Abstract
More than three decades ago, the presence of dendritic cells (DCs) was described in the nasal mucosa. From then on, there was a gradual increase of knowledge on the basic aspects of DC function. DCs in the airways sample the airway lumen for incoming antigen and present it to the various players of the immune system subsequently, so that an optimal response is induced to clear pathogens. The enormous interest of immunologists in the role that DCs play in the induction and regulation of immune responses is currently beginning to integrate into the framework of disease pathogenesis. DCs have been implicated in causing allergic sensitization as well as ongoing allergic inflammation. DCs play a crucial role in the development and maintenance of allergic rhinitis (AR). The depletion of DCs in AR abrogates the features of AR. Consequently, DCs and several molecules on DCs are ideal targets for therapeutic intervention.
The nose is the part of the human body with mucosal tissue that gets in contact with inhaled air. It functions like an air conditioner that filters air that is inhaled. Because of this function, the nose is continuously exposed to pathogens. These dangers need to be prevented by good defense mechanisms that protect against pathogens. Epithelial cells form the first cellular barrier, with an important barrier role for tight junctions in between epithelial cells. In cases in which this is insufficient, there is another defense mechanism caused by immune cells. These immune cells stay in the epithelium until they have been activated by a stimulus that is recognized by that particular cell. The most important immune-competent cells in the epithelium are the dendritic cells (DCs). DCs play an important role in allergic inflammation that was first described by Fokkens et al. (Citation1, Citation2). In steady-state condition, a few of these professional antigen presenting cells (APCs) are present (Citation1, Citation2). These DCs form an intensive network of cells in between epithelial cells Citation3–(Citation5). Intraepithelial location of DCs is often seen. The DCs seem to be anchored in the epithelium by the use of tight junction protein claudin-1, and DCs can penetrate beyond the most apical tight junction protein, occludin. DCs are able to open and make connections with the tight junctions, enabling dendrite sprouting beyond the epithelium (Citation6). The tight junction mechanisms behind have two important advantages: 1) the DC itself is anchored; and, due to the penetration, 2) the epithelial tight junction barrier stays the same (Citation7, Citation8).
Triggers of DC activation in the nasal mucosa
When a DC is triggered, it gets activated and changes from an immature DC into a mature DC phenotype. The sentinel function of DCs is very important for inducing an immune response. The (in)direct activation of DCs occurs due to the so-called pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) (Citation9). Both epithelial cells and DCs express a broad range of PAMPs and DAMPs [for a review, see Willart et al. (Citation10)] for the epithelium, with the consequence of cytokine production and release that can activate the innate immune system, including DCs. Important chemokines are the so-called alarmins (Citation11), including cytokines like IL-33, thymic stromal lymphopoietin (TSLP), and other members of the interleukin (IL)-1 family, including IL-1α, IL-1β, and the IL-7 family () (Citation12). TSLP, IL-33, and IL-33R–T1ST2 have been associated with asthma genome-wide, but typically the impact of each of these genes is mild Citation13–(Citation15). The effect of these alarmins in general is the activation of DCs, with the consequence of migration to the draining lymph node (LN). IL-33 is one of the epithelial-derived cytokines that supports Th2 immunity via the binding of the ST2 receptor that is detectable on immune-competent cells, including DCs, in the nasal mucosa (Citation16, Citation17). This receptor is also expressed by DCs, and activation of IL-33 leads to increased expression of MHCII, co-stimulatory molecules, and proinflammatory cytokines, chemokines (including CCL17, CCL22) and support the Th2 response Citation18–(Citation20). IL-33 is detectable in the nasal mucosa of allergic rhinitis (AR) patients and elevated in allergen-challenged AR patients (Citation21). Moreover, IL-33 plays a critical role in experimental AR (Citation22). IL-25 (IL17E), which has similar functions as IL-33, is important in the intestine (Citation23) and is not crucial for the development of AR (Citation12). Also, TSLP is expressed in the nasal mucosa of AR and chronic rhinosinusitis patients (Citation24), and it is important to stimulate DCs in AR (Citation25). Moreover, TSLP-activated DCs play a role to induce naïve CD4+ T-cell differentiation into Th2 cells (Citation26).
