Abstract
Most cases of lower extremity limb loss in the United States occur among people with diabetes who have a diabetic foot ulcer (DFU). These DFUs and the associated limb loss that may occur lead to excess healthcare costs and have a large negative impact on mobility, psychosocial well-being, and quality of life. The strategies for DFU prevention and management are evolving, but the implementation of these prevention and management strategies remains challenging. Barriers to implementation include poor access to primary medical care; patient beliefs and lack of adherence to medical advice; delays in DFU recognition; limited healthcare resources and practice heterogeneity of specialists. Herein, we review the contemporary outcomes of DFU prevention and management to provide a framework for prioritizing quality improvement efforts within a resource-limited healthcare environment.
Approximately 84% of non-traumatic major amputations among people with diabetes are preceded by a diabetic foot ulcer (DFU) (Citation1). These DFUs – defined as any necrosis, gangrene, or full-thickness skin defect occurring distal to the ankle in a diabetic patient (Citation2) – serve as the portal of entry for severe foot infections, and the end-stage complication may be limb loss through major (above-ankle) amputation (). DFUs are analogous to many cancers in that the diagnosis and management of certain identifiable/visible precursor states may halt progression of disease and reduce end-stage complications (Citation3) ().
Fig. 1 The clinical states leading to limb loss among patients with diabetes mellitus and the risk factors that influence the transition between these states. DFU=diabetic foot ulcer.
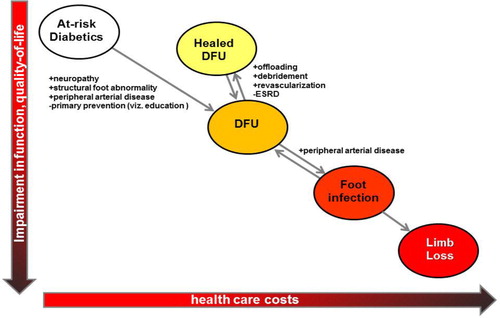
Table 1 A comparison of the burden of disease, detection, and management of colorectal cancer and diabetes-associated limb loss
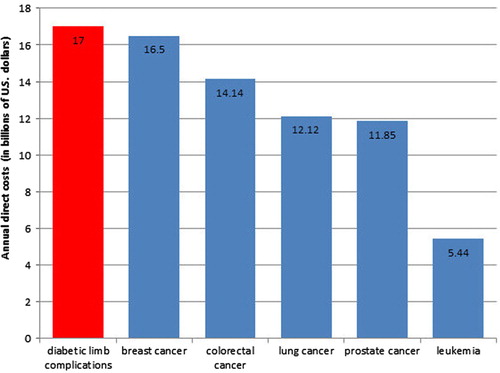
Multiple large-scale studies of patient self-reported quality of life have shown that limb loss has a larger negative impact on quality of life than any other complication of diabetes, including end-stage renal disease or blindness (Citation6, Citation7). In addition to the loss of mobility and independence (Citation8, Citation9), depression and anxiety are very prevalent among people with diabetes who have experienced limb loss (Citation10–Citation12). The economic costs associated with diabetic foot care – including amputation care – represent the single largest category of excess medical costs associated with diabetes (Citation13). The total cost for diabetic foot care for those with neuropathy has been estimated to be $11 billion (Citation14). Even conservatively extrapolating these figures to include those with diabetes and peripheral arterial disease (PAD) would increase the total cost estimate to $17 billion, comparable to the annual costs of breast cancer and colorectal cancer (Citation5) ().
Fig. 2 The estimated annual direct costs of diabetic limb complications in comparison to the annual direct costs of the five most costly cancers in the United States.
Diabetic foot care has indeed improved significantly over the past decade. There has been a clearer understanding of the causal factors leading to limb loss and increasing consensus on the management of various aspects of diabetic foot care (Citation15–Citation17). Overall rates of limb loss among people with diabetes appear to be decreasing in the US (Citation18–Citation20) and elsewhere (Citation21–Citation24). At least part of this decrease may be due to improved coordination of care and more frequent interdisciplinary collaboration between specialty providers (Citation21, Citation22) (Citation25–Citation30). Reform of the US healthcare system through recent legislation (Citation31) designed to improve access to care, encourage more preventative services, and align provider reimbursement with patient-oriented outcomes may provide additional opportunities for US healthcare providers to further improve the system of care for people with diabetes and diabetic limbs.
The current reality in most US healthcare systems is still marked by many significant challenges, however. Limited patient understanding of the potential health significance of a DFU and limited access to care may both have negative impacts (Citation32, Citation33). Primary care providers perform complete foot examinations only infrequently (Citation34–Citation36) and may lack the time or training (Citation35) to educate at-risk people with diabetes. Adherence to guidelines is uneven (Citation35), and referrals to specialty care can be sporadic (Citation37–Citation39). Beliefs regarding the utility and cost-effectiveness of limb preservation efforts may range from doubt to pessimism and nihilism, even among specialists (Citation40, Citation41). The resources within any healthcare systems are finite, and the requests for additional providers or funds for quality improvement efforts may be approved only based on the priorities within that healthcare system (Citation35). With these realities and challenges in mind, this review presents a review not only of the patient-related factors but also the provider- and healthcare system-related factors that influence the development of DFUs, the response of DFUs to various treatment modalities, and the progression to limb loss. Through this review, we hope to provide a framework for optimizing patient-oriented outcomes through healthcare system-wide improvements in diabetic foot care in a resource-limited healthcare environment.
