Abstract
Background
The protective effect of farm upbringing on allergic rhinitis is well known, but how upbringing in other environments influences the development of allergic rhinitis is scarcely investigated. The aim of this study was to investigate the association between place of upbringing and pet keeping in childhood and allergic rhinitis and nasal symptoms in adulthood.
Methods
The population-based Respiratory Health in Northern Europe study includes subjects from Denmark, Norway, Sweden, Iceland, and Estonia born in 1945–1973. This paper analyses 13,376 participants of the third study wave. Six categories of place of upbringing were defined: farm with livestock, farm without livestock, village in rural area, small town, city suburb, and inner city. Pets in the home at birth and during childhood were recorded. Data were analysed using adjusted logistic regression models.
Results
Livestock farm upbringing predicted less adult allergic rhinitis [odds ratio (OR) 0.68, 0.54–0.85] and nasal symptoms (OR 0.82, 0.68–0.99) than city upbringing, and an urban–rural gradient with decreasing risk per level of urbanisation was observed (OR 0.92, 0.88–0.94). Pets in the home at birth (OR 0.78, 0.68–0.88) and during childhood (OR 0.83, 0.74–0.93) were associated with less subsequent allergic rhinitis. Pet keeping did not explain the protective effect of place of upbringing.
Conclusion
Risk of allergic rhinitis and nasal symptoms in adulthood was inversely associated with the level of urbanisation during upbringing. Pets at birth decreased the risk further, but did not explain the urban–rural gradient. Persistent beneficial effects of microbial diversity in early life might be an explanation for the findings.
The prevalence of allergic diseases has increased rapidly since the mid-20th century and has become a major public health problem, particularly in modern industrialised countries (Citation1–Citation3). Allergic rhinitis is the most common of all allergic diseases, and it severely affects the quality of life (Citation4). Allergic rhinitis also represents a substantial economic burden for both individuals and the society (Citation5).
Several studies have shown that the prevalence of allergic rhinitis is lower among people born and raised on a farm (Citation6–Citation9). The microbial load and diversity accompanied with farm living has been suggested as one of the most important reasons for the beneficial effects on allergy (Citation10). However, it is still unclear whether this effect is also present in other environments where the microbial load and diversity may be different.
Elholm et al. showed that the risk of allergic sensitisation and allergic rhinitis in adulthood was gradually decreasing across four levels of upbringing ranging from city to farm (Citation11). However, the study only included inhabitants from Denmark (Citation11). The Danish study confirmed results from a Swedish study also using four categories of urbanisation among adults between 16 and 75 years of age (Citation12), and similar results were suggested in a Finnish study using three categories of urbanisation (Citation13). Recently, an international study with three levels of urbanisation also suggested an urban–rural gradient in allergic rhinitis (Citation14). In the current international study, we included six categories of urbanisation and explored the association with both allergic rhinitis and nasal symptoms, taking pet keeping into consideration.
We hypothesise that the prevalence of allergic rhinitis and nasal symptoms is negatively associated with microbial load and diversity in childhood. To investigate this hypothesis, we used place of upbringing as a proxy for microbial load and diversity in early life and also took pet keeping into consideration.
Materials and methods
Study population
This study is conducted on a subpopulation of the European Community Respiratory Health Survey (ECRHS). During recruitment in 1989–1992, the original study population was randomly selected from 22 countries (Citation15, Citation16). ECRHS Stage 1 included over 130,000 men and women in the age range of 20–44 years (Citation15, Citation16).
The Respiratory Health in Northern Europe (RHINE) study comprises 21,659 ERCHS subjects from Northern Europe: Norway, Sweden, Iceland, Estonia and Denmark (Citation17). In 1999–2001, a postal questionnaire (RHINE II) was sent out, and 16,105 subjects (74%) responded. At follow-up in 2010–2012 (RHINE III), 13,499 subjects (62%) responded to the postal questionnaire. At both follow-ups, two reminders were sent out (Citation16). The ethics committees for research approved the study for each study centre, and informed consent was obtained from all the study subjects.
Definition of outcomes, exposures and covariates
Outcomes were derived from RHINE III using the following questions: ‘Do you have any nasal allergies including hay fever?’ and ‘Have you ever experienced nasal symptoms such as nasal congestion, rhinorrhoea (runny nose) and/or sneezing attacks without having a cold?’.