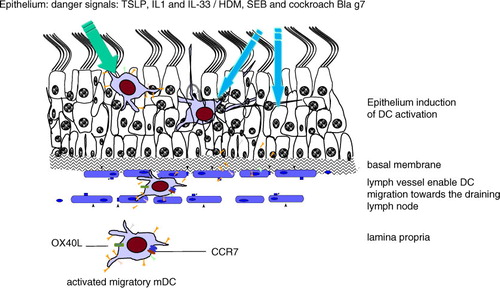
Fig. 1. Dendritic cells as critical players and sentinels of the immune system in the nasal mucosa of allergic rhinitis. The presence of DCs in the epithelium fulfills a sentinel function in the steady-state condition. During inflammation and allergic inflammation, DCs were activated due to the so-called alarmins: TSLP and members of the IL-1 family, including IL-1α and IL-33. Also, nonphysiological factors like staphylococcal enterotoxin B antigens act as super-antigens, influencing the epithelium and indirect DCs. Furthermore, the nonphysiological presence of allergens like house dust mites (HDMs) and the cockroach allergen Bla G 7 influences the epithelium and DCs. Once epithelial DCs are triggered, they get activated and migrate from the epithelium via the lymph vessel to the draining lymph node. Moreover, activated DCs are also present in the lamina propria and are seen in close proximity to T-cells, suggesting the support of a local immune response. An important molecule for DC migration is CCR7.
The presence of certain antigens could influence the number of DCs. The superantigen staphylococcal enterotoxin (SEB) in the nasal mucosa of AR impairs the numbers of tolerogenic DCs. In this active process, the epithelial integrin Avb6 plays a role. Experimental confirmation of this relation is seen in experiments in which neutralization of SEB by antibodies in epithelial culture experiments could impair the suppressive effect of SEB on Avb6 levels of mRNA and protein (Citation27).
There are also examples of direct DC activation by allergens. In the case of cockroach allergy in AR patients, it became clear that the cockroach allergen rBla g 7 induces DC-dictated Th2 polarization of CD4+ T cells through a mechanism in which TIM4 is involved (Citation28). Moreover, rBla g 7 induces IL-13 production of human DCs, which has implications for the isotype switch of B-cells into immunoglobulin E (IgE)-producing B-cells (Citation28).
DC migration from peripheral tissue to the draining LN
Once DCs have been activated, they express receptors reacting to chemokine signaling. Then, DCs start to migrate in the direction of an LN. Data strongly suggest that these activated maturated DCs migrate via the lymphatic vessel and appear in the draining LN. From textbooks, it is known that when DCs are activated, they express CCR7, which is important for the migration from peripheral tissue sites toward the draining LN. Blocking CCR7 impairs the pathogenic contribution of DCs in allergy (Citation29). Once they have arrived in the draining LN, they present antigen to antigen-specific T-cells and decide in which of them the immune response should occur. This process is generally the same for all immune responses.
Studying the deposition of airborne particles indicated that more than 80% are trapped in the nasal mucosa, and the minority is trapped in the lower airway (Citation30). Dyes used as models to study flow in the nasal mucosa illustrated that dyes could not penetrate the nasal mucosa easily, revealing the physical barrier function of the nasal mucosa Citation31–(Citation34). Introduction of carboxyfluorescein succinimidyl ester (CFSE)-labeled DCs or DiY Dye in the nasal mucosa in droplet form resulted in detectable CFSE-labeled DCs and DiY in the nasal mucosa in the nasal-associated lymphoid tissue (NALT) and in the cervical LN, which drains the nose. No CFSE-labeled cells could be detected in either the mediastinal LN or the lung (Citation35). In a human migration study of the respiratory tract, it is indicated that antigen pulsed DCs that are introduced by injection in the nasal inferior turbinates can be traced back to the regional LNs (Citation36). Although DCs used in this study were monocyte-derived DCs, they still clearly show a localized draining and migration pattern of the introduced DCs, as observed with the DiY and allergen-loaded DCs in murine studies (Citation4).
