Abstract
The last decades have seen a major gain in understanding the action of cannabinoids and the endocannabinoid system in reward processing and the development of addictive behavior. Cannabis-derived psychoactive compounds such as Δ9-tetrahydrocannabinol and synthetic cannabinoids directly interact with the reward system and thereby have addictive properties. Cannabinoids induce their reinforcing properties by an increase in tonic dopamine levels through a cannabinoid type 1 (CB1) receptor–dependent mechanism within the ventral tegmental area. Cues that are conditioned to cannabis smoking can induce drug-seeking responses (ie, craving) by eliciting phasic dopamine events. A dopamine-independent mechanism involved in drug-seeking responses involves an endocannabinoid/glutamate interaction within the corticostriatal part of the reward system. In conclusion, pharmacological blockade of endocannabinoid signaling should lead to a reduction in drug craving and subsequently should reduce relapse behavior in addicted individuals. Indeed, there is increasing preclinical evidence that targeting the endocannabinoid system reduces craving and relapse, and allosteric modulators at CB1 receptors and fatty acid amide hydrolase inhibitors are in clinical development for cannabis use disorder. Cannabidiol, which mainly acts on CB1 and CB2 receptors, is currently being tested in patients with alcohol use disorder and opioid use disorder.
En las últimas décadas se ha observado un gran progreso en el conocimiento acerca de la acción de los cannabinoides y del sistema endocannabinoide en el procesamiento de recompensas y el desarrollo de conductas adictivas. Los compuestos psicoactivos derivados del cannabis como el Δ9-tetrahidrocannabinol y los cannabinoides sintéticos interactúan directamente con el sistema de recompensa y, por lo tanto, tienen propiedades adictivas. Los cannabinoides inducen sus propiedades reforzadoras mediante un aumento en los niveles de dopamina tónica a través de un mecanismo dependiente del receptor cannabinoide 1 (CB1) dentro del área tegmental ventral. Las señales que están condicionadas con fumar cannabis pueden inducir respuestas de búsqueda de drogas (es decir, craving) al provocar liberación fásica de dopamina. Un mecanismo independiente de la dopamina implicado en las respuestas de búsqueda de droga incluye una interacción endocannabinoide / glutamato dentro de la parte cortico-estriatal del sistema de recompensa. En conclusión, el bloqueo farmacológico de la señalización endocannabinoide debería conducir a una reducción del craving por droga y, posteriormente, debería reducir las recaídas en las personas adictas. De hecho, existe una creciente evidencia preclínica de que el elegir como blanco el sistema endocannabinoide reduce el craving y la recaída. Los moduladores alostéricos de los receptores CB1 y los inhibidores de amida hidrolasa de ácidos grasos están en desarrollo clínico para el trastorno por consumo de cannabis. Actualmente se está probando el cannabidiol, que actúa principalmente sobre los receptores CB1 y CB2, en pacientes con trastorno por consumo de alcohol y de opioides.
Les avancées de ces 10 dernières années nous ont permis de mieux comprendre l'action des cannabinoïdes et du système endocannabinoïde dans le processus de récompense et le développement de l’addiction. Le Δ9-tétrahydrocannabinol, comme les autres composés psychoactifs dérivés du cannabis, et les cannabinoïdes synthétiques interagissent directement avec le système de récompense et ont donc des propriétés addictives. La capacité de renforcement des cannabinoïdes s’exerce par un mécanisme dépendant du récepteur cannabinoïde 1 (CB1R) dans la zone tegmentale ventrale qui augmente les taux de dopamine en mode d’activation tonique. La consommation de cannabis entraîne des signaux qui peuvent induire des réactions toxicomaniaques (sensation de manque) en provoquant le mode d’activation phasique dopaminergique. Dans les réponses toxicomaniaques, le mécanisme d’action est indépendant de la dopamine et implique une interaction endocannabinoïde/glutamate dans la partie corticostriatale du système de récompense. En conclusion, bloquer pharmacologiquement la signalisation des endocannabinoïdes devrait diminuer la sensation de manque et donc diminuer les rechutes chez les personnes dépendantes. En effet, de plus en plus de données précliniques montrent qu’en ciblant le système endocannabinoïde, la sensation de manque et les rechutes diminuent. Des modulateurs allostériques au niveau des récepteurs CB1 et des inhibiteurs de l'hydrolase des amides d'acides gras sont en cours de développement clinique pour les troubles liés à la consommation de cannabis. Agissant principalement sur les récepteurs CB1 et CB2, le cannabidiol est actuellement testé chez des patients souffrant de troubles liés à la consommation d'alcool et d'opiacés.
