Abstract
Background
In diabetic persons with painless neuropathic foot ulceration, foot skin was found to be insensate to noxious pinprick stimulation (stimulation area less than 0.05 mm2), while compression of deep subcutaneous foot tissues by Algometer II® (stimulation area 1 cm2) could evoke a deep dull aching. To elucidate this discrepancy, the Algometer II stimulation technique was critically reviewed by varying probe sizes and anatomical sites in the same study population 3 years later.
Methods
Ten control subjects without neuropathy and 11 persons with painless diabetic neuropathy (PLDN, seven of whom with diabetic foot syndrome, i.e., past painless foot ulcer, or inactive Charcot arthropathy) were re-examined using Algometer II. Deep pressure pain perception threshold (DPPPT) was measured in random sequence with stimulation areas of 0.5 cm2, 1 cm2, and 2 cm2 (separated by 5 min intervals), at the plantar forefoot, the instep, and the hindfoot of both legs.
Results
In the control and PLDN groups, median DPPPTs differed significantly between stimulation areas (highest with 0.5 cm2, intermediate with 1 cm2, lowest with 2 cm2; p<0.001), and varied moderately by anatomical site. Between-group differences were relatively small. Results of the 1 cm2 assessments repeated 3 years apart were similar.
Conclusions
Algometer II readings represent spatial summation of low-threshold pressure-receptor rather than of high-threshold nociceptor stimulation and are, thus, unhelpful for assessing PLDN. Reproducibility of the measurements is good.
In previous studies of feet with painless diabetic neuropathy (PLDN) (Citation1–Citation5), the authors have encountered unexplained discrepancies between deep pressure pain perception thresholds (DPPPTs) which were partly normal and cutaneous ‘pinprick’ pressure pain perception thresholds (CPPPTs) which were always extremely elevated (due to degeneration of intraepidermal nociceptors).
Blunt deep tissue pressure pain stimulation is part of established protocols of quantitative sensory testing (QST (Citation6–Citation8)). DPPPT or pressure pain tolerance threshold has long been measured using a pressure algometer (Citation7, Citation8). Conventional pressure algometers are hand-held devices furnished with a mechanical or electronic force gauge and a plunger, which can be supplied with circular flat blunt rubber tips of various surface areas (e.g. 0.5 cm2, 1 cm2, 2 cm2) (). The operator presses the plunger perpendicularly to skin and underlying tissues with a steadily increasing force. For a DPPPT to be determined, the study subject has to report when his/her pressure sensation turns to pain.
Fig. 1 Picture of the deep tissue pressure algometer with electronic force gauge (Algometer II®, SB-Medic, Sweden) used in the study.

Blunt indentation of skin and subdermal tissues, whereby touch, pressure, and pain modalities are co-stimulated simultaneously, evokes deep tissue pressure pain or discomfort. The physical properties of the indented tissues, namely the compliance, affect the performance of the algometer. The pain quality evoked by an algometer with 1 cm2 stimulation area is described as dull or aching (intolerable pressure discomfort). Pain intensity at reaching DPPPT is rated around 3/10 on visual analogue scale by healthy subjects (Citation9).
Other types of pressure algometry stimulate by an inflatable cuff for compressing a limb over a contact area of approximately 400 cm2 (Citation9–Citation11), or by two branches of forceps-like devices for squeezing a portion of skin and subcutaneous tissue (with stimulation areas on both branches (Citation12, Citation13) or on one branch only (Citation14)). However, these different stimulators will produce different DPPPTs within one person and at the same anatomical region due to different area sizes being stimulated, ranging from 1.5 N/cm2 (with 400 cm2 pressure area) to 60 N/cm2 (with 1 cm2 pressure area) and to 300 N/cm2 (with 3 mm2 pressure area) (Citation15). Stimulation area–related DPPPT is caused by spatial summation, which is the augmented perception of the central nervous system (CNS) through collective input from a multitude of simultaneously stimulated receptors. As a result, the pain perception threshold in the CNS decreases, and the perceived pain intensity increases.
