Abstract
Psychiatric patients have a greater risk of premature mortality, predominantly due to cardiovascular diseases (CVDs). Convincing evidence shows that psychiatric conditions are characterized by an increased risk of metabolic syndrome (MetS), a clustering of cardiovascular risk factors including dyslipidemia, abdominal obesity, hypertension, and hyperglycemia. This increased risk is present for a range of psychiatric conditions, including major depressive disorder (MDD), bipolar disorder (BD), schizophrenia, anxiety disorder, attention-deficit/hyperactivity disorder (ADHD), and posttraumatic stress disorder (PTSD). There is some evidence for a dose-response association with the severity and duration of symptoms and for a bidirectional longitudinal impact between psychiatric disorders and MetS. Associations generally seem stronger with abdominal obesity and dyslipidemia dysregulations than with hypertension. Contributing mechanisms are an unhealthy lifestyle and a poor adherence to medical regimen, which are prevalent among psychiatric patients. Specific psychotropic medications have also shown a profound impact in increasing MetS dysregulations. Finally, pleiotropy in genetic vulnerability and pathophysiological mechanisms, such as those leading to the increased central and peripheral activation of immunometabolic or endocrine systems, plays a role in both MetS and psychiatric disorder development. The excess risk of MetS and its unfavorable somatic health consequences justifies a high priority for future research, prevention, close monitoring, and treatment to reduce MetS in the vulnerable psychiatric patient.
Los pacientes psiquiátricos tienen un mayor riesgo de mortalidad prematura, principalmente por enfermedades cardiovasculares (ECVs). Existe evidencia convincente que muestra que las condiciones psiquiátricas están caracterizadas por un aumento del riesgo del síndrome metabólico (SMet), un conjunto de factores de riesgo cardiovascular que incluyen dislipidemia, obesidad abdominal, hipertensión e hiperglicemia. Este riesgo aumentado se presenta en diversas condiciones psiquiátricas como trastorno depresivo mayor (TDM), trastorno bipolar (TB), esquizofrenia, trastorno de ansiedad, trastorno por déficit de atención con hiperactividad (TDAH) y trastorno por estrés postraumático (TEPT). Al parecer existe alguna evidencia de una asociación dosis-respuesta entre la gravedad y duración de los síntomas y el impacto longitudinal bidireccional entre los trastornos psiquiátricos y el SMet. En general las asociaciones parecen más potentes con la obesidad abdominal y la dislipidemia que con la hipertensión. Los mecanismos que contribuyen a esto son un estilo de vida poco saludable y una pobre adherencia al tratamiento médico, condiciones que son prevalentes entre los pacientes psiquiátricos. Los medicamentos psicotrópicos específicos también han demostrado un impacto importante en el aumento de las fallas en la regulación del SMet. Por último, la pleiotropía en la vulnerabilidad genética y los mecanismos fisiopatológicos, como los que conducen a una mayor activación central y periférica de los sistemas inmunometabólico y endocrino, tienen un papel tanto en el desarrollo del SMet como del trastorno psiquiátrico. El riesgo aumentado del SMet y las consecuencias desfavorables en la salud somática justifican una prioridad alta para la investigación, prevención, monitorización estricta y tratamiento para reducir a futuro el SMet en el paciente psiquiátrico vulnerable.
Les patients psychiatriques ont un risque plus élevé de mortalité prématurée, surtout en raison des maladies cardiovasculaires (MCV). D'après des données convaincantes, les troubles psychiatriques se caractérisent par un risque augmenté de syndrome métabolique (SM), un ensemble de facteurs de risque cardiovasculaire comprenant une dyslipidémie, une obésité abdominale, une hypertension et une hyperglycémie. Dans ces troubles psychiatriques, on trouve le trouble dépressif caractérisé (TDC), le trouble bipolaire (TB), la schizophrénie, le trouble anxieux, le trouble déficit de l'attention/hyperactivité (TDAH) et le trouble de stress post-traumatique (TSPT). Il semble exister une association dose-réponse entre la sévérité et la durée des symptômes et l'impact longitudinal bidirectionnel entre les troubles psychiatriques et le SM. Ces associations paraissent généralement plus fortes avec l'obésité abdominale et les dyslipidémies qu'avec l'hypertension. Un mode de vie malsain et une mauvaise adhésion au traitement médical, fréquents chez les patients psychiatriques, y participent. Les traitements psychotropes spécifiques influent fortement sur l'augmentation des dysrégulations du SM. Enfin, la pléiotropie de la vulnérabilité génétique et des mécanismes physiopathologiques, comme de ceux qui augmentent l'activation centrale et périphérique des systèmes endocriniens ou immunométaboliques, joue un rôle dans le développement à la fois du SM et des troubles psychiatriques. Ce risque majoré de SM et ses conséquences négatives sur la santé somatique justifient une priorité élevée pour la recherche, la prévention, la surveillance étroite et le traitement afin de diminuer dans l'avenir le SM chez les patients psychiatriques vulnérables.
