development—factors that are also critical to the design of clinical trials but often not considered at the preclinical level. It is therefore argued that researchers should attach greater importance to issues of data robustness and data generalizability when designing preclinical studies.
Furthermore, there are also concerns about the design of clinical trials and divergences in primary and secondary outcomes of preclinical and clinical studies, insufficient target engagement in the human condition, too-low dosing in order to avoid unwanted side effects, and augmented placebo effects; these may in many cases be the reasons for failure in clinical trials.Citation3
Despite these obvious concerns, the generally held opinion is that animal models in psychiatric research do not provide translatable information for the clinical situation and are therefore misleading. Is this conclusion correct for psychiatric disorders, and in particular for alcohol-use and substance-use disorders? Although for some complex human mental disorders, such as schizophrenia, this conclusion might be correct, it is argued here that animal models of voluntary drug intake and addiction models based on the Diagnostic and Statistical Manual of Mental Disorders (DSM) are crucial and highly informative tools for the identification of pathological mechanisms, target identification, and drug development. The precondition for each animal model is a high degree of face validity, defined here as characteristic behavioral features that are seen both in laboratory animals and in humans.
Face validity of animal models in addiction research
Humans and laboratory animals, such as monkeys, rats, and mice, voluntarily take drugs by different routes of administration, be it orally or intravenously. If unlimited voluntary intravenous access to heroin or cocaine is provided, laboratory animals can easily overdose to death. Lethal overdosing also frequently happens in drug users. Mice and rats can also voluntarily drink large quantities of alcohol, which leads to strong intoxication. These characteristic features seen in drug-taking behavior in laboratory animals resemble drug-taking behavior in humans and suggests a high degree of face validity. However, face validity is often a result of anthropomorphic interpretations of an animal's behavior. If, however, behavioral features are evolutionarily developed, real face validity is inferred. For example, behavioral fear responses are critical to most species for survival and developed over millions of years. As such, freezing behavior in response to a threatening stimulus, as seen in mice and humans alike, has real face validity. Since it is generally believed that psychoactive drug use in humans is a novel feature of our environment and cultural development,Citation4 one wonders whether drug-taking behavior in laboratory animals actually resembles drug taking in humans?
Given the fact that the evolution of our human ancestors and of animals proceeded in a world rich in drugs, an alternative theory favors the idea that drug and alcohol intake by mammals and other species has always been an everyday occurrence.Citation5 Thus, occasional and even long-term intake of psychoactive drugs produced by a variety of plants or alcohol ingestion through sugar-rich plant products susceptible to fermentation might be a behavior that has been shaped over millions of years.Citation6 Interestingly, it was found that hominids adapted to metabolize alcohol long before human-directed fermentation. Using a paleogenetics approach, Carrigan and colleaguesCitation7 resurrected digestive alcohol dehydrogenases (ADH4) from our primate ancestors to explore the history of primate-alcohol interactions and identified a single mutation occurring roughly 10 million years ago that endowed our ancestors with a markedly enhanced ability to metabolize ethanol. This change occurred around the time that our ancestors adopted a terrestrial lifestyle. Because fruit collected from the forest floor contains higher concentrations of fermenting yeast and alcohol than similar fruits hanging from trees, this transition may also be the first time our ancestors were exposed to and adapted to substantial amounts of alcohol. These discoveries favor the idea that from an evolutionary perspective, alcohol and drug-intake behavior has been shaped over millions of years and should be considered a part of our normal behavioral repertoire. These evolutionary roots of alcohol- and drug-taking behavior in mammals support the real face validity of animal models of drug self-administration.
Animal models of drug self-administration
Self-administration-based animal models are widely used in preclinical addiction research and are extensively reviewed by in ref 8. As explained, these models have excellent face validity, and it is assumed that the neurochemical and neuroanatomical substrates involved in drug-intake behavior are similar in laboratory rodents and humansCitation8,Citation9 (which is often defined as construct validity). Consequently, self-administration-based animal models are helpful in unraveling the molecular and neurobiological mechanisms of drug-related behaviors and therefore critical in identifying strategies useful in the intervention of human drug consumption; ie, they provide predictive validity. For example, pharmacological opioid-receptor blockade reduces alcohol consumption in many self-administration studies in different strains of rats and mice under different conditions,Citation10 which provides generalizability of preclinical data that translates to the human situation, in which the opioid-receptor blocker nalmefene also reduces alcohol consumption.Citation11
Self-administration procedures can be classified according to different criteria. From a pharmacokinetic perspective, they can be classified according to the route of administration by which the drug is delivered to the organism. Pharmacokinetic aspects that are determined by the route of administration are of critical importance in modeling of human drug-taking behavior. For example, researchers have used oral self-administration procedures to model human opioid-taking behavior.Citation12 However, most opioid users inject the drug intravenously. The oral and intravenous route of opioid administration yield completely different pharmacokinetics and, consequently, different pharmacodynamics cascades are to be expected. Therefore, the route of administration is of critical importance for modeling human behavior.
From a behavioral perspective, drug self-administration can be classified into operant and nonoperant procedures. Drug self-administration based on operant and nonoperant responses differ in procedural characteristics, but may also differ in their sensitivity to the manipulation of specific brain substrates.Citation13
Nonoperant drug self-administration
Nonoperant procedures are restricted to oral self-administration. This kind of procedure is very common in the context of alcohol research, but has also been used with other drugs of abuse, such as cannabinoids, nicotine, cocaine, amphetamine, and opioids.Citation12 However, when those drugs are orally self-administered, they show reduced motivational efficacy.Citation12 As the oral route of administration in humans applies mainly to alcohol, 3,4-methylenedioxymethamphetamine (MDMA), and related designer drugs that are usually taken as pills, it is incongruous to use a nonoperant-based procedure of self-administration to model human cannabis, nicotine, cocaine, or opioid use—drugs that are either inhaled or intranasally or intravenously applied. Therefore, the following paragraphs will focus solely on oral alcohol self-administration procedures.