Place of upbringing and pets at birth and in childhood were used as a proxy for load and diversity of microbial exposure. Information on place of upbringing was derived from RHINE III and defined from the following question: ‘What term best describes the place you lived most of the time when you were under the age of 5 years?’ The response categories were 1) farm with livestock, 2) farm without livestock, 3) village in rural area, 4) small town, 5) suburb of city and 6) inner city.
Information on pets at birth and in childhood were derived from RHINE II using the following questions: ‘When you were born, were any of the following in your home?’ and ‘When you were a child, were any of the following in your home?’, with dog, cat and/or other furry animals as response categories for both questions.
Other covariates were derived from the RHINE III questionnaire and included age, sex, smoking, parental smoking in offspring childhood, parental asthma and household size. These were selected a priori on the basis of existing evidence about risk factors for allergic rhinitis. Asthma was not included as a covariate in the analyses in order to avoid overadjustment.
Statistical methods
Data were analysed using logistic regression models and presented as odds ratios (ORs) with corresponding 95% confidence intervals (CI). To be included in the analyses, the subjects had to answer at least one of the two outcome questions. Additional analyses included test for trend and stratified analysis by year of birth and sex. Age stratification was performed before and after 1960 (median birth year).
All statistical analyses were performed using Stata Version 13.1 (StataCorp LP, College Station, TX, USA).
Results
Basic characteristics for the study population (N=13,376) are shown in .
Table 1 Characteristics of the study population
Subjects who grew up in a city were more likely to be current smokers and exposed to parental smoking in childhood. Pet ownership was more prevalent among subjects from farms and rural areas. Sex and parental asthma status were similar across the six exposure groups.
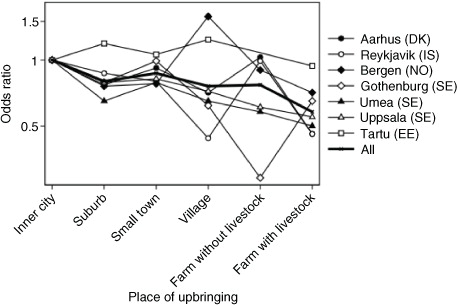
Place of upbringing was not equally distributed across centres. Fewer subjects from Umeå were brought up in the inner city, and in both Umeå and Uppsala, fewer subjects were brought up in suburbs. Gothenburg and Tartu stand out when it comes to small towns as being less represented, and the same was true for Reykjavik and Bergen for village in a rural area. On the other hand, Bergen was over-represented among subjects from farm without livestock, and Umeå was over-represented for farm with livestock. Gothenburg and Reykjavik were the two centres with the lowest proportion brought up on farms with livestock.
The study population included 3,195 cases with allergic rhinitis and 6,307 cases with nasal symptoms. This corresponds to a prevalence of 24% for allergic rhinitis ranging from 20% on livestock farms to 26% in inner cities, and 47% for nasal symptoms ranging from 45% on livestock farms to 49% in inner cities (). In adjusted logistic regression models, livestock farm upbringing predicted less adult allergic rhinitis compared with city upbringing (OR 0.58, 0.48–0.71), and a significant decreased risk per level of urbanisation was observed (OR 0.92, 0.88–0.94). Further adjustment for pets and household size resulted in slightly decreased estimates for all six exposure groups. The same pattern was observed for nasal symptoms, although it was less prominent ().
Fig. 1 Allergic rhinitis and nasal symptoms in relation to place of upbringing presented as OR adjusted for sex, age, smoking, parental asthma, parental smoking in offspring childhood and centre.
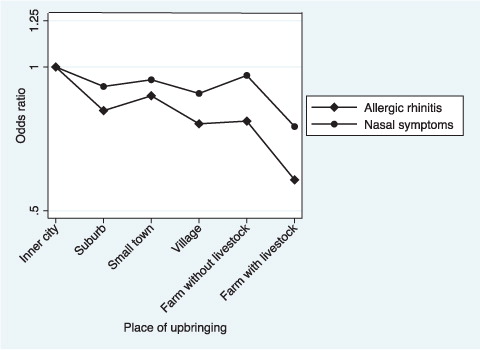
Table 2 Logistic regression analyses on allergic rhinitis and nasal symptoms in relation to place of upbringing, OR (95% CI)
Sub-analysis showed no differences between men and women, or between subjects born before and after 1960 (). No significant interaction between sex or year of birth and place of upbringing was found.
Table 3 Adjusted logistic regression analyses on allergic rhinitis and nasal symptoms in relation to place of upbringing stratified by sex and birth year, OR (95% CI).