Nasal mucosa dendritic cell type and quantification
The use of markers for DCs includes CD1a as one of the most important characteristic markers. Other markers for myeloid DCs (mDCs) are CD1c, MHCII, CD11c, CD207, and CD208, and markers for plasmacytoid DCs (pDCs) are CD123, CD303, and CD304. Several of these markers were widely in use as blood DC markers. For histology, the use of an appropriate marker along with a dendritic morphology is essential to characterize cells as DCs (Citation4) (). This is important because the markers used are often expressed on other cell types as well. Ratio calculations between mDCs and pDCs were performed to reflect active allergic inflammation and/or a steady state in nasal mucosa tissue (Citation37). Moreover, an active role for pDCs in allergic inflammation was also confirmed in allergen challenge studies with an important role for the DC stimulation of TNFα (Citation38). Elevated numbers of DCs (CD209 DC-Sign) were observed in allergic fungal rhinosinusitis (Citation39). Models of AR showed an elevated number of DCs (CD11c-positive cells with a dendritic morphology) (Citation4, Citation40, Citation41). Moreover, the tolerogenic number of DCs as measured as CD11c+ ADLH1/2+ cells was shown to be reduced in AR mice, and the corresponding number of FoxP3 shows the same reduction, indicating an impaired tolerogenic response in AR mice (Citation41). A nice example of increased levels of HLADR+ CD209+ CD14+ DCs of other nasal disease pathogens is seen in the circulation of rhinitis patients with or without nasal polyps. Moreover, the proportion of CD4 +CCR8+ T-cells reflecting Th2 cells is higher in rhinitis when compared to controls (Citation42). Moreover, this observation could be confirmed in the nasal mucosa tissue of allergic fungal rhinosinusitis patients (Citation39).
Table 1 Human DC antibodies
Nasal mucosal mDCs and pDCs numbers were significant increase in relevant allergen challenged nasal mucosa in AR which was not seen in antigen-challenged control subjects at 8 hours following allergen challenge. In the skin of AR patients only an increase in mDCs was observed 48 h post allergen challenge and not for pDCs. Allergen challenge induces the production of IL-10 in nasal mucosa of control subject which was not seen in AR (Citation43). Tolerogenic DCs identified as CD11c+ transforming growth factor (TGF)-β-positive cells were less present in the nasal mucosa of AR patients when compared to non-AR controls. Along this, the Foxp3+ CD4+CD3+ T-cells were also less present in the nasal mucosa of AR patients when compared to non-AR controls. The levels of IL4 and IgE in nasal mucosa tissue homogenates were higher in AR patients than in non-allergics. The number of mast cells was higher in AR patients than in non-allergics (Citation26). The aforementioned examples illustrate the close correlation between disease pathogenesis DCs and Th2 cells.
DCs express immunologically relevant molecules; therapeutic targets
Myeloid DCs from AR were constitutively primed to induce Th2 cytokines and TNF in allogenic CD4+ T cells, whereas no differences were observed for interferon (IFN)-γ or IL-10 (Citation44). These DCs, obtained from either human blood or nasal mucosa tissue DCs, express lower levels of the ICOS ligand in AR than in controls. ICOS is a co-stimulatory molecule on T-cells that is important for a stable DC–T-cell interaction; it is associated with Th1 response, and the expression of ICOS on DCs is reduced in allergic inflammation models (Citation45). Regarding the cytokine production by DCs, no differences in DC-derived IL-10 and IL-12 were seen between AR and control mDCs. Targeting by blocking antibodies of ICOSL in control DCs upregulated IL-13 but not IFN-γ in co-cultures with T-cells, whereas PD-L1 blockade upregulated both IL-13 and IFN-γ (Citation44). OX40 ligand (OX40l) has already been long known as a co-stimulatory molecule. OX40l plays a sentinel role in the adaptive immune response by promoting Th2 polarization of naïve T cells within the LN. The OX40–OX40l axis might be an interesting treatment opportunity; although the first in vivo trails were not as good as expected for all patients, in some patients it seemed to be beneficial (Citation46, Citation47). Some recent studies targeting indirect DCs with antibodies against OX40l or TSLP in asthma might have also beneficial effects on AR (Citation47, Citation48) ().
Table 2 Novel DC targets in allergic rhinitis
Pharmacological interaction of in vitro cultured DCs in the presence of the Clara cell 10-kDa (CC10) showed reduced levels of OX40l expression, lowered the production of IL-23 and IL-6, and enhanced the expression of CD86 and the production of TGF-β. DCs treated with CC10 protein inhibit Th17 responses through modulating DCs in the setting of AR (Citation49).
Sphingosine 1 phosphate (S1P) and its analogs are important mediators for immune cell migration. DCs express S1P receptors 1–5 (S1P1–5). Sphingosine lyase (SL) is important for the breakdown of S1P; FTY720 is an inhibitor of SL and an analog of S1P. S1P reduces the migration and the capacity of the antigen presentation function of DCs (Citation50). Topical intranasal treatment of FTY720 may be mediated by affecting and impairing the DC function. We found that topical treatment with FTY720 reduces the features of AR. When OVA antigen was applied intranasally, OVA-specific T-cell divisions were seen in the lymphoid structures draining the upper airway, including the NALT. The division was blocked partially in the NALT by FTY720 treatment when compared to saline treatment. This effect was not observed in the cervical nodes. This illustrates the functional effect of FTY720 on DC migration and also might influence the type of Th immune response (Citation40). Moreover, T-cell differentiation pursuant to treatment with S1P (Citation40) is such that Th17 cell development is almost unaffected, whereas the outgrowth of other Th populations is reduced (Citation51). In our hands, FTY720 showed a significant reduction in IL-4-producing CD4-positive T-cells cultured under Th2 conditions when FTY720 was added to the culture. IFN-γ-producing CD4 T-cells and IL-17-producing Th17 cells were not affected by FTY720 treatment (Citation40).