In nonoperant alcohol self-administration, two bottles are usually offered to a laboratory animal in its home cage, with one containing an aqueous solution of alcohol and another bottle containing water. Different factors can affect alcohol consumption, such as the number of available bottles, the temporal accessibility to alcohol, the alcohol concentration, etc. In general, it has been shown that alcohol consumption increases when more than two bottles with different concentrations of alcohol solutions are presented or when subjects are given restricted access to them.Citation14 Alcohol concentration is a critical issue in these procedures, because low or overly high concentrations can be orally consumed or rejected because of their mild-sweet or aversive tasting properties, respectively. Moreover, as the amount of ingested fluid is limited by physiological constraints, too low of an alcohol concentration may result in negligible brain alcohol levels. Thus, it is usually considered that ethanol concentrations below 4% (v/v) are not pharmacologically relevant and that a concentration in the range of 10% to 12% is a suitable standard for consumption by rats and mice. In contrast, when initially offered, most rodent strains will most likely not drink much of a highly concentrated alcohol solution. Consequently, several procedures have been developed for “training” rodents to orally self-administer pharmacologically relevant amounts of alcohol, including the presentation of ascending concentrations of ethanol, the addition of a sweet flavor agent (ie, sucrose) that can be progressively faded out or not, or the inclusion of a time period of forced exposure to ethanol.Citation12,Citation14 Using these procedures, laboratory rodents drink up to 40% alcohol solutions and can become highly intoxicated. An alternative strategy has been the development of rodent strains with a high inborn alcohol consumption/preference,Citation15,Citation16 but this strategy reduces data generalizability, and results from these genetically selected animal strains may therefore not translate well to the human situation. However, rodent strains for high versus low alcohol consumption and recombinant inbred rodent strains are powerful tools to identify genetic risk alleles that predispose to excessive alcohol consumption.Citation17
Another very efficient procedure to induce excessive oral alcohol consumption is the exposure to intermittent alcohol vapor. Long-term intermittent intoxication with alcohol vapor leads to long-lasting neuronal and behavioral adaptations that persist even in the absence of the drug.Citation18 Although this model is based on experimenter-controlled intoxication—as opposed to largely voluntary drinking in humans—it has been valuable in identifying potential molecular mechanisms underlying excessive alcohol consumption.Citation19,Citation20 This rat model of excessive alcohol consumption after intermittent vapor exposure was also instrumental in the discovery that enhanced corticotropin-releasing-hormone (CRH) signaling within the amygdala is a key molecular mechanism mediating excessive alcohol consumption and alcohol seeking.Citation21,Citation22 However, the human application of two different selective pharmacological CRH-receptor 1 (CRH-R1) antagonists failed in clinical trials,Citation23,Citation24 establishing doubt in the translational and predictive value of this rat model of excessive alcohol drinking. In this context, one must admit that intermittent alcohol vapor exposure over several weeks lacks face validity for the human drinking situation and may as such produce false-positive signals that do not translate to excessive human drinking behavior.
The measures in nonoperant models of alcohol self-administration are the amount of pure ethanol consumed, alcohol preference, and total fluid intake. The recent introduction of drinkometer systems along with advanced mathematical time-series analysis of drinking dataCitation25,Citation26 are providing additional information on alcohol consumption. In particular, microdrinking patterns, circadian and ultradian drinking patterns, can be identified.Citation27,Citation28 Knowledge on individual microdrinking patterns may help to forecast excessive drinking episodes and, as such, hold great potential for the development of new preventive strategies to reduce drinking. The use of a drinkometer system also allows the measurement of additional behavioral features, such as licking and wanting, that are relevant to the development of alcohol-use disorders.Citation25,Citation28,Citation29
Operant drug self-administration
Different operant schedules are routinely used in drug and addiction research. The most common schedule of reinforcement is the fixed ratio (FR) schedule. Under a FR schedule, the drug is delivered each time a preselected number of responses have been completed; eg, under an FR1 schedule, a single active-lever response results in the delivery of a small, previously defined quantity of a drug. Under a progressive ratio (PR) schedule, the required ratio increases after a predefined, usually arithmetic, progression. The most common index of performance under a PR schedule is the breaking point, defined as the highest response rate accomplished to obtain a single reinforcer. PR measurements are indicators for the motivation of a subject to obtain a drug. For example, the harder a subject is willing to work under a PR, the higher the breaking point will be, which is then indicative of an incentive motivation for the drug. Not all drugs are equally self-administered under FR or PR schedules, and several factors modify reinforcing efficacy, such as food restriction, previous drug exposure, stress, etc. FR1 performance is less affected by those factors than by other schedules of reinforcement. Therefore, when the objective is to assess the potential liability of a drug or its initial intake as a result of its unconditioned psychopharmacological effects, the FR1 procedure may be a first choice. However, when considering changes in later stages of drug intake, such as the development of addicted behavior interval schedules in which the drug follows a response after a given period of time has elapsed may be more appropriate.Citation8,Citation30 Especially in fixed-interval (FI) schedules, high response rates can be achieved even by drug doses that normally disrupt performances under FR schedules. This is seen in a variety of species and over a wide range of FI valuesCitation30; therefore, FI schedules should be used to study the development of addictive behavior. FR and FI schedules of drug injection can also be combined in secondorder schedules. On a second-order schedule, a subject responds according to one schedule for a brief presentation of a stimulus, such as a light. Responding by the subject on this initial schedule is then reinforced according to another schedule of reinforcement. Second-order schedules of drug injection allow the study of more complex behavioral sequences than do simple schedules and may more accurately reflect the human drug-intake situation.Citation31 Derived from studies of conditioned reinforcement, an important feature of second-order schedules is that responding is maintained by conditioned stimuli. They are thus well-suited to examine cue-induced drug-seeking behavior, including its behavioral, neural, and neurochemical basis.Citation32 Experiments with second-order schedules have, for example, been instrumental for the discovery of the so-called spiraling striatonigrostriatal circuitry,Citation33 which is critically involved in the development and performance of drug habits that are a key characteristic of drug addiction.