Pets at birth was associated with a lower prevalence of allergic rhinitis (22% versus 26%) and nasal symptoms (46% versus 48%). In logistic regression models, pets at birth was associated with a decreased risk of both allergic rhinitis (OR 0.78, 0.68–0.88) and nasal symptoms (OR 0.87, 0.78–0.97) (). Pets during childhood was also associated with a decreased risk of both allergic rhinitis (OR 0.83, 0.74–0.93) and nasal symptoms (OR 0.90, 0.78–1.05). Similar results were found for cat and dog keeping, respectively (results not shown). The results persisted after mutual adjustment for pets at birth and pets during childhood, respectively. Place of upbringing did not modify the association between allergic rhinitis or nasal symptoms and pets in the home (data not shown).
Table 4 Logistic regression analyses on allergic rhinitis and nasal symptoms in relation to pets in the home stratified by sex presented as OR (95% CI)
In general, all centres showed an urban–rural gradient. suggests variation between centres, especially for village and for farm without livestock, but no significant difference between centres was revealed (p=0.88 for interaction between centre and place of upbringing). For all centres, a decreased risk per level of urbanisation was observed, although not statistically significant for all centres: Aarhus (OR 0.88, 0.82–0.96), Reykjavik (OR 0.86, 0.78–0.95), Bergen (OR 0.98, 0.90–1.08), Gothenburg (OR 0.91, 0.81–1.03), Umeå (OR 0.88, 0.81–1.14), Uppsala (OR 0.90, 0.50–0.81) and Tartu (OR 0.97, 0.86–1.08).
Discussion
In this population-based study, the risk of allergic rhinitis and nasal symptoms was gradually decreasing across six upbringing levels of urbanisation ranging from inner city to livestock farm. The results were most pronounced for allergic rhinitis. As expected, subjects living on a farm with livestock the first 5 years of their life had significantly less allergic rhinitis and nasal symptoms compared to those living in the inner city. Having pets in the home at birth and during childhood reduced the risk of allergic rhinitis later in life. For nasal symptoms, the protective effect was only statistically significant for pets in the home at birth. Pet keeping did not explain the effect of place of upbringing on allergic rhinitis or nasal symptoms.
Our results are consistent with the findings of Elholm et al., showing an urban–rural gradient for allergic sensitisation and allergic rhinitis among young Danish adults (Citation11), and also support earlier findings from Sweden (Citation12) and Finland (Citation13). Furthermore, an urban–rural gradient has been suggested in the RHINE cohort for inflammatory bowel diseases (Citation18) and asthma (Citation19).
The findings on place of upbringing were comparable across different study centres, although the farming industry and structure differs significantly. In Denmark, relatively big industrialised farms are seen, whereas Norway is known for its smaller farms, often with only one person employed. In addition, Estonia was less urbanised compared to the other countries during the period when the study population was born.
Our findings are not consistent with the findings in the GABRIEL study, where they revealed some centre-specific patterns, that is, the protective effects of farm upbringing on allergic rhinitis were less pronounced in Poland than in Germany, Switzerland and Austria (Citation20).
An important strength of this study is the population-based design with more follow-ups. A limitation of the study is that all variables of interest are self-reported, and therefore a potential risk of recall bias occurs because of the retrospective collection of data. However, we expect that the participants were able to correctly remember their place of upbringing, and therefore we do not consider recall bias to be an issue with the main predictor in our analysis. More critically with regards to recall bias is probably pets in the home at birth. The difficulty is reflected in the low response rate (19–24% missing) for specific question(s) about pet keeping.
In this study, no clinical information about allergic rhinitis was available. This is a clear limitation, and additional analyses on allergic sensitisation would have improved the study. However, a high correlation between immunoglobulin E (IgE)-mediated sensitisation and self-reported allergic rhinitis has been shown in several other studies (Citation21, Citation22). Our results are more pronounced for allergic rhinitis than for nasal symptoms, which probably reflects that the term ‘nasal symptoms’ is unspecific and covers a range of symptoms. Furthermore, the temporally presence of outcomes differs between the two questions, as the question about allergic rhinitis is on present allergies and the question about nasal symptoms is on ever symptoms.
All centres used a random sample recruitment strategy for including participants. The recruitment strategy may however have varied between the five countries (Citation23).
Only 53% of the original study population responded to all three questionnaires (Citation17). However, a non-response analysis for the three follow-up waves did not show any significant variation in associations between selected exposures (e.g. smoking) and outcomes (e.g. allergic rhinitis) at baseline between long-term responders and only baseline responders (Citation17).