Introduction
Psychiatric patients have a greater risk of premature all-cause mortality than the general population. Epidemiological studies show that the life expectancy of patients with major psychiatric disorders is reduced by 7 to 24 years.Citation1 Psychiatric illness takes a toll at least as great as the 8-year difference exacted by heavy smoking.Citation1 About 60% of the excess mortality observed in psychiatric patients is due to physical comorbidities, predominantly cardiovascular diseases (CVDs).Citation2 Indeed, the CVD risk, but also that of the related comorbidities of diabetes, stroke, and obesity, has proven to be significantly increased in a multitude of psychiatric conditions, including depression,Citation3 schizophrenia,Citation4 bipolar disorder (BD),Citation5 and anxiety disorder.Citation6
To assist clinicians in identifying and treating patients at an increased risk of CVD, the concept of metabolic syndrome (MetS) was introduced. MetS is defined by a combination of abdominal obesity (also known as central obesity), high blood pressure, low high-density lipoprotein cholesterol (HDL-C), elevated triglycerides, and hyperglycemia. MetS indicates a preclinical state for the development of CVD and diabetes.Citation7 Various definitions for MetS have been proposed and are all aimed at being easy to use in clinical settings and all share similar diagnostic thresholds.Citation8 However, abdominal obesity is central to the MetS definition of the International Diabetes Federation, whereas it is not a mandatory criterion in the MetS definition of the National Cholesterol Education Program (NCEP) Adult Treatment Panel III.Citation8 One should be aware that MetS is a heterogeneous concept: hypertension, dyslipidemia, and hyperglycemia are highly comorbid and intercorrelated, but their pathophysiologies do not necessarily overlap. However, as a prevalent condition and predictor of CVD across racial, gender, and age groups, MetS provides the opportunity to identify high-risk populations and prevent the progression of some major causes of morbidity and mortality.
In line with an increased cardiovascular mortalityrisk, a recent meta-analysis showed that the prevalence of MetS is 58% higher in psychiatric patients than in the general population.Citation2 This increased prevalence is seen independently of the MetS definition used and is consistently observed for each of the MetS components, although to a lesser extent in hypertension. The risk of MetS was similarly elevated in those with schizophrenia, BD, and major depressive disorder (MDD), which suggests that MetS is a general comorbidity seen in different psychiatric patient groups. Consequently, it is likely that in large part, general, nonspecific disease mechanisms contribute to the MetS-psychiatric disease comorbidity. Below we describe the current evidence base of MetS dysregulations in various individual psychiatric conditions. Mechanisms inherent to psychiatric disorders that contribute to an increased MetS risk are then discussed. We conclude by describing clinical implications and future directions.
MetS and major depression
Pan et al systematically reviewed 29 cross-sectional studies involving 155 333 subjects and found depression (either defined through self-reported symptoms or a psychiatric disorder) and MetS to be modestly associated (adjusted odds ratio [OR]=1.34).Citation9 In the approximately 3000 subjects in the Netherlands Study of Depression and Anxiety conducted among psychiatric patients, we confirmed an association between major depression and MetS and showed evidence for a dose-response association between the two.Citation10 Prospective evidence is scarce but does confirm a bidirectional relationship, with depression predicting the onset of MetS, and MetS predicting the onset of depression over time.Citation9 The most consistent evidence exists between depression and obesity-related components (abdominal obesity, low HDL-C, hypertriglyceridemia), whereas associations with hyperglycemia and hypertension are confirmed less frequently.Citation11 Three longitudinal studies among depressed patients found that a combination of multiple metabolic dysregulations contributes to the sustained chronicity of depression.Citation12-Citation14 Once both are present, MetS abnormalities may contribute to patients maintaining a depressed state.
To what extent does antidepressant utilization contribute to an increased MetS risk among depressed individuals? Several studies have illustrated that antidepressants have an impact on (subtle) metabolic dysregulations. There is consistent evidence that antidepressant medications, especially tricyclic antidepressants (TCAs) and serotonin and norepinephrine reuptake inhibitors (SNRIs), increase cardiac vagal control,Citation11,Citation15 which contributes to elevated systolic and diastolic blood pressure and hypertension among medication users.Citation16 Autonomic activity differences diminished when antidepressant medication use was stopped.Citation15 In a meta-analysis of treatment trials, Serretti and MandelliCitation17 evaluated short-term weight change after antidepressant treatment. Amitriptyline, mirtazapine, and paroxetine were associated with a greater risk of weight gain. In contrast, weight loss seems to occur with fluoxetine and bupropion, although the effect of fluoxetine appears to be limited to the acute phase of treatment. Other compounds were found to have a transient or negligible effect on body weight in the short term. It is important to indicate that —despite some detrimental metabolic impact— a favorable impact of antidepressant use on body weight has been illustrated. Hannestad and colleaguesCitation18 meta-analyzed 22 studies and found that antidepressants, mainly selective serotonin reuptake inhibitors (SSRIs), reduced cytokine levels during treatment.
In a 6-year observational study with three assessment waves, antidepressant use was consistently associated with metabolic dysregulation at all assessment waves, and it exerted a negative, longitudinal impact on subsequent metabolic health.Citation19 Compared with antidepressant nonusers, TCA use was associated with lower HDL-C, and the use of most types of antidepressants (TCA, SSRI, SNRI) was associated with higher waist circumference, triglycerides, and a number of MetS abnormalities. Effect sizes observed were somewhat stronger for TCA use than for use of SSRIs and SNRIs, particularly for waist circumference. As this study also included drug-naive depressed patients, it could illustrate that both symptom severity and antidepressant use exerted independent effects on MetS and that patients without antidepressant medication have an increased MetS risk. Finally, it is important to point out that the prevalence of MetS abnormalities may partly depend on the depression symptom profile. Recent studies point more toward MetS abnormalities in depressed persons with many atypical, neurovegetative symptoms, including hyperphagia, hypersomnia, lack of energy, and leaden paralysis.Citation11,Citation20,Citation21
MetS and bipolar disorder
In line with observations on MDD, MetS prevalence has been found to be higher in BD. In a study among 972 younger bipolar and unipolar depressed patients in a current depressive episode, both patient groups showed a comparably higher MetS prevalence than population controls.Citation22 Patients had higher body mass index (BMI), higher levels of glucose, total cholesterol, low-density-lipoprotein cholesterol (LDL-C), and lower HDL-C levels, but did not differ in hypertension from healthy controls. Other studies have indicated that the increased MetS risk extends to BD patients who are not in a current depressive episode. Vancampfort et al meta-analyzed 37 studies involving around 7000 BD patients and found an overall MetS rate of 37.3%. Citation23 When compared with general population groups, BD patients had 1.98-times higher MetS rates.
