Abstract
The significance of bats as sources of emerging infectious diseases has been increasingly appreciated, and new data have been accumulated rapidly during recent years. For some emerging pathogens the bat origin has been confirmed (such as lyssaviruses, henipaviruses, coronaviruses), for other it has been suggested (filoviruses). Several recently identified viruses remain to be ‘orphan’ but have a potential for further emergence (such as Tioman, Menangle, and Pulau viruses). In the present review we summarize information on major bat-associated emerging infections and discuss specific characteristics of bats as carriers of pathogens (from evolutionary, ecological, and immunological positions). We also discuss drivers and forces of an infectious disease emergence and describe various existing and potential approaches for control and prevention of such infections at individual, populational, and societal levels.
Introduction
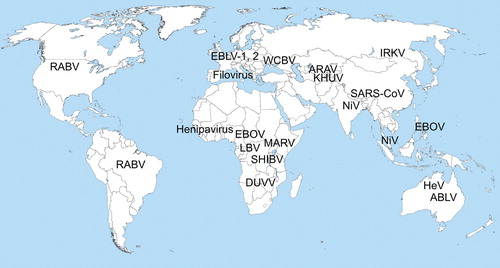
Infectious diseases continue to emerge and the majority of these are zoonotic in origin Citation1 Citation2. Zoonotic infections, particularly those caused by RNA viruses, have been recognized as a significant human health threat, particularly in developing countries Citation3. The significance of bats as reservoirs of such emerging infectious diseases (EIDs) has been increasingly appreciated Citation4. New pathogens are documented in bats every year and most of these new agents still require characterization. The majority of studies performed to date have focused on infections of significant public health and veterinary concern. In this paper, we summarize the available information on several selected pathogens, including lyssaviruses, coronaviruses, henipaviruses, and filoviruses, for which a considerable amount of information has been collected to date on a global basis ().
Fig. 1. Bat-associated and presumable bat-associated EIDs. Abbreviations: RABV,=rabies virus; EBLV-1,2=European bat lyssaviruses type 1 and 2; WCBV=West Caucasian bat virus; ARAV=Aravan virus; KHUV=Khujand virus; IRKV=Irkut virus; LBV=Lagos bat virus; SHIBV=Shimoni bat virus; DUVV=Duvenhage virus; MARV=Marburg virus; EBOV=Ebola virus; Filovirus=unclassified filovirus detected in bats in Europe; HeV=Hendra virus; NiV=Nipah virus; Henipavirus=unclassified henipavirus; SARS-CoV=SARS coronavirus.
Bat rabies—a global threat
Rabies is an acute progressive encephalitis caused by viruses in the Genus Lyssavirus, Family Rhabdoviridae, with the highest fatality rate among conventional infectious diseases. Known in bats for well over a century, rabies is the best studied infection associated with the Chiroptera. Bats are the principal reservoirs for 10 of the 11 recognized lyssavirus species and are suspected as hosts of other putative species Citation5. Only one lyssavirus, Mokola virus (MOKV), has never been isolated from bats to date. However, the principal reservoir for MOKV is unknown Citation6. Another viral species, rabies virus (RABV), circulates in bats and other mammals (predominantly carnivores). Interestingly, RABV circulates in bats only in the Americas, whereas in carnivores, the disease circulates globally. In the Old World, bats maintain circulation of other lyssavirus species, such as Lagos bat virus (LBV), Duvenhage virus (DUVV), European bat lyssaviruses type 1 (EBLV-1) and 2 (EBLV-2), Australian bat lyssavirus (ABLV), Aravan virus (ARAV), Khujand virus (KHUV), Irkut virus (IRKV), West Caucasian bat virus (WCBV), and Shimoni bat virus (SHIBV). For these viruses, bats are the principal hosts, with only a few spillover infections documented in other mammals. Isolation of RABV from Eurasian bats has been suggested several times, but never confirmed (reviewed in Kuzmin and Rupprecht Citation7). Indeed, the surveillance data from developing countries is very limited. We do not know which lyssaviruses circulate in bats of northern Africa and southern Asia, although historical reports Citation8 Citation9 along with more recent serological findings Citation10–Citation12 indicate that bats do maintain lyssavirus circulation in these territories.
A paralytic disease in cattle and sporadically in humans bitten by a vampire bat has been reported from the time of the Spanish first colonized Latin America. However, the diagnosis of rabies was first confirmed by the identification of Negri bodies in the brain of cattle during an outbreak in Brazil in 1911 Citation13. Vampire bats probably maintained rabies virus circulation for a long time prior to the arrival of Europeans in the Americas. The association between vampire bites and the disease was understood by natives, who cauterized or washed the bites to prevent the disease Citation14. However, historical antecedents might be some other progenitor virus, quite different from those ones that circulate in bat populations presently.
Economic losses due to vampire bat rabies in livestock are tremendous. In the enzootic area there is an at-risk population of more than 70 million head of cattle. Vampire bats usually bite many animals in a herd. The proportion of animals bitten may vary from 6 to 52% Citation15. Significant outbreaks of vampire bat rabies were documented in Amazon area (Brazil, Peru) during recent years. Up to 23–55% of respondents interviewed had vampire bat bites during the last year. During the outbreaks, up to 15% of such bites caused rabies in humans (reviewed in Kuzmin and Rupprecht Citation7).
An idea that vampire bats may be asymptomatic rabies carriers, shedding the virus in their saliva for months, was popular during initial studies of vampire bat rabies Citation16. However, in a well-documented experimental study by Moreno and Baer Citation17, the disease in vampire bats was similar to rabies observed in other mammals. The bats that developed signs of disease and excreted the virus via saliva soon died, whereas those that survived the inoculation without clinical signs never excreted the virus or had it in the brain as demonstrated upon euthanasia. More recently, the asymptomatic excretion of RABV in the saliva of experimentally infected vampire bats, which survived the challenge during at least 2 years of observation, was documented again Citation18. Clearly, this phenomenon requires additional investigation.