Occurrence and management of an initial DFU
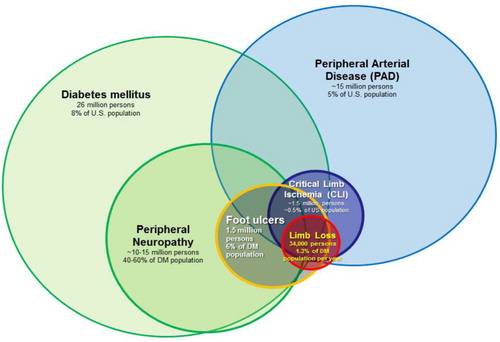
Three factors consistently play an important role in the development of DFUs: structural foot abnormalities, sensory neuropathy, and PAD (). First, the feet of people with diabetes often undergo characteristic structural changes that are the consequence of autonomic and motor neuropathies, intrinsic muscle atrophy, and reduced joint/tendon mobility. Previous minor amputations (i.e. amputations below the level of the ankle, such as toe amputations or partial foot amputations) also result in structural abnormalities (Citation42). The end result of these structural abnormalities is an unequal distribution of stress on the plantar surface of the foot during the gait cycle (Citation43), which in turn predisposes prominent areas of the foot to repetitive trauma and ultimately full-thickness skin ulceration (Citation44). Peripheral sensory neuropathy (also referred to as loss of protective sensation) is a second important factor that leads to DFUs in that it decreases or eliminates the nociceptive response to repetitive trauma that would typically be protective against repetitive trauma occurring during the gait cycle. The prevalence of sensory neuropathy in diabetic populations in the United State and United Kingdom typically ranges between 40 and 60% (Citation45–Citation49) and denotes up to a twofold higher relative risk of DFU incidence (Citation49, Citation50). The presence of PAD also has a major influence on the development of DFUs (Citation50, Citation51). The incidence of PAD in the general diabetic population is 20–30%, at least twofold higher than that of non-diabetics. Among those with DFUs, the incidence reaches 50%; (Citation52–Citation54). PAD, diabetes, and peripheral neuropathy are three important risk factors that frequently overlap in patients at risk for limb loss (). Other important risk factors include end-stage renal disease (Citation55, Citation56), visual impairment (Citation49), improperly fitted shoes (Citation57, Citation58), autonomic neuropathy (Citation59, Citation60), and depressive symptoms (Citation61, Citation62). Poor glycemic control has a well-recognized role in the development of peripheral sensory neuropathy (Citation63) and therefore has at least some causal role in the development of DFUs. It is unclear if glycemic control has a significant impact of limb loss risk independent of the presence or absence of neuropathy, however (Citation64, Citation65).
Fig. 3 The overlapping relationship of risk factors associated with non-traumatic limb loss in the United States. Estimates of total affected US population, US prevalence and annual incidence rates are shown.
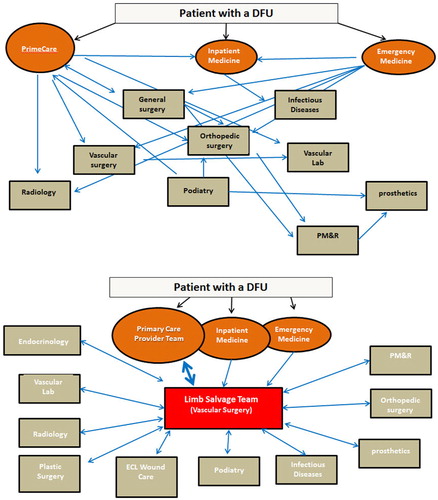
DFU treatment is best done through interdisciplinary management (Citation21, Citation22) (Citation25, Citation30) (Citation66, Citation67). Primary care physicians, podiatrists, vascular surgeons, and prosthetists/orthotists typically comprise the core of these teams in most healthcare systems, but wound care clinicians, interventional radiologists and cardiologists, general surgeons, plastic surgeons, orthopedic surgeons, physical therapists, physical medicine and rehabilitation physicians, endocrinologists, and infectious disease specialists may also be involved to varying degrees in many healthcare systems.
Up to 60% of foot ulcers present with some clinical signs of infection (Citation54, Citation68). The spectrum of infection may include cellulitis, abscess, tenosynovitis, myositis, fasciitis, septic arthritis, osteomyelitis, or some combination thereof (Citation16). Foot infections in patients with diabetes lead to increased healthcare costs and increased risk of limb loss. Thus identifying and treating any infection present is an important initial step in the management of a DFU. Treatment of infection may range from oral antibiotics alone to minor amputations/aggressive foot debridement with wide-spectrum intravenous antibiotics.
Once infection is controlled, further DFU management generally consists of three important components: (i) ensuring/establishing adequate arterial perfusion to the foot; (ii) offloading; and (iii) local wound care (Citation69) (). Evaluating the arterial perfusion to the foot should be the first component undertaken, as offloading and local wound care are unlikely to achieve durable healing if this is not present (Citation54, Citation70). Revascularization, either in the form of endovascular intervention (i.e. angioplasty) or surgical bypass, increases the likelihood of DFU healing and significantly decreases the risk of limb loss (Citation71). Offloading – reducing pressure in the area of ulceration during the gait cycle – is a second critical component of DFU management (Citation72–Citation75). This may best be achieved by total contact casts or non-removable controlled-ankle movement walkers (Citation43), though surgical procedures [Achilles tendon lengthening (Citation76) and other means (Citation77)] may also be useful in limiting pressure to ulcerated areas of the foot. Diligent local wound care is also important, with debridement done as needed (Citation78, Citation79). Certain wound care adjuncts – including recombinant platelet-derived growth factors (Citation80–Citation82), negative pressure wound therapy (Citation83–Citation85), and human skin equivalents (Citation86) – may produce improved healing rates compared to standard gauze dressing materials (Citation87). Even with diligent attention to these three components of DFU management, however, healing is often slow [24% healed within 12 weeks (Citation87)] and incomplete [10–20% remaining unhealed at 1 year (Citation88)].
DFU primary prevention: identifying and modifying DFU risk factors to avoid DFU occurrence
Structural foot abnormalities, PAD, and neuropathy are irreversible. Primary prevention efforts have therefore focused on the identification of risk factors, patient education and the promotion of certain health behaviors to minimize foot trauma and avoid delayed presentation. Foot-protective health behaviors often taught to patients with diabetes focus on minimizing the foot trauma through avoidance of barefoot walking, wearing shoes with improper fit, and stepping into bath water without checking the temperature, and monitoring the variability of walking and other activities (Citation89). Between 40 and 90% of patients with neuropathy are unaware of having it (Citation90, Citation91); these foot protective behaviors might be especially beneficial to these patients who are unaware of their impaired nociception and its potential consequences.