Vancampfort's meta-analysis also investigated the role of clinical characteristics.Citation23 As five studies investigated patients with bipolar 1 disorder (BD-1), and others concerned mixed or unspecified diagnostic groups, it was possible to compare MetS risks. The BD-1 patients had a significantly lower risk of MetS. However, as the mixed or unspecified diagnostic groups were older on average, this result could simply reflect age differences. Six of the included studies in the meta-analysisCitation23 also reported MetS prevalence in BD patients using antipsychotic medication compared with antipsychotic-free BD patients. BD patients using antipsychotic medication were at a significantly 1.72 times greater risk of MetS relative to antipsychotic-free patients. Individual studies that directly compared BD patients on medication with those using placebo or no medication confirm the role of medication on metabolic dysregulations. A meta-analysis demonstrated that patients receiving lithium gained more weight than those receiving a placebo.Citation24 Similarly, in a pooled analysis of placebo-controlled trials in patients with acute mania associated with BD-1, olanzapine, quetiapine, risperidone, and valproic acid were all associated with greater weight gain than the placebo.Citation25 As most studies examined BD patients on psychotropic medications, there is not much literature on the MetS risk in drug-naive BD patients. However, some recent studies do suggest that the increased MetS risk extends to drug-naive BD patients.Citation22,Citation26,Citation27
MetS and schizophrenia
Also in schizophrenia, CVD is the leading cause of death.Citation28 Vancampfort's meta-analysis found schizophrenia patients to have a significantly higher risk of abdominal obesity (OR=4.43), hypertension (OR=1.36), low HDL-C (OR=2.35), hypertriglyceridemia (OR=2.73), and MetS (OR=2.35).Citation29 Metabolic disturbances in schizophrenia do increase with illness durationCitation30 and with age.Citation31 Schizoaffective disorder has shown slightly higher rates of MetS than has schizophrenia.Citation32
Quite a number of studies have been dedicated to the contribution of antipsychotics to MetS dysregulations. In particular, second-generation antipsychotics have been shown to cause weight gain, abdominal obesity, lipid and glucose metabolism alterations, and insulin resistance,Citation33 which are side effects that lead to high rates of medication discontinuation. Second-generation antipsychotics associated with extensive weight gain are also the ones associated with most metabolic alterations, with weight gain reported in up to 72% of all antipsychotic-receiving patients.Citation34 However, some studies have reported metabolic alterations to occur even without weight gain.Citation28 The antipsychotics clozapine and olanzapine have the highest weight gain potential through the dysregulation of adipose tissue homeostasis.Citation35 A common target of all antipsychotics is the dopamine D2 receptor, located in the brain where dopamine has an effect on food intake and body weight, as well as in the body where dopamine impacts insulin-producing β-cells of the pancreas.Citation36 Pathways through oxidative stress reactions,Citation35 altered ghrelin and leptin release,Citation35 dysfunctions in the autonomic nervous system activity,Citation37 inflammatoryCitation35 and other signaling pathways (eg, those involving dopamine, histamine, serotonin, muscarinic mechanisms, cannabinoids, and adiponectin)Citation35 have also been implicated as important contributing processes leading to MetS comorbidity with the use of antipsychotics.Citation35
Controversial results have been reported on whether schizophrenia itself confers an inherent risk for metabolic alterations or whether the impact is solely due to antipsychotic use. Some individual studies could not detect metabolic alterations in first-episode, drug-naive patients,Citation30 but these nonsignificant findings could also be due to small sample sizes, low severity, and short disease duration. In fact, several recent findings clearly point toward metabolic alterations due to schizophrenia itself, in the absence of medication and chronic (behavioral) alterations. Glucose homeostasis,Citation38 waist:hip ratio,Citation31 and visceral fatCitation31 have been shown to be altered from illness onset and also in the absence of antipsychotic use. Others reported MetS alterations in at-risk populations who do not have schizophrenia themselves, such as in family members and in ultra-high-risk groups for psychosis.Citation30
MetS and other psychiatric disorders
Anxiety is part of a symptom continuum ranging from a comorbid symptom to a full-blown separate diagnosis. Both sides of this continuum seem to have influence on MetS risk,Citation39 although reported associations are not always robust.Citation40,Citation41 Within-study comparisons of associations between anxiety and depression with MetS found similar,Citation19 as well as weaker,Citation40,Citation41 associations for anxiety. A recent meta-analysis summarizing 18 cross-sectional studies examining MetS risk in persons with high anxiety found a weak, but significantly increased risk (OR=1.07).Citation42 The two longitudinal studies in this metaanalysis could not confirm a prospective association. In a medical records analysis,Citation43 patients with anxiety had higher cardiometabolic risks: diabetes (OR=1.31), hypertension (OR=1.21), hyperlipidemia (OR=1.25), and obesity (OR=1.09).Citation44 These odds, although significant, are still slightly lower than those found for some other psychiatric conditions.