Rabies of insectivorous bats was first documented in 1953 in Florida. Later it was documented across the United States, in Canada, and Latin America. Several RABV lineages were documented, and in general, they correspond to particular host species (reviewed in Kuzmin and Rupprecht Citation7). Moreover, widely distributed bat species, such as Mexican free-tailed bat (Tadarida brasiliensis) and big brown bat (Eptesicus fuscus), maintain circulation of several RABV variants across their geographic range. Insectivorous bats are the major source of human rabies in the United States and Canada, which became especially prominent after elimination of RABV circulation among dogs. During 1958–2009, a total of 49 naturally acquired human rabies cases caused by bat RABV variants were reported in the United States and Canada (excluding four rabies cases caused by organ transplantation from a donor who died of unrecognized rabies) Citation19 Citation20. In 19 of these cases the exposure was ‘cryptic’, as the patients did not recall any contact with animals or a bat was seen flying in the residence but no direct physical contact was reported.Appears that some bat bites, especially if they were inflicted by small bat species, may be ignored or not recognized as dangerous by people (such as a previously unattended child, mentally disabled, or intoxicated person).
Two closely related RABV variants (previously considered as one), associated with the silver-haired bat (Lasionycteris noctivagans) and eastern tri-colored bat (Perimyotis subflavus) have caused about 60% of human rabies cases, where the virus variant could be identified. These bats are relatively small, do not form large colonies, and usually do not roost in close proximity to human dwellings. In contrast, the big brown bat RABV variant and the Myotis RABV variant caused one human case each, even if these bats frequently occupy house attics and crevices in men-made constructions. Furthermore, big brown bats constitute about 90% of all rabid bats, submitted to diagnostic laboratories in the United States and definitely have more contacts with humans Citation21 Citation22. The Mexican free-tailed bat RABV variant caused several human rabies cases as well, including four cases that occurred in 2004 after transplantation of organs and vessel from a donor who died of rabies Citation23. Several versions were suggested to explain the disproportional prevalence of the silver-haired bat and eastern tri-colored bat RABV variant among human rabies cases. Investigations suggested that these viruses have enhanced pathogenicity to humans, for example they may have a greater ability to replicate in fibroblasts and epithelial cells, being delivered into a superficial bat bite Citation24.
In the Old World, the significance of bat rabies for veterinary and public health is well addressed only in the countries with developed surveillance systems, such as Western Europe and Australia. The EBLV-1 and EBLV-2 circulate in Europe among insectivorous bats Eptesicus fuscus and Myotis spp, respectively. These viruses caused at least three cases of human rabies, where the virus was characterized, in Finland, Russia, and in the UK (reviewed in Kuzmin and Rupprecht Citation7). The IRKV, first identified in insectivorous bat Murina leucogaster in eastern Siberia during 2002 Citation25, was known by this only one isolate until 2007, when it caused a human death after a bite of unidentified insectivorous bat in the Russian Far East Citation26. Moreover, at least three other cases, where the viruses were not identified but the disease was compatible with rabies and developed after bat exposure, were reported from the Ukraine and China Citation27–Citation29. A few cases of spillover EBLV-1 infections were documented in terrestrial mammals, including domestic cats Citation30, and they represent a potential exposure risk for humans.
The EBLVs, as well as IRKV, are covered by the commercially available rabies biologics Citation31 Citation32, therefore the disease can be efficiently prevented by administration of standard rabies post-exposure prophylaxis (PEP). This is not the case for WCBV. This virus, isolated from insectivorous bat Miniopterus schreibersii in south-eastern Europe, is the most divergent member of the Lyssavirus genus, and rabies biologics are incapable of providing significant protection against it Citation32. Because of lacking surveillance, there is only one isolate of WCBV available to date. Ecology of this virus and its significance for public health are unknown. However, laboratory animals and bats, infected with WCBV, developed typical rabies and died Citation33.
A variety of bat lyssaviruses have been documented in Africa. The LBV, first documented in Nigeria in 1956 Citation34, was further isolated in many sub-Saharan countries Citation35. Moreover, in 1999 it was imported into France with fruit bats Rousettus aegyptiacus captured in Togo or Egypt Citation36. Fruit bats of several species serve as reservoir hosts for LBV, with infrequent spillover infections documented in dogs, cats, and a mongoose Citation37. The viruses, currently included into LBV, represent several divergent lineages and there is a possibility that further taxonomic efforts may facilitate separation of these viruses into two or three species Citation5 Citation38 Citation39. Another divergent lyssavirus, SHIBV, was isolated from insectivorous bat Hipposideros commersoni in Kenya in 2009. The SHIBV demonstrates similarity to MOKV and LBV, but cannot be included into any of these species Citation5. Significance of these viruses for public health is unknown however, as in the case of WCBV, they are pathogenic for laboratory animals, which develop rabies and die after intracranial or peripheral inoculation Citation5 Citation35 Citation40. Furthermore, due to their antigenic differences, they are not covered by current rabies biologics Citation32 Citation41.
Recently, serologic reactivity to WCBV was detected in Miniopterus bats of several species from Kenya Citation42. Given that WCBV does not cross-react serologically with other known lyssaviruses, this seroprevalence indicates that WCBV or some other antigenically similar virus circulates in Africa as well (and probably more broadly, corresponding to the distribution range of Miniopterus bats).
Another African bat lyssavirus, DUVV, is covered by rabies biologics, but still kills people because of insufficient knowledge, either in general public and health professionals. The DUVV is perhaps the most mysterious African lyssavirus. Of four isolates available, three came from humans who died of rabies after bat exposures and only one was isolated from an insectivorous bat, presumptive Miniopterus sp Citation43. The most recent human case occurred in 2007 in Kenya, where a Dutch tourist was attacked by a bat in a campsite of Tsavo West national park. The patient applied for medical help, but a local physician assured that bat rabies does not exist in Kenya and PEP was not administered. Several weeks later, back in the Netherlands, the patient developed rabies and died. The virus was identified as DUVV Citation44.
The discovery of ABLV in 1996 in the ‘rabies-free’ Australia was surprising. Following the discovery that flying foxes were a reservoir of Hendra virus, surveillance of these animals was increased. During this activity, ABLV was identified first in a sick black flying fox (Pteropus alecto). The second case was diagnosed retrospectively in another bat of the same species, sampled in 1995 with signs of unusual aggressiveness Citation45. Later ABLV was documented in each of the four flying fox species, present in continental Australia. Furthermore, a genetically divergent variant of ABLV was discovered in insectivorous bats Saccolaimus flaviventris Citation46.
Two human cases of ABLV infection have been documented to date. Both were fatal and clinical symptoms were compatible with rabies. The first one was reported very shortly after the virus discovery in 1996. The patient was a 39-year-old female presumably infected by a S. flaviventris bat in her care. The virus that was isolated was compatible with this bat species Citation46 Citation47. The second case occurred in a 37-year-old female who developed rabies in 1998, approximately 27 months after presumable exposure from a bite by an unspecified flying fox. This isolate belonged to the pteropid ABLV variant Citation48 Citation49.
