Abstract
The significance of early life for the long-term programming of mental health is increasingly being recognized. However, most psychotropic medications are currently intended for adult patients, and early psychopharmacological approaches aimed at reverting aberrant neurodevelopmental trajectories are missing. Psychopharmacologic intervention at an early age faces the challenge of operating in a highly plastic system and requires a comprehensive knowledge of neurodevelopmental mechanisms. Recently the systems biology approach has contributed to the understanding of neuroplasticity mechanisms from a new perspective that interprets them as the result of complex and dynamic networks of signals from different systems. This approach is creating opportunities for developmental psychopharmacology, suggesting novel targets that can modulate the course of development by interfering with neuroplasticity at an early age. We will discuss two interconnected systems—the immune and gut microbiota—that regulate neurodevelopment and that have been implicated in preclinical research as new targets in the prevention of aberrant brain development.
Cada vez se reconoce más la importancia de la vida temprana para la programación a largo plazo de la salud mental. Sin embargo, la mayoría de los medicamentos psicotrópicos está destinada actualmente a pacientes adultos, y faltan enfoques psicofarmacológicos en niños, orientados a revertir trayectorias del neurodesarrollo aberrantes. La intervención psicofarmacológica a una edad temprana enfrenta el desafío de operar en un sistema altamente plástico y requiere de un conocimiento completo de los mecanismos del neurodesarrollo. Recientemente, el enfoque de la biología de sistemas ha contribuido a la comprensión de los mecanismos de neuroplasticidad desde una nueva perspectiva que los interpreta como el resultado de redes complejas y dinámicas de señales de diferentes sistemas. Este enfoque está creando oportunidades para la psicofarmacología del desarrollo, sugiriendo nuevos objetivos que pueden modular el curso del desarrollo al interferir con la neuroplasticidad a una edad temprana. Discutiremos dos sistemas interconectados, el inmunológico y la microbiota intestinal, que regulan el neurodesarrollo y que han sido implicados en la investigación preclínica como nuevos objetivos en la prevención del desarrollo cerebral aberrante.
L’importance des premières années pour la programmation à long terme de la santé mentale est de plus en plus reconnue. Cependant, la plupart des médicaments psychotropes sont à destination des patients adultes, et des approches psychopharmacologiques précoces ayant pour but de corriger des neurodévelopements aberrants font défaut. Une intervention psychopharmacologique à un âge précoce doit faire face au défi d’agir dans un système hautement plastique et requiert une connaissance exhaustive des mécanismes du neurodévelopement. Récemment l’approche par la biologie des systèmes a participé à la compréhension des mécanismes de la neuroplasticité dans une nouvelle perspective qui les interprète comme le résultat des signaux de réseaux complexes et dynamiques provenant de différents systèmes. Cette approche crée des opportunités pour la psychopharmacologie pédiatrique, suggérant de nouvelles cibles qui pourraient moduler le cours du développement en interférant avec la neuroplasticité à un âge précoce. Nous discuterons deux systèmes interconnectés – le système immunitaire et le microbiote intestinal – qui régulent le neurodévelopement et qui ont été étudiés dans des recherches précliniques en tant que nouvelles cibles dans la prévention du développement aberrant du cerveau.
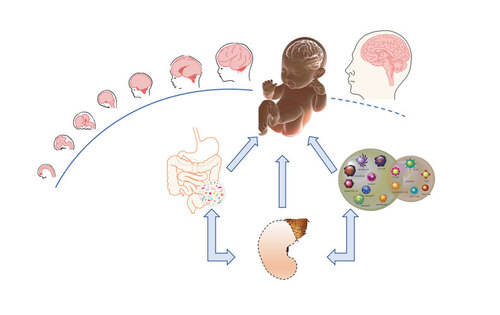
The regulation of neurodevelopment from conception to maturity is the result of extensive cooperation and bidirectional interactions between environmental-interfacing systems. In the figure, the immune, endocrine, and gut-microbiota systems are represented as the most active participants in the dialogue between the environment and the developing brain.
Introduction
Mental health is, to a significant extent, decided in early life. The importance of neurodevelopment in establishing the risk for mental disorders is increasingly being recognized Citation1 and most psychopathologies are ultimately the manifestation of aberrant development of the brain.