Several studies have examined the prevalence of MetS in patients with posttraumatic stress disorder (PTSD). A meta-analysis of nine studies compared MetS across 9673 PTSD patients with 6852 controls.Citation45 The pooled MetS prevalence in PTSD patients was 38.7%. In comparison with matched general population controls, patients with PTSD had a 1.82-times higher risk for MetS.Citation45 This risk was found to be consistent across geographical regions and populations (war veterans or not).
Personality disorders (PD), and in particular borderline PD, are associated with multiple cardiovascular risk factors, but the inherent impact of PD itself on MetS risk has not yet been examined. The use of second-generation antipsychotics in PD is common practice and comorbid psychiatric disorders are prevalent, making PD patients a group vulnerable to MetS abnormalities.Citation46 A lack of data is also an issue for obsessive compulsive disorder. An Italian study of 104 patients found MetS to be present in 21.2% of cases, but the confidence interval encompassed that of the general population estimate.Citation47
A recent meta-analysis showed that 1 out of 5 patients (21.5%) with alcohol-use disorders (AUDs) had MetS.Citation48 The prevalence of MetS was found to be especially high in patients with high psychiatric comorbidity. An important moderator for the MetS risk in AUDs is chronic liver disease, which has a profound influence on lipid metabolism. Heroin and methadone users showed a MetS prevalence of 29.5% with exposure time to methadone use as a significant predictor of MetS.Citation49 Drugs themselves, for instance (meth)amphetamine, have an important influence on glucose metabolism, have immunosuppressive or proinflammatory properties, and are cardiotoxic.Citation49 For drug- and alcohol-dependence disorders, studies including a healthy reference group are necessary to indicate more precisely to what extent these disorders increase the overall MetS risk when compared with the general population.
Even childhood developmental disorders have been related to some extent to MetS dysregulations, although large-scale studies examining the entire concept of MetS are absent. A medical record study indicated that children with autism spectrum disorders are at an increased risk of obesity and obesity-related disorders (OR=1.85).Citation50 This study also indicated that autistic patients may be at risk partly because they are commonly using antipsychotics, antidepressants, or antiepileptics for extended periods of time.Citation50 Whereas hardly any research has been done in children with attention-deficit/ hyperactivity disorder (ADHD), some reports in adults indicate that ADHD may involve an increased BMI and alterations in lipid profiles, although findings seem inconsistentCitation51,Citation52 and well-powered studies are lacking.
Mechanisms connecting MetS and psychiatric disorders
How may we explain the increased MetS risk in persons with a psychiatric disorder? As illustrated above, the use of psychotropic medications can partly contribute to the observed increased MetS risk in psychiatric patients. However, it is also clear that the increased risk of MetS is present in patient groups using very different types of treatments and also extends to patients not on treatment. Consequently, the increased MetS risk is also very likely to be inherent to the presence of psychiatric disease itself. Considering that the increased MetS risk is seen in various different psychiatric patient populations, it is likely that general (nonspecific) mechanisms are in play. Some important potential connecting mechanisms are discussed below.
Poorer lifestyle and medical care
General factors predisposing psychiatric patients to MetS include unhealthy lifestyle choices such as smoking, excessive alcohol intake, poor sleep hygiene, physical inactivity, and unhealthy nutritional patterns, which are all more common in different psychiatric patient groups.Citation11,Citation35 These are known behavioral risk factors that contribute to poorer cardiovascular health and an increased risk of MetS. Various individual studies examining MetS in psychiatric patients have often attempted to adjust for basic lifestyle factors such as smoking habits and activity patterns. When doing so, MetS associations are generally not much reduced. However, it is notoriously difficult to fully quantify the impact of lifestyle in psychiatric patients, as it can only be partly captured using self-report information, and therefore residual confounding likely exists. Considering the huge impact of lifestyle on MetS, and the fact that psychiatric patients generally live unhealthier lives across many aspects, poor lifestyle plays a role in the psychiatric disorder MetS association. In addition, the reduced likelihood of psychiatric patients receiving standard (optimal) levels of medical care likely contributes to an unhealthier metabolic profile among psychiatric patients.Citation53
Central and peripheral immune, metabolic, and endocrine dysregulations
Accumulating evidence suggests that different psychiatric patient groups share inherent pathophysiological features of deregulated homeostasis systems, including the hypothalamic-pituitary-adrenal (HPA)-axis and inflammatory response. These pathophysiological features all have links to MetS development as well.
Regarding the HPA-axis, disturbances of glucocorticoid sensitivity accompanied with a systemic Cortisol action is a well-recognized characteristic in patients with stress-related disorders.Citation54 HPA-axis hyperactivation determines visceral fat accumulation via increased lipid storage and adipogenesis.Citation55 This glucocorticoidmediated effect is amplified in abdominal adipose tissue expressing a high density of glucocorticoid receptors. Hypercortisolemia induces lipolysis, the release of fatty acids, and synthesis of very-low-density lipoprotein (VLDL), resulting in hypertriglyceridemia.
White adipose tissue, especially in the abdominal area, is an active endocrine organ producing inflammatory cytokines and hormones (eg, leptin) and, therefore, a major contributor to pathogenic immunometabolic responses in the central nervous system, as well as in the rest of the body. Cytokines produced peripherally can access the brain —either directly crossing the bloodbrain barrier through saturable active transport systems or indirectly via microglia activation— and result in decreased neurogenesis in emotion-regulating brain structures.Citation56 Cytokines also catalyze the synthesis of kynurenine from tryptophan, resulting in the reduced synthesis of serotonin and increased synthesis of tryptophan catabolites, which perturb neurotransmission and lead to neuronal damage.Citation57 The activation of a proinflammatory response stimulates the release of lipids in the bloodstream, resulting in a reduction in HDL-C and phospholipids and an increase in triglycerides. Both the sustained HPA-axis and inflammatory activation may affect insulin sensitivity and alter glucose metabolism acting directly on pancreatic β-cells.