Filoviruses in bats
Filoviruses, such as Lake Victoria Marburg virus (MARV) and Ebola virus (EBOV), cause severe hemorrhagic fever with a high fatality case rate in humans (80–90%). Furthermore, they are easily transmitted between humans, and several significant outbreaks were reported from sub-Saharan Africa Citation50–Citation52. The index cases of MARV infection occurred during 1967 among laboratory workers in Germany and the former Yugoslavia, who handled tissues and blood of African non-human primates Citation53. However, the natural reservoirs of filoviruses have been unknown for many years, in spite of significant international efforts to determine their natural relationships. Until recently, these viruses were identified only in moribund humans and apes. The situation changed between 2001 and 2005, when antibodies to EBOV were detected in four species of tree-roosting fruit bats from Gabon: 4 of 17 Hypsignathus monstrosus, 8 of 117 Epomops franqueti, and 4 of 58 Myoncteris torquata. Viral RNA was detected in the liver and spleen of other bats from the same populations: 4 of 21, 5 of 117, and 4 of 141, respectively Citation50. However, no direct link between human disease and bat exposure could be established. More recently, an epidemiologic investigation putatively linked the index case of EBOV outbreak in the Democratic Republic of the Congo (DRC) in 2007 to a contact with freshly killed fruit bats, which were migrating in close proximity to the outbreak villages and represented an important food source for local people Citation54.
Furthermore, retrospective analysis demonstrated that the majority of human cases of MARV infection could be linked to visitation of caves and mines. Recently, it was re-iterated by fatal cases of MARV infection in tourists who visited caves in Uganda where multiple bats were present Citation55 Citation56. Surveillance of a variety of animals, collected in the Durba mine (DRC) during the MARV outbreak, demonstrated the presence of MARV RNA in insectivorous bats from the Rhinolophus and Miniopterus genera and in Egyptian fruit bats (Rousettus aegyptiacus), but not in animals from many other taxa including vertebrates and invertebrates Citation51. Similarly, MARV RNA was detected in R. aegyptiacus from Gabon, Uganda, and Kenya, whereas in other bat species it was detected only occasionally. Moreover, in Uganda, infectious virus was isolated from R. aegyptiacus with a high RNA load detected by the polymerase chain reaction (PCR). Gene sequences of MARV strains identified in bats were identical to those in humans Citation52 Citation57 Citation58.
However, the detailed ecology of filoviruses is still unknown. Reports on seroprevalence in bats are somewhat controversial. Colonies of R. aegyptiacus in caves often consist of tens of thousands of bats (). The opportunity for conspecific exposure rates in such colonies appears quite high and, therefore, bat populations should have a significant seroprevalence rate to these viruses. For example, seroprevalence to lyssaviruses in some colonial bat species was reported as high as 60–70% Citation35. In contrast, the seroprevalence of MARV neutralizing antibodies in colonies of R. aegyptiacus where PCR-positive bats were collected was only approximately 12% or as low as 2.4% Citation52 Citation57. It is still unclear whether bats are the principal reservoir hosts of filoviruses, or if they represent a spillover infection from some other source. In fact, the identity of gene sequences from bat and human isolates does not necessarily mean that humans were infected from bats. Potentially, bats and humans could be independently and simultaneously infected from some other source in mines and caves.
Fig. 2. A dense colony of the Egyptian fruit bats (Rousettus aegyptiacus) in cave (Photo by Ivan V. Kuzmin).

Presently, it appears that the likelihood of filovirus spillover into humans is limited. Nevertheless, as transmission mechanisms and the sources of such spillover infections are poorly understood, public awareness must be increased, and health authorities informed about the documented and suspected presence of filoviruses in bats. Enhanced ecological and epidemiological study of wildlife, such as bats and their associated pathogens, will assist in the eventual prevention and control of these highly pathogenic EIDs.
Henipaviruses and other paramyxoviruses: a recent emergence with severe consequences
In 1994, at the Brisbane suburb of Hendra, Australia, infection with a previously undescribed member of the Paramyxoviridae family caused the deaths of 13 horses and one human from an acute respiratory disease Citation59 Citation60. The virus, known initially as equine morbillivirus, was later renamed Hendra virus (HeV). Thereafter, in 1995, a farmer from Mackay, in Queensland, developed fatal HeV encephalitis, attributed to exposure to two HeV-infected horses that had died more than a year ago Citation61 Citation62. The HeV outbreak stimulated enhanced surveillance to find the natural reservoir of the virus. Fruit bats (Pteropus spp.) were found to have a high seroprevalence to HeV, indicating that they may be a wildlife reservoir. Serological evidence of HeV infection has not been found in any animal species, other than bats in the Pteropus (so-called flying foxes) genus Citation63 Citation64. As was demonstrated experimentally, Pteropus bats develop subclinical infection after inoculation with HeV with transient viremia Citation65 Citation66.
Another related paramyxovirus, Nipah virus (NiV), was first recognized in a large human outbreak that affected 283 persons and caused 109 deaths in Malaysia during 1999. The outbreak was preceded by a large NiV outbreak among pigs, which resulted in a culling of over a million swine Citation67 Citation68. Genetic similarity between NiV and HeV suggested creation of the Henipavirus genus within the viral family Paramyxoviridae Citation69 and a search of NiV reservoir among fruit bats. Antibodies against NiV virus were identified in two native Pteropus species in Malaysia Citation70. The virus was subsequently isolated from urine samples from a P. hypomelanus colony on Tioman Island Citation71. The initial porcine outbreak was thought to be caused by transmission of NiV from fruit bats to pigs. One of the scenarios suggested that an infected fruit bat might drop a piece of contaminated fruit within a pig sty or, alternatively, an infected sick or dead bat might be eaten by pigs.
Further antibodies to NiV were detected in Pteropus bats from Cambodia, Thailand, China, and Bangladesh Citation72–Citation75. In Bangladesh, several severe outbreaks of NiV encephalitis in humans were documented starting from the early 2000s, with case fatality rates about 70–90%. In a few initial outbreaks, contacts with sick livestock were suggested as a source of the infection, whereas for other outbreaks direct transmission of NiV from Pteropus giganteus bats to index cases was suggested via consumption of contaminated fruits or drinking of contaminated date palm sap with further human-to-human transmission Citation74 Citation76 Citation77.