Recent novelties; Lessens from outside the nasal mucosa
In the development of allergen-specific immunotherapy, several advantages were performed in preclinical and clinical research. Due to the use of adjuvants like toll-like receptor agonist and vectors like liposomes and microspheres in clinical trials, advantages were seen in Phase II and Phase III trials (Citation52). Nasal mucosa administration of antigens in the presence of adjuvants like nano-emulsion induced distinct pathways of humoral and cellular immunity (Citation53).
CD40, CD80, and CD86 are molecules expressed by DCs that are important for a well-established immune response. Recently accumulating data appeared from the Notch pathway. Notch ligands expressed by DCs are important players for the outcome of immune responses. The DC Notch ligand molecules Jagged 1 and Jagged 2 were important for Th2 responses, whereas delta-like ligand 1 and 4 were important for Th1 responses (Citation54). In vivo data by our group in asthma models indicate the importance of the Notch pathway. Mice that lack recombination signal binding protein for the immunoglobulin kappa J region (RBPj), which is an important protein of the intracellular Notch complex that binds Gata3, showed impaired allergic airway inflammation, including less eosinophilic inflammation, lower levels of Th2 cytokines in BAL fluid, and lower levels of IgE. This implies that the Notch pathway is important in allergic airway inflammation.
Conclusions
DCs together with epithelial cells play an important role in the connection between innate and adaptive immune responses of the upper airways. The DCs are the migratory cells that ideally could be targeted by inhibiting their migratory capacity and by therapeutically preventing their interaction with cells of the adaptive immune system.
Conflict of interest and funding
The author declares no conflict of interest and funding.
References
- Fokkens WJ Vroom TM Rijntjes E Mulder PG. Fluctuation of the number of CD-1(T6)-positive dendritic cells, presumably Langerhans cells, in the nasal mucosa of patients with an isolated grass-pollen allergy before, during, and after the grass-pollen season. J Allergy Clin Immunol. 1989;84(1):39-43.
- Fokkens WJ Vroom TM Rijntjes E Mulder PG. CD-1 (T6), HLA-DR-expressing cells, presumably Langerhans cells, in nasal mucosa. Allergy. 1989;44(3):167-72.
- Jahnsen FL Farkas L Lund-Johansen F Brandtzaeg P. Involvement of plasmacytoid dendritic cells in human diseases. Hum Immunol. 2002;63(12):1201-5.
- KleinJan A Willart M van Rijt LS Braunstahl GJ Leman K Jung S et al An essential role for dendritic cells in human and experimental allergic rhinitis. J Allergy Clin Immunol. 2006;118(5):1117-25.
- Takano K Kojima T Go M Murata M Ichimiya S Himi T et al HLA-DR- and CD11c-positive dendritic cells penetrate beyond well-developed epithelial tight junctions in human nasal mucosa of allergic rhinitis. J Histochem Cytochem. 2005;53(5):611-9.
- Hammad H Lambrecht BN. Dendritic cells and airway epithelial cells at the interface between innate and adaptive immune responses. Allergy. 2011;66(5):579-87.
- Kojima T Go M Takano K Kurose M Ohkuni T Koizumi J et al Regulation of tight junctions in upper airway epithelium. Biomed Res Int. 2013;2013:947072: [PubMed Abstract] [PubMed CentralFull Text]
- Ogasawara N Kojima T Go M Takano K Kamekura R Ohkuni T et al Epithelial barrier and antigen uptake in lymphoepithelium of human adenoids. Acta Otolaryngol. 2011;131(2):116-23.
- Lundberg K Rydnert F Greiff L Lindstedt M. Human blood dendritic cell subsets exhibit discriminative pattern recognition receptor profiles. Immunology. 2014;142(2):279-88.
- Willart MA Hammad H. Alarming dendritic cells for allergic sensitization. Allergol Int. 2010;59(2):95-103.