The effectiveness patient education on the prevention of DFUs has been analyzed in a Cochrane database review [most recently updated in 2012 (Citation92)]. This review included 12 randomized clinical trials that assessed the impact of patient education interventions ranging from a 10–20 min educational session to multiple sessions covering various aspects of diabetes management with or without supplementary written materials and/or mailed care reminders for both patients and clinicians. The review concluded that patient education interventions may improve patients’ understanding of foot complications and adherence to certain health behaviors, but there was no consistent evidence to suggest a reduction in the incidence of DFU formation or lower extremity amputation. Only one trial (Citation93) included in the review focused exclusively on primary prevention (i.e. the avoidance of DFU formation among at-risk patients without a previous history of DFU), with the remaining 11 trials including patients with previous DFUs or whose baseline characteristics were not described. A similar review of ‘complex interventions’ (defined as ‘two or more prevention strategies on at least two different levels of care’) also failed to find any clear evidence of benefit in reducing DFU incidence, but the few trials included in the review were small and differed somewhat in the interventions studied.
Mitigating the effects of structural foot abnormalities has been another approach to DFU primary prevention efforts. One randomized clinical trial (Citation94) and at least two non-randomized studies (Citation95, Citation96) have examined the use of insoles among people with diabetes without a history of previous DFU but who were considered high risk because of pronation, neuropathy, and/or elevated peak plantar pressures. The use of insoles does appear to decrease peak plantar pressures in these studies, but it has not been clearly demonstrated that this translates to a significant reduction in DFU formation.
In addition to the lack of evidence for benefit, there are other obstacles to primary prevention efforts that exist in clinical practice. First, many primary care providers fail to examine the feet of patients with diabetes and infrequently assess for risk factors. In one study of primary care clinic in San Antonio, foot examinations that included an evaluation for neuropathy and PAD occurred in as few as 14% of clinic visits. The foot examination rate was no higher among patients who were known to have PAD, a history of previous foot ulcers, or microvascular complications in other organ systems. A clinic-wide program to improve surveillance for DFUs in this study increased the foot examination rate to only 62% (Citation36). The difficulty of diagnosing PAD in people with diabetes [especially the popliteal and tibial-level distribution most commonly seen in this patient population (Citation97, Citation98)] may also contribute to this problem. Palpating for pedal pulses does not have good interobserver agreement (Citation99), and even the ankle-brachial indices can be of limited utility in the diagnosis of PAD in the diabetic population (Citation100). Finally, persistent but incorrect beliefs – such as ‘small-vessel disease’ having a causal role in diabetic limb loss (Citation44) – may persist among healthcare providers and lead to a poor understanding of the risk factors involved in DFU formation and limb loss.
The scale of efforts needed for consistent primary prevention efforts is another obstacle. The healthcare system of two of the authors, for example, provides primary care for approximately 110,000 people, approximately 28,000 of whom have diabetes. Even focusing prevention efforts on only moderate- and high-risk individuals would require thorough and accurate risk stratification of all 28,000 patients with diabetes and prevention efforts for approximately 8,000. As in many US healthcare systems, primary care providers at our institution are already challenged to provide basic primary care to patients in a busy outpatient clinic setting; adding additional primary prevention for foot care would simply not be possible without additional personnel and other resources.
DFU secondary prevention: avoiding delays in the recognition of DFUs
Once a DFU has developed, management is best provided by a collaborative team of multidisciplinary specialty providers (Citation21, Citation22) (Citation25, Citation30) (Citation66, Citation67). The provision of this multidisciplinary care is predicated on both the identification of a DFU and access to medical care. Primary care providers and specialty providers (especially podiatrists, vascular surgeons, and endocrinologists) may identify DFUs not previously noted or treated, patients themselves are the most important persons who can identify a new DFU and seek treatment for it. Many patient-related barriers to prompt recognition of DFUs exist, however. One study of veterans with diabetes found that only 32% examined their feet on a daily basis (Citation101). A daily self-foot examination is not likely to be done or be helpful if patients have not been instructed to do these examinations or if the patient does not known what an ‘ulcer’ or other foot abnormalities looks like (Citation33). Visual impairment, poor balance/equilibrium, decreased limb flexibility, and obesity may also limit a patient's ability to examine the plantar aspect of his or her foot and recognize the abnormalities. Even when abnormalities are found, patients may not immediately seek medical attention because they are unaware of the relationship between DFUs and limb loss, have limited access to medical care (Citation102), or do not know what type of provider to see (Citation32). Even after adjusting for DFU incidence, demographics, and other important variables, US patients with lower socioeconomic status have a higher risk of limb loss (Citation103), and this does not appear to be related to the prevalence of physicians and/or podiatrists in a given area (Citation103).
Patients with recognized DFUs should generally be referred for multidisciplinary specialist foot care, but these referrals are frequently delayed or absent. In the EuroDIALE study, for example, 27% of the patients were referred to a specialist foot clinic only after the DFU had been present for more than 3 months. Specialist referrals varied widely not only between countries but also among centers within a country (Citation39). Such delays can negatively impact outcomes (Citation37). Access to the specialist clinic may remain a problem even after a referral is made, as long delays between referral and appointments are common. The creation of open access facilities – i.e. clinics or centers where ‘walk in’ appointments or urgent referrals are seen whenever needed – may be one method to avoid delays in treatment and significantly improve clinical outcomes (Citation104).
The wide array of specialist providers involved in various aspects of DFU management may introduce some confusion into the referral process. The relationship between the various specialist providers involved in any given patient's care may range from an ad hoc collection of specialists with no or infrequent communication within some healthcare systems to a formalized team with robust, regular communication in others. The providers may be located in separate clinics scattered across a geographical area, located in separate clinics of a large hospital, or physically co-located in one clinic. Following from this, the referral of patients with DFUs to particular specialists may range from uncertain, sporadic and determined on an individual basis by the primary provider the patient happens to be seeing to a consistent multidisciplinary team with a formalized referral protocol ().