In 2001, an outbreak of febrile illness in humans, associated with an altered sensorium, was observed in Siliguri, India. Laboratory investigations at the time of the outbreak did not identify an infectious agent. Because Siliguri is in close proximity to Bangladesh, where outbreaks of NiV infection were recently described, clinical material obtained during the Siliguri outbreak was retrospectively analyzed for evidence of viral infection. The presence of NiV antibodies and RNA were detected in ∼50% of the patients. As in Bangladesh, direct human-to-human transmission was observed between family members of the patients and hospital staff Citation78.
The distribution of Pteropus bats is limited to islands of the Pacific and Indian Oceans and continental areas from Pakistan east across Southeast Asia to Australasia. By inference, this area might be considered enzootic for henipavirus distribution. However, recent discoveries changed this interpretation. Antibodies to NiV were detected in several fruit bat species in Madagascar including Pteropus rufus, Eidolon dupreanum, and Rousettus madagascariensis. The two latter species are not members of Pteropus genus and their exposure to NiV might occur via contact with P. rufus Citation79. However, later seroprevalence to henipaviruses was detected in 22–39% of Eidolon helvum fruit bats from Ghana, out of the Pteropus genus range Citation80. Moreover, divergent henipavirus RNA was detected in fecal samples of these bats. One of the obtained gene sequences was most related to NiV and another two represented novel genetic lineages within the Henipavirus genus Citation81. This is remarkable because E. helvum is highly abundant in sub-Saharan Africa and form large colonies, which conduct annual transcontinental migration following the rainfall gradient to suitable feeding grounds Citation82. These animals frequently roost in urban settings and, in several African countries, are routinely hunted and consumed by humans as a supplementary source of protein.
During the search for NiV on the Tioman Island, two other bat viruses were isolated in addition to the NiV, which was the main target. These were Tioman virus and Pulau virus Citation83 Citation84. Tioman virus was a novel paramyxovirus in the genus Rubulavirus, whereas Pulau virus was a novel reovirus in the genus Orthoreovirus. At the time of discovery, both viruses were orphans in terms of their significance for veterinary and public health.
In 1997, during the investigation of a swine disease outbreak a new paramyoxvirus, named Menangle virus, was isolated from stillborn piglets with deformities at a large commercial piggery in New South Wales, Australia Citation85. Serological investigation of persons in contact with pigs revealed that two humans, who were in close contact with infected pigs and suffered an influenza-like illness, had high levels of neutralizing antibodies to Menangle virus Citation86. Antibodies to Menangle virus were detected in all four species of flying foxes in Australia Citation87.
Molecular and antigenic studies indicated that Tioman virus is very closely related to Menangle virus, which indirectly confirmed the bat origin of Menangle virus Citation83 Citation88. The potential of Tioman virus to infect and cause disease in human or other animals is unknown. However, recent studies have demonstrated that pigs are susceptible for infection by Tioman virus Citation89 and that there is serological evidence for infection of humans by this virus on Tioman Island Citation90.
SARS-like and other coronaviruses in bats
Severe acute respiratory syndrome (SARS) emerged during November 2002 in southern China, and a SARS coronavirus (SARS-CoV) was identified as the etiologic agent Citation91. This epidemic, and the identification of SARS-CoV in animals, associated with the wildlife trade in southern China particularly in civets and raccoon dogs Citation92 stimulated increased CoV surveillance. As demonstrated from the outbreak, none of the suspect animals were a direct source of SARS-CoV. Furthermore, surveillance led to identification of SARS-like CoVs in horseshoe bats (Rhinolophus spp) in China. These CoVs shared similar genomic organization and identity with SARS-CoV, except for the spike protein gene S, which is responsible for binding to the receptor on susceptible cell surface Citation93 Citation94. The level of nucleic acid sequence difference (∼8%) from SARS CoV in multiple genes was too great for SARS-like CoV in Rhinolophus bats to be the parent to the outbreak virus. The presence of multiple SARS CoV-like viruses, the inability to detect SARS CoV-like viruses in other species of wild animals, and the detection of a wide range of other coronaviruses in bats suggests that bats are a rich source of CoVs, however, the evolutionary pathway of SARS-CoV remains to be fully identified.
Further surveillance and characterization of bat CoVs identified close members of many known mammalian CoV species as well as several species exclusively present in the bat. These studies revealed high genetic diversity bat CoVs across a large geographic distribution. Moreover, the same species of bat from different geographic locations can also contain the same type of CoV Citation95. In China, high CoV prevalence was detected in the Vespertilionidae and Rhinolopidae families of bats. The overall prevalence was about 6%, but in certain bat colonies it was as high as 35–55%. Such diversity was significant and not only SARS-like CoVs (from Betacoronavirus, former group 2) but also additional putative novel subgroups from Alphacoronavirus (former group 1) were identified in bats Citation96. Similar results were reported from Hong Kong Citation97. Further studies demonstrated that CoV-positive bats appeared healthy, with only a limited reduction of body weight, with viral clearance occurring between 2 weeks and 4 months Citation98. In addition, the authors reported that co-infection of the same bat species by two different coronaviruses, a SARSr-Rh-BatCoV Rp3 from Guangxi, China, and a Rf1 from Hubei, China, may have allowed the opportunities for recombination via a breakpoint at the nsp16/spike region and possibly generated a recombinant virus—the Civet SARSr-CoV.
After the discovery of SARS-like CoVs in bats in Asia, a number of bat CoVs were identified in Europe, North America, South America, Australia, and Africa with an overall prevalence of 9 to 20%. In Europe, the alphacoronaviruses and betacoronaviruses, identified in Vespertilionidae bats, were genetically similar to the CoVs identified in bats from China Citation99. In the United States, 17% of Eptesicus fuscus and 50% of Myotis occultus were positive for CoVs. Phylogenetically these viruses belonged to the same alphacoronavirus group but formed distinct clusters from Asian CoVs Citation100. In Canada, an alphacoronavirus identified in Myotis lucifugus bat is probably a variant of alphacoronaviruses identified in Myotis occultus in the United States. In South America, the Trinidadain CoVs identified in Phyllostomidae bats were clustered with alphacoronavirus from North America. In Africa, enhanced surveillance demonstrated significant divergence of CoVs in bats from Kenya Citation101. In that study, SARS-like CoV was identified in a Chaerephon sp. bat (Molossidae). Furthermore, in contrast to China and Hong Kong, various CoVs were detected in bats from the families Hipposideridae and Pteropidae. Overall CoV prevalence in Kenya bats was approximately 19%, and CoV diversity was greater compared to that documented in Asia, Europe, North and South Americas, and Australia.