Other linking pathophysiological mechanisms may also be relevant. For instance, the leptin-melanocortin pathway has an important role in lipid and glucose homeostasis, as it is a key neuroendocrine regulator of energy homeostasis. This pathway has also been shown to play a role in mood regulation through the enhancement of neurogenesis and neuroplasticity in hippocampal and cortical structures and the modulation of HPA-axis and immune system activity.Citation58 In addition, higher levels of oxidative and nitrosative stress could be a further linking mechanism, as these have been shown to be involved in both the development of psychiatric diseases and metabolic dysregulations.Citation59 In sum, various immunometabolic and endocrine homeostasis systems show bidirectional interplay with both psychiatric and somatic health, and dysregulations in these may contribute to a psychiatric disorder-MetS comorbidity.
Shared genetic vulnerability
The last decade has given rise to large-scale studies in which genome-wide association studies (GWAs) and candidate gene studies have identified genetic variants that are associated with CVD, MetS dysregulations, and psychiatric disorders. A recent reviewCitation60 revealed 24 pleiotropic genes that seem to be shared between mood disorders and cardiometabolic conditions. These genes included among others CACNA1D (encoding calcium voltage-gated channel subunit α1 D), FTO (encoding fat mass and obesity-associated protein), BDNF (encoding brain-derived neurotrophic factor), POMC (encoding proopiomelanocortin), and IGF1 (encoding insulin-like growth factor 1). A pathway analysis revealed shared genetic pathways involving corticotropin-releasing hormone signaling, axonal guidance signaling, serotonin and dopamine receptors signaling, circadian rhythm signaling, and leptin signaling. Further confirmation for the overlap in mood disorders and MetS-relevant genes comes from observations that genome-wide genetic risk scores for obesity and inflammation are associated with a significantly increased risk of MDD, especially those with neurovegetative symptoms.Citation61 Similarly, a recent review article found robust and multiple-study evidence for fat mass and obesity associated genes (including FTO), leptin genes, MTHFR (encoding methylenetetrahydrofolate reductase), and serotonin receptor 2C genes to be involved in the pathogenesis of both MetS and schizophrenia.Citation62 These recent studies clearly suggest that the MetS-psychiatric disorder comorbidity may partly arise from a shared genetic vulnerability involving pathophysiological processes that impact on metabolic as well as mental health.
Gut microbiome alterations
The gut is colonized by trillions of microorganisms collectively called the microbiome. It is increasingly clear that this microbiome plays a critical role in many aspects of health, including metabolism, immunity, and even neurobiology. Consequently, it is possible that commensal bacteria are also a connecting factor to both metabolic and psychiatric health. The penetration of bacteria across the gut epithelium may modulate a range of neurotrophins and proteins involved in brain development and plasticity, resulting in chronic lowgrade inflammation, which further induces MetS.Citation63,Citation64 This research area is currently growing at a rapid rate and will teach us more about the importance of microbiome alterations as an underlying mechanism for the MetS comorbidity in psychiatric patients.
Clinical implications and future perspectives
The abovementioned evidence that MetS is more prevalent among psychiatric patients indicates the relevance of considering the diagnosis and treatment of MetS simultaneously with the management of psychiatric conditions. Such simultaneous treatment could decelerate the somatic consequences of MetS, but possibly also the progression of psychiatric conditions, as MetS has been associated with a more chronic and progressive disease course. For some psychotropic medications, such as antidepressants and mood stabilizers, there is evidence that MetS dysregulations are predictive of treatment resistance.Citation12,Citation65 This may even suggest that the reduction in MetS prevalence could potentially improve the response to psychotropic medications; however, this point deserves future confirmatory research. Overall, the clinical evaluation and treatment of MetS in psychiatric patients is very worthwhile both for somatic and mental health of patients.
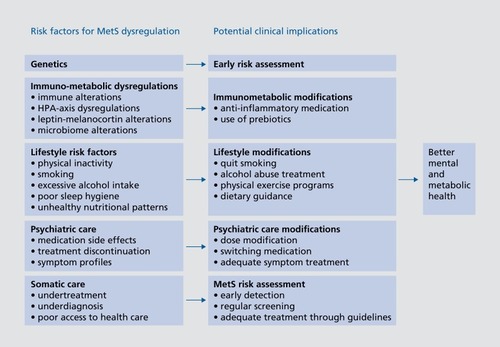
One obvious question is how to best prevent and treat MetS in psychiatric patients. summarizes the main risk factors for MetS comorbidity in psychiatric patients and the potential (future) clinical implications that could be considered to prevent or reduce the impact of MetS risk. As described above, it is important to be aware of specific psychotropic medications that raise the MetS risk more than others and to be able to adapt the prescription of a particular medication to the cardiovascular risk profile of patients. This is especially important in patients who are already at an increased risk due to obesity or preexisting somatic conditions. Dose reduction or the switching of antipsychotic medication has proven to be safe and beneficial.Citation66 Evidence has been reported for concomitant metformin and statin use to be effective in the treatment of antipsychotic-induced weight gain and metabolic adversities.Citation67 In addition, adequate monitoring of MetS and its potential deterioration during treatment is necessary in order to provide timely treatments when clinically relevant MetS deterioration occurs. Multiple studies have reported considerable underdiagnosis and undertreatment of somatic comorbidity in psychiatric patients.Citation68,Citation69 Early detection, identifying high-risk patients, and early treatment based on existing somatic guidelines should be part of daily practice. Another obvious route to prevent or improve the risk of MetS is by directly modifying the lifestyle of psychiatric patients. Lifestyle interventions, changing sedentary lifestyle behavior, and reducing smoking have been shown to improve depressive and psychotic symptom severityCitation70,Citation71 and favorably affect metabolic parameters and cardiorespiratory status.Citation72,Citation73 A pilot trial indicated that subjects with BD, MDD, and schizophrenia who underwent a program of dietary changes, exercise, and modules of wellness had a lower waist circumference and better mental health.Citation74 Lifestyle-improving programs could and should become much more integrated and accessible in standard clinical practice.