- Willart MA Deswarte K Pouliot P Braun H Beyaert R Lambrecht BN et al Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209(8):1505-17.
- Nakanishi W Yamaguchi S Matsuda A Suzukawa M Shibui A Nambu A et al IL-33, but not IL-25, is crucial for the development of house dust mite antigen-induced allergic rhinitis. PLoS One. 2013;8(10):e78099
- Ober C Yao TC. The genetics of asthma and allergic disease: A 21st century perspective. Immunol Rev. 2011;242(1):10-30.
- Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8(3):169-82.
- Wjst M Sargurupremraj M Arnold M. Genome-wide association studies in asthma: What they really told us about pathogenesis. Curr Opin Allergy Clin Immunol. 2013;13(1):112-8.
- Baba S Kondo K Kanaya K Suzukawa K Ushio M Urata S et al Expression of IL-33 and its receptor ST2 in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2014;124(4):E115-22.
- Baumann R Rabaszowski M Stenin I Tilgner L Gaertner-Akerboom M Scheckenbach K et al Nasal levels of soluble IL-33R ST2 and IL-16 in allergic rhinitis: Inverse correlation trends with disease severity. Clin Exp Allergy. 2013;43(10):1134-43. [PubMed Abstract]
- Rank MA Kobayashi T Kozaki H Bartemes KR Squillace DL Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123(5):1047-54.
- Besnard AG Togbe D Guillou N Erard F Quesniaux V Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011;41(6):1675-86.
- Kurokawa M Matsukura S Kawaguchi M Ieki K Suzuki S Watanabe S et al Interleukin-33-activated dendritic cells induce the production of thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Arch Allergy Immunol. 2013;161(Suppl 2):52-7.
- Rogala B Gluck J. The role of interleukin-33 in rhinitis. Curr Allergy Asthma Rep. 2013;13(2):196-202.
- Haenuki Y Matsushita K Futatsugi-Yumikura S Ishii KJ Kawagoe T Imoto Y et al A critical role of IL-33 in experimental allergic rhinitis. J Allergy Clin Immunol. 2012;130 1 184–94, e11
- Buning C Genschel J Weltrich R Lochs H Schmidt H. The interleukin-25 gene located in the inflammatory bowel disease (IBD) 4 region: No association with inflammatory bowel disease. Eur J Immunogenet. 2003;30(5):329-33.
- Nagarkar DR Poposki JA Tan BK Comeau MR Peters AT Hulse KE et al Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132 3 [PubMed CentralFull Text] 593–600, e12
- Melum GR Farkas L Scheel C Van Dieren B Gran E Liu YJ et al A thymic stromal lymphopoietin-responsive dendritic cell subset mediates allergic responses in the upper airway mucosa. J Allergy Clin Immunol. 2014
- Liu T Liang X Li TL Ma J Yang JF Yang PC. Staphylococcal enterotoxin B compromises the immune tolerant status in the airway mucosa. Clin Exp Allergy. 2012;42(3):375-82.
- Ito T Wang YH Duramad O Hori T Delespesse GJ Watanabe N et al TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202(9):1213-23.
- Xu L Zhang M Ma W Jin S Song W He S. Cockroach allergen Bla g 7 promotes TIM4 expression in dendritic cells leading to Th2 polarization. Mediators Inflamm. 2013;2013:983149: [PubMed Abstract] [PubMed CentralFull Text]
- Schlereth S Lee HS Khandelwal P Saban DR. Blocking CCR7 at the ocular surface impairs the pathogenic contribution of dendritic cells in allergic conjunctivitis. Am J Pathol. 2012;180(6):2351-60.
- Hellings PW Ceuppens JL. Mouse models of global airway allergy: What have we learned and what should we do next?. Allergy. 2004;59(9):914-9.
- Tomaki M Zhao LL Lundahl J Sjostrand M Jordana M Linden A et al Eosinophilopoiesis in a murine model of allergic airway eosinophilia: Involvement of bone marrow IL-5 and IL-5 receptor alpha. J Immunol. 2000;165(7):4040-50.
- Saito H Howie K Wattie J Denburg A Ellis R Inman MD et al Allergen-induced murine upper airway inflammation: Local and systemic changes in murine experimental allergic rhinitis. Immunology. 2001;104(2):226-34.
- McCusker C Chicoine M Hamid Q Mazer B. Site-specific sensitization in a murine model of allergic rhinitis: Role of the upper airway in lower airways disease. J Allergy Clin Immunol. 2002;110(6):891-8.