DFU tertiary prevention: ensuring adequate DFU treatment
Other barriers to optimal care persist even after a patient with a DFU is seen by a specialist. Perhaps the most important is specialty referral or practice heterogeneity. Only 41% of patients with PAD in the EuroDIALE study received vascular imaging, and only 43% of patients with severe limb ischemia underwent revascularization (Citation39). The possibility that such practice heterogeneity affects major amputation rates has been suggested by multiple studies of US administrative data demonstrating decreased revascularization among blacks with DFUs (Citation105–Citation107). Some of the variation in amputation rates is due to variations in patient presentation (Citation108), and geographic variations in amputation rates are seen even in nationalized healthcare systems in the US (Citation109) and the UK (Citation110). Regardless, the findings of the Medicare studies and others (Citation103, Citation111) (Citation112) do suggest practice heterogeneity may have at least some negative impact on patient outcomes.
Additional provider-related factors influence diabetic foot outcomes. Providers may not be aware of national or international consensus treatment guidelines, may not have established local consensus guidelines (Citation35), or may have differing opinions on the proper treatment for an individual patient (Citation113, Citation114). Patients who undergo revascularization at low volume medical centers may have a small but significant increase in the risk of limb loss and mortality compared to those who undergo revascularization at high volume centers (Citation115). US President Barack Obama had suggested that the higher relative monetary reimbursement for major amputation versus revascularization may lead to a higher propensity to perform amputation (Citation116). The relative reimbursement amount as measured by relative value units per median procedural times for infrainguinal revascularization is indeed much lower than that of major amputations (Citation117), and although there are no data to suggest that this differential reimbursement rate does indeed affect propensity to pursue limb preservation efforts over major amputation, many others have suggested the need to better align provider reimbursement with the provision of patient-oriented outcomes (Citation118).
Reducing ulcer recurrence
In large observational series of patients with DFUs, the risk of DFU recurrence reaches 40–60% (Citation119, Citation120). Two factors seem to be largely responsible for this high recurrence rate. First, a healed DFU remains at increased risk because of abnormal epidermis/dermis structure. Second, DFUs most often occur in patients with known risk factors; although the DFU may heal, these risk factors typically remain.
Studies of patient education interventions to prevent DFU recurrence have reported somewhat contradictory results (Citation120, Citation121), but as mentioned above there is overall no clear evidence that patient education has a significant impact on reducing DFU recurrence (Citation92). The continued use of modified therapeutic shoes appears to have a significant impact on reducing DFU recurrence compared to normal shoes (28% vs. 58% recurrence at 1 year, respectively) (Citation122). Daily thermography has been demonstrated to significantly reduce reulceration rates. With daily thermography, patients plantar foot skin temperature gradients by standing on a thermography scale or using a specialized cutaneous temperature probe. If a gradient of ≥5°F is found, walking is minimized until the foot temperatures equilibrate. Three randomized trials in 483 patients with a history of previous DFU have demonstrated rather impressive reductions: from 6-month DFU incidence rates of 20% with standard care to 2% with daily thermography (Citation123–Citation125).
Conclusions: prioritizing quality improvement efforts for diabetic foot care
Optimal management of the diabetic foot for the minimization of limb loss truly requires a robust system of multidisciplinary care (). This system of care is not just a single provider or specialty but an array of providers, ideally working in a cohesive, multidisciplinary team; it is not simply management of the DFU but also screening, education, and surveillance; and it should target not only those referred for care but also patients not currently receiving care but who are in need of it. So can such robust systems of care be established? It can be challenging even within nationalized healthcare systems (Citation35); it can be even more challenging where healthcare systems are composed of individuals or groups that each have a selective area of focus and lack incentive (financial or otherwise) to organize and provide care across the full spectrum of disease.
Table 2 Components of diabetic foot care and respective objectives
First, the role of primary prevention needs to be clarified. Few randomized trials have examined the impact of primary prevention efforts. Larger, better quality trials – perhaps with accompanying economic analyses – may be needed to more convincingly demonstrate the benefit of various forms of primary prevention. Recurrence of DFU is frequent, and better methods for reducing this recurrence rate are needed (Citation126).
Delays in the recognition of PAD appear to be common. Given the impact of PAD on DFU outcomes, efforts to reduce delays in the recognition and treatment of PAD among patients with DFUs are well deserved” (Citation2). In spite of the higher initial costs associated with treatment, limb preservation efforts appear to be cost-effective and, in some situations, may provide cost-savings (Citation127). The number of publications from healthcare systems reporting significant decreases in major amputations rates continues to grow (Citation21, Citation22) (Citation25, Citation30) (Citation67), but still the existence of robust systems of multidisciplinary care is somewhat sporadic in the United States. Quantitative studies that assess not only the impact of various prevention and treatment strategies but also the impact of delays might help provide a further (esp. economic) argument for establishing a robust system of diabetic foot care.
Finally, while the economic, functional and psychosocial impacts of diabetic foot complications are difficult to overstate, there exists a yawning gap between the impact of this problem and funding for research to improve management. Of more than 22,000 diabetes-related research projects with US federal funding between 2002 and 2011, only 33 (0.15%) were related to DFU care. Although diabetic foot complications may comprise as much as 30% of the excess medical costs of patients with diabetes (Citation128), the cumulative funding for these projects accounted for only 0.17% of the total US funding for diabetes-related research, a >600-fold difference (Citation129). More support through improved federal funding for DFU-related research may help produce meaningful therapeutic improvements in this area.
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this review.
Acknowledgements
The authors appreciate the help of Avo Artinyan, MD, in reviewing data on the costs and epidemiology of colorectal cancer used in this article.
References
- Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990; 13: 513–21.
- Schaper NC, Andros G, Apelqvist J, Bakker K, Lammer J, Lepantalo M, etal. Specific guidelines for the diagnosis and treatment of peripheral arterial disease in a patient with diabetes and ulceration of the foot 2011. Diabetes Metab Res Rev. 2012; 28(Suppl 1): 236–7.