Another important modification in operant drug self-administration procedures is the reinstatement model of drug-seeking,Citation34 which became a standard model to assess some properties of addictive behavior and to test potential anticraving and antirelapse compounds.Citation35
Reinstatement and incubation of drug-seeking: animal models that are indicative of craving
In a typical drug reinstatement experiment, an animal is trained to self-administer a drug, and the behavior is then subjected to extinction—that is, the animal is tested under conditions of nonreinforcement until operant responding appears to be extinguished. When the animal reaches some criterion of unresponsiveness, various stimuli are presented. A stimulus is said to reinstate the drug-seeking behavior if it causes renewed responding, ie, lever pressing, without any further response-contingent drug reinforcement. At least three events can reinstate responding: (i) drug priming, that is, the injection of a small dose of the drug; (ii) stress; and (iii) conditioned stimuli. Importantly, after the presentation of a conditioned cue or stress, drug-seeking responses are measured under a drug-free condition. This allows the study of drug-seeking behavior without the interference produced by the psychoactive effects of the drug (ie, a drug that increases locomotor behavior could produce spurious increases in lever pressing), and the increase in the number of operant responses compared with that observed during extinction is inferred to be an enhancement of the subject's drug-seeking behavior.
An alternative method to study drug-seeking behavior relates to the conditioned place preference (CPP) paradigm.Citation8,Citation36 In a typical CPP experiment, subjects are injected daily with the drug and paired with a distinguishable compartment in a conditioning box. A second compartment is paired with vehicle injections. After several days of conditioning, a drug-conditioned place preference is achieved. Then, this acquired preference is extinguished with repeated saline injections in both the previously drug-paired compartment and the saline-paired compartment. Following the extinction phase, the reinstatement of CPP is initiated by drug priming. In comparison with reinstatement testing in an operant self-administration paradigm, this modified CPP procedure allows rapid screening of drug-seeking behavior, eg, in transgenic mice,Citation37 with the disadvantage that stress- or cue-induced drug-seeking responses cannot be assessed.
The reinstatement of drug-seeking behavior can be used to study the neurobiological and molecular basis of drug craving and cue reactivity, as there appears to be a good correspondence between the events that induce drug-seeking in laboratory animals and those that provoke craving and cue reactivity in humans.Citation35 Data derived from over 1200 studies using the reinstatement model suggest that the neuronal substrates that mediate drug-, stress-, and cue-induced reinstatement are not identical.Citation35 The reinstatement model also allows drug testing for potential anticraving properties,Citation35 but the predictive validity is still questionable. All reinstatement procedures include an extinction phase; however, human addicts usually do not undergo extinction, and no systematic study has been conducted to assess its possible consequences on the predictive validity of these procedures. Most important, in this model, the operant response is reinstated, but the subjects, strictly speaking, do not relapse because they do not actually resume drug consumption. Thus, reinstatement testing of drug-seeking behavior is to some degree indicative of drug craving and cue reactivity, but not relapse behavior, in human drug users. Since drug craving in humans involves a very strong urge to seek out the drug, a more appropriate animal model to assess craving-like responses in laboratory animals involves the incubation procedure developed by Shaham and coworkers.Citation38,Citation39
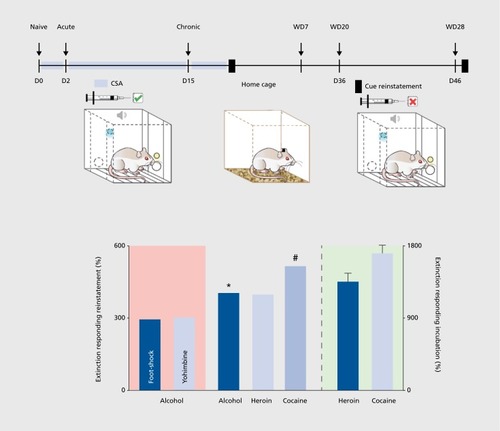
In humans, drug craving can occur in response to environmental stimuli previously associated with drug use, even after extended periods of abstinence. In rats, cue-elicited cocaine seeking has been shown to increase progressively during the first 2 months of abstinence from drug self-administrationCitation38 () This phenomenon, referred to as the incubation of cocaine seeking, is consistent with the hypothesis that in humans, craving increases over time and remains high after a prolonged period of abstinence.Citation40 Time-dependent changes in neuroplasticity in several structures of the mesolimbic brain reward system have been demonstrated during cocaine withdrawal and probably mediate the incubation of cocaine seeking.Citation41,Citation42 It is remarkable that the incubation phenomenon of drug seeking was first discovered in laboratory rats and later also demonstrated to occur in smokers,Citation43 alcoholics,Citation44 and cocaine addictsCitation45—such a direct translation to the human situation indicates high face, construct, and predictive validity of the incubation model of drug craving.