In general, the diversity of CoVs in bats appears greater than in other animals tested to date. This observation suggests that bats are likely the primary hosts of this viral family. Because attempts to isolate CoVs from bats by multiple international groups have failed and only viral RNA was detected (predominantly in fecal swabs), this limitation significantly reduces the possibility to investigate CoV pathobiology, evolution, and adaptive mechanisms in vitro and in vivo.
Bats: are they special?
Why are bats the reservoirs of so many EIDs? Bats have several unique features that may account for their importance in EID transmission and maintenance. Bats are the second largest order of mammals. Currently, there are ∼1,200 recognized bat species worldwide, accounting for approximately 25% of all mammalian species Citation102. The diversity of bat species alone, along with their worldwide distribution, contributes to the biodiversity of their pathogens.
Bats are unique in their mobility as they are the only mammals capable of flight, allowing them to transmit EIDs during their foraging flights and during seasonal migrations. This extensive mobility, coupled with roosting plasticity and broad food range, means that bats could transport viral material to many different animal species in various locations per unit time Citation103.
The ability to fly also has immunological implications. Flight requires a low body mass and bats have evolved to have hollow bones to decrease their body mass. The hollow bones allow them to fly, but as a result they do not have bone marrow as similar to non-volant mammals and must produce B-cells in different locations Citation104. Whereas basic immunological commonalities are shared among all mammals, certain unique anatomical and physiological parameters peculiar to bats may also help to explain the plethora of agents associated with this mammalian order.
Besides their ecological vagility, bats are considered one of the most social groups of mammals (). Many bat species roost together in very large and dense colonies. This dense clustering of individuals provides ample opportunities for viral exchange within bat populations Citation103. Bats with high levels of interspecies contact, such as Myotis, have been found to harbor a diverse range of RABV, suggesting that increased contact between species increases viral transmission Citation105. Several infectious agents, including NiV, have been isolated from the urine of fruit bats and during mutual grooming fur contaminated by urine may allow for viral transmission between individuals Citation68 Citation106.
Regarding ecological flexibility, bats inhabit a wide variety of ecological niches. Some species are flexible in roost preferences, including caves, trees, and many man-made structures, other are more restricted to specific roosting. The ability of bats to occupy men-made structures is of particular importance, because it increases the opportunities for interactions between bats, domestic animals, and humans. For example, the big brown bat (Eptesicus fuscus) and the serotine bat (Eptesicus serotinus), both of which are known to harbor lyssaviruses, commonly roost in men-made structures. Bats often inhabit and feed in agricultural areas, which brings them into closer contact with humans and domesticated animals. In the tropics, frugivorous bats can be found roosting urbanistically and feeding on fruit trees in plantations Citation107.
Not only are bats able to inhabit a variety of diverse locations, but they also have a number of trophic specializations. The majority of bat species are frugivorous or insectivorous. In addition, three bat species, all found in Central and South America, are hematophagous (vampire bats). These dietary habits affect rabies transmission risks as described above. Depletion of environmental resources and urban expansion into bat habitats can deplete natural food sources. When their natural food sources are scarce, vampire bats will switch preferences and feed on humans and domestic animals Citation14. Frugivorous bats often leave behind half-eaten fruits that may be contaminated with viral particles from their saliva; if the viral levels are high enough, an animal may develop infection following consumption of these fruits. It is hypothesized that the consumption of half-eaten fruits may have caused the transmission of NiV from fruit bats to pigs and humans, as well as sharing of the raw date palm sap from the tree collectors Citation67 Citation68 Citation76 Citation77. Similarly, insectivorous bats will discard contaminated insect parts, which can then be consumed by foraging animals Citation107, although mechanisms for such route of pathogen transmission from insectivorous bats has not been corroborated to date. Omnivorous bats, such as Phyllostomidae, will consume nectar, plants, arthropods, and small vertebrates as food sources as necessary. This ability to utilize a wide variety of food sources may lead to increased biodiversity in a small area, enhancing the opportunities for multiple species to interact and share infectious pathogens. Environmental factors can shape pathogen transmission and spillover into a new species as well; periods of resource limitation may bring together diverse species. During the dry season, primates and bats may come into closer contact as they search for limited food supplies, enhancing opportunities for cross-species transmission of filoviruses Citation107.
Although their mobility, sociality, and ability to inhabit a variety of niches likely influence the importance of bats as sources of EIDs, there are some additional characteristics of bats that may contribute into this phenomenon. For example, Microchiroptera possess the ability to echolocate, to produce laryngeal vocalizations for navigational purposes. Echolocation may cause aerosolization of viral particles in the nasal mucosa and saliva, enhancing transmission to other individuals Citation4. However, this mode of transmission has not been experimentally verified to date. Additionally, when adjusted for body mass, Chiroptera are the longest-living mammalian order Citation108. Although long-term viral persistence in bats remains to be determined, a long-lived carrier would have even more opportunities to transmit the infection within bats populations and to other species.
The long evolutionary history of bats may also play a role in their association with EIDs, because of long co-evolution between bats and the viruses. Pathogens could have evolved to utilize cellular receptors that are conserved across a wide range of animal species, providing a mechanism for interspecific infections Citation4 Citation109. For example, henipaviruses are capable of infecting species in six mammalian orders and SARS-CoV uses an enzyme receptor that is conserved among animals Citation109.
The specific immunological parameters that are involved in agent evolution leading to persistence or perpetuation of EIDs in bat populations are not fully understood. Recent studies have examined innate, antiviral, and interferon genes from several species of bat, and suggest that certain alleles may be associated with increased parasite burden Citation110. Cells expressing surface immunoglobulin were identified in Pteropus indicating that lymphoid development in bats, as well as immune system components, like IgG, IgM, IgA, macrophages, B- and T-lymphocyte-like cells, are similar to other mammals Citation111 Citation112. In studies examining leukocyte response to the phytohemagglutinin (PHA) skin test, a technique used to measure delayed-type cellular immune response in many vertebrates, diverse leukocyte traffic was observed in the 6–24 hours following PHA injection Citation113. Bat interferon alpha and beta are homologous to other mammalian interferons, but there is low homology of these interferons specifically between bats and humans, which could indicate different antiviral activity between the two and contribute to the high pathogenicity of bat agents in humans Citation114.