This study only provides information about place of residence for the first 5 years of life. Douwes et al. have suggested that farming exposure might be protective even after 5 years of age when it comes to the development of allergic rhinitis (Citation24). The strongest protection was seen in adults with current and childhood exposure, although the childhood exposure was the strongest protective factor (Citation24). This was supported by Elholm et al. (Citation25).
In this study, we hypothesised that rural upbringing with high microbial load and diversity protects against development of allergic rhinitis and nasal symptoms. It could also be argued that exposures more prevalent in urban areas, for example, air pollution, could explain our findings, and it has been suggested that allergic rhinitis is more common in urban areas with more traffic pollution (Citation26). Even though we would not expect the air pollution level to differ across all six urbanization levels, for example between farm with and farm without livestock, we cannot preclude that others exposures but microbial load and diversity can explain our findings.
It has been argued that allergic rhinitis has a strong genetic component (Citation27), and the results from this study can be explained by selection towards urbanised environments for parents with allergic diseases. In this study, parental asthma status was used as a proxy for the genetic predisposition as data on parental allergic rhinitis were not available. However, adjusting for parental asthma did not change the results. In a recent study by Eduard et al., no difference in asthma prevalence was found among farming apprentices and their non-farmer siblings, which suggests that selection into farming is not substantial (Citation28). In line with that, a study in 1999 found that early farm exposure protects against allergic rhinitis and that it was rather unlikely that it was caused by selection bias from the atopic status of the parents (Citation7). Conversely, a Swedish register study with a large study population showed that subjects with asthma and atopic diseases more often chose to move from farms compared with those without asthma and atopic diseases (Citation29).
In this study, place of upbringing in early childhood is used as a proxy for the microbial load and diversity. However, the study gives no information about how much time the children spent in the stables, at the hayloft, etc. Riedler found that the time spent in the stables plays a dominant role for the protective effect on allergic rhinitis later in life (Citation30). This indicates that the microbial exposure itself is important for the protection against allergic rhinitis and not the livestock farm per se as suggested by others (Citation8, Citation31).
On the other hand, data from the GABRIEL study among children from Switzerland, Austria and Southern Germany indicated that the protective effect against allergic rhinitis was only seen for subjects exposed to livestock animal sheds, haylofts and raw milk in early childhood, but still they found less allergic rhinitis among children growing up on a farm without contact to livestock compared with those growing up in a rural area but not on a farm (Citation32).
Farm children differ in many ways from children growing up elsewhere, which raise the question whether the protective effects are because of other coexisting factors. Strachan et al. argued that the number of (especially older) siblings played a protective role for the development of allergic diseases (Citation33), which has been confirmed by others (Citation34). Farm children in this study and other studies have more siblings than children growing up in urban areas (Citation35–Citation38). However, we adjusted for household size, and this did not change our results.
Pet exposure in early childhood is also an important factor to take into consideration when investigating allergic rhinitis and nasal symptoms. It is debated whether pet exposure is a protective factor or a risk factor for the development of allergic rhinitis (Citation37, Citation39). We found pet exposure in early life to prevent allergic rhinitis and nasal symptoms, but furthermore we confirmed the results from Elholm et al., who also found an independent effect of place upbringing after adjusting for pet exposure (Citation11).
Conclusion
Subjects growing up on a farm the first 5 years of life had significantly less allergic rhinitis and nasal symptoms compared with subjects growing up in the inner city. The risk of allergic rhinitis and nasal symptoms was gradually decreasing across each level of urbanisation, resulting in a statistically significant urban–rural gradient. Having pets during childhood protected against the development of allergic rhinitis and nasal symptoms later in life, but did not explain the effect of place of upbringing. These results suggest that early childhood exposures associated with the level of urbanisation play a persistent role for allergic rhinitis and nasal symptoms later in life. Further understanding of exposures associated with urbanisation may contribute to the future prevention of allergic diseases.
Authors’ contributions
VS, SHC and CS take responsibility for the overall integrity of the reported results. VS, CS, SHC and ST developed the aims of the study and drafted the plan for analysis. CJ, BB, BF, MH, RJ, AJ, EO, TS, CS and VS participated in the data collection. SHC, ST and VS performed the data analysis. All authors interpreted the results. SHC drafted the manuscript. All authors critically commented the manuscript and accepted the final draft.