The incubation model has been used for studying neurobiological aspects of craving. Most notable are adaptations in the glutamate system. In particular, calcium permeable GluR2-lacking -αamino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors—which exhibit higher conductance than calcium-impermeable GluA2-containing AMPA receptors—are produced in the nucleus accumbens during prolonged abstinence from cocaine self-administration. These compensatory changes in AMPA-receptor subunit composition alter the properties of neuronal networks and exacerbate disease processes by increasing the reactivity of accumbens neurons to cocaine-associated cues that promote craving.Citation41 At the neural-circuit level, glutamatergic projection neurons from the basolateral amygdala to the nucleus accumbens that impinge on silent synapses—which are excitatory synapses that contain N-methyl-D-aspartate (NMDA) receptors with absent or labile AMPA receptors—play a critical role in the incubation phenomenon.Citation46 Thus, increased silent synapses are seen shortly after cocaine self-administration but not after prolonged abstinence. The unsilencing process that occurs with prolonged cocaine abstinence is associated with the insertion of calcium-permeable AMPA receptors. Re-silencing those synapses, by in vivo optogenetic stimulation, causes downregulation of calcium permeable AMPA receptors and abolishes the incubation of cocaine craving.Citation46 These results are consistent with a causal role of calcium-permeable AMPA receptors in the nucleus accumbens in cocaine craving.Citation41
Although drug craving may not be directly responsible for all compulsive drug use,Citation47 it is a core feature of substance-use disorders and often precedes relapse. Animal models that are based on diagnostic criteria defined in DSM-IV/5 and that also involve the measurement of relapse behavior are discussed below.
DSM-based animal models of relapse and addictive behavior
The current psychiatric diagnostic classification systems—DSM-5 and International Classification of Diseases, 10th revision (ICD10)—are based upon clinical observations and symptom reports by patients and are by nature built on anthropomorphic terms. Thus, it is argued that contemporary psychiatry uses a syndrome-based disease classification that is not based on mechanisms and does not guide treatment.Citation48 As a result, a number of different psychiatric diagnoses largely overlap in terms of their symptoms, underlying neurobiological mechanisms, and genetic risk factors. For example, cue-mediated craving involves largely overlapping neuronal substrates of reactivity to drug, food, and sexual cuesCitation49 and is therefore not exclusive for addictive disorders, but rather resembles the intersection of information pathways for processing reward, emotional responses, nondeclarative memory, and obsessive-compulsive behavior. This highlights the ambiguities associated with the classification of mental disorders by DSM-5 or ICD10.
Despite all of this criticism of our current psychiatric diagnostic classification system and recent efforts toward a new diagnosis system, such as the Research Domain Criteria project (RDoC),Citation50 we should realize that DSM-5 and ICD10 are used worldwide and will certainly be used in the coming decades. Therefore it is argued that if we are making our diagnoses according to DSM-5, it is logical that we must gear our research toward DSM-5. Therefore, our animal models should be based on DSM-5 criteria—is this possible? Modeling the entire spectrum of a complex human mental disorder, such as addiction, in animals is not possible because of its complexity. However, we can translate anthropomorphic terminology into objectively and behaviorally measurable parameters and can thereby model at least some key criteria of the disorder. Especially when it comes to relapse behavior, this is a straightforward endeavor, as a relapse is defined as the recurrence of a past condition, namely excessive and uncontrolled drug intake after a phase of abstinence. In particular, the alcohol-deprivation model provides excellent face validity to relapse behavior seen in alcoholics.
The alcohol-deprivation-effect model for relapse
In animals with voluntary access to alcohol for a certain period of time and that are then deprived of alcohol for several days/weeks/months, the representation of alcohol leads to a robust but temporally limited increase in alcohol intake over baseline drinking, a relapse-like drinking referred to as the alcohol deprivation effect (ADE).Citation51 The ADE can be achieved under both operantCitation52 and nonoperant home-cage free-choice drinking conditions.Citation14,Citation53 There are several experimental and biological factors that influence the magnitude and duration of the ADE. As said, concurrent access to more than one alcohol concentration (eg, 5%, 10%, and 20% v/v ethanol solutions vs water) enhances the magnitude and duration of the ADE.Citation14 The magnitude of the ADE also depends on the duration of access to alcohol and on the length of abstinence. It has been demonstrated that only long-lasting alcohol consumption, for at least 6 to 8 weeks, will lead to a reliable ADE, and that at least 2 days of withdrawal are needed to increase alcohol consumption by more than 50%.Citation14 Data from different rat strains and alcohol-preferring rat lines show that levels of baseline alcohol intake do not correlate with the robustness of the ADE, suggesting that baseline alcohol drinking behavior and relapse-like drinking behavior are controlled, at least in part, via different brain systems.Citation9,Citation53
Long-term alcohol consumption with repeated deprivation phases in rats—a model of compulsive drinking in a relapse situation
This animal model is designed to demonstrate compulsive drinking during a relapse situation. Alcohol, unlike cocaine or opioids, is a weak reinforcer and as such requires long-term exposure to induce compulsiveness during a relapse situation. Such conditions might be difficult to achieve with relatively short operant training procedures. Therefore, voluntary long-term oral alcohol consumption is a prerequisite for the development of compulsive drinking; ie, rats must have free access to alcohol for at least 8 months. In addition, this access should be interrupted repeatedly with forced abstinence phases. At the end of this procedure, compulsive drinking during an ADE can be measured. For this purpose, two procedures are used—taste adulteration testing with quinine and monitoring of the circadian drinking rhythmicity. In the taste adulteration test, the taste of alcohol solutions is altered with bitter quinine; alternatively, animals could be offered a highly palatable sucrose solution instead of water.Citation14,Citation54 An animal is expected to naturally choose a more palatable (or less aversive) fluid as a drinking source. Those animals that exhibit an ADE despite alcohol taste adulteration with quinine or a competitive choice of a highly palatable fluid are classified as compulsive animals in this experimental setting. It has been demonstrated that taste adulteration reduced post-abstinence drinking in Wistar rats after a short-term alcohol experience, suggesting at least partial control of the behavior at this stage.Citation55 However, long-term chronic alcohol consumption repeatedly interrupted with deprivation phases was shown to lead to an animal's complete loss of control over behavior, as taste adulteration procedures no longer modified the ADE.Citation55 This behavior resembles the continuation of drug use despite clear evidence of overtly harmful consequences and neglect of alternative interests. Another sign of compulsive alcohol drinking is an alteration of the normal circadian drinking pattern.Citation25,Citation28 Orcadian disturbances have been reported in human addicts.Citation56 In long-term drinking rats repeatedly deprived of alcohol for several weeks, re-exposure leads to increased drinking frequency and a loss of diurnal drinking rhythmicity during the first post-abstinence days. Specifically, rats show increased wanting of more highly concentrated alcohol solutions to more rapidly achieve high blood alcohol concentrations.Citation25,Citation28,Citation29
Many drug targets and more than 50 different putative anti-relapse compounds have already been tested in this model.Citation57 In alcoholics, medications that reduce relapse rates, such as naltrexone and acamprosate, also reduce compulsive drinking during an ADE,Citation58,Citation59 demonstrating the predictive power of this animal model. Drugs such as agomelatine or melatonin, which normalize circadian drinking activity during the ADE,Citation28 could easily be transferred to the clinical situation. Agomelatine is clinically used as an antidepressive medication and is already approved in many countries.Citation60 Given that there is a high comorbidity between alcoholism and depressive behavior, general practitioners can easily prescribe agomelatine for this comorbid condition.