In addition, recent sequencing of genome fragments to infer genes within the interferon alpha family in both Pteropus and Myotis bats has revealed that both have up to 24 IFNW genes, while humans, mice, and pigs have only one Citation115. The enormous size of this gene family within bats compared to other mammals suggests that it may still be involved in host immune defense, even though its function may have been lost in other vertebrates. He et al. Citation116 suggested that the bat interferon alpha gene family is under positive selection, which most likely reflects an evolutionary arms race, between pathogens evolving to block immune recognition and host immune systems responding to maintain effective response to these pathogens. However, based on latitude, some bats undergo hibernation during winter, which has been shown to decrease levels of neutrophils, monophils, and lymphocytes, leading to immunosuppression in other small mammals Citation117. If so, how do related infectious agents overwinter in bats? These and many other questions about basic bat immunology and pathobiology of bat-adapted pathogens still remain unanswered. Serological assays have shown though that some virus specific adaptive T- and B-cell responses do occur, despite the suggestion of persistent infection with viruses including HeV, SARSs-CoV, and EBOV (reviewed in Calisher et al. Citation4). In addition, bats are capable of harboring large numbers of genetically diverse viruses within a geographic location and within a taxonomic group Citation118. Several viruses for which bats act as a reservoir, including the paramyxo-, filo-, and rhabdoviruses, appear phylogenetically related and grouped in the order Mononegavirales, possibly indicating a more fundamental connection between bats and these specific RNA agents Citation119.
Persistence or perpetuation of bat-associated EIDs
The essential pathobiology of bat agents contributes inherently to their persistence or perpetuation in reservoir individuals, colonies, and populations. Based on current research, there is very little evidence to suggest that any of the major bat-associated EIDs persist within the host, and it is therefore most likely that these viruses are maintained in nature by perpetuation within and between bat colonies and through multiple spillover events into other hosts due to the extreme mobility and highly social nature of the bat hosts. Among these various agents, lyssaviruses have been most thoroughly characterized. For example, RABV, the representative species of this genus, perpetuates through bite transmission between infected animals. In essence, RABV is characterized by a rather low basic reproductive rate and a short infectious period. Bats mount both an innate and adaptive immune response to peripheral RABV infection. Helper and cytotoxic T-cells activate upon infection, to recognize and clear the virus both outside and inside of infected cells. However, once the virus reaches the CNS, the host adaptive immune response is less able to clear infection.
Pathogenic RABV may limit its replication rate and produce fewer infectious particles to completely evade, or only minimally activate, the peripheral host response. Single exposures do not always confer protection against successive infections, leading to perpetuation in bat populations, but repeated exposure has been shown to provide long-term immunity (up to a year) and reduced susceptibility Citation120. These findings have been corroborated by other studies that show that colony-wide mortality does not increase significantly after episodes of infection with EBLV-1 Citation121.
In affected bat colonies, a relatively low point prevalence of rabies infection has been usually observed, varying from <1 to 4%. In contrast, prevalence levels of RABV-neutralizing antibodies have been documented between 65 and70% (reviewed in Kuzmin and Rupprecht Citation7). In bats, cave colonies show strong seasonal fluctuations with increased seroprevalence in adult females and juveniles directly following parturition Citation122. These seasonal shifts may be due to the birth pulse, adding large numbers of susceptible juveniles into the population and increased contact rates between adult females and pups while nursing. Recently, employing a model that integrated immunological parameters, epizootiology, and disease demography, Dimitrov et al. Citation123 showed that total colony immunity is actually strengthened by perpetuating RABV infection. This model predicted that low removal rates of infected individuals (due to death) led to a colony with a stronger total immune profile while high removal rates, like that seen in other carnivores, led to the epizootics normally associated with RABV.
Little is known about how NiV and HeV are maintained in bat populations. These viruses encode V proteins that bind to signal transducer and activators of transcription 1 (STAT1) and STAT2 proteins of host cells to block alpha, beta, and gamma interferon responses Citation124–Citation126. Such V proteins may facilitate evasion of the host immune system, although it is not well known how viral proteins will affect potential interferon responses to virus infections in bats. The NiV V protein can prevent IFN signaling in cells from multiple species and other proteins may also have specific activities Citation126. The presence of multiple anti-IFN mechanisms may relate to the zoonotic nature of henipaviruses but this remains to be explored in Pteropodid bats, which are seemingly natural reservoir of these viruses have likely passed a long-term co-evolution.
Ecological studies suggest that NiV spillover events may fluctuate seasonally. All outbreaks, with the exception of the initial spillover event in Malaysia, occurred during the first 5 months of the year Citation74–Citation78. Wacharapluesadee et al. Citation127 found that this time period coincided with the time in which the greatest amount of viral RNA could be recovered from wild populations of Pteropus lylei. Horizontal transmission via urine, feces, and saliva is thought to be the primary route of intra-specific and spillover infection for HeV Citation128 Citation129. Spillover events of HeV into horses are associated with the flying fox birthing season, when pregnant and lactating females are at a higher risk for HeV infection (reviewed in Halpin et al. Citation130). These events are hypothesized to occur through contact with either infected birthing material or exposure to an increased number of infected individuals. Models of HeV infection dynamics suggest that the pathogen is not endemic in local populations, but persists broadly due to meta-population dynamics. Further, it has also been hypothesized that immunity in Pteropus scapulatus, the principal reservoir of HeV, wanes over short time scales and this could enhance the persistence of infection in P. scapulatus populations. Nutritional stress in response to decreases in fruit and nectar availability has also been associated with increasing risk of transmission Citation128 Citation129.
In fact, bats were not the direct origin of the human SARS epidemic but the diversity of bat coronaviruses is fascinating. Modes of perpetuation of CoVs have not been established, but an increase in prevalence within lactating adult female bats has been demonstrated for several vespertilionid species Citation99. As has been recently shown, bats experimentally infected with CoV did not develop clinical signs of disease, although viral RNA was detected in their intestines and feces. In addition, reduced susceptibility of bats to a CoV isolated from another bat species has been demonstrated, suggesting that certain CoV variants are well adapted to certain host species Citation131.