Introduction
The endocannabinoid system is comprised of cannabinoid CB 1 and CB 2 receptors and endogenous agonists of these receptors—so-called endocannabinoids—and the processes playing a role in biosynthesis, release, transport, and metabolism of these endogenous lipid-signaling molecules. Endocannabinoids such as anandamide and 2-arachidonylglycerol (2-AG) are highly lipophilic compounds that are not stored in vesicles after production. After their release on demand from depolarized postsynaptic neurons, endocannabinoids act retrogradely, activating CB 1 receptors on presynaptic terminals, leading to either transient endocannabinoid-mediated short-term depression or long-term depression (LTD) of synaptic transmission. Citation1 Their overall effect is either excitatory or inhibitory depending on the presynaptic inhibition of GABA or glutamatergic transmission. This powerful modulatory action on synaptic transmission of the main transmitter systems has significant functional implications on many physiological functions including reward processing. The last decades have seen a major gain in understanding the involvement of the endocannabinoid system in reward processing and development of addictive behavior. Citation2
The endocannabinoid system with its two cannabinoid receptors is also a target for psychoactive compounds such as Δ 9 -tetrahydrocannabinol (Δ 9 -THC) derived from Cannabis sativa or for synthetic cannabinoids. More than 182 million people regularly consume cannabis products, and this nonmedical cannabis use is associated with a high health burden. Citation3 Although only a small proportion of individuals who use cannabis products develop cannabis use disorder (CUD), the treatment of those patients is becoming an increasing problem in psychiatry and addiction medicine. Epidemiological studies have found that of those people who regularly consume cannabis, approximately 9% develop a CUD; in comparison, approximately 20% of those who drink alcohol or use cocaine on a regular base develop an alcohol use disorder (AUD) or cocaine addiction. Citation4,Citation5 In contrast to the negative consequences of nonmedical cannabis use, the application of medical cannabis or medicinal products derived from cannabis is generating increasing interest in the domain of treatment for psychiatric disorders (posttraumatic stress disorder [PTSD] and attention-deficit/hyperactivity disorder [ADHD] in adults), including substance use disorders (SUDs). Citation6 In addition, synthetic compounds (eg, antagonists and allosteric modulators) that interfere with the endocannabinoid system in many ways are also promising for the treatment of SUDs and AUDs.
Here, I will summarize our knowledge of the interaction of the endocannabinoid system with the reward system, then focus on the addictive properties of cannabis products and synthetic cannabinoids and the development of CUD, and finally discuss the potential use of cannabinoid drugs for the treatment of addictive behavior.
The interaction of endocannabinoid signaling and the reward system
Endocannabinoids activate CB 1 and/or CB 2 receptors to modulate a variety of physiological functions. The distribution of these receptors within the central nervous system and periphery correlates with its role in the control of motor function, cognition and memory, appetite, immune function, sleep, stress response, thermoregulation, analgesia, and reward processing. Citation7
The CB 1 receptor, which is one of the most abundant G-protein-coupled receptors (GPCRs) in the brain, is highly expressed in the basal ganglia nuclei, hippocampus, cortex, and cerebellum. Citation8 CB 1 receptors are primarily localized on the terminals of neurons, where they mediate inhibition of neurotransmitter release. Citation9 CB 1 receptors are found at significantly higher levels on GABAergic than glutamatergic neurons in various brain regions. Citation10 CB 1 receptors are also present on astrocytes, where they are expressed at much lower levels than on neurons, but where they have been shown to modulate synaptic transmission and plasticity. Citation11
The CB 2 receptor is abundantly expressed in peripheral organs with immune function, including macrophages, spleen, tonsils, thymus, and leukocytes, as well as the lung and testes. Citation12 However, functional CB 2 receptors have been also found in healthy and diseased brain cells and seem to be involved in several neuropsychiatric disorders, including addiction. Citation13
The crystal structures of the cannabinoid receptors have recently been revealed, providing further insight into complex ligand-receptor interactions. Citation14-Citation17 For example, the CB 1 receptor has considerable agonist-independent constitutive activity and exhibits paradoxical pharmacological interactions Citation18 ; eg, the CB 1 receptor is antagonized by cannabidiol (CBD), a molecule that is nearly identical to the CB 1 receptor agonist Δ 9 -THC. Citation15 The new atomistic framework helps understanding of the constitutive activity of these receptors and also provides a molecular basis for predicting the binding modes and actions of Δ 9 -THC, CBD, and other endogenous and synthetic cannabinoids.
Although CB 1 and CB 2 receptors are the primary targets of cannabinoids, it is generally accepted that at least some endocannabinoids, as well as Δ 9 -THC and several synthetic CB 1 /CB 2 -receptor agonists and antagonists, can interact with a number of established non-CB 1 / non-CB 2 GPCRs, ligand-gated ion channels, ion channels, and nuclear receptors. Citation19 One prominent example of a noncannabinoid receptor target is the transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, which can be modulated by several endogenous, phytogenic, and synthetic cannabinoids. Citation20
The endocannabinoid system participates in natural and drug reward through interaction with the dopaminergic reward system. The reward pathway originates in the ventral tegmental area (VTA) and A10 dopamine neurons mainly project to the nucleus accumbens (NAc) where dopamine is released in response to rewards. All drugs of abuse, including Δ 9 -THC and other cannabinoids, as well as natural (eg, food and sex) and social rewards, increase dopamine levels within the NAc. Citation21,Citation22 Dopamine neurons have two modes of activity, tonic and phasic firing. Citation23 Tonic activity consists of pacemaker-like spontaneous single spikes (1-5 Hz), whereas phasic activity is characterized by rapid transient increases in dopamine levels that result from high-frequency bursts (>20 Hz). Citation23 Phasic activity of dopamine neurons is necessary to establish long-term memories associating predictive stimuli with rewards, whereas tonic activity of these neurons determines the motivation to respond to such cues. Citation24
Cannabinoids increase both tonic dopamine levels by an increase in the firing rate of dopamine A10 neurons Citation25,Citation26 as well as phasic dopamine events through a CB 1 -receptor–dependent mechanism within the VTA. Citation27,Citation28 However, dopamine cell bodies lack CB 1 receptors, Citation8 so where do cannabinoids act within the VTA to enhance dopaminergic activity? Peters et al Citation28,Citation29 propose the following disinhibition mechanism: similar to a mechanism described for opioids, Citation30 cannabinoids act via GABAergic interneurons within the VTA to disinhibit dopamine neurons.