To summarize, the model of long-term alcohol consumption with repeated deprivation phases allows distinguishing between addicted and non-addicted animals and examining the transition from controlled drug use to compulsive drug wanting. A similar model has been developed for cocaine addiction in rats.
A DSM-based animal model of cocaine addiction
A diagnosis of cocaine addiction is given when an individual shows multiple signs of loss of control over drug use. Signs of loss of control can be indicated by the following: (i) the inability to refrain from drug seeking; (ii) high motivation for the drug; and (iii) maintained drug use despite negative consequences. These three signs constitute the clinical-based theoretical background of the multisymptomatic 0/3 criteria (0/3crit) model of cocaine addiction.Citation61-Citation65 Matching prevalence rates of cocaine-addicted individuals,Citation62 15% to 20% of a rat cohort loses control over cocaine intake after prolonged training, showing addict-like behavior (3crit), whereas the majority of animals maintain control over cocaine intake, showing nonaddict-like behavior (0crit). Hence, the 0/3crit model of addiction is a multidimensional experimental approach aimed at identifying subpopulations of rats possessing vulnerability (3crit) and resilience (0crit) toward cocaine-addiction-like behavior. In the 0/3crit model, the three clinical signs of loss of control listed above are modeled by three corresponding addiction-like criteria: (i) persistence in drug seeking when the drug is signaled but not available; (ii) motivation to self-administer cocaine under a PR schedule of reinforcement; and (iii) maintenance of cocaine drug seeking and taking despite punishment by an electric footshock () 0crit and 3crit rats represent the two opposite extremes in each of these behaviors. The 0crit rats show nonaddict-like behavior, characterized by refraining from drug seeking when cocaine is not available, a lack of motivation to produce increasing effort to get their dose, and avoidance of cocaine self-administration during punishment. On the contrary, 3crit rats show addict-like behavior, being unable to refrain from drug seeking when cocaine is unavailable, requires increasing effort, or is punished.Citation62,Citation64 Therefore, the 0/3crit model of addiction allows us to correlate behavioral subdimensions of cocaine addiction with specific brain alterations, either on the molecularCitation65,Citation66 or neuroanatomical level.Citation67 Although very time- and labor-intensive, drug testing in the 0/3crit model provides valuable preclinical information for drug development,Citation68 and similar to the findings in the ADE model, melatonin reduces motivation for cocaine self-administration and prevents relapse behavior in cocaineaddicted rats.Citation69
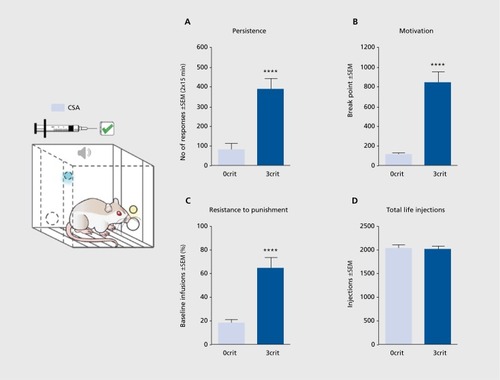
Most strikingly, the 0/3 crit model of addiction provided new insight into the neurobiological understanding of a transition into a pathological state. During the acquisition of cocaine self-administration, NMDA-receptor-mediated long-term depression (LTD) in the nucleus accumbens is essential for learning new rewardresponse associations. Once the learning has been consolidated and with further cocaine self-administration, LTD is suppressed in all rats. However, after prolonged cocaine self-administration, LTD is progressively recovered in animals that maintain a controlled drug intake (0crit), whereas it is persistently lost in animals undergoing the transition to addiction (3crit).Citation65 This persistent impairment in LTD could explain the loss of control on drug intake observed in 3crit rats. LTD in the nucleus accumbens is considered important in rescaling synapses that are enhanced during acquisition of cue-reward associations, allowing those synapses to encode future associations and restore flexibility to neuronal circuits. The persistent inability to rescale synapses in 3crit rats may render drug-seeking behavior consistently resistant to modulation by environmental contingencies, finally resulting in loss of control over drug intake. Thus, the major behavioral difference between 3crit and 0crit, ie, between addicted vs nonaddicted subjects, is their capacity to adjust their drug intake as a function of environmental contingencies. Nonaddicts can stop seeking drugs if they know that the drug is not available, if it requires an excessively high workload, or if taking the drug results in negative consequences. Addicts have lost this ability and continue to seek drugs independently of environmental conditions. In conclusion, the transition to addiction is associated, at least in the nucleus accumbens, with a form of anaplasticity, ie, the incapacity of addicted subjects to counteract initial drug-induced impairments.Citation65 The anaplasticity of addicted rats is relevant to revising conceptualizations of the transition to addiction, currently seen as the progressive development of specific brain adaptations that lead to loss of control over drug intake. In fact, anaplasticity means the loss of neuronal and molecular adaptations that characterize an addicted brain—a new concept that can be applied to most brain pathologies.