The maturation of the brain during perinatal life is a highly regulated process and results from the interplay between environmental instructions that drive adequate neuronal connections toward their final form and individual genetic profiles that provide the latitude in which to operate. Citation2 , Citation3 Thus, consistent with the multifactorial etiologies of most mental disorders, Citation4 environmental perturbations, such as stress exposure during perinatal life, can elicit long-term behavioral dysregulations and increase the vulnerability to psychopathologies. Citation4 , Citation5
Despite the significance of neurodevelopment, the majority of psychotropic medications are intended for adult patients, Citation6 with primarily symptomatic effects and poor therapeutic efficacy, and are thus often associated with high relapse rates.
Due to the plastic properties of immature circuits, psychopharmacologic interventions at an early age can influence brain development, with unpredictable long-term effects on brain function that discourage the use of classical psychotropic drugs in the young population and restrict it to seriously invalidating early symptomatic conditions. Citation7
Bridging psychopharmacology and brain development
Recent data on neurodevelopmental trajectories and their regulatory systems are guiding early pharmacological interventions that may reverse the course of abnormal development, with long-term benefits for adult behavior. To this end, research is moving in two parallel directions: first, toward the identification of early biosignatures that could reliably predict the risk of developing a psychopathology before symptoms manifest. Secondly, studies are focusing on the understanding of the genetic vulnerability to mental disorders and the mechanisms that underlie the environmental impact on brain development. Citation8 These aspects have converged in the study of epigenetic mechanisms, such as DNA methylation, which translate environmental disruptions during sensitive periods into enduring modifications to individual gene transcriptional profiles. Citation9 Indeed, epigenome modifications have been consistently associated with the risk for psychopathologies in adulthood. Citation10 - Citation14
The reversible nature of the epigenetic changes marks them as potential targets for therapeutic interventions. Citation12 - Citation14 However, pharmacological interference with the function of epigenetic machinery during sensitive periods may alter the global epigenome, with unpredictable pleiotropic effects. Citation12 Nevertheless, the identification of key genes that are susceptible to epigenetic modifications and are important contributors to the risk for psychopathologies, Citation15 - Citation18 combined with the cutting-edge technology that applies gene-specific epigenomic editing, Citation19 , Citation20 will engender promising therapeutic opportunities.
The “systems approach” to treating the brain
We have recently entered a new era in which the integrated approach of systems biology has led us to reconsider health or disease as a result of perturbations in the complex homeostasis of biological networks and their dynamic interactions between systems. Citation21 - Citation23 The “systems approach” has increased the recognition that neurodevelopmental regulation is not restricted to internal neuronal mechanisms, but instead is the result of extensive cooperation and bidirectional interactions between systems, including the immune, endocrine, and gut-microbiota systems (Figure 1) . The cooperation of these systems, now commonly referred to as the gut-immune-brain axis, participates actively in the dialogue between the environment and brain development and is emerging as an important early determinants of adult mental health. Citation24 This holistic approach is thus offering unprecedented opportunities for the construction of a new “perinatal psychopharmacology” aimed at reverting the aberrant development of the brain by instructing different modulatory systems during perinatal or late developmental stages. Here, we will briefly discuss two highly connected systems—the immune and gut microbiota—as new targets, that can physiologically modulate the course of brain development by interfering with neuroplasticity at an early age.