Conflict of interest and funding
The authors have no conflicts of interest to declare. The RHINE III was supported financially by the Norwegian Research Council (grant number 214123), the Bergen Medical Research Foundation, the Western Norwegian Regional Health Authorities (grant numbers 911 892 and 911 631), the Norwegian Labour Inspection, the Norwegian Asthma and Allergy Association, the Faculty of Health of Aarhus University (project number 240008), the Wood Dust Foundation (project number 444508795), the Danish Lung Association, the Swedish Heart and Lung Foundation, the Vårdal Foundation for Health Care Science and Allergy Research, the Swedish Council for Working Life and Social Research, the Bror Hjerpstedt Foundation, the Swedish Asthma and Allergy Association, the Icelandic Research Council and the Estonian Science Foundation (grant number 4350).
References
- Brooks C, Pearce N, Douwes J. The hygiene hypothesis in allergy and asthma: an update. Curr Opin Allergy Clin Immunol. 2013; 13: 70–7.
- Braun-Fahrlander C. Mikrobielles Umfeld und allergische Erkrankungen im Kindesalter. Ther Umsch. 2013; 70: 714–19. [In German].
- Bornelöv S, Sääf A, Melén E, Bergström A, Torabi Moghadam B, Pulkkinen V, etal. Rule-based models of the interplay between genetic and environmental factors in childhood allergy. PLoS One. 2013; 8: 1–12.
- Linneberg A, Dam Petersen K, Hahn-Pedersen J, Hammerby E, Serup-Hansen N, Boxall N. Burden of allergic respiratory disease: a systematic review. Clin Mol Allergy. 2016; 14: 12.
- Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011; 106: 12–6.
- Riedler J, Eder W, Oberfeld G, Schreuer M. Austrian children living on a farm have less hay fever, asthma and allergic sensitization. Clin Exp Allergy. 2000; 30: 194–200.
- Braun-Fahrländer C, Gassner M, Grize L, Neu U, Sennhauser FH, Varonier HS, etal. Prevalence of hay fever and allergic sensitization in farmer's children and their peers living in the same rural community. SCARPOL team. Swiss Study on Childhood Allergy and Respiratory Symptoms with Respect to Air Pollution. Clin Exp Allergy. 1999; 29: 28–34.
- Von Ehrenstein O, Von Mutius E, Illi S, Baumann L, Bohm O, Von Kries R. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy. 2000; 30: 187–93.
- Nicolaou N, Siddique N, Custovic A. Allergic disease in urban and rural populations: increasing prevalence with increasing urbanization. Allergy. 2005; 60: 1357–60.
- Ege M, Mayer M, Normand A, Genuneit J, William O, Braun-Fahrländer C, etal. Exposure to enviromental microorganisms and childhood asthma. N Engl J Med. 2011; 364: 701–9.
- Elholm G, Linneberg A, Husemoen LLN, Omland Ø, Grønager PM, Sigsgaard T, etal. The Danish urban-rural gradient of allergic sensitization and disease in adults. Clin Exp Allergy. 2016; 46: 103–11.
- Eriksson J, Ekerljung L, Lötvall J, Pullerits T, Wennergren G, Rönmark E, etal. Growing up on a farm leads to lifelong protection against allergic rhinitis. Allergy. 2010; 65: 1397–403.
- Kilpelaine M, Terh E, Helenius H, Koskenvuo M. Farm environment in childhood prevents the development of allergies. Clin Exp Allergy. 2000; 30: 201–8.
- Campbell B, Raherison C, Lodge CJ, Lowe AJ, Gislason T, Heinrich J, etal. The effects of growing up on a farm on adult lung function and allergic phenotypes: an international population-based study. Thorax. 2016. [Epub ahead of print]
- Omenaas E, Svanes C, Janson C, Toren K, Jogi R, Gislason T, etal. What can we learn about asthma and allergy from the follow-up of the RHINE and the ECRHS studies?. Clin Respir J. 2008; 2(Suppl 1): 45–52.
- Burney PG, Luczynska C, Chinn S, Jarvis D. The European community respiratory health survey. Eur Respir J. 1994; 7: 954–60.
- Johannessen A, Verlato G, Benediktsdottir B, Forsberg B, Franklin K, Gislason T, etal. Longterm follow-up in European respiratory health studies – patterns and implications. BMC Pulm Med. 2014; 14: 63.
- Timm S, Svanes C, Janson C, Sigsgaard T, Johannessen A, Gislason T, etal. Place of upbringing in early childhood as related to inflammatory bowel diseases in adulthood: a population-based cohort study in Northern Europe. Eur J Epidemiol. 2014; 29: 429–37.