Rats are a better model system than mice to study addictive behavior
Despite major efforts, the multisymptomatic model of cocaine addiction has not been successfully established in mice. The attempt to establish a model of compulsive drinking behavior during a relapse situation (as described above) also failed in mice.Citation52 Establishing such mouse models has potential value in behavioral neuroscience and genetics due to the availability of a large number of genetically modified mouse lines that could be tested in this model in order to contribute to our understanding of the mechanisms involved in the transition from controlled to compulsive drug use and to functionally validate genetic risk variants derived from genome-wide association studies.Citation70 However, with the introduction of new gene editing tools, such as CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats [CRISPR]/CRISPR-associated protein-9 nuclease),Citation71 any specific rat mutant of interest can be generated. Thus, genetic manipulations are no longer an argument to use mice. There are also other technical considerations in favor of using rats in addiction research.Citation72 Brain size matters for performing in vivo electrophysiological recording in conjunction with behavior and translational neuroimaging.The use of different modalities in magnetic resonance imaging has great potential for translation. For example, glutamate spectroscopy in alcohol-dependent rats and alcohol-dependent humans has yielded identical results: during withdrawal, both rats and humans demonstrate hyperglutamatergic activity in different brain sites that diminishes when withdrawal symptomatology subsides.Citation73 Furthermore, intracranial surgery produces less damage to brain tissue in rats than in mice. Intravenous surgery and catheter handling are also more straightforward in rats; for example, it would be impossible to maintain catheter function for months in mice as required for the multisymptomatic model of cocaine addiction. Certainly, these technical advantages come with costs. Rat breeding colonies, especially with the use of double transgenic animals, are much more cost intensive and take more space in the animal facility. The smaller size of mice also means that a lower amount of drug or virus can be administered, which is more cost effective.
There are also biological factors to be considered. Rats can more readily learn complex operant training schedules relative to mice, which often require more training and higher numbers of animals per group due to larger individual variability.Citation72 Considering the number of responses on the inactive lever, mice and rats should be viewed differently, since in the former, lever pressing per se seems to be a reinforcing activity.Citation8 Thus, in a reinstatement test, mice may exhibit high inactive lever responding. Rats are social animals and are the first choice when considering social factors in drug and addiction studies. Mice in the wild are nonsocial and spend significantly less time interacting with conspecifics and many even find such interactions aversive, whereas rats are social animals; in fact, rats find social interaction even more rewarding than cocaine.Citation74
Most importantly, the pharmaceutical industry has built its pharmacological databases upon rats, and mice can sometimes produce paradoxical drug effects when compared with humans. Ellenbroeck and Youn have recently summarized fundamental differences between rats and mice in drug development and addiction research and conclude that rats are the optimal rodent model for studies of human addictive behavior.Citation72
Conclusions and future perspectives
In my brief overview of nonoperant and operant models of drug-taking behavior, incubation of drug-seeking, relapse, and DSM-based animal models, some readers may miss their favorite procedure or model. For example, intracranial self-stimulationCitation75—an excellent test procedure to measure the rewarding value of a drug or dysphoria during withdrawalCitation76 and to evaluate the abuse potential of a drug—has not been discussed here. This experimental method is a “test” that does not have a direct resemblance to the human situation and can therefore not be considered as an animal model of drug use or addiction. This statement also relates to the phenomenon of behavioral sensitization, which refers to the ability of addictive drugs to progressively increase locomotion after repeated administration in a variety of laboratory animals.Citation29 Although this phenomenon plays an important role in the incentive-sensitization theory of addiction,Citation29 the possible relevance of drug-induced sensitization in humans is still unclear. This does not devaluate the importance of testing the phenomenon of drug-induced sensitization, but I do suggest that the models described here have a more direct translational value to the human situation.
Twenty years ago Alan Leshner, the former director of the National Institute on Drug Abuse, proclaimed that “addiction is a brain disease and it matters.”Citation77 Although this statement gave an enormous boost to addiction research from a neuroscience perspective, it is my strong belief that the whole organism has to be taken into consideration to provide the best therapy for our patients. In the future, heart, liver, and other organ physiology should also be taken into consideration when developing new animal models. Most important, addiction researchers should focus more on data robustness and data generalizability when designing preclinical studies.
The author would like to thank Rick E. Bernardi for text editing. This work was supported by the Deutsche Forschungsgemeinschaft (DFG): Reinhart-Koselleck Award SP 383/5-1 and the ERANET COCADDICT grant. The author reports no conflicts of interest.