Drug-conditioned cues, eg, cues that are conditioned to cannabis smoking, increase phasic dopamine events through a CB 1 -receptor–dependent mechanism within the VTA. Citation27,Citation28 , Citation31 The phasic dopamine events that are induced by conditioned drug cues play a critical role in drug-seeking behavior, and disrupting endocannabinoid signaling decreases cue-evoked phasic dopamine events. Citation27 If a drug-conditioned cue leads to dopamine neuron firing in high-frequency bursts, increased intracellular calcium levels within dopamine cell bodies activate, primarily, diacylglycerol lipase (DAGL), which leads to the synthesis of the endocannabinoid 2-AG. Citation32 2-AG then acts retrogradely on CB 1 receptors at presynaptic terminals of GABA neurons. Therefore, CB 1 -receptor activation leads to an inhibition of GABA transmission. This GABA suppression results in disinhibition of dopamine neurons, which further promotes their phasic firing activity ( Figure 1 ). Disrupting endocannabinoid signaling within the VTA thus reduces these cue-evoked phasic dopamine responses and therefore interrupts reward-seeking behavior. This mechanism applies to all cue-reward/drug associations and thus provides the foundation for a mechanism-based intervention of drug-seeking responses (ie, craving).
Endocannabinoids not only act on the level of dopamine cell bodies within the VTA to interfere with primary and secondary reinforcement processes, but also on projection sites within the NAc. This interaction involves medium spiny neurons (MSNs) and prefrontal glutamate afferents, especially glutamate release at the prelimbic cortex–NAc synapses. Citation33 Stimulation of these prefrontal glutamate afferents can cause LTD of NAc glutamatergic synapses, an effect mediated also by 2-AG release and presynaptic CB 1 -receptor activation. Citation34-Citation36 This form of endocannabinoid-mediated synaptic plasticity in the NAc depends on postsynaptic metabotropic glutamate receptor 5 (mGluR5). In mice, conditional ablation of mGluR5 in dopamine D1-receptor– but not D2-receptor–expressing MSNs (D1 or D2-MSN) by cell-type specific RNA interference Citation37 abolishes 2-AG-dependent LTD and prevents the expression of drug, natural reward, and brain stimulation–seeking behavior. Citation36 Pharmacological enhancement of 2-AG within the NAc restores both endocannabinoid-dependent-LTD and reward-seeking behavior in these conditional mice. Citation36 These findings extend the disinhibition model and show that endocannabinoid/glutamate interaction within the NAc also contributes to reward-seeking responses ( Figure 1 ). Citation36
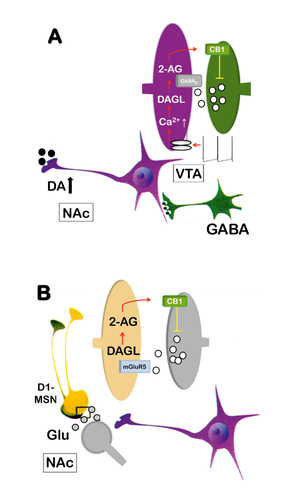
The disinhibition mechanism within the VTA and the endocannabinoid-based mechanism within D1-MSNs provide the rationale that blockade of CB 1 receptors should lead to a reduction in drug-induced increases in tonic dopamine levels, drug-cue–associated phasic firing, and of 2-AG–dependent LTD within the NAc (ie, mechanism-based intervention). As a consequence of these neurochemical and physiological events, drug-seeking behavior (craving), drug memories, and subsequent relapse should be reduced. In the next paragraphs, interventions based on the disruption of endocannabinoid signaling and the consequences on addictive behavior are described.