Spatial summation associated with conventional pressure algometry as described above (Citation16–Citation18) is well known. In healthy humans, spatial summation of pressure discomfort or pain perception (mediated by low-threshold mechanoreceptors at the endings of A-beta fibres) is of greater magnitude than that of sharp or burning pain (mediated by high-threshold mechanoreceptors at the endings of A-delta and C-fibres) (Citation19). Both types of receptors, mechanoreceptors as well as nociceptors, may be co-stimulated by conventional pressure algometry (Citation20, Citation21). In persons with PLDN, loss of deep tissue nociceptors had been anticipated, and the findings of DPPPTs in the normal range seemed inconsistent (Citation1–Citation5, Citation22). To examine whether anatomical sites of the foot, or the effects of spatial summation, would play a role, the impact of the stimulation area on algometer performance was studied at different sites of the feet in subjects with and without PLDN.
Materials and methods
Study design
A prospective observational clinical study was performed. Deep pressure pain was measured with the Algometer II device, a clinically established method of QST (Citation6–Citation8, Citation13) (Citation14, Citation16–Citation18), approved by the local ethics commission. Written informed consent was obtained from every participant.
Participants
Healthy persons (n=10) and persons with PLDN (n=11), whose pressure pain perception had already been studied in our institution in 2011, were re-examined. Their former findings were published in this journal and elsewhere (Citation1–Citation5, Citation22). In particular, the PLDN-persons’ CPPPTs measured by pinprick-stimulators at a plantar skinfold of the foot were unmeasurably elevated. Subjects with bleeding disorders; capillary fragility; mental disorders; cancer; rheumatic arthritis; foot ischaemia from peripheral artery disease; fever; hypoglycaemia; neuropathic pains; allodynia; multiple sclerosis; concurrent administration of anticoagulant, analgesic, antidepressant, or antiepileptic drugs were excluded, as were patients with an active ulcer or foot inflammation from infection or from active Charcot arthropathy grade 0 or 1 (Citation23). Of the 11 persons with PLDN, seven had inactive diabetic foot syndrome (DFS, see Definitions section).
Definitions
PLDN was defined according to common clinical practice by a vibration perception threshold <5/8 at the first metatarsophalangeal joint, measured with the graded Rydel–Seiffer tuning fork in subjects with established type-1 or type-2 diabetes mellitus, in whom neuropathic pains were absent. DFS was defined as past painless foot ulcer, or inactive Charcot foot grade 0 or grade 1 (Citation23). DPPPT was defined as minimum force of pressure that produces pain or discomfort.
Measurements
For measuring DPPPT, a hand-held electronic pressure algometer with a strain pressure gauge (Algometer II, Sbmedic Electronics, Solna, Sweden) was used, see . The device has a digital readout of ramp rate and peak pressure force (in kPa), and holds peak pressure until reset. Three measurements were subsequently taken at three different locations of each foot, requiring almost 40 min to complete. The algometer probe was furnished with a stimulation area of 0.5 cm2, 1 cm2, or 2 cm2. Each area size was randomly applied – with intermissions of 5 min – at the instep, a MTP joint, and below the medial ankle. The largest stimulation area was tried first, and the smallest area last, with the study persons being blinded to the area sizes. According to the manufacturer's instructions, the recording scale of the algometer was adapted to the stimulation surface by operating a switch on the meter. The probe was pressed perpendicularly on the skin over the targeted structures, so as to increase indentation steadily by a rate of approximately 50 kPa per second. Care was taken not to place the probe on callosities.
The subjects were lying on a hospital bed for assessing DPPPT. They were asked to keep their eyes closed during the measurements and to report the onset of pressure discomfort, or pressure pain, whereupon the stimulation was stopped. In particular, they were asked to respond verbally as soon as they felt that the – steadily increased – algometer pressure became painful or uncomfortable (‘the moment of transition between strong and painful pressure’Citation9), to describe their perceptions at reaching DPPPT (either ‘dull and soaring’ or ‘sharp and stinging’), and to rate pain intensity on a 10-point numerical rating scale (NRS).