- Anichini R, Zecchini F, Cerretini I, Meucci G, Fusilli D, Alviggi L, etal. Improvement of diabetic foot care after the implementation of the International Consensus on the Diabetic Foot (ICDF): results of a 5-year prospective study. Diabetes Res Clin Pract. 2007; 75: 153–8.
- National Cancer Institute. SEER stat fact sheet: colon and rectum. Available from: http://seer.cancer.gov/statfacts/html/colorect.html [cited 27 November 2012].
- National Cancer Institute. The cost of cancer. Available from: http://www.cancer.gov/aboutnci/servingpeople/cancer-statistics/costofcancer [cited 23 November 2012].
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making. 2002; 22: 340–9.
- Laiteerapong N, Karter AJ, Liu JY, Moffet HH, Sudore R, Schillinger D, etal. Correlates of quality of life in older adults with diabetes: the diabetes & aging study. Diabetes Care. 2011; 34: 1749–53.
- Taylor SM, Kalbaugh CA, Blackhurst DW, Hamontree SE, Cull DL, Messich HS, etal. Preoperative clinical factors predict postoperative functional outcomes after major lower limb amputation: an analysis of 553 consecutive patients. J Vasc Surg. 2005; 42: 227–35.
- Nehler MR, Coll JR, Hiatt WR, Regensteiner JG, Schnickel GT, Klenke WA, etal. Functional outcome in a contemporary series of major lower extremity amputations. J Vasc Surg. 2003; 38: 7–14.
- Darnall BD, Ephraim P, Wegener ST, Dillingham T, Pezzin L, Rossbach P, etal. Depressive symptoms and mental health service utilization among persons with limb loss: results of a national survey. Arch Phys Med Rehabil. 2005; 86: 650–8.
- Singh R, Ripley D, Pentland B, Todd I, Hunter J, Hutton L, etal. Depression and anxiety symptoms after lower limb amputation: the rise and fall. Clin Rehabil. 2009; 23: 281–6.
- Williams LH, Miller DR, Fincke G, Lafrance J-P, Etzioni R, Maynard C, etal. Depression and incident lower limb amputations in veterans with diabetes. J Diabetes Complications. 2011; 25: 175–82.
- American Diabetes Association. Economic costs of diabetes in the U.S. In 2007. Diabetes Care. 2008; 31: 596–615.
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003; 26: 1790–5.
- Frykberg RG, Armstrong DG, Giurini J, Edwards A, Kravette M, Kravitz S, etal. Diabetic foot disorders: a clinical practice guideline. American College of Foot and Ankle Surgeons. J Foot Ankle Surg. 2000; 39(5 Suppl): S1–60.
- Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJG, Armstrong DG, etal. Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012; 54: e132–73.
- Apelqvist J, Bakker K, van Houtum WH, Nabuurs-Franssen MH, Schaper NC. International consensus and practical guidelines on the management and the prevention of the diabetic foot. International Working Group on the Diabetic Foot. Diabetes Metab Res Rev. 2000; 16(Suppl 1): S84–92.
- Tseng CL, Rajan M, Miller DR, Lafrance JP, Pogach L. Trends in initial lower extremity amputation rates among Veterans Health Administration health care System users from 2000 to 2004. Diabetes Care. 2011; 34: 1157–63.
- Jones WS, Patel MR, Dai D, Subherwal S, Stafford J, Calhoun S, etal. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000–2008. J Am Coll Cardiol. 2012; 60: 2230–6.
- Li Y, Burrows NR, Gregg EW, Albright A, Geiss LS. Declining rates of hospitalization for nontraumatic lower-extremity amputation in the diabetic population aged 40 years or older: U.S., 1988–2008. Diabetes Care. 2012; 35: 273–7.
- Larsson J, Apelqvist J, Agardh CD, Stenström A. Decreasing incidence of major amputation in diabetic patients: a consequence of a multidisciplinary foot care team approach?. Diabet Med. 1995; 12: 770–6.
- Krishnan S, Nash F, Baker N, Fowler D, Rayman G. Reduction in diabetic amputations over 11 years in a defined U.K. population: benefits of multidisciplinary team work and continuous prospective audit. Diabetes Care. 2008; 31: 99–101.
- Vamos EP, Bottle A, Majeed A, Millett C. Trends in lower extremity amputations in people with and without diabetes in England. 1996–2005. Diabetes Res Clin Pract. 2010; 87: 275–82.
- López-de-Andrés A, Martínez-Huedo MA, Carrasco-Garrido P, Hernández-Barrera V, Gil-de-Miguel A, Jiménez-García R. Trends in lower-extremity amputations in people with and without diabetes in Spain, 2001–2008. Diabetes Care. 2011; 34: 1570–6.
- Hellingman AA, Smeets HJ. Efficacy and efficiency of a streamlined multidisciplinary foot ulcer service. J Wound Care. 2008; 17: 541–4.
- Wrobel JS, Charns MP, Diehr P, Robbins JM, Reiber GE, Bonacker KM, etal. The relationship between provider coordination and diabetes-related foot outcomes. Diabetes Care. 2003; 26: 3042–7.
- Pogach L, Charns MP, Wrobel JS, Robbins JM, Bonacker KM, Haas L, etal. Impact of policies and performance measurement on development of organizational coordinating strategies for chronic care delivery. Am J Manag Care. 2004; 10: 171–80.
- Rogers LC, Andros G, Caporusso J, Harkless LB, Mills JL Sr , Armstrong DG. Toe and flow: essential components and structure of the amputation prevention team. J Vasc Surg. 2010; 52(3 Suppl): 23S–7S.
- Carls GS, Gibson TB, Driver VR, Wrobel JS, Garoufalis MG, Defrancis RR, etal. The economic value of specialized lower-extremity medical care by podiatric physicians in the treatment of diabetic foot ulcers. J Am Podiatr Med Assoc. 2011; 101: 93–115.
- Armstrong DG, Bharara M, White M, Lepow B, Bhatnagar S, Fisher T, etal. The impact and outcomes of establishing an integrated interdisciplinary surgical team to care for the diabetic foot. Diabetes Metab Res Rev. 2012; 28: 514–18.