Cannabis and synthetic cannabinoids and the development of CUD
Cannabis is the most commonly used illegal drug in Europe. New forms of highly potent cannabis have been developed in recent years due to advances in cultivation, extraction, and production techniques. Hybrid multistem plants that provide high-potency cannabis have started to replace established forms of the plant in both Europe and Morocco, where much of the cannabis resin used in Europe comes from. Citation45 Data provided by the European Union Member States show that the Δ 9 -THC concentration of cannabis products found in Europe over the last decade has increased, raising concerns about potential harm. In Europe, the estimated mean potency of herbal cannabis doubled from 5% to 10% Δ 9 -THC, and cannabis resin potency increased from 8% to 17% Δ9-THC in the last decade. Similar trends in cannabis potency have been observed in the United States over the last two decades. Citation46
Most worry is due to the increased abuse of synthetic cannabinoids. In Europe, about 15 years ago, this problem mainly started with the use of spice products. It has been claimed that the smoking of these “healthy” spice products produces cannabinoid-like effects, even though they do not contain cannabis. However, withdrawal phenomena such as inner unrest, profuse sweating, and tremor, and a dependence syndrome after the consumption of spice products were soon described, Citation47 and when the admixture of the synthetic cannabinoid substances JWH-018 and CP-47-497 were found, it became clear that spice can be a dangerous product. Citation48 Synthetic cannabinoids are often sprayed onto plant matter and are usually smoked and have been marketed as “herbal smoking blends” under common names like spice. Citation49 The spice era marked the beginning of an increased use of strongly potent synthetic cannabinoids that leads not only to bizarre intoxication, as for example the “zombie” outbreak in New York City, but also to a high mortality rate. On July 12, 2016, a synthetic cannabinoid caused mass intoxication of 33 persons in one New York City neighborhood in an event described in the popular press as a “zombie” outbreak because of the appearance of the intoxicated persons. Citation50 It was found that the herbal spice product “Karat Gold,” which was implicated in the outbreak, contained the ultra-potent synthetic cannabinoid methyl 2-(1-(4-fluorobenzyl)-1 H -indazole-3-carboxamido)-3-methylbutanoate (AMB-FUBINACA). In the past 10 years, almost 170 different new synthetic cannabinoids have entered the market; there are new compounds on the market with up to 100-fold potency compared with Δ 9 -THC, thus carrying a high health risk and having considerable mortality rates. Citation51 One myth around cannabis is that this is a safe drug; high-potency cannabis varieties and new synthetic ultra-potent cannabinoids—some of which may also have long half-lives leading to a prolonged psychoactive effect—tell another story. They can lead to severe intoxication and death, disrupt neurodevelopmental processes, induce psychotic behavior, and lead to a rapid onset of CUD. Citation51-Citation53 Cannabis products and synthetic cannabinoids interact with the reward system and lead to CUD through this interaction. As outlined in the previous chapter, we have a good understanding of the molecular interactions of cannabinoids with the reward system and can therefore provide mechanism-based interventions for CUD.
Current and future treatment interventions for CUD
Panlilio and Justinova Citation54 have recently provided an excellent summary of preclinical studies for pharmacological treatment development for CUD, and Sloan et al Citation55 have summarized the experimental clinical studies and randomized clinical trials (RCTs) for CUD. I will reflect on these two reviews and discuss the most recent RCTs and developments in terms of behavioral and neuromodulatory interventions.
One approach is substitution therapy with dronabinol, which is an approved drug for other indications (AIDS-induced anorexia, chemotherapy-induced nausea and vomiting). Dronabinol is the principal psychoactive constituent enantiomer form, -Δ 9 -THC, found in cannabis. Although substitution therapy is a great success for opioid-use disorder, dronabinol substitution has not yielded promising results. Citation55 One possible explanation for the lack of an effect of dronabinol on cannabis use is a low motivation to quit. CUD patients usually have no immediate or dramatic socioeconomic or psychosocial problems, which are often seen with cocaine, heroin, or alcohol dependence. Consequences of use are often long term and more subtle. Citation56 Thus, trying to initiate change over a relatively short period (eg, patients in the trials conducted thus far were maintained on dronabinol for only a few weeks) may have been inadequate. Clearly, low motivation to quit in CUD patients applies to any other intervention and is thus an inherent problem for treatment.
An alternative approach to substitution therapy is the blockade of the CB 1 receptor by antagonists, inverse agonists, or allosteric modulators. The application of rimonabant is the classic approach for a CB 1 -receptor blockade. Despite having an atomistic framework of CB 1 -receptor–ligand interactions, Citation14-Citation17 the molecular mode of action of rimonabant is still not fully understood—at high micromolar concentrations, rimonabant behaves as an inverse agonist at CB 1 receptors. This inverse agonistic effect probably results from an off-target effect, namely by a direct inhibition of G-protein signaling. Citation57 However, the CB 1 -receptor antagonist/inverse agonist rimonabant is not an option for the treatment of CUD as it produces serious psychiatric side effects, including anxiety, depression, and even suicidal ideation. Citation58 Several strategies are currently being pursued to circumvent the mechanisms leading to these serious side effects by developing neutral antagonists or allosteric modulators.
One promising approach goes along with the recent discovery in preclinical studies that the hormone pregnenolone acts as an allosteric CB 1 -receptor inhibitor and in doing so markedly reduces the effects of cannabis-like drugs. Citation59 Out of this discovery, the pregnenolone derivative AEF0117 was developed, which has a long half-life, is orally available, is not converted into downstream active steroids, and potently attenuates all of Δ 9 -THC’s effects in preclinical behavioral models. Importantly, the allosteric modulator AEF0117 produces none of the problems associated with rimonabant, ie, precipitated withdrawal and mood-related side effects. Based on these findings this AEF0117 is now in clinical development for CUD (ClinicalTrials.gov identifier: NCT03717272).