Targeting neuroimmune communication during brain development: microglial cells
Historically, the brain has been considered an immune- privileged organ, but in the past two decades, however, the importance of neuroimmune communication in the homeostasis and function of the central nervous system has become clear. This communication is active throughout life, but in prenatal and early postnatal life it appears to be crucial in regulating neurodevelopment. Citation25 In this context, microglial cells and their released mediators, such as cytokines and chemokines, have emerged as principal mediators of these effects. Citation26 Microglial cells are the resident mononuclear phagocytes of the brain, and together with participating in the immune defense of the brain, they contribute intensively to shaping embryonic and postnatal brain circuits by regulating neurogenesis, the remodeling of synaptic networks and connectivity, and the modulation of synaptic and neuronal activity. Citation26 - Citation28 Notably, during perinatal age, microglia differ morphologically compared with those in adults and present distinct gene expression profiles according to the stage of development of various regions of the brain. Citation29 This suggests a specific function of microglia during development. Consistently, preclinical studies have shown that, although temporarily depleting microglia in adulthood has little impact on behavior, Citation30 this treatment during the neonatal period induces persistent changes in social and mood-related behaviors. Citation31 , Citation32
As innate immune cells, microglia are sensors of the surrounding environment and are highly responsive to environmental perturbations, such as stress and immune challenges, that often drive morphological and functional changes of microglia into a proinflammatory state. Citation31 , Citation33 , Citation34 Thus, the contributions of microglia during development can be impacted by changes in the prenatal and postnatal environment, causing developmental dysregulation that predisposes one to psychopathologies later in life. Citation34 - Citation36 In our recent study we found that microglial proinflammatory activation, induced by the exposure to adversities in early age, had a long-term impact on brain structures subserving reward and stress-response behaviors, leading to the increased vulnerability to cocaine-related behaviors later in life. Importantly, the pharmacological prevention of microglial activation in postnatal life—but not during adulthood—was sufficient to restore the functional and behavioral phenotypes. Citation36
Given the specific developmental features of microglial cells and their key function in instructing the immature brain about external perturbations, targeting microglia in early life is a potential intervention that can counteract programmed long-term behavioral dysregulation. Instrumental to this end, different pharmacological tools are being tested for their impact on microglial function, including classical anti-inflammatory drugs, tetracycline antibiotics that counteract microglial proinflammatory response, Citation37 or inhibitors of the Colony-stimulating factor1 receptor, that enable a temporary depletion of microglia. Citation38
Treating the gut to change the brain: the role of microbiota
A second promising system for modulatory interventions on brain development is the gut microbiota. The gut microbiota—the set of bacteria that constitute the intestinal flora—has been demonstrated to have a critical influence on the host physiology in the past decade. One of its most studied effects, is the regulation of normal and pathological host behavior in rodents and humans, demonstrating extensive communication with the central nervous system. Citation39 , Citation40 To mediate its effects on the brain the gut microbiota recruits important components of different systems, including the hypothalamic–pituitary–adrenal (HPA) axis, the immune system, and the autonomic nervous system. Citation39
An individual’s microbiota is primarily derived from the maternal one Citation41 and it is influenced by the perinatal environment, which appears to be crucial in establishing the long-term basic composition of the microbiota. It can take up to 3 years for an infant to develop the microbiota composition of an adult. Citation42 , Citation43 Gut-brain communication during the first weeks of life contributes to proper neurodevelopment. Citation44 , Citation4 5 Using germ-free mice, several studies have demonstrated that the absence of intestinal bacteria in mice evokes anxiety-like behavior and exaggerated stress responses with effects on neuronal activity and plasticity genes and changes to the serotonergic system. Citation46 - Citation48 Notably, normal stress responses are restored by fecal transplantation during the first 6 weeks of life, but not in adulthood, implicating critical developmental time windows for the long-term programming of behavioral phenotypes by microbiota. Citation48 Moreover, in preclinical studies, the dysbiosis due to early environmental disruptions, like the antibiotic exposure during perinatal periods, Citation49 or a high-fat maternal dietary regimen, Citation50 can influence the immature plastic brain and has been associated with adult behavioral deficits. Citation51 Consistently, the microbiota contributes to the long-term behavioral dysregulations that are induced by early-life traumatic experiences. Citation52
In light of the burgeoning literature, the microbiota is emerging as a viable target that could be easily manipulated during prenatal or postnatal life to favor proper neurodevelopment. However, studies have failed to identify the beneficial or harmful microbiome composition but support the appropriate composition in individuals. Thus, to ensure good outcomes with microbiota-based therapies, research has to put effort into the stratification of patients in order to identify the proper composition for the specific gut.
Conclusions
Psychopharmacology during brain development faces the great challenge of operating in a highly plastic system. This may represent a strong advantage for the design of intervention aimed at restoring the course of pathogenetic mechanisms, but efforts should be dedicated to improving the definition of neurodevelopmental trajectories and the specific sensitive time windows. Moreover, crucial to the application of psychopharmacology in early age will be the identification of early biomarkers that could predict the development of a psychopathology before symptoms manifest.