Scientific research is currently exploring novel types of interventions that could help reduce the combination of MetS dysregulations as well as psychiatric symptoms. One novel route is through adjunctive anti-inflammatory agents such as nonsteroidal anti-inflammatory drugs (NSAIDs) or N-acetylcyteine, which may have direct symptom-reducing effects in psychiatric patients or improve efficacy of psychotropic medication. Such first evidence exists for patients with BD,Citation75 depressionCitation76 and schizophrenia.Citation77 This proof-of-concept evidence shows that additional, more robust studies evaluating immuno-modulating agents for larger groups of psychiatric patients are merited. A second novel route of intervention may be targeting the gut-brain axis. Animal research provided first evidence that the broad-scale alteration of the microbiome via use of selective dietary microbial growth substrates, or prebiotics, may directly affect the expression of brain-relevant proteins such as brain-derived neurotrophic factor (BDNF) and N-methyl-D-aspartic acid (NMDA) as well as on various metabolic processes including insulin resistance and inflammation.Citation63 In line with this, the administration of probiotics in healthy women improved brain connectivity and emotional processing and reduced stress.Citation63 Studies targeting the gut-brain axis in psychiatric patients are ongoing and will show us whether this is an efficacious route to improve both MetS and psychiatric health outcomes for the future.
Conclusion
The risk of MetS is increased in a range of psychiatric patients. This increased risk is due to an intricate combination of pathways acting synergistically and having a negative effect on the course of psychiatric diseases. MetS changes in psychiatric disorders could be causal, consequential, or due to an underlying same set of causes. A likely multitude of factors interact, including iatrogenic effects of psychotropic medication, an unhealthy lifestyle, poorer medical care of psychiatric patients, and genetic and pathophysiological vulnerability. Because of the adverse consequences of MetS on somatic as well as psychiatric outcomes, coordinated pharmacological interventions managing mental health and metabolic dysregulations coupled with behavioral approaches may help lessen the disease burden. Treating psychiatric disorders and MetS simultaneously is merited in order to enhance treatment outcomes for both conditions. Future research is required to further our understanding of underlying mechanisms and the effectiveness of such interventions.
BP has received research funding (not related to contents of current paper) from Jansen Research and Boehringer Ingelheim. SL has nothing to disclose.
REFERENCES
- ChesneyE.GoodwinGM.FazelS.Risks of all-cause and suicide mortality in mental disorders: a meta-review.World Psychiatry.201413215316024890068
- VancampfortD.StubbsB.MitchellAJ.et al.Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis.World Psychiatry.201514333934726407790
- PenninxBW.MilaneschiY.LamersF.VogelzangsN.Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile.BMC Med.20131112923672628
- MitchellAJ.VancampfortD.SweersK.VanWinkel R.YuW.De HertM.Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders — a systematic review and meta-analysis.Schizophr Bull.201339230631822207632
- MclntyreRS.SoczynskaJK.BeyerJL.et al.Medical comorbidity in bipolar disorder: re-prioritizing unmet needs.Curr Opin Psychiatry.200720440641617551358
- BatelaanNM.SeldenrijkA.BotM.van BalkomAJ.PenninxBW.Anxiety and new onset of cardiovascular disease: critical review and metaanalysis.Br J Psychiatry.2016208322323126932485
- MottilloS.FilionKB.GenestJ.et al.The metabolic syndrome and cardiovascular Risk.J Am Coll Cardiol.201056141113113220863953
- EckelRH.AlbertiK.GrundySM.ZimmetPZ.The metabolic syndrome.Lancet.2010375971018118320109902
- PanA.KeumN.OkerekeOI.et al.Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies.Diabetes Care.20123551171118022517938
- van Reedt PortlandAK.GiltayEJ.van VeenT.ZitmanFG.PenninxBW.Metabolic syndrome abnormalities are associated with severity of anxiety and depression and with tricyclic antidepressant use.Acta Psychiatr Scand.20101221303920456284
- PenninxBW.Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms.Neurosci Biobehav Rev.201774pt B27728627461915
- VogelzangsN.BeekmanAT.van Reedt DortlandAK.et al.Inflammatory and metabolic dysregulation and the 2-year course of depressive disorders in antidepressant users.Neuropsychopharmacology.20143971624163424442097
- VogelzangsN.BeekmanAT.BoelhouwerIG.et al.Metabolic Depression.J Clin Psychiatry.201172559860421535996
- MarijnissenRM.VogelzangsN.MulderME.van den BrinkRH.ComijsHC.Oude VoshaarRC.Metabolic dysregulation and late-life depression: a prospective study.Psychol Med.20174761041105227938429
- LichtCM.de GeusEJ.van DyckR.PenninxBW.Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability.Biol Psychiatry.201068986186820843507
- LichtCM.de GeusEJ.SeldenrijkA.et al.Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension.Hypertension.200953463163819237679
- SerrettiA.MandelliL.Antidepressants and body weight: a comprehensive review and meta-analysis.J Clin Psychiatry.201071101259127221062615
- HannestadJ.DellaGioiaN.BlochM.The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a metaanalysis.Neuropsychopharmacology.201136122452245921796103