REFERENCES
- HymanSE.Psychiatric drug development: diagnosing a crisis.Cerebrum.20132013523720708
- BespalovA.StecklerT.AltevogtB.et alFailed trials for central nervous system disorders do not necessarily invalidate preclinical models and drug targets.Nat Rev Drug Discov.2016157516520
- CookD.BrownD.AlexanderR.et alLessons learned from the fate of AstraZeneca's drug pipeline: a five-dimensional framework.Nat Rev Drug Discov.201413641943124833294
- NesseRM.BerridgeKG.Psychoactive drug use in evolutionary perspective.Science.1997278533563669311928
- SullivanRJ.HagenEH.Psychotropic substance-seeking: evolutionary pathology or adaptation?Addiction.200297438940011964056
- WiensF.ZitzmannA.LachanceMA.et alChronic intake of fermented floral nectar by wild treeshrews.Proc Natl Acad Sci U SA.2008105301042610431
- CarriganMA.UryasevO.FryeCB.et alHominids adapted to metabolize ethanol long before human-directed fermentation.Proc Natl Acad Sci USA.2015112245846325453080
- Sanchis-SeguraC.SpanagelR.Behavioural assessment of drug reinforcement and addictive features in rodents: an overview.Addict Biol.200611123816759333
- VengelieneV.BilbaoA.MolanderA.SpanagelR.Neuropharmacology of alcohol addiction.Br J Pharmacol.2008154229931518311194
- HerzA.Endogenous opioid systems and alcohol addiction.Psychopharmacology.19971292991119040115
- MannK.BladstromA.TorupL.GualA.van den BrinkW.Extending the treatment options in alcohol dependence: a randomized controlled study of as-needed nalmefene.Biol Psychiatry.201373870671323237314
- MeischRA.Oral drug self-administration: an overview of laboratory animal studies.Alcohol.200124211712811522433
- SalamoneJD.CorreaM.MingoteSM.WeberSM.Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine.Curr Opin Pharmacol.200551344115661623
- SpanagelR.HolterSM.Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism?Alcohol Alcohol.199934223124310344783
- QuintanillaME.IsraelY.SapagA.TampierL.The UChA and UChB rat lines: metabolic and genetic differences influencing ethanol intake.AddictBiol.2006113-4310323
- SommerW.HyytiäP.KiianmaaK.The alcohol-preferring AA and alcohol-avoiding ANA rats: neurobiology of the regulation of alcohol drinking.Addict Biol.2006113-428930916961760
- CrabbeJC.PhillipsTJ.BelknapJK.The complexity of alcohol drinking: studies in rodent genetic models.Behav Genet.201040673775020552264
- MeinhardtMW.SommerWH.Postdependent state in rats as a model for medication development in alcoholism.Addict Biol.201520112125403107
- MeinhardtMW.HanssonAC.Perreau-LenzS.et alRescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence.J Neurosci.20133372794280623407939
- HirthN.MeinhardtMW.NooriHR.et alConvergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence.Proc Natl Acad Sci US A.20161131130243029
- HeiligM.KoobGF.A key role for corticotropin-releasing factor in alcohol dependence.Trends Neurosci.200730839940617629579
- SommerWH.RimondiniR.HanssonAC.et alUpregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala Crhr1 expression following a history of dependence.Biol Psychiatry.200863213914517585886
- KwakoLE.SpagnoloPA.SchwandtML.et alThe corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study.Neuropsychopharmacology.20154051053106325409596
- SchwandtML.CortesCR.KwakoLE.et alThe CRF1 antagonist verucerfont in anxious alcohol-dependent women: translation of neuroendocrine, but not of anti-craving effects.Neuropsychopharmacology.201641122818282927109623
- VengelieneV.NooriHR.SpanagelR.The use of a novel drinkometer system for assessing pharmacological treatment effects on ethanol consumption in rats.Alcohol Clin Exp Res.201337E322E32822757984
- Villarín PildaínL.VengelieneV.MatthausF.New measurement criteria for studying alcohol drinking and relapse in rodents.In Silico Pharmacol.201311325505658
- EisenhardtM.LeixnerS.SpanagelR.BilbaoA.Quantification of alcohol drinking patterns in mice.Addict Biol.20152061001101126515884
- VengelieneV.NooriHR.SpanagelR.Activation of melatonin receptors reduces relapse-like alcohol consumption.Neuropsychopharmacology.201540132897290625994077
- BerridgeKC.RobinsonTE.Liking, wanting, and the incentive-sensitization theory of addiction.Am Psychol.201671867067927977239
- SpealmanRD.GoldbergSR.Drug self-administration by laboratory animals: control by schedules of reinforcement.Annu Rev Pharmacol Toxicol.197818313339348062
- SchindlerCW.PanlilioLV.GoldbergSR.Second-order schedules of drug self-administration in animals.Psychopharmacology (Berl).20021633432734412373434
- EverittBJ.RobbinsTW.Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour.Psychopharmacology (Berl) .20001531173011255926
- BelinD.EverittBJ.Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum.Neuron.200857343244118255035
- de WitH.StewartJ.Reinstatement of cocaine-reinforced responding in the rat.Psychopharmacology.19817521341436798603
- BossertJM.MarchantNJ.CaluDJ.ShahamY.The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research.Psychopharmacology.2013229345347623685858
- TzschentkeTM.Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade.Addict Biol.2007123422746217678505
- EngblomD.BilbaoA.Sanchis-SeguraC.et alGlutamate receptors on dopamine neurons control the persistence of cocaine seeking.Neuron.200859349750818701074
- GrimmJW.HopeBT.WiseRA.ShahamY.Neuroadaptation. Incubation of cocaine craving after withdrawal.Nature.2001412684314114211449260
- VenniroM.CaprioliD.ShahamY.Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence.Prog Brain Res.2016224255226822352
- GawinFH.KleberHD.Abstinence symptomatology and psychiatric diagnosis in cocaine abusers.Arch Gen Psychiatry19864321071133947206