Although both EBOV and MARV are hypothesized to have a bat reservoir, no conclusive evidence has been obtained to date. Though it is not known if or how EBOV persists in bat populations, trends in great ape mortality suggest the seasonal component. Death rates of great apes have been observed to increase at the end of the rainy season, which may lead to increased contact rates between apes and other animals, including bats, competing for food Citation132–Citation134. However, contacts between bats and apes have also been observed during the dry season when fruit is abundant and many animals share the same food source locations Citation50 Citation135.
Making ourselves sick: drivers of bat-associated EIDs
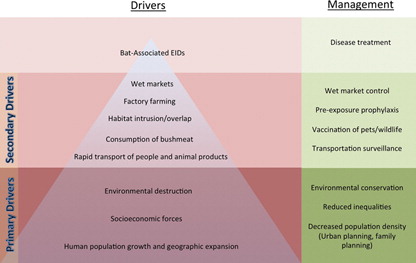
As with other zoonotic EIDs, emergence of diseases from bat reservoirs is primarily associated with ecological changes that influence the host or parasite. More specifically, changes that increase the duration or frequency of host-pathogen interaction give rise to greater opportunities for transmission Citation119. The underlying causes of bat EIDs can be organized in a hierarchical fashion () as macro-scale societal changes lead to increased animal/human interactions, which in turn lead to increased disease emergence. Bat-associated EIDs appear as a ‘tip of the iceberg’ regarding a much more dynamic complex of interacting variables.
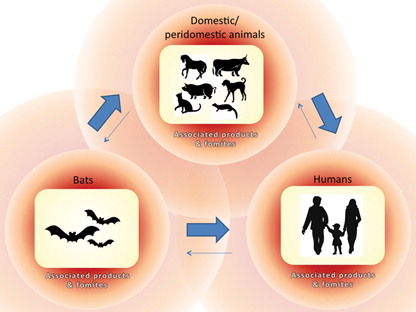
The pathways of disease transmission between bats, peridomestic/domestic animals, and humans are summarized in . Although we are predominantly concerned with human pathogens, it should be noted that infectious diseases emergence also occurs in non-human hosts, including bats themselves, as a result of ecological changes brought by human activity. For example, the recent epizootic of White Nose Syndrome (WNS) among several bat species in the Northeastern United States may be due to the translocation of a fungus from Europe to North America by humans Citation136. Furthermore, it is highly probable that the emergence of pathogens in bats also occurs via contact with domestic/peridomestic animals. However, evidence in support of this pathway is limited due to of lack of research in this area.
Fig. 4. Possible routes of disease transmission between bats, peridomestic/domestic animals, and humans. Thick arrows represent the most significant pathways for bat-associated EIDs. Thin arrows represent pathways about which less known or that are less common (as in the case of transmission of pathogens directly from bats to humans).
The primary drivers of bat-associated EIDs include overpopulation, environmental degradation, and socioeconomical forces. The emergence of new pathogens is associated with growth and increased density of humans and other mammals. In recent history, the human population has exploded, with an increase from 1 billion to 6.8 billion in the past 110 years. As populations grow, humans begin to inhabit previously untouched, often biodiverse areas. Research suggests that diseases are more likely to emerge in such regions Citation2. Coupled with this is the increased utilization of natural resources to meet the human demand for food and support consumption of goods.
Environmental degradation causes habitat disturbance and reduction, resulting in changes in species range and density. For example, land use changes such as mining and deforestation for farming and the construction of human habitats in the Amazon Basin have likely contributed to the re-emergence of vampire bat-derived rabies in humans. In 2004, at least 46 deaths were caused by vampire bat rabies (predominantly in Brazil and Colombia), whereas only 20 cases were transmitted by dogs in all of Latin America Citation137 Citation138.
Economic forces unquestionably fuel environmental destruction. Much of the deforestation and habitat intrusion in the Amazon, for example, is a result of the increasing demand for oil and minerals. Moreover, economic forces contribute to secondary drivers such as increased transportation, agricultural practices, food availability, and choice regarding food consumption. Socioeconomic inequalities, meanwhile, perpetuate disease transmission through disparities in health care access, education, food security, and access to clean water. In addition, the increase in human population density and mobility, together with environmental destruction and economic forces, are also contributing to pathogen emergence. These primary drivers not only contribute to risk by increasing the abundance of human hosts, but also fuel more immediate causes of disease emergence such as those discussed below.
Economic prosperity has led to advances in technology, which has fostered the development of an expansive global trade network. Increased mobility of people, animals, and goods allows for the rapid spread of novel diseases and outbreaks of existing diseases. The cross-continental spread of infectious disease is most likely to occur through air travel Citation139, while local transportation networks may be important for sustaining epidemics within continents Citation140. For example, the SARS outbreak was first reported in Guandong, China in November of 2002 and within months a cluster of cases appeared in Hong Kong Citation141. Due to air transit, SARS cases were reported as far away as Canada (in addition to several Southeast Asian countries) by the end of March of 2003 Citation142. As previously mentioned, the spread of WNS throughout bat populations may serve as an example of how human movement also influences the emergence of EIDs in wildlife Citation136.
Large societal changes have led to smaller, more regional phenomena that increase the emergence of infectious diseases. For example, ecotourism likely increases the rate of disease exposure through the direct intrusion of humans into wildlife habitats (such as recent cases of MARV infection in tourists after visitation of caves in Africa Citation55 Citation56, or DUVV infection of a tourist bitten by a bat during a safari trip Citation44).
Human practices surrounding the production and consumption of food can contribute significantly to the risk of new pathogen emergence. For example, wet markets (in which live animals are sold and butchered on the spot) are an ideal environment for microbial exchange due to the high density of people, and the diversity of wild and farmed animals sold at these sites. Live animal markets appear to have contributed to the emergence of SARS in China in 2002. With primary bat origin, several intermediate hosts have been suggested (such as Chinese ferret-badgers, Melogale moschata, and raccoon-dogs, Nyctereutes procyonoides), although palm civets (Paguma larvata) are suspected to be the most important for the transmission of the virus to humans Citation92. In Southern China, civets are both hunted and farmed for eating. In fact, civets tested in wet markets have a higher rate of seropositivity for SARS than those tested on farms, suggesting that the markets may serve as centers for viral transmission Citation143. In addition, food handlers and persons employed at wet markets are more likely to be seropositive than those with other occupations Citation144.
Consumption of bush meat is known to amplify the risk of pathogen emergence and this is also true for the transmission of bat-associated EIDs. Epidemiological evidence suggests the direct transmission of EBOV from bats to humans in the Democratic Republic of Congo, where the migratory fruit bats, Hypsignathus monstrosus and Epomops franqueti, are hunted and sold in markets for consumption Citation54. Additionally, Eidolon helvum fruit bats, a natural reservoir for LBV and henipaviruses, are consumed in several regions of West Africa Citation80.