Diabetic blood glucose concentrations (except for hypoglycaemia) were not accounted for, as they had no influence on pain measurements in previous studies. The maximum force to stimulate DPPPT was limited to 1,400 kPa (=140 N/cm2 ~ 14 kg) as in our previous studies, in order not to overstress hypoaesthetic/anaesthetic tissues in patients with painless neuropathy.
Anatomic regions exposed to stimulation
Hindfoot
The area below the medial malleolus was chosen to target the deltoid ligament. The following anatomical structures and tissues were exposed to the algometer pressure probe: glabrous skin, subcutaneous fat tissue; medial plantar vein, artery and nerve; the tendons and tendon sheaths of mm.tibialis posterior, flexor digitorum longus, and flexor hallucis longus; the tibialis posterior artery; and the fascia (retinaculum musculorum flexorum). Previous studies with 1 cm2 pressure area had reported that healthy subjects’ DPPPT averages around 40 to 90 N/cm2 over lateral foot ligaments (anterior talofibular ligament, calcaneofibular ligament) (Citation24–Citation26).
Midfoot
The instep was chosen to target the abductor hallucis muscle. The following structures were co-stimulated: glabrous skin and subdermal fat tissue, plantar fascia; medial plantar vein artery and nerve. Previous studies had reported that healthy subjects’ 1 cm2 DPPPT averages around 20 to 50 N/cm2 over abductor hallucis muscle (Citation1, Citation4) (Citation5, Citation27) (Citation28).
Forefoot
The second or third MTP joint was chosen as target and was stimulated from the plantar side. Thereby, the following anatomical structures and tissues were co-compressed: glabrous skin; subdermal fat tissue; superficial and deep transverse ligaments; plantar aponeurosis; tendons and tendon sheaths of the flexor digitorum brevis muscle; common plantar digital arteries, veins, and nerves; and joint capsule. Previous studies had reported that healthy subjects’ DPPPT over MTP joints averages around 40 to 70 N/cm2 (Citation1, Citation22) (Citation27, Citation28).
Data analyses
Forces are presented as N/cm2 (1 N/cm2=10 kPa~0.1 kg). Measurements from both feet were averaged for further analysis, as previous studies had shown no differences between both feet, neither in healthy nor in diabetic subjects. In order not to lose values exceeding the safety range (140 N/cm2), a constant of 1 was added (giving 141 N/cm2) prior to analysis. Data were displayed as median (range), unless stated otherwise. Friedman analysis of variance and 3×3 factorial ANOVA for independent samples was applied for descriptive purposes, as appropriate. A two-sided Bonferroni-corrected p<0.0125 was considered significant.
Results
Demographic data of the participants are summarised in , showing that the PLDN and control study groups were not quite homogeneous. Median DPPPTs were in the healthy range at each of the sites under study (MTP joint, foot instep, below the medial malleolus) in the PLDN group (p>0.0125), and tended to be higher over the MTP joint than over the foot instep and below the medial malleolus (p>0.0125) in both groups. In PLDN patients, DPPPTs exceeded the safety limit of 140 N/cm2 with a stimulation area of 0.5 cm2 in seven measurements, with a stimulation area of 1 cm2 in four measurements, and with a stimulation area of 2 cm2 in two measurements, compared with one measurement (with 0.5 cm2 stimulation area) in the control persons. In both PLDN and control groups, median DPPPTs were lowest with 2 cm2 stimulation area, intermediate with 1 cm2 stimulation area, and highest when 0.5 cm2 stimulation area was applied (p<0.0002), see and . There was no interaction between measurement site and stimulation area in controls (ANOVA: p=0.24) and PLDN patients (ANOVA: p=0.77). Pain intensity at reaching DPPPT was similar in the control and PLDN group (NRS 3.75 (Citation2–Citation7) controls versus 4 (0–5.5) PLDN). The smallest algometer stimulation area evoked pain that was more of a ‘stinging’ nature in the control (n=5) rather than the PLDN (n=3) group, while the largest stimulation area in both groups evoked ‘dull pressure discomfort’ only. Adverse effects of the measurements like bruising were not evident.