- Patient Protection and Affordable Care Act. PL 111-148. 3-23-2010. PL 111-148. 3-23-2010.
- Mirmiran R, Page JC, Armstrong JR, Killian R. Barriers to podiatric care among diabetic patients in the San Francisco Bay area. J Foot Ankle Surg. 2000; 39: 301–4.
- Gale L, Vedhara K, Searle A, Kemple T, Campbell R. Patients’ perspectives on foot complications in type 2 diabetes: a qualitative study. Br J Gen Pract. 2008; 58: 555–63.
- Wylie-Rosett J, Walker EA, Shamoon H, Engel S, Basch C, Zybert P. Assessment of documented foot examinations for patients with diabetes in inner-city primary care clinics. Arch Fam Med. 1995; 4: 46–50.
- Winocour PH, Morgan J, Ainsworth A, Williams DRR. Association of British Clinical Diabetologists (ABCD): survey of specialist diabetes care services in the UK, 2000. 3. Podiatry services and related foot care issues. Diabet Med. 2002; 19(Suppl 4): 32–8.
- O'Brien KE, Chandramohan V, Nelson DA, Fischer JR Jr , Stevens G, Poremba JA. Effect of a physician-directed educational campaign on performance of proper diabetic foot exams in an outpatient setting. J Gen Intern Med. 2003; 18: 258–65.
- Mills JL, Beckett WC, Taylor SM. The diabetic foot: consequences of delayed treatment and referral. South Med J. 1991; 84: 970–4.
- Del Aguila MA, Reiber GE, Koepsell TD. How does provider and patient awareness of high-risk status for lower-extremity amputation influence foot-care practice?. Diabetes Care. 1994; 17: 1050–4.
- Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, etal. Delivery of care to diabetic patients with foot ulcers in daily practice: results of the Eurodiale Study, a prospective cohort study. Diabet Med. 2008; 25: 700–7.
- Taylor SM. Current status of heroic limb salvage for critical limb ischemia. Am Surg. 2008; 74: 275–84.
- Bradbury AW. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial in perspective. J Vasc Surg. 2010; 51(5 Suppl): 1S–4S.
- Quebedeaux TL, Lavery LA, Lavery DC. The development of foot deformities and ulcers after great toe amputation in diabetes. Diabetes Care. 1996; 19: 165–7.
- Cavanagh PR, Bus SA. Off-loading the diabetic foot for ulcer prevention and healing. J Vasc Surg. 2010; 52(3 Suppl): 37S–43S.
- LoGerfo FW, Coffman JD. Current concepts. Vascular and microvascular disease of the foot in diabetes. Implications for foot care. N Engl J Med. 1984; 311: 1615–9.
- Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993; 36: 150–4.
- Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, etal. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993; 43: 817–24.
- Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care. 2000; 23: 606–11.
- Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003; 26: 491–4.
- Boyko EJ, Ahroni JH, Cohen V, Nelson KM, Heagerty PJ. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care. 2006; 29: 1202–7.
- Lavery LA, Peters EJG, Williams JR, Murdoch DP, Hudson A, Lavery DC. Reevaluating the way we classify the diabetic foot: restructuring the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care. 2008; 31: 154–6.
- Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999; 22: 1036–42.
- Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006; 47: 921–9.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007; 45(Suppl S): S5–67.
- Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, etal. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008; 51: 747–55.
- Margolis DJ, Hofstad O, Feldman HI. Association between renal failure and foot ulcer or lower-extremity amputation in patients with diabetes. Diabetes Care. 2008; 31: 1331–6.
- Ndip A, Rutter MK, Vileikyte L, Vardhan A, Asari A, Jameel M, etal. Dialysis treatment is an independent risk factor for foot ulceration in patients with diabetes and stage 4 or 5 chronic kidney disease. Diabetes Care. 2010; 33: 1811–6.
- Nixon BP, Armstrong DG, Wendell C, Vazquez JR, Rabinovich Z, Kimbriel HR, etal. Do US veterans wear appropriately sized shoes?: the Veterans Affairs shoe size selection study. J Am Podiatr Med Assoc. 2006; 96: 290–2.
- Harrison SJ, Cochrane L, Abboud RJ, Leese GP. Do patients with diabetes wear shoes of the correct size?. Int J Clin Pract. 2007; 61: 1900–4.
- Tentolouris N, Marinou K, Kokotis P, Karanti A, Diakoumopoulou E, Katsilambros N. Sudomotor dysfunction is associated with foot ulceration in diabetes. Diabet Med. 2009; 26: 302–5.
- Tentolouris N, Voulgari C, Liatis S, Kokkinos A, Eleftheriadou I, Makrilakis K, etal. Moisture status of the skin of the feet assessed by the visual test neuropad correlates with foot ulceration in diabetes. Diabetes Care. 2010; 33: 1112–4.
- Gonzalez JS, Vileikyte L, Ulbrecht JS, Rubin RR, Garrow AP, Delgado C, etal. Depression predicts first but not recurrent diabetic foot ulcers. Diabetologia. 2010; 53: 2241–8.
- Williams LH, Rutter CM, Katon WJ, Reiber GE, Ciechanowski P, Heckbert SR, etal. Depression and incident diabetic foot ulcers: a prospective cohort study. Am J Med. 2010; 123: 748–54.
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993; 329: 977–86.
- Litzelman DK, Marriott DJ, Vinicor F. Independent physiological predictors of foot lesions in patients with NIDDM. Diabetes Care. 1997; 20: 1273–8.
- Lavery LA, Armstrong DG, Vela SA, Quebedeaux TL, Fleischli JG. Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Intern Med. 1998; 158: 157–62.
- Driver VR, Madsen J, Goodman RA. Reducing amputation rates in patients with diabetes at a military medical center: the limb preservation service model. Diabetes Care. 2005; 28: 248–53.
- Driver VR, Goodman RA, Fabbi M, French MA, Andersen CA. The impact of a podiatric lead limb preservation team on disease outcomes and risk prediction in the diabetic lower extremity: a retrospective cohort study. J Am Podiatr Med Assoc. 2010; 100: 235–41.