Today the move toward integrated biological systems in understanding brain pathophysiology is advancing us into a new era of psychopharmacology, for which the possibility of manipulating behaviors through the intestine or to programming long-term mental health using anti- inflammatory drugs is being realized. To fulfill these expectations, researchers across diverse disciplines will have to join forces to disentangle the complex dynamics of neurodevelopment, identify sensitive periods for interventions and reliable biomarkers, and direct these findings toward the design of new drugs.
The authors declare no conflicts of interest.
REFERENCES
- NewmanLJuddFOlssonCAEarly origins of mental disorder - risk factors in the perinatal and infant period.BMC Psychiatry.20161627027473074
- BinderEB.Dissecting the molecular mechanisms of gene x environment interactions: implications for diagnosis and treatment of stress-related psychiatric disorders.Eur J Psychotraumatol.20178suppl 5S1412745
- KolbBGibbR.Brain plasticity and behaviour in the developing brain.J Can Acad Child Adolesc Psychiatry.201120426527622114608
- UherRZwickerA.Etiology in psychiatry: embracing the reality of poly-gene-environmental causation of mental illness.World Psychiatry.201716212112928498595
- Lo IaconoLCarolaV.The impact of adolescent stress experiences on neurobiological development.Semin Cell Dev Biol.2018779310328982627
- KoelchMSchnoorKFegertJM.Ethical issues in psychopharmacology of children and adolescents.Curr Opin Psychiatry.200821659860518852568
- AndersenSLNavaltaCP.Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs.Int J Dev Neurosci.2004225-642344015380841
- SaleABerardiNMaffeiL.Environment and brain plasticity: towards an endogenous pharmacotherapy.Physiol Rev.201494118923424382886
- BurnsSBSzyszkowiczJKLuheshiGNLutzPETureckiG.Plasticity of the epigenome during early-life stress.Semin Cell Dev Biol.20187711513229017800
- HoffmannASportelliVZillerMSpengler D.Epigenomics of major depressive disorders and schizophrenia: early life decides.Int J Mol Sci.2017188
- NestlerEJPenaCJKundakovicMMitchell AAkbarianS.Epigenetic basis of mental illness.Neuroscientist.201622544746326450593
- BoksMPde JongNMKasMJCurrent status and future prospects for epigenetic psychopharmacology.Epigenetics.201271202822207355
- DayJJSweattJD.Epigenetic treatments for cognitive impairments.Neuropsychopharmacology.201237124726021593731
- RicqELHookerJMHaggartySJ.Toward development of epigenetic drugs for central nervous system disorders: Modulating neuroplasticity via H3K4 methylation.Psychiatry Clin Neurosci.2016701253655027485392
- FarrellCDoolinKO’Leary NDNA methylation differences at the glucocorticoid receptor gene in depression are related to functional alterations in hypothalamic-pituitary-adrenal axis activity and to early life emotional abuse.Psychiatry Res.201826534134829793048
- FeifelAJShairHNSchmaussC.Lasting effects of early life stress in mice: interaction of maternal environment and infant genes.Genes Brain Behav.201716876878028557378
- Lo IaconoLVisco-ComandiniFValzaniaAAdversity in childhood and depression: linked through SIRT1.Transl Psychiatry.20155e62926327687
- PenaCJKronmanHGWalkerDMEarly life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2.Science.201735663431185118828619944
- HamiltonPJLimCJNestlerEJHellerEA.Neuroepigenetic Editing.Methods Mol Biol.2018176711313629524131
- PfluegerCTanDSwainTA modular dCas9-SunTag DNMT3A epigenome editing system overcomes pervasive off-target activity of direct fusion dCas9-DNMT3A constructs.Genome Res.20182881193120629907613
- GeschwindDHKonopkaG.Neuroscience in the era of functional genomics and systems biology.Nature.2009461726690891519829370
- HoodLHeathJRPhelpsMELinB.Systems biology and new technologies enable predictive and preventative medicine.Science.2004306569664064315499008
- HostinarCENusslockRMillerGE.Future directions in the study of early-life stress and physical and emotional health: implications of the neuroimmune network hypothesis.J Clin Child Adolesc Psychol.201847114215628107039