Fig. 2 DPPPTs (N/cm2, medians) attained with various stimulation area sizes. DPPPT=deep pressure pain perception threshold, MTP=metatarsophalangeal, mall. med.=medial malleolus, PLDN=painless diabetic neuropathy.
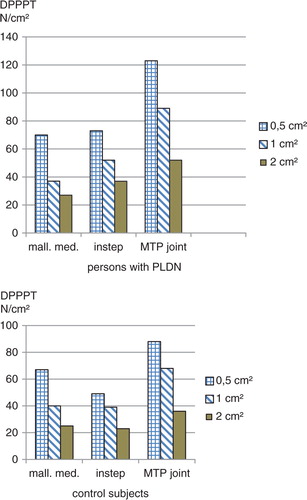
Table 1 Demographic data of subjects under study
Table 2 Deep pressure pain perception thresholds (DPPPTs) attained with different stimulation areas
At MTP joint, 1 cm2 DPPPTs measured 3 years before were not different (n.s., data not shown). Since the first DPPPT study, of the PLDN patients, one sustained a Charcot arthropathy grade 1 and one patient had a relapse of a healed Charcot foot; seven patients had relapse of healed feet ulcer (duration of follow-up: 3 years on average).
Discussion
The present data corroborate our previous preliminary findings that conventional algometer pressure stimulation evokes deep pain or discomfort at relatively low force per area even in patients with PLDN, whose foot skin is completely insensitive to any noxious impact. When compared with pinprick stimulation which aims to activate single cutaneous nociceptors (i.e. intraepidermal nerve endings of A-delta or C-fibres), deep pressure stimulation by conventional algometer technique inevitably stimulates low-threshold pressure receptors in subepidermal tissues (Merkel's disks, Meissner's corpuscles, Pacinian corpuscles, feeding A-beta fibres), and probably together with nociceptors. Spatial summation of low-threshold pressure-receptor activation induces a pain percept like ‘dull pressure discomfort’, which differs markedly from ‘stinging or burning’ evoked by pinprick stimulation of cutaneous high-threshold nociceptors. In cases of PLDN, where the endings of A-delta and C-fibres degenerate and eventually vanish before the A-beta fibres are affected (Citation22), DPPPT at the foot stimulated by conventional pressure algometry may be normal although the foot skin proves to be insensate to painful pressure stimuli (although not necessarily to touch with the palm of a hand; EAC, unpublished observation). This finding is consistent with human experiments applying skin anaesthesia (Citation29–Citation32). Lidocaine blockade of cutaneous nociceptors, increasing mechanical pain thresholds, but not tactile detection thresholds (Citation32), changes the character of mechanically stimulated pain from sharp pricking pain to dull pressure discomfort (Citation15). Interestingly, the mechanoreceptors stimulated by conventional algometer are subject to sensitisation from inflammation due to a single strong trauma (Citation4), or chronic repetitive submaximal trauma (Citation33, Citation34), since DPPPT and mechanical detection threshold decrease in response to these conditions in healthy subjects.
The lack of a measurable deterioration of DPPPT over 3 years on average corroborates the data by Gibbons et al. (Citation35) who showed no changes in heat pain, cold pain, and vibration perception thresholds at the foot in diabetic patients with and without neuropathy over a period of 3 years.
Study limitations
Unequivocally, the study has limitations due to the small sample size precluding potential statistical differences to become significant. Due to lack of pre-existing data on the effect of stimulation area size in PLDN, a power-calculation before planning the study was not feasible. Other weaknesses of the study relate to this method of quantitative pain sensitivity testing as such, which is only semi-objective and prone to biases. And last, but not least, pain in deep tissues (visceral pain) is not normally evoked by stimuli evoking somatic pain-like thermal and mechanical energies.
Conclusion
In conclusion, spatial summation of a multitude of low-threshold pressure receptors simultaneously activated by innocuous force during conventional pressure algometry explains the seeming discrepancy between absence of pinprick-perception and presence of deep pressure pain perception in persons with PLDN and painless foot ulcers. Our previous hypothesis is, thus, unlikely that ‘residual C-fibre nociceptors inside musculoskeletal structures may contribute to deep dull aching’ (Citation3) as stimulated by Algometer II in feet with PLDN. Conventional deep pressure algometry is unsuitable for assessing nociceptive dysfunction at the diabetic foot. Other methods seem more promising in this respect, for example, quantitative pinprick stimulation or intraepidermal electrical stimulation (Citation36, Citation37).