CBD is hyped as a panacea in the public press, and due to its pharmacological profile, it may also be effective in the treatment of CUD, but is there any preclinical/clinical evidence for the efficacy of CBD in this indication? CBD acts as a negative allosteric modulator at CB 1 receptors Citation60 and also acts at several other receptors such as CB 2 receptors, serotonin 1A (5-HT 1A ) receptors, and opioid receptors. In its function as a negative allosteric modulator, CBD inhibits endocannabinoid signaling; hence cannabis varieties rich in CBD content counterbalance the psychotropic effect of Δ 9 -THC. However, preclinical and human studies do not indicate efficacy of CBD treatment in CUD. In rodents, CBD does not alter the discriminative stimulus properties of Δ 9 -THC nor does it affect self-administration of Δ 9 -THC. Citation61 However, rodents do not reliably self-administer Δ 9 -THC; only if combined with CBD do they show a low rate of self-administration in comparison with other drugs of abuse. Citation62,Citation63 Therefore, it is a challenging task to test a CBD intervention in a rodent model of cannabinoid self-administration. A case report shows that CBD reduced self-reported cannabis use; however, in a human laboratory study, oral CBD did not reduce the reinforcing or positive subjective effects of smoked cannabis. Citation64
Another possible pharmacological intervention is the use of fatty acid amide hydrolase (FAAH) inhibitors. FAAH is the principal catabolic enzyme of endogenous cannabinoids. In a recently published RCT, treatment with the novel FAAH inhibitor PF-04457845 reduced symptoms of cannabis withdrawal and also reduced self-reported cannabis use at 4 weeks of treatment with no serious adverse events. Citation65 Not only is this a promising finding for further clinical development for CUD, it also shows that FAAH inhibitors can have a good safety profile. This is notable, as the safety of FAAH inhibitors was questioned after the observation of very severe neurological deficits after trial treatment with BIA 10-2474, an orally administered reversible FAAH inhibitor given to healthy volunteers in a phase 1 study designed to assess safety. Citation66 The promising safety profile of PF-04457845, then, suggests that perhaps BIA 10-2474 inhibits a protein other than FAAH and that specific FAAH inhibitors are safe. Nevertheless, after the BIA 10-2474 catastrophe, most pharmaceutical companies closed their FAAH-inhibitor program; however, the D’Souza et al Citation65 study may stimulate new interest. Indeed, the promising finding with PF-0447845 is currently being followed up by a well-powered multsite RCT, and results are expected by end of 2022 (ClinicalTrials.gov identifier: NCT03386487).
Other approaches refer to behavioral therapies and neuromodulatory intervention strategies. It is recognized that biases in cognitive processing of drug-related stimuli are central to the development and maintenance of addiction. In a recent proof-of-principle laboratory experiment, a four-session computerized approach-bias-modification training protocol led to blunted cannabis-cue–induced craving at the end of training, as well as to reduced cannabis use. Citation67 This promising approach of bias-modification training should be followed up as an adjunct to psychosocial treatments for treatment-seeking adults with CUD. Neuromodulation via neurofeedback approaches Citation68 — currently discussed as a useful add-on tool in the management of AUD to enhance the cognitive abilities required to maintain abstinence—or repetitive transcranial magnetic stimulation (rTMS) may further offer a treatment alternative. A preliminary study in a few CUD patients showed that 20 sessions of rTMS targeting the left dorsolateral prefrontal cortex reduced craving and cannabis use in a 4-week follow-up period. Citation69
In summary, several promising treatment approaches targeting the endocannabinoid system—especially allosteric modulators at CB 1 receptors and FAAH inhibitors—are in clinical development for CUD. In combination with behavioral and neuromodulatory approaches and psychosocial support, these pharmacological interventions might provide useful therapies in the near future.
As already described, disrupting endocannabinoid signaling reduces cue-evoked phasic dopamine responses within the reward pathway and thereby blocks drug memories and reward-seeking behavior (ie, craving). As a result, relapse behavior should be reduced as well. This cascade of events applies to all drug/cue responses, and, therefore, several preclinical and clinical attempts have been undertaken to interfere with the endocannabinoid system for treatment development for AUD, nicotine use disorder, and opioid use disorder. Citation2,Citation42,Citation44,Citation55 These endocannabinoid system–based intervention approaches will be discussed in the following section.
The endocannabinoid system as a target for AUD and SUD treatment
Rimonabant was a very promising candidate as a smoking cessation therapy. Convincing preclinical evidence was obtained that rimonabant can reduce conditioned place preference, nicotine self-administration, and cue-induced reinstatement behavior. Citation44 These preclinical studies led to a series of clinical trials showing that a high dose of rimonabant significantly increased abstinence rates and reduced smoking-cessation–related weight gain. Citation55,Citation70 Already described in the previous section, rimonabant has severe side effects and is not an option for further clinical development. Nevertheless, rimonabant provides the clinical proof of principle that pharmacological interventions, being it by neutral antagonists or by allosteric modulators at the CB 1 receptor are a promising target for the treatment of nicotine-dependent patients, especially in patients for whom smoking-cessation–induced weight gain is a deterrent to quit smoking and enter a treatment program.