- DelaneySHornigM.Environmental exposures and neuropsychiatric disorders: what role does the gut-immune-brain axis play?Curr Environ Health Rep.20185115816929423662
- BilboSDSchwarzJM.The immune system and developmental programming of brain and behavior.Front Neuroendocrinol.201233326728622982535
- FrostJLSchaferDP.Microglia: architects of the developing nervous system.Trends Cell Biol.201626858759727004698
- PaolicelliRCFerrettiMT.Function and dysfunction of microglia during brain development: consequences for synapses and neural circuits.Front Synaptic Neurosci.20179928539882
- WeinhardLdi BartolomeiGBolascoGMicroglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction.Nat Commun.201891122829581545
- Matcovitch-NatanOWinterDRGiladiAMicroglia development follows a stepwise program to regulate brain homeostasis.Science.20163536301aad867027338705
- ElmoreMRNajafiARKoikeMAColony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain.Neuron.201482238039724742461
- NelsonLHLenzKM.Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female ratsBehav Brain Res201731627929327613230
- VanRyzinJWYuSJPerez-PouchoulenMMcCarthyMM.Temporary depletion of microglia during the early postnatal period induces lasting sex-dependent and sex-independent effects on behavior in rats.eNeuro.201636
- BranchiIAlboniSMaggiL.The role of microglia in mediating the effect of the environment in brain plasticity and behavior.Front Cell Neurosci.2014839025477783
- LenzKMNelsonLH.Microglia and beyond: innate immune cells as regulators of brain development and behavioral function.Front Immunol.2018969829706957
- KimJWHongJYBaeSM.Microglia and autism spectrum disorder: overview of current evidence and novel immunomodulatory treatment options.Clin Psychopharmacol Neurosci.201816324625230121973
- Lo IaconoLCataleCMartiniAFrom traumatic childhood to cocaine abuse: the critical function of the immune system.Biol Psychiatry.2018841290591630029767
- KobayashiKImagamaSOhgomoriTMinocycline selectively inhibits M1 polarization of microglia.Cell Death Dis.20134e525
- HortiAGNaikRFossCAPET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R).Proc Natl Acad Sci U S A.2019
- TogniniP.Gut microbiota: a potential regulator of neurodevelopment.Front Cell Neurosci.2017112528223922
- WarnerBB.The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders.Pediatr Res.2019 -85221622430283047
- JasarevicEHowertonCLHowardCDBale TL.Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain.Endocrinology.201515693265327626079804
- BaileyMTLubachGRCoeCL.Prenatal stress alters bacterial colonization of the gut in infant monkeys.J Pediatr Gastroenterol Nutr.200438441442115085020
- GolubevaAVCramptonSDesbonnetLPrenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood.Psychoneuroendocrinology.201560587426135201
- Diaz HeijtzRWangSAnuarFNormal gut microbiota modulates brain development and behavior.Proc Natl Acad Sci U S A.201110873047305221282636
- HsiaoEYMcBrideSWHsienSMicrobiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders.Cell.201315571451146324315484
- ClarkeGGrenhamSScullyPThe microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner.Mol Psychiatry.201318666667322688187
- NeufeldKMKangNBienenstockJFosterJA.Reduced anxiety-like behavior and central neurochemical change in germ-free mice.Neurogastroenterol Motil.201123325526421054680
- SudoNChidaYAibaYPostnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice.J Physiol.2004558Pt 126327515133062
- TochitaniSIkenoTItoTSakuraiAYamauchiTMatsuzakiH.Administration of non-absorbable antibiotics to pregnant mice to perturb the maternal gut microbiota is associated with alterations in offspring behavior.PLoS One.2016111e013829326789865
- SullivanELNousenEKChamlouKA.Maternal high fat diet consumption during the perinatal period programs offspring behavior.Physiol Behav.201412323624223085399
- GurTLShayLPalkarAVPrenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring.Brain Behav Immun.201764505828027927
- CongXHendersonWAGrafJMcGrath JM.Early life experience and gut microbiome: the brain-gut-microbiota signaling system.Adv Neonatal Care.201515531432326240939