Conflict of interest and funding
The author has no conflict of interest to declare and has not received any funding of his work.
References
- Chantelau EA , Wienemann T , Richter A . Pressure pain thresholds at the diabetic Charcot-foot: an exploratory study. J Musculoskelet Neuronal Interact. 2012; 12: 95–101.
- Wienemann T , Chantelau E , Richter A . Pressure pain perception at the injured foot: the impact of diabetic neuropathy. J Musculoskelet Neuronal Interact. 2012; 12: 254–61.
- Chantelau EA, Wienemann T. Pressure pain perception in the diabetic Charcot foot: facts and hypotheses. Diabet Foot Ankle. 2013; 4: 20981. , doi: http://dx.doi.org/10.3402/dfa.v4i0.20981.
- Wienemann T, Chantelau EA, Koller A. Effect of painless diabetic neuropathy on pressure pain hypersensitivity (hyperalgesia) after acute foot trauma. Diabet Foot Ankle. 2014; 5: 24926. , doi: http://dx.doi.org/10.3402/dfa.v5.24926.
- Wienemann T , Chantelau EA . The diagnostic value of measuring pressure pain perception in patients with diabetes mellitus. Swiss Med Wkly. 2012; 142: w13682.
- Rolke R , Baron R , Maier C , Tölle TR , Treede RD , Beyer A , etal. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006; 123: 231–43.
- Rolke R , Campbell KA , Magerl W , Treede RD . Deep pain thresholds in the distal limbs of healthy human subjects. Eur J Pain. 2005; 9: 39–48.
- Treede RD , Rolke R , Andrews K , Magerl W . Pain elicited by blunt pressure: neurobiological basis and clinical relevance. Pain. 2002; 98: 235–40.
- Polianskis R , Graven-Nielsen T , Arendt-Nielsen L . Computer-controlled pneumatic pressure algometry- a new technique for quantitative sensory testing. Eur J Pain. 2001; 5: 267–77.
- Polianskis R , Graven-Nielsen T , Arendt-Nielsen L . Pressure-pain function in desensitized and hypersensitized muscle and skin assessed by cuff-algometry. J Pain. 2002; 3: 28–37.
- Polianskis R , Graven-Nielsen T , Arendt-Nielsen L . Spatial and temporal aspects of deep tissue pain assessed by cuff algometry. Pain. 2002; 100: 19–26.
- Le Quesne PM , Fowler CJ . A study of pain threshold in diabetics with neuropathic foot lesions. J Neurol Neurosurg Psychiatry. 1986; 49: 1191–4.
- Georgoudis G , Oldham J , Watson PJ , Grammatopoulou E . Reliability measures of subcutaneous pressure pain threshold measurements: a proposed method of assessing painful musculoskeletal disorders. J Nov Physiother. 2014; 4: 5.
- Brennum J , Kjeldsen M , Jensen K , Jensen TS . Measurement of human pressure pain thresholds on fingers and toes. Pain. 1989; 38: 211–17.
- Andresen T , Pfeiffer-Jensen M , Brock C , Drewes AM , Arendt-Nielsen L . A human experimental bone pain model. Basic Clin Pharmacol Toxicol. 2013; 112: 116–23.
- Defrin R , Ronat A , Ravid A , Peretz C . Spatial summation of pressure pain: effect of body region. Pain. 2003; 106: 471–80.
- Nie HL , Graven-Nielsen T , Arendt-Nielsen L . Spatial and temporal summation of pain evoked by mechanical pressure stimulation. Eur J Pain. 2009; 13: 592–9.
- Finocchietti S , Graven-Nielsen T , Arendt-Nielsen L . Bone hyperalgesia after mechanical impact stimulation: a human experimental pain model. Somatosens Mot Res. 2014; 31: 178–85.