Rimonabant did not produce a significant reduction in relapse rate in an RCT of alcohol-dependent patients, Citation71 and approved pharmacological treatments for AUD are limited in their effectiveness. New drugs that can easily be introduced into the clinic are needed. Currently, great hope lies in the potential of CBD to effectively treat AUD and associated somatic harm. Thus, a recent systematic review of preclinical studies shows that CBD attenuates cue-elicited and stress-elicited alcohol seeking, alcohol self-administration, withdrawal-induced convulsions, and impulsive discounting of delayed rewards in rodents. Citation72 Moreover, CBD is neuroprotective against adverse alcohol effects and attenuates alcohol-induced hepatotoxicity in rodent models. Citation72 Clearly, the effect of CBD in AUD patients now has be to rigorously tested, and indeed, a double-blind, randomized proof-of-concept study is registered (ClinicalTrials.gov identifier: NCT03252756) that is currently recruiting patients to test CBD vs placebo.
Chye et al Citation73 recently summarized all preclinical evidence on CBD in withdrawal, reward facilitation, self-administration, and reinstatement paradigms and provided a quite convincing profile of CBD for further clinical development for nicotine and opioid use disorders; however, the very few studies conducted so far in humans generated mixed results. Citation71 Most promising is a recent exploratory RCT were the acute and long-lasting effects of different doses of CBD were tested on drug-cue–induced craving in abstinent individuals with heroin use disorder. Acute CBD administration, in contrast to placebo, significantly reduced cue-induced craving, and long-lasting beneficial effects on craving were also reported. Citation74 Consequently, several new clinical trials have been initiated to test the effects of CBD on opioid withdrawal and abstinence.
Finally, recent findings revealing a role of CB 2 receptors in mediating the addictive properties of several drug classes have also opened up a promising new avenue for the clinical development of novel therapeutic approaches, including CB 2 -receptor allosteric modulators. Citation2 Although CB 2 as well as CB 1 receptors are promising targets, we are a long way from clinical development of a new molecule that would act at these targets; hence CBD, which acts on both sites as well as at other receptors and also has a good safety profile, currently has the best potential for clinical development in AUD and opioid use disorder.
Summary and future perspectives
The last decades have seen a major gain in understanding of the action of cannabinoids and the endocannabinoid system in reward processing and the development of addictive behavior. This basic knowledge provides the rationale that pharmacological or genetic interference with the endocannabinoid system—be it on the level of CB 1 /CB 2 -receptor blockade or the inhibition of endocannabinoid synthetizing enzymes, especially FAAH inhibitors—may reduce drug craving and subsequent relapse in addicted patients. Unfortunately, the interest of major pharmaceutical industries for clinical development of new compounds targeting the endocannabinoid system has been severely dampened by the worldwide withdrawal of the already approved antiobesity medication rimonabant (Acomplia) due to serious psychiatric side effects. Therefore, only small biotechnology companies and academic-driven clinical developments will further drive medication development. In contrast to these slowly ongoing future medication developments, massive investments are being made in medical cannabis products including CBD. However, whether substitution therapy with medical cannabis—as proposed for CUD—is a promising approach is questionable. The same is true of CBD; it has a good safety profile, but preclinical and clinical evidence is mixed, and only large RCTs, especially in alcohol and opioid addiction, will give us conclusive insights into its effectiveness. A caveat for all these drug development efforts is that the endocannabinoid system does not only mediate primary and secondary reinforcing properties for drugs of abuse but is itself involved in reward processing. Therefore, any interference with this system may not only block craving and relapse for a given drug but may also interfere with any natural reward, such as eating, libido, social rewards, and many other rewards that drive our daily activities.
Financial support for this work was provided by the Bundesministerium für Bildung und Forschung (BMBF) funded ERA-NET program: Psi-Alc (FKZ: 01EW1908), the BMBF-funded SysMedSUDs consortium (FKZ: 01ZX1909A), and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 402170461 – TRR 265.Citation75 The author RS declares no conflict of interest.