- Lavery LA, Armstrong DG, Murdoch DP, Peters EJG, Lipsky BA. Validation of the Infectious Diseases Society of America's diabetic foot infection classification system. Clin Infect Dis. 2007; 44: 562–5.
- Armstrong DG, Lavery LA, Nixon BP, Boulton AJM. It's not what you put on, but what you take off: techniques for debriding and off-loading the diabetic foot wound. Clin Infect Dis. 2004; 39(Suppl 2): S92–9.
- Faglia E, Clerici G, Caminiti M, Quarantiello A, Curci V, Morabito A. Predictive values of transcutaneous oxygen tension for above-the-ankle amputation in diabetic patients with critical limb ischemia. Eur J Vasc Endovasc Surg. 2007; 33: 731–6.
- Barshes NR, Belkin M. A framework for the evaluation of “value” and cost-effectiveness in the management of critical limb ischemia. J Am Coll Surg. 2011; 213: 552–66.
- Mueller MJ, Diamond JE, Sinacore DR, Delitto A, Blair VP 3rd , Drury DA, etal. Total contact casting in treatment of diabetic plantar ulcers. Controlled clinical trial. Diabetes Care. 1989; 12: 384–8.
- Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Off-loading the diabetic foot wound: a randomized clinical trial. Diabetes Care. 2001; 24: 1019–22.
- Armstrong DG, Lavery LA, Wu S, Boulton AJ. Evaluation of removable and irremovable cast walkers in the healing of diabetic foot wounds: a randomized controlled trial. Diabetes Care. 2005; 28: 551–4.
- Katz IA, Harlan A, Miranda-Palma B, Prieto-Sanchez L, Armstrong DG, Bowker JH, etal. A randomized trial of two irremovable off-loading devices in the management of plantar neuropathic diabetic foot ulcers. Diabetes Care. 2005; 28: 555–9.
- Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of Achilles tendon lengthening on neuropathic plantar ulcers. A randomized clinical trial. J Bone Joint Surg Am. 2003; 85-A: 1436–45.
- Frykberg RG, Bevilacqua NJ, Habershaw G. Surgical off-loading of the diabetic foot. J Vasc Surg. 2010; 52(3 Suppl): 44S–58.
- Tan JS, Friedman NM, Hazelton-Miller C, Flanagan JP, File TM Jr. Can aggressive treatment of diabetic foot infections reduce the need for above-ankle amputation?. Clin Infect Dis. 1996; 23: 286–91.
- Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 2010; 003556.
- Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg. 2002; 137: 822–7.
- Niezgoda JA, Van Gils CC, Frykberg RG, Hodde JP. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005; 18: 258–66.
- Landsman A, Agnew P, Parish L, Joseph R, Galiano RD. Diabetic foot ulcers treated with becaplermin and TheraGauze, a moisture-controlling smart dressing: a randomized, multicenter, prospective analysis. J Am Podiatr Med Assoc. 2010; 100: 155–60.
- Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005; 366: 1704–10.
- Akbari A, Moodi H, Ghiasi F, Sagheb HM, Rashidi H. Effects of vacuum-compression therapy on healing of diabetic foot ulcers: randomized controlled trial. J Rehabil Res Dev. 2007; 44: 631–6.
- Apelqvist J, Armstrong DG, Lavery LA, Boulton AJ. Resource utilization and economic costs of care based on a randomized trial of vacuum-assisted closure therapy in the treatment of diabetic foot wounds. Am J Surg. 2008; 195: 782–8.
- Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001; 24: 290–5.
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis. Diabetes Care. 1999; 22: 692–5.
- Pickwell KM, Siersma VD, Kars M, Holstein PE, Schaper NC. Diabetic foot disease: impact of ulcer location on ulcer healing. Diabetes Metab Res Rev. 2013. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23390115 [cited 6 March 2013].
- Armstrong DG, Lavery LA, Holtz-Neiderer K, Mohler MJ, Wendel CS, Nixon BP, etal. Variability in activity may precede diabetic foot ulceration. Diabetes Care. 2004; 27: 1980–4.
- Holewski JJ, Moss KM, Stess RM, Graf PM, Grunfeld C. Prevalence of foot pathology and lower extremity complications in a diabetic outpatient clinic. J Rehabil Res Dev. 1989; 26: 35–44.
- Bongaerts BW, Rathmann W, Heier M, Kowall B, Herder C, Stöckl D, etal. Older subjects with diabetes and prediabetes are frequently unaware of having distal sensorimotor polyneuropathy: The KORA F4 study. Diabetes Care. 2013; 36: 1141–6.
- Dorresteijn JA, Kriegsman DM, Assendelft WJ, Valk GD. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev. 2012; 10: CD001488.
- Corbett CF. A randomized pilot study of improving foot care in home health patients with diabetes. Diabetes Educ. 2003; 29: 273–82.
- Weintraub MI, Wolfe GI, Barohn RA, Cole SP, Parry GJ, Hayat G, etal. Static magnetic field therapy for symptomatic diabetic neuropathy: a randomized, double-blind, placebo-controlled trial. Arch Phys Med Rehabil. 2003; 84: 736–46.
- Albert S, Rinoie C. Effect of custom orthotics on plantar pressure distribution in the pronated diabetic foot. J Foot Ankle Surg. 1994; 33: 598–604.
- Lobmann R, Kayser R, Kasten G, Kasten U, Kluge K, Neumann W, etal. Effects of preventative footwear on foot pressure as determined by pedobarography in diabetic patients: a prospective study. Diabet Med. 2001; 18: 314–9.
- Graziani L, Silvestro A, Bertone V, Manara E, Andreini R, Sigala A, etal. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg. 2007; 33: 453–60.
- Rueda CA, Nehler MR, Perry DJ, McLafferty RB, Casserly IP, Hiatt WR, etal. Patterns of artery disease in 450 patients undergoing revascularization for critical limb ischemia: implications for clinical trial design. J Vasc Surg. 2008; 47: 995–9. discussion 999–1000.