- ConradKL.TsengKY.UejimaJL.et alFormation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving.Nature.2008454720011812118500330
- LiX.CaprioliD.MarchantNJ.Recent updates on incubation of drug craving: a mini-review.Addict Biol.201520587287625440081
- BediG.PrestonKL.EpsteinDH.et alIncubation of cue-induced cigarette craving during abstinence in human smokers.Biol Psychiatry.201169770871120817135
- LiP.WuP.XinX.et alIncubation of alcohol craving during abstinence in patients with alcohol dependence.Addict Biol.201520351352224698092
- ParvazMA.MoellerSJ.GoldsteinRZ.Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography.JAMA Psychiatry.201673111127113427603142
- LeeBR.MaYY.HuangYH.et alMaturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving.Nat Neurosci.201316111644165124077564
- TiffanyST.A cognitive model of drug urges and drug use behavior: role of automatic and nonautomatic processes.Psychol Rev.19909721471682186423
- StephanKE.BachDR.FletcherPC.et alCharting the landscape of priority problems in psychiatry, part 1: classification and diagnosis.Lancet Psychiatry.201631778326573970
- NooriHR.Cosa LinanA.SpanagelR.Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: a comprehensive meta-analysis.Eur Neuropsychopharmacol.20162691419143027397863
- CuthbertBN.InselTR.Toward the future of psychiatric diagnosis: the seven pillars of RDoC BMCMed.201311126
- SinclairJD.SenterRJ.Increased preference for ethanol in rats following alcohol deprivation.Psychon Sci.196781112
- HölterSM.LandgrafR.ZieglgansbergerW.SpanagelR.Time course of acamprosate action on operant ethanol self-administration after ethanol deprivation.Alcohol Clin Exp Res.19972158628689267536
- VengelieneV.BilbaoA.SpanagelR.The alcohol deprivation effect model for studying relapse behavior: a comparison between rats and mice.Alcohol.201448331332024811155
- WolffgrammJ.HeyneA.From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat.Behav Brain Res.199570177948519431
- SpanagelR.HölterSM.AllinghamK.LandgrafR.ZieglgansbergerW.Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat.Eur J Pharmacol.19963051-339448813529
- DanelT.ToitouY.Chronobiology of alcohol: from chronokinetics to alcohol-related alterations of the circadian system.Chronobiol Int.200421692393515646239
- SpanagelR.Alcoholism: a systems approach from molecular physiology to addictive behavior.Physiol Rev.200989264970519342616
- HölterSM.SpanagelR.Effects of opiate antagonist treatment on the alcohol deprivation effect in long-term ethanol-experienced rats.Psychopharmacology.1999145436036910460312
- BachtelerD.EconomidouD.DanyszW.CiccocioppoR.SpanagelR.The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat.Neuropsychopharmacology.20053061104111015668725
- TaylorD.SparshattA.VarrnaS.OlofinjanaO.Antidepressant efficacy of agomelatine: meta-analysis of published and unpublished studies.BMJ.2014348g188824647162
- Deroche-GamonetV.BelinD.PiazzaPV.Evidence for addiction-like behavior in the rat.Science.200430556861014101715310906
- VanderschurenLJ.EverittBJ.Drug seeking becomes compulsive after prolonged cocaine self-administration.Science.200430556861017101915310907
- Deroche-GamonetV.PiazzaPV.Psychobiology of cocaine addiction: contribution of a multi-symptomatic animal model of loss of control.Neuropharmacology.201476Pt B43744923916478
- Belin-RauscentA.FouyssacM.BonciA.BelinD.How preclinical models evolved to resemble the diagnostic criteria of drug addiction.Biol Psychiatry.2016791394625747744
- KasanetzF.Deroche-GamonetV.BersonN.et alTransition to addiction is associated with a persistent impairment in synaptic plasticity.Science.201032859861709171220576893
- KasanetzF.LafourcadeM.Deroche-GamonetV.et alPrefrontal synaptic markers of cocaine addiction-like behavior in rats.Mol Psychiatry.201318672973722584869
- CannellaN.Cosa-LinanA.BüchlerE.Falfan-MelgozaC.Weber-FahrW.SpanagelR.In vivo structural imaging in rats reveals neuroanatomical correlates of behavioral sub-dimensions of cocaine addiction. Addict Biol. 2017 Feb 23. Epub ahead of print. doi:10.1111/adb.1 2500.
- CannellaN.HalboutB.UhrigS.EvrardL.et alThe mGluR2/3 agonist LY379268 induced anti-reinstatement effects in rats exhibiting addiction-like behavior.Neuropsychopharmacology.201338102048205623624743
- TakahashiTT.VengelieneV.SpanagelR.Melatonin reduces motivation for cocaine self-administration and prevents relapse-like behavior in rats.Psychopharmacology (Berl).2017234111741174828246896
- SpanagelR.Convergent functional genomics in addiction research - a translational approach to study candidate genes and gene networks.In Silico Pharmacol.201311825505662
- ShaoY.GuanY.WangL.et alCRISPR/Cas-mediated genome editing in the rat via direct injection of one-cell embryos.NatProtoc.201491024932512
- EllenbroekB.YounJ.Rodent models in neuroscience research: is it a rat race?Dis Model Mech.20169101079108727736744
- HermannD.Weber-FahrW.SartoriusA.et alTranslational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats.Biol Psychiatry.201271111015102121907974
- FritzM.El RawasR.SaltiA.et alReversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats.Addict Biol.201116227328421309948
- NegusSS.MillerLL.Intracranial self-stimulation to evaluate abuse potential of drugs.Pharmacol Rev.201466386991724973197
- Epping-JordanMP.WatkinsSS.KoobGF.MarkouA.Dramatic decreases in brain reward function during nicotine withdrawal.Nature.1998393668076799590692
- LeshnerAl.Addiction is a brain disease, and it matters.Science.1997278533545479311924