- Wang Y McCusker CT. Interleukin-13-dependent bronchial hyper-responsiveness following isolated upper-airway allergen challenge in a murine model of allergic rhinitis and asthma. Clin Exp Allergy. 2005;35(8):1104-11.
- KleinJan A. The crucial role of dendritic cells in rhinitis. Curr Opin Allergy Clin Immunol. 2011;11(1):12-7.
- Horiguchi S Matsuoka T Okamoto Y Sakurai D Kobayashi K Chazono H et al Migration of tumor antigen-pulsed dendritic cells after mucosal administration in the human upper respiratory tract. J Clin Immunol. 2007;27(6):598-604.
- Reinartz SM van Tongeren J van Egmond D de Groot EJ Fokkens WJ van Drunen CM. Dendritic cells in nasal mucosa of subjects with different allergic sensitizations. J Allergy Clin Immunol. 2011;128(4):887-90.
- Ekman AK Erjefalt JS Jansson L Cardell LO. Allergen-induced accumulation of CD68−, CD123+ dendritic cells in the nasal mucosa. Int Arch Allergy Immunol. 2011;155(3):234-42.
- Ayers CM Schlosser RJ O'Connell BP Atkinson C Mulligan RM Casey SE et al Increased presence of dendritic cells and dendritic cell chemokines in the sinus mucosa of chronic rhinosinusitis with nasal polyps and allergic fungal rhinosinusitis. Int Forum Allergy Rhinol. 2011;1(4):296-302.
- Kleinjan A van Nimwegen M Leman K Hoogsteden HC Lambrecht BN. Topical treatment targeting sphingosine-1-phosphate and sphingosine lyase abrogates experimental allergic rhinitis in a murine model. Allergy. 2013;68(2):204-12.
- Xie MQ Liu J Long Z Tian DF Zhao CQ Yang PC. Modulation of immune tolerance with a Chinese traditional prescription inhibits allergic rhinitis in mice. N Am J Med Sci. 2011;3(11):503-7.
- O'Connell BP Schlosser RJ Wentzel JL Nagel W Mulligan JK. Systemic monocyte-derived dendritic cells and associated Th2 skewing in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2014;150(2):312-20.
- Pilette C Jacobson MR Ratajczak C Detry B Banfield G VanSnick J et al Aberrant dendritic cell function conditions Th2-cell polarization in allergic rhinitis. Allergy. 2013;68(3):312-21.
- Shen C Hupin C Froidure A Detry B Pilette C. Impaired ICOSL in human myeloid dendritic cells promotes Th2 responses in patients with allergic rhinitis and asthma. Clin Exp Allergy. 2014;44(6):831-41.
- van Rijt LS Jung S Kleinjan A Vos N Willart M Duez C et al In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201(6):981-91.
- Kaur D Brightling C. OX40/OX40 ligand interactions in T-cell regulation and asthma. Chest. 2012;141(2):494-9.
- Gauvreau GM Boulet LP Cockcroft DW FitzGerald JM Mayers I Carlsten C et al OX40L blockade and allergen-induced airway responses in subjects with mild asthma. Clin Exp Allergy. 2014;44(1):29-37.
- Gauvreau GM O'Byrne PM Boulet LP Wang Y Cockcroft D Bigler J et al Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370(22):2102-10.
- Liu Y Yu HJ Wang N Zhang YN Huang SK Cui YH et al Clara cell 10-kDa protein inhibits T(H)17 responses through modulating dendritic cells in the setting of allergic rhinitis. J Allergy Clin Immunol. 2013;131 2 387-94. [PubMed Abstract] [PubMed CentralFull Text] e1–12
- Idzko M Hammad H van Nimwegen M Kool M Muller T Soullie T et al Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest. 2006;116(11):2935-44.
- Liao JJ Huang MC Goetzl EJ. Cutting edge: Alternative signaling of Th17 cell development by sphingosine 1-phosphate. J Immunol. 2007;178(9):5425-8.
- Pfaar O Cazan D Klimek L Larenas-Linnemann D Calderon MA. Adjuvants for immunotherapy. Curr Opin Allergy Clin Immunol. 2012;12(6):648-57.
- Bielinska AU Makidon PE Janczak KW Blanco LP Swanson B Smith DM et al Distinct pathways of humoral and cellular immunity induced with the mucosal administration of a nanoemulsion adjuvant. J Immunol. 2014;192(6):2722-33.
- Amsen D Antov A Flavell RA. The different faces of Notch in T-helper-cell differentiation. Nat Rev Immunol. 2009;9(2):116-24.