REFERENCES
- LutzBMarsicanoGMaldonadoRHillardCJThe endocannabinoid system in guarding against fear, anxiety and stressNat Rev Neurosci2015161270571826585799
- ManzanaresJCabañeroDPuenteNGarcía- GutiérrezMSGrandesPMaldonadoRRole of the endocannabinoid system in drug addictionBiochem Pharmacol201815710812130217570
- The health and social effects of nonmedical cannabis use (2016) World Health Organization Available at:
- WagnerFAAnthonyJCInto the world of illegal drug use: exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaineAm J Epidemol2002155918925
- Lopez-QuinteroCPérez de los CobosJHasinDSet alProbability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC)Drug Alcohol Depend20111151-212013021145178
- SarrisJSinclairJKaramacoskaDDavidsonMFirthJMedicinal cannabis for psychiatric disorders: a clinically-focused systematic reviewBMC Psychiatry20202012431948424
- LigrestiADe PetrocellisLDi MarzoVFrom phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacologyPhysiol Rev20169641593165927630175
- HerkenhamMLynnABJohnsonMRet alCharacterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic studyJ Neurosci19911125635831992016
- SzaboBSchlickerEEffects of cannabinoids on neurotransmissionHandb Exp Pharmacol2005 16832736516596780
- PiomelliDThe molecular logic of endocannabinoid signallingNat Rev Neurosci200341187388414595399
- Oliveira da CruzJFRobinLMDragoFMarsicanoGMetna-LaurentMAstroglial type-1 cannabinoid receptor (CB1): a new player in the tripartite synapseNeuroscience2016323354225967266
- MunroSThomasKLAbu-ShaarMMolecular characterization of a peripheral receptor for cannabinoidsNature1993365644161657689702
- JordanCJXiZXProgress in brain cannabinoid CB2 receptor research: from genes to behaviorNeurosci Biobehav Rev20199820822030611802
- HuaTVemuriKPuMet alCrystal structure of the human cannabinoid receptor CB1Cell2016167375076227768894
- HuaTVemuriKNikasSPet alCrystal structures of agonist-bound human cannabinoid receptor CB1Nature2017547766446847128678776
- LiXHuaTVemuriKHoJHet alCrystal structure of the human cannabinoid receptor CB2Cell2019176345946730639103
- ShaoZYinJChapmanKet alHigh-resolution crystal structure of the human CB1 cannabinoid receptorNature2016540763460260627851727
- Console-BramLMarcuJAboodMECannabinoid receptors: nomenclature and pharmacological principlesProg Neuropsychopharmacol Biol Psychiatry201238141522421596
- PertweeRGHowlettACAboodMEet alInternational Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2Pharmacol Rev201062458863121079038
- MullerCMoralesPReggioPHCannabinoid ligands targeting TRP channelsFront Mol Neurosci20191148730697147
- Di ChiaraGImperatoADrugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving ratsProc Natl Acad Sci U S A19888514527452782899326
- SpanagelRWeissFThe dopamine hypothesis of reward: past and current statusTrends Neurosci1999221152152710529820
- RoeperJDissecting the diversity of midbrain dopamine neuronsTrends Neurosci201336633634223582338
- WiseRARobbleMADopamine and addictionAnnu Rev Psychol2020717910631905114
- FrenchEDΔ9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptorsNeurosci Lett199722631591629175591
- GessaGLMelisMMuntoniALDianaMCannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptorsEur J Pharmacol1998341139449489854
- CheerJFWassumKMHeienMLPhillipsPEWightmanRMCannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake ratsJ Neurosci200424184393440015128853
- PetersKZOlesonEBCheerJFA brain on cannabinoids: the role of dopamine release in reward seeking and addictionCold Spring Harb Perspect Med202021piia03930
- LupicaCRRiegelACEndocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addictionNeuropharmacology20054881105111615878779
- SpanagelRHerzAShippenbergTSOpposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathwayProc Natl Acad Sci U S A1992896204620501347943
- OlesonEBBeckertMVMorraJTet alEndocannabinoids shape accumbal encoding of cue-motivated behavior via CB1 receptor activation in the ventral tegmentumNeuron20127336037322284189
- AlgerBEKimJSupply and demand for endocannabinoidsTrends Neurosci201134630431521507493
- RobbeDAlonsoGDuchampFBockaertJManzoniOJLocalization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbensJ Neurosci200121110911611150326
- RobbeDKopfMRemauryABockaertJManzoniOJEndogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbensProc Natl Acad Sci U S A200299128384838812060781
- ZlebnikNECheerJFDrug-induced alterations of endocannabinoid-mediated plasticity in brain reward regionsJ Neurosci20163640102301023827707960
- BilbaoANeuhoferDSepersMet alEndocannabinoid LTD in accumbal D1 neurons mediates reward-seeking behavioriScience202023310095132179475
- NovakMHalboutBO’ConnorECet alIncentive learning underlying cocaine-seeking requires mGluR5 receptors located on dopamine D1 receptor-expressing neuronsJ Neurosci20103036119731198220826661
- AugierEDulmanRSDamadzicRPillingAHamiltonJPHeiligMThe GABAB positive allosteric modulator ADX71441 attenuates alcohol self-administration and relapse to alcohol seeking in ratsNeuropsychopharmacology20174291789179928294133
- van den BrinkWAddoloratoGAubinHJet alEfficacy and safety of sodium oxybate in alcohol-dependent patients with a very high drinking risk levelAddict Biol201823496998630043457
- BäckströmPBachtelerDKochSHyytiäPSpanagelRmGluR5 antagonist MPEP reduces ethanol-seeking and relapse behaviorNeuropsychopharmacology20042992192814735132