- Greenspan JG , Thomadaki M , McGillis SLB . Spatial summation of perceived pressure, sharpness and mechanically evoked cutaneous pain. Somatosens Mot Res. 1997; 14: 107–12.
- Handwerker HO , Kobal G . Psychophysiology of experimentally induced pain. Physiol Rev. 1993; 73: 639–71.
- Baumgärtner U , Greffrath W , Treede RD . Contact heat and cold, mechanical, electrical and chemical stimuli to elicit small fibre-evoked potentials: merits and limitations for basic science and clinical use. Neurophysiol Clin. 2012; 42: 267–80.
- Chantelau EA . Nociception at the diabetic foot, an uncharted territory. World J Diabetes. 2015; 6: 391–402.
- Chantelau EA , Grützner G . Is the Eichenholtz classification still valid for the diabetic Charcot foot?. Swiss Med Wkly. 2014; 144: w13948.
- Algafly AA , George KP . The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. Br J Sports Med. 2007; 41: 365–9.
- Ramiro-Gonzalez MD , Cano-de-la-Cuerda R , De-la-Llave-Rincon AI , Miangolarra-Page JC , Zarzoso-Sanchez R , Fernandez-de-las-Penas C . Deep tissue hypersensitivity to pressure pain in individuals with unilateral acute inversion ankle sprain. Pain Med. 2012; 13: 361–7.
- Truyols-Dominguez S , Salom-Moreno J , Abian-Vicen J , Cleland JA , Fernandez-de-las-Penas C . Efficacy of thrust and nonthrust manipulation and exercise with or without the addition of myofascial therapy for the management of acute inversion ankle sprain: a randomized clinical trial. J Orthop Sports Phys Ther. 2013; 43: 300–9.
- Xiong S , Goonetilleke RS , Jiang Z . Pressure thresholds of the human foot: measurement reliability and effects of stimulus characteristics. Ergonomics. 2011; 54: 282–93.
- Xiong S , Goonetilleke RS , Rodrigo WD , Zhao J . A model for the perception of surface pressure on human foot. Appl Ergon. 2013; 44: 1–10.
- Kosek E , Ekholm J , Hansson P . Pressure pain thresholds in different tissues in one body region. The influence of skin sensitivity in pressure algometry. Scand J Rehabil Med. 1999; 31: 89–93.
- Graven-Nielsen T , Mense S , Arendt-Nielsen L . Painful and non-painful pressure sensations from human skeletal muscle. Exp Brain Res. 2004; 159: 273–83.
- Takahashi K , Taguchi T , Itoh K , Okada K , Kawakita K , Mizumura K . Influence of surface anesthesia on the pressure pain threshold measured with different-sized probes. Somatosens Mot Res. 2005; 22: 299–305.
- Krumova EK , Zeller M , Westermann A , Maier C . Lidocaine patch (5%) produces a selective, but incomplete block of A-delta and C-fibres. Pain. 2012; 153: 273–80.
- Messing K , Kilbom A . Standing and very slow walking: foot pain-pressure threshold, subjective pain experience and work activity. Appl Ergon. 2001; 32: 81–90.
- Alfuth M , Rosenbaum D . Are diurnal changes in foot sole sensation dependent on gait activity?. Neurosci Lett. 2011; 504: 247–51.
- Gibbons CH , Freeman R , Tecilazich F , Dinh T , Lyons TE , Gnardellis C , etal. The evolving natural history of neurophysiologic function in patients with well controlled diabetes. J Peripher Nerv Syst. 2013; 18: 153–61.
- Kukidome D , Nishikawa T , Sato M , Igata M , Kawashima J , Shimoda S , etal. Measurement of small fibre pain threshold values for the early detection of diabetic polyneuropathy. Diabet Med. 2016; 33: 62–9.
- Suzuki C , Kon T , Funamizu Y , Ueno T , Haga R , Nishijima H , etal. Elevated pain threshold in patients with asymptomatic diabetic neuropathy: an intra-epidermal electrical stimulation study. Muscle Nerve. 2016; 54: 146–9.