- HilesSA.RévészD.LamersF.GiltayE.PenninxBW.Bidirectional prospective associations of metabolic syndrome components with depression, anxiety, and antidepressant use.Depress Anxiety.201633875476427120696
- LamersF.BursteinM.HeJP.AvenevoliS.AngstJ.MerikangasKR.Structure of major depressive disorder in adolescents and adults in the US general population.Br J Psychiatry.2012201214315022700082
- LasserreAM.GlausJ.VandeleurCL.et al.Depression with atypical features and increase in obesity, body mass index, waist circumference, and fat mass: a prospective, population-based study.JAMA Psychiatry.201471888088824898270
- MoreiraFP.JansenK.CardosoTA.et al.Metabolic syndrome in subjects with bipolar disorder and major depressive disorder in a current depressive episode: population-based study.J Psychiatr Res.20179211912328433948
- VancampfortD.VansteelandtK.CorrellCU.et al.Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators.Am J Psychiatry.2013170326527423361837
- McKnightRF.AdidaM.BudgeK.StocktonS.GoodwinGM.GeddesJR.Lithium toxicity profile: a systematic review and meta-analysis.Lancet.2012379981772172822265699
- CorrellCU.SheridanEM.DelBelloMP.Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials.Bipolar Disord.201012211614120402706
- GuhaP.BhowmickK.MazumderP.GhosalM.ChakrabortyI.BurmanP.Assessment of insulin resistance and metabolic syndrome in drug naive patients of bipolar disorder.Indian J Clin Biochem.2014291515624478549
- SilarovaB.GiltayEJ.Van Reedt DortlandA.et al.Metabolic syndrome in patients with bipolar disorder: comparison with major depressive disorder and non-psychiatric controls.J Psychosom Res.201578439139825742722
- NewcomerJW.Antipsychotic medications: metabolic and cardiovascular risk.J Clin Psychiatry.200768suppl 4813
- VancampfortD.WampersM.MitchellAJ.et al.A meta-analysis of cardio-metabolic abnormalities in drug naive, first-episode and multiepisode patients with schizophrenia versus general population controls.World Psychiatry.201312324025024096790
- CarneyR.CotterJ.BradshawT.FirthJ.YungAR.Cardiometabolic risk factors in young people at ultra-high risk for psychosis: a systematic review and meta-analysis.Schizophr Res.20161702-329030026794596
- EmulM.KaleliogluT.Etiology of cardiovascular disease in patients with schizophrenia: Current perspectives.Neuropsychiatr Dis Treat.2015112493250326491327
- BartoliF.CrocamoC.CasliniM.ClericiM.CarràG.Schizoaffective disorder and metabolic syndrome: a meta-analytic comparison with schizophrenia and other non-affective psychoses.J Psychiatr Res.201566-6712713426004300
- RojoLE.GasparPA.SilvaH.et al.Metabolic syndrome and obesity among users of second generation antipsychotics: a global challenge for modern psychopharmacology.Pharmacol Res.2015101748526218604
- FonsekaTM.MüllerDJ.KennedySH.Inflammatory cytokines and antipsychotic-induced weight gain: review and clinical implications.Mol Neuropsychiatry.2016211427606316
- HendersonDC.VincenziB.AndreaNV.UlloaM.CopelandPM.Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses.Lancet Psychiatry.20152545246426360288
- NashAL.Crosstalk between insulin and dopamine signaling: a basis for the metabolic effects of antipsychotic drugs.J Chem Neuroanat.201783-84596827480675
- LeungJY.BarrAM.ProcyshynRM.HonerWG.PangCC.Cardiovascular side-effects of antipsychotic drugs: the role of the autonomic nervous system.Pharmacol Ther.2012135211312222565090
- PillingerT.BeckK.GobjilaC.DonocikJG.JauharS.HowesOD.Impaired glucose homeostasis in first-episode schizophrenia.JAMA Psychiatry.201774326126928097367
- ScottKM.Depression, anxiety and incident cardiometabolic diseases.Curr Opin Psychiatry.201427428929324840158
- ButnorieneJ.BuneviciusA.NorkusA.BuneviciusR.Depression but not anxiety is associated with metabolic syndrome in primary care based community sample.Psychoneuroendocrinology.20144026927624485498
- SkiltonMR.MoulinP.TerraJL.BonnetF.Associations between anxiety, depression, and the metabolic syndrome.Biol Psychiatry.200762111251125717553465
- TangF.WangG.LianY.Association between anxiety and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies.Psychoneuroendocrinology.20177711212128027497
- Pérez-PiñarM.AyerbeL.GonzálezE.MathurR.Foguet-BoreuQ.AyisS.Anxiety disorders and risk of stroke: a systematic review and meta-analysis.Eur Psychiatry.20174110210828135591
- Perez-PinarM.MathurR.FoguetQ.AyisS.RobsonJ.AyerbeL.Cardiovascular risk factors among patients with schizophrenia, bipolar, depressive, anxiety, and personality disorders.Eur Psychiatry.20163581527061372
- RosenbaumS.StubbsB.WardPB.SteelZ.LedermanO.VancampfortD.The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: a systematic review and meta-analysis.Metabolism.201564892693325982700
- KahlKG.GreggersenW.SchweigerU.et al.Prevalence of the metabolic syndrome in patients with borderline personality disorder: results from a cross-sectional study.Eur Arch Psychiatry Clin Neurosci.2013263320521322777277
- AlbertU.AgugliaA.ChiarleA.BogettoF.MainaG.Metabolic syndrome and obsessive-compulsive disorder: a naturalistic Italian study.Gen Hosp Psychiatry.201335215415923158675
- VancampfortD.HallgrenM.MugishaJ.et al.The prevalence of metabolic syndrome in alcohol use disorders: a systematic review and metaanalysis.Alcohol Alcohol.201651551552127337988
- VallecilloG.RoblesMJ.TorrensM.et al.Metabolic syndrome among individuals with heroin use disorders on methadone therapy: prevalence, characteristics, and related factors.SubstAbus. 2017 August 3. Epub ahead of print. doi:10. 1080/08897077.2017.1363122.