- Timm S, Frydenberg M, Janson C, Campbell B, Forsberg B, Gislason T, etal. The urban-rural gradient in asthma: a population-based study in Northern Europe. Int J Environ Res Public Health. 2016; 13: 93–107.
- MacNeill SJ, Sozanska B, Danielewicz H, Debinska A, Kosmeda A, Boznanski A, etal. Asthma and allergies: is the farming environment (still) protective in Poland? The GABRIEL Advanced Studies. Allergy. 2013; 68: 771–9.
- Olivieri M, Heinrich J, Schlünssen V, Antó J, Forsberg B, Janson C, etal. The risk of respiratory symptoms on allergen exposure increases with increasing specific IgE levels. Allergy. 2016; 6: 859–68.
- Bodtger U, Poulsen LK, Linneberg A. Rhinitis symptoms and IgE sensitization as risk factors for development of later allergic rhinitis in adults. Allergy. 2006; 61: 712–6.
- Toren K, Gislason T, Omenaas E, Jogi R, Forsberg B, Nystrom L, etal. A prospective study of asthma incidence and its predictors: the RHINE study. Eur Respir J. 2004; 24: 942–6.
- Douwes J, Travier N, Huang K, Cheng S, McKenzie J, Le Gros G, etal. Lifelong farm exposure may strongly reduce the risk of asthma in adults. Allergy. 2007; 62: 1158–65.
- Elholm G, Schlünssen V, Doekes G, Basinas I, Bibby BM, Hjort C, etal. Become a farmer and avoid new allergic sensitization: adult farming exposures protect against new-onset atopic sensitization. J Allergy Clin Immunol. 2013; 132: 1239–41.
- Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, etal. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015; 70: 245–56.
- Chiarella SE, Fernandez R, Avila PC. The genes and the environment in nasal allergy. Curr Opin Allergy Clin Immunol. 2015; 15: 440–5.
- Eduard W, Schlünssen V, Sigsgaard T, Omland Ø, Pearce N, Douwes J. Can selection explain the protective effects of farming on asthma?. Ann Agr Environ Med. 2015; 22: 467–9.
- Bråbäck L, Hjem A, Rasmussen F. Selective migration to a healthy worker effect in the farming population. J Clin Epidemiol. 2006; 59: 102–5.
- Riedler J. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. J Am Med Assoc. 2001; 286: 1129–33.
- Horak F, Studnicka M, Gartner C, Veiter A, Tauber E, Urbanek R, etal. Parental farming protects children against atopy: longitudinal evidence involving skin prick tests. Clin Exp Allergy. 2002; 32: 1155–9.
- Horak E, Morass B, Ulmer H, Genuneit J, Braun-Fahrländer C, von Mutius E. Prevalence of wheezing and atopic diseases in Austrian schoolchildren in conjunction with urban, rural or farm residence. Wien Klin Wochenschr. 2014; 126: 532–6.
- Strachan DP. Hay fever, hygiene, and household size. Br Med J. 1989; 299: 1259–60.
- Karmaus W, Botezan C. Does a higher number of siblings protect against the development of allergy and asthma?. A review. J Epidemiol Community Health. 2002; 56: 209–17.
- Leynaert B, Neukirch C, Jarvis D, Chinn S, Burney P, Neukirch F. Does living on a farm during childhood protect against asthma, allergic rhinitis, and atopy in adulthood?. Am J Respir Crit Care Med. 2001; 164: 1829–34.
- Gassner-Bachmann M, Wüthrich B. Bauernkinder leiden selten an Heuschnupfen und asthma. Dtsch Med Wochenschr. 2000; 125: 924–31. [In German].
- Matheson MC, Dharmage SC, Abramson MJ, Walters EH, Sunyer J, de Marco R, etal. Early-life risk factors and incidence of rhinitis: results from the European community respiratory health study. An international population-based cohort study. J Allergy Clin Immunol. 2011; 128: 816–23.
- Genuneit J, Strachan DP, Büchele G, Weber J, Loss G, Sozanska B, etal. The combined effects of family size and farm exposure on childhood hay fever and atopy. Pediatr Allergy Immunol. 2013; 24: 293–8.
- Svanes C, Heinrich J, Jarvis D, Chinn S, Omenaas E, Gulsvik A, etal. Pet-keeping in childhood and adult asthma and hay fever: European community respiratory health survey. J Allergy Clin Immunol. 2003; 112: 289–300.