- OliveMFMetabotropic glutamate receptor ligands as potential therapeutics for addictionCurr Drug Abuse Rev20092839819630739
- WangXMoussawiKKnackstedtLShenHKalivasPWRole of mGluR5 neurotransmission in reinstated cocaine-seekingAddict Biol201318404922340009
- De VriesTJShahamYHombergJRet alA cannabinoid mechanism in relapse to cocaine seekingNat Med20017101151115411590440
- CippitelliABilbaoAHanssonAet alEuropean TARGALC Consortium. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in ratsEur J Neurosci2005212243225115869521
- European Drug Report 2019 European Monitoring Centre for Drugs and Drug Addiction Available at:
- ElSohlyMAMehmedicZFosterSGonCChandraSChurchJCChanges in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United StatesBiol Psychiatry20167961361926903403
- ZimmermannUSWinkelmannPRPilhatschMNeesJASpanagelRSchulzKWithdrawal phenomena and dependence syndrome after the consumption of “spice gold.”Dtsch Arztebl Int20091062746446719652769
- AuwärterVDresenSWeinmannWMüllerMPützMFerreirosN‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs?J Mass Spectrom200944583283719189348
- SpadernaMAddyPHD’SouzaDCSpicing things up: synthetic cannabinoidsPsychopharmacology (Berl)2013228452554023836028
- AdamsAJBanisterSDIrizarryLTreckiJSchwartzMGeronaR“Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New YorkN Engl J Med2017376323524227973993
- 2017 Synthetic cannabinoids in Europe (perspectives on drugs)2017 July 2020 Available at: https://www.emcdda.europa.eu/publications/pods/synthetic-cannabinoids
- AlexandreJCarmoHCarvalhoFSilvaJPSynthetic cannabinoids and their impact on neurodevelopmental processesAddict Biol2020252e1282431441196
- HindleyGBeckKBorganFet alPsychiatric symptoms caused by cannabis constituents: a systematic review and meta-analysisLancet Psychiatry20207434435332197092
- PanlilioLVJustinovaZPreclinical studies of cannabinoid reward, treatments for cannabis use disorder, and addiction-related effects of cannabinoid exposureNeuropsychopharmacology201831116141
- SloanMEGowinJLRamchandaniVAHurdYLLe FollBThe endocannabinoid system as a target for addiction treatment: trials and tribulationsNeuropharmacology2017124738328564576
- BudneyAJRadonovichKJHigginsSTWongCJAdults seeking treatment for marijuana dependence: a comparison with cocaine-dependent treatment seekersExp Clin Psychopharmacol1998644194269861556
- PorcuAMelisMTurecekRet alRimonabant, a potent CB1 cannabinoid receptor antagonist, is a Gαi/o protein inhibitorNeuropharmacology201813310712029407764
- NguyenTThomasBFZhangYCurrent approaches for therapeutics development. overcoming the psychiatric side effects of the cannabinoid CB1 receptor antagonistsCurr Top Med Chem201919161418143531284863
- ValléeMVitielloSBellocchioLet alPregnenolone can protect the brain from cannabis intoxicationScience20143436166949824385629
- LaprairieRBBagherAMKellyMEDenovan-WrightEMCannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptorBr J Pharmacol2015172204790480526218440
- WakefordAGPWetzellBBPomfreyRLet alThe effects of cannabidiol (CBD) on Δ9-tetrahydrocannabinol (THC) self-administration in male and female Long-Evans ratsExp Clin Psychopharmacol201725424224828682102
- SpencerSNeuhoferDChiomaVCet alA model of Δ9-tetrahydrocannabinol self-administration and reinstatement that alters synaptic plasticity in nucleus accumbensBiol Psychiatry201884860161029861097
- SpanagelRAnimal models of addictionDialogues Clin Neurosci201719324725829302222
- HaneyMMalcolmRJBabalonisSet alOral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabisNeuropsychopharmacology20164181974198226708108
- D’SouzaDCCortes-BrionesJCreaturaGet alEfficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trialLancet Psychiatry201961354530528676
- KerbratAFerréJCFillatrePet alAcute neurologic disorder from an inhibitor of fatty acid amide hydrolaseN Engl J Med2016375181717172527806235
- ShermanBJBakerNLSquegliaLMMcRae-ClarkALApproach bias modification for cannabis use disorder: a proof-of-principle studyJ Subst Abuse Treat201887162229471922
- KirschMGruberIRufMKieferFKirschPReal-time functional magnetic resonance imaging neurofeedback can reduce striatal cue-reactivity to alcohol stimuliAddict Biol201621498299226096546
- SahlemGLCarusoMAShortEBet alA case series exploring the effect of twenty sessions of repetitive transcranial magnetic stimulation (rTMS) on cannabis use and cravingBrain Stimul202031265266
- RobinsonJDCinciripiniPMKaram-HageMet alPooled analysis of three randomized, double-blind, placebo controlled trials with rimonabant for smoking cessationAddict Biol201823129130328429843
- SoykaMKollerGSchmidtPet alCannabinoid receptor 1 blocker rimonabant (SR 141716) for treatment of alcohol dependence: results from a placebo-controlled, double-blind trialJ Clin Psychopharmacol200828331732418480689
- TurnaJSyanSKFreyBNet alCannabidiol as a novel candidate alcohol use disorder pharmacotherapy: a systematic reviewAlcohol Clin Exp Res201943455056330698831
- ChyeYChristensenESolowijNYücelMThe endocannabinoid system and cannabidiol’s promise for the treatment of substance use disorderFront Psychiatry2019106330837904
- HurdYLSpriggsSAlishayevJet alCannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trialAm J Psychiatry20191761191192231109198
- HeinzAKieferFSmolkaMNet alAddiction Research Consortium: losing and regaining control over drug intake (ReCoDe) – from trajectories to mechanisms and interventionsAddict Biol2020252e1286631859437