- ShedlockK.SusiA.GormanGH.Hisle-GormanE.Erdie-LalenaCR.NylundCM.Autism spectrum disorders and metabolic complications of obesity.J Pediatr.201617816
- SpencerTJ.FaraoneSV.TarkoL.McDermottK.BiedermanJ.Attention-deficit/hyperactivity disorder and adverse health outcomes in adults.J Nerv Ment Dis.20142021072573125211634
- WynchankD.BijlengaD.LamersF.et al.The association between metabolic syndrome, obesity-related outcomes, and ADHD in adults with comorbid affective disorders.J Atten Disord.201822546047127422611
- MitchellAJ.LordO.MaloneD.Differences in the prescribing of medication for physical disorders in individuals with v. without mental illness: meta-analysis.Br J Psychiatry .2012201643 5443
- OtteC.GoldSM.PenninxBW.et al.Major depressive disorder.Nat Rev Dis Prim.201621606527629598
- van RossumEF.KoperJW.HuizengaNA.et al.A polymorphism in the glucocorticoid receptor gene, which decreases sensitivity to glucocorticoids in vivo, is associated with low insulin and cholesterol levels.Diabetes.200251103128313412351458
- SheltonRC.MillerAH.Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression.Prog Neurobiol.201091427529920417247
- SubletteME.PostolacheTT.Neuroinflammation and depression.Psychosom Med.201274766867222923699
- Paz-FilhoG.WongML.LicinioJ.The procognitive effects of leptin in the brain and their clinical implications.Int J Clin Pract.201064131808181221070531
- LoprestiDrummondPD.Obesity and psychiatric disorders: commonalities in dysregulated biological pathways and their implications for treatment.Prog Neuropsychopharmacol Biol Psychiatry.201345929923685202
- AmareAT.SchubertKO.Klingler-HoffmannM.Cohen-WoodsS.BauneBT.The genetic overlap between mood disorders and cardiometabolic diseases: a systematic review of genome wide and candidate gene studies.Transl Psychiatry.201771e100728117839
- MilaneschiY.LamersF.PeyrotWJ.et al.Polygenic dissection of major depression clinical heterogeneity.Mol Psychiatry.201621451652226122587
- Malan-MüllerS.KilianS.van den HeuvelLL.et al.A systematic review of genetic variants associated with metabolic syndrome in patients with schizophrenia.Schizophr Res.2016170111726621002
- RogersGB.KeatingDJ.YoungRL.WongML.LicinioJ.WesselinghS.From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways.Mol Psychiatry.201621673874827090305
- LeyRE.TurnbaughPJ.KleinS.GordonJI.Microbial ecology: human gut microbes associated with obesity.Nature.200644471221022102317183309
- KempDE.CalabreseJR.TranQV.PikalovA.EudiconeJM.BakerRA.Metabolic syndrome in patients enrolled in a clinical trial of aripiprazole in the maintenance treatment of bipolar I disorder.J Clin Psychiatry.20107191138114420492838
- ShulmanM.MillerA.MisherJ.TentlerA.Managing cardiovascular disease risk in patients treated with antipsychotics: a multidisciplinary approach.J Multidiscip Health.20147489501
- de SilvaVA.SuraweeraC.RatnatungaSS.DayabandaraM.WanniarachchiN.HanwellaR.Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis.BMC Psychiatry.201616134127716110
- MitchellAJ.DelaffonV.VancampfortD.CorrellCU.De HertM.Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices.Psychol Med.201242112514721846426
- LaursenTM.NordentoftM.MortensenPB.Excess early mortality in schizophrenia.Annu Rev Clin Psychol.20141042544824313570
- KvamS.KleppeCL.NordhusIH.HovlandA.Exercise as a treatment for depression: a meta-analysis.J Affect Disord.2016202678627253219
- RosenbaumS.TiedemannA.SherringtonC.CurtisJ.WardPB.Physical activity interventions for people with mental illness.J Clin Psychiatry.201475996497424813261
- ChurchTS.EarnestCP.SkinnerJS.BlairSN.Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure.JAMA.200729719208117507344
- Sari-SarrafV.AliasgarzadehA.NaderaliMM.EsmaeiliH.NaderaliEK.A combined continuous and interval aerobic training improves metabolic syndrome risk factors in men.Int J Gen Med.2015820321026056487
- Van CittersAD.PrattSI.JueK.et al.A pilot evaluation of the In SHAPE individualized health promotion intervention for adults with mental illness.Community Ment Health J.201046654055220012197
- RosenblatJD.KakarR.BerkM.et al.Anti-inflammatory agents in the treatment of bipolar depression: a systematic review and meta-analysis.Bipolar Disord.20161828910126990051
- KöhlerO.BenrosME.NordentoftM.et al.Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects.JAMA Psychiatry.20147112138125322082
- LezaJC.García-BuenoB.BioqueM.et al.Inflammation in schizophrenia: a question of balance.Neurosci Biobehav Rev.20155561262626092265