- Lawson IR, Ingman SR, Masih Y, Freeman B. Reliability of palpation of pedal pulses as ascertained by the kappa statistic. J Am Geriatr Soc. 1980; 28: 300–3.
- Potier L, Abi Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg. 2011; 41: 110–16.
- Olson JM, Hogan MT, Pogach LM, Rajan M, Raugi GJ, Reiber GE. Foot care education and self management behaviors in diverse veterans with diabetes. Patient Prefer Adherence. 2009; 3: 45–50.
- Feinglass J, Shively VP, Martin GJ, Huang ME, Soriano RH, Rodriguez HE, etal. How ‘preventable’ are lower extremity amputations? A qualitative study of patient perceptions of precipitating factors. Disabil Rehabil. 2012; 34: 2158–65.
- Margolis DJ, Hoffstad O, Nafash J, Leonard CE, Freeman CP, Hennessy S, etal. Location, location, location: geographic clustering of lower-extremity amputation among Medicare beneficiaries with diabetes. Diabetes Care. 2011; 34: 2363–7.
- Wrobel JS, Davies ML, Robbins JM. Does open access improve the process and outcome of podiatric care?. J Clin Med Res. 2011; 3: 101–5.
- Tunis SR, Bass EB, Klag MJ, Steinberg EP. Variation in utilization of procedures for treatment of peripheral arterial disease. A look at patient characteristics. Arch Intern Med. 1993; 153: 991–8.
- Regenbogen SE, Gawande AA, Lipsitz SR, Greenberg CC, Jha AK. Do differences in hospital and surgeon quality explain racial disparities in lower-extremity vascular amputations?. Ann Surg. 2009; 250: 424–31.
- Henry AJ, Hevelone ND, Belkin M, Nguyen LL. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia. J Vasc Surg. 2011; 53: 330–9.
- van Battum P, Schaper N, Prompers L, Apelqvist J, Jude E, Piaggesi A, etal. Differences in minor amputation rate in diabetic foot disease throughout Europe are in part explained by differences in disease severity at presentation. Diabet Med. 2011; 28: 199–205.
- Tseng CL, Helmer D, Rajan M, Tiwari A, Miller D, Crystal S, etal. Evaluation of regional variation in total, major, and minor amputation rates in a national health-care system. Int J Qual Health Care. 2007; 19: 368–76.
- Moxey PW, Hofman D, Hinchliffe RJ, Jones K, Thompson MM, Holt PJ. Epidemiological study of lower limb amputation in England between 2003 and 2008. Br J Surg. 2010; 97: 1348–53.
- Robinson WP, Owens CD, Nguyen LL, Chong TT, Conte MS, Belkin M. Inferior outcomes of autogenous infrainguinal bypass in Hispanics: an analysis of ethnicity, graft function, and limb salvage. J Vasc Surg. 2009; 49: 1416–25.
- Flavin NE, Mulla ZD, Bonilla-Navarrete A, Chedebeau F, Lopez O, Tovar Y, etal. Health insurance and the development of diabetic complications. South Med J. 2009; 102: 805–9.
- Connelly J, Airey M, Chell S. Variation in clinical decision making is a partial explanation for geographical variation in lower extremity amputation rates. Br J Surg. 2001; 88: 529–35.
- Fincke BG, Miller DR, Christiansen CL, Turpin RS. Variation in antibiotic treatment for diabetic patients with serious foot infections: a retrospective observational study. BMC Health Serv Res. 2010; 10: 193.
- Moxey PW, Hofman D, Hinchliffe RJ, Poloniecki J, Loftus IM, Thompson MM, etal. Volume-outcome relationships in lower extremity arterial bypass surgery. Ann Surg. 2012; 256: 1102–7.
- American College of Surgeons. Statement from the American College of Surgeons regarding recent comments from President Obama. Available from: http://www.facs.org/news/obama081209.html [cited 9 January 2012].
- Barshes NR, Nguyen LL, Belkin M. Do Relative Value Units (RVUs) accurately reflect the work done by vascular surgeons?. J Vasc Surg. 2010; 52: 1121.
- Porter M, Teisberg EO. Redefining health care: creating value-based competition on results. 2006; Boston, MA: Harvard Business Press.
- Ghanassia E, Villon L, Thuan Dit Dieudonne JF, Boegner C, Avignon A, Sultan A. Long-term outcome and disability of diabetic patients hospitalized for diabetic foot ulcers: a 6.5-year follow-up study. Diabetes Care. 2008; 31: 1288–92.
- Lincoln NB, Radford KA, Game FL, Jeffcoate WJ. Education for secondary prevention of foot ulcers in people with diabetes: a randomised controlled trial. Diabetologia. 2008; 51: 1954–61.
- Malone JM, Snyder M, Anderson G, Bernhard VM, Holloway GA Jr , Bunt TJ. Prevention of amputation by diabetic education. Am J Surg. 1989; 158: 520–3. discussion 523–4.
- Uccioli L, Faglia E, Monticone G, Favales F, Durola L, Aldeghi A, etal. Manufactured shoes in the prevention of diabetic foot ulcers. Diabetes Care. 1995; 18: 1376–8.
- Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Armstrong DG, etal. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004; 27: 2642–7.
- Armstrong DG, Holtz-Neiderer K, Wendel C, Mohler MJ, Kimbriel HR, Lavery LA. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med. 2007; 120: 1042–6.
- Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Athanasiou KA, etal. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care. 2007; 30: 14–20.
- Armstrong DG, Mills JL. Toward a change in syntax in diabetic foot care: prevention equals remission. J Am Pod Med Assoc. 2013; 103: 161–2.
- Barshes NR, Chambers JD, Cohen J, Belkin M. Cost-effectiveness in the contemporary management of critical limb ischemia with tissue loss. J Vasc Surg. 2012; 56: 1015–24.
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013; 36: 1033–46.
- Armstrong DG, Kanda VA, Lavery LA, Marston W, Mills JL Sr, Boulton AJ. Mind the gap: the disparity between research funding and costs of care for diabetic foot ulcers. Diab Care. 2013; 36: 1815–7.