Furthermore, agribusiness has overshadowed small-scale farming across the globe, particularly in Southeast Asia. Industrialized agriculture involves the mass-production of a single species of animal or plant. Monoculture increases susceptibility to pathogens due to the widespread availability of hosts of the same species. This is exemplified by the NiV outbreaks in Malaysia during 1998–1999. Deforestation and the increased farming of pigs and fruit-producing trees are suspected to have contributed to the swine infection with NiV. Of course, the intensification and expansion of agriculture is a serious concern throughout the world, as it is a driver of many other EIDs (not associated with bats), the most notorious of which are the avian influenza viruses.
Ironically, the achievements of human civilizations over the past century have been the indirect drivers of new classes of zoonotic diseases. These issues are not only difficult to solve, but they are also expensive and politically unpopular to address, as human economic progress is often in inherent conflict with environmental conservation.
Pathogen emergence occurs as a result of complex interactions of many factors, requiring a multidisciplinary approach to research and prevention. The drivers of disease emergence are studied by professionals trained in fields as diverse as disease ecology, anthropology, geography, economics, wildlife population biology, and wildlife veterinary medicine Citation1–Citation3. A better understanding of drivers of EID emergence is essential to inform effective policies that address both the immediate and underlying causes of disease emergence.
Control and prevention of bat-associated EIDs
The previous section focused on the mechanisms that drive disease emergence from bats, progressing from primary to more proximate factors. This section will examine control in the opposite direction, progressing from the individual level, proceeding through population-wide approaches, and ending with society-wide suggestions for primary prevention of bat-associated EIDs.
At the individual level
No specific medical therapy has proven beneficial once people become ill from bat EIDs (at least of viral origin). For example, although rabies is an ancient disease, effective therapeutic treatment of rabies in humans continues to be very challenging. Rapid early diagnosis in the biting animal is critical, since identification of rabies before its fulminant stage allows for effective prophylaxis. Fulminant rabies continues to carry a very poor prognosis. The first case of the successful experimental treatment of rabies in a naïve patient was a 15-year-old girl bitten by a bat in 2004 Citation145. However, extension of the ‘Milwaukee Protocol’ (i.e., therapeutic coma, antiviral drugs, intensive medical care) in other patients has been much less successful (see for example Rupprecht Citation146 and Rubin et al. Citation147).
Prophylaxis, after exposure but well in advance of illness, has a much higher success rate. Appropriate post-exposure wound cleansing has been shown to reduce significantly the likelihood of RABV transmission Citation148. Besides washing the wound with soap and water, unvaccinated persons should receive both rabies immune globulin and four doses of cell-culture vaccine. Globally, more than 12 million persons receive post-exposure prophylaxis each year Citation149.
Besides rabies, novel treatment strategies are being developed for other bat EIDs. The use of RNA interference has been suggested for the treatment of henipaviruses Citation150. These currently untreatable infections may be ameliorated by the introduction of small interfering RNA molecules homologous to the RNA in these pathogens. While promising in theory for many agents, this line of treatment is still in its preliminary stages, and issues such as efficacy in humans, delivery, and cost have yet to be addressed.
The potential for filoviruses to be used as bioweapons has spurred research efforts for an effective vaccine that could be used in an outbreak. For example, in a mouse model of hemorrhagic EBOV infection, a vesicular stomatitis virus-based vaccine has been shown to be safe and effective in preventing clinical presentation of disease Citation151. Furthermore, the possibility that this vaccine may be deliverable through mucosal surfaces offers potential as a rapid vaccination agent during an outbreak.
At the population level
At the population level, rabies is the quintessential bat EID that has been studied most intensively. Public health guidelines recommend rabies vaccination for humans in high-risk groups, vaccination of pets as well as animals on public display, isolation of domestic animals from the wildlife reservoirs of rabies, and public health education on appropriate precautions. Current guidelines recommend that pre-exposure prophylaxis be offered to those in high-risk groups including veterinarians, animal handlers, rabies researchers, and some laboratory workers. In addition, the vaccine can be offered to long-term travelers to endemic areas, especially if immediate medical attention will be unavailable Citation148 Citation152. Routine vaccination of the general population is currently not recommended, mostly due to cost.
Despite advances in determining best practices for animal vaccination, control of rabies in domestic and wild reservoirs remains challenging in resource-limited settings. Control of rabies in bats has proven challenging. Bat rabies has been reported in every state except Hawaii and 1,806 rabid bats were documented in the United States during 2009 Citation19. Of all animals, bats in particular pose a serious risk for rabies and should be excluded from structures to prevent contact with humans Citation148 Citation152. However, widespread reductions in bat populations to control rabies is neither feasible nor desirable. Instead, some novel methods have been explored to control infection in bat populations. Vampire bats can efficiently digest only coagulated blood and they die if the consumed blood is not coagulated. Application of anticoagulant-containing ointment on the fur of captured vampire bats (with their subsequent release) leads to consumption of the coagulant by several roost mates via mutual grooming. Similarly, anticoagulation of livestock is another useful approach to control vampire bat populations where rabies is a threat (reviewed in Kuzmin and Rupprecht Citation7). As another approach, it has been suggested that oral vaccination of wildlife may limit the spread of rabies by bats Citation153. Finally, we know that some species of moths are able to disrupt bat echolocation using ultrasonic clicks of their own Citation154 Citation155. The use of similar, artificially produced, sounds to ward off bats from human and livestock habitats should be explored.
At the societal level
The recent emergence of SARS coronavirus and Henipavirus from bat reservoirs has spurred thinking on how to control future disease emergence. As we noted in the previous section, the primary drivers of emergence are growing global mobility, environmental degradation, and overpopulation.
We may be able to confront the threat presented by increased global mobility with practical measures such as transportation surveillance. Monitoring ports and borders for ill passengers and animals and providing care to them would not only benefit the ill, but also the populations they are moving into. Likewise, pre-trip vaccinations and post-trip health monitoring not only benefit the international travelers but also the population to which they return.
Environmental conservation has long been the domain of those hoping to preserve biodiversity and the magnificence the natural world. Given the emerging evidence that environmental degradation leads to increased rates of disease emergence, it may be time for those in the public health field to also advocate environmental conservation. Since the effects of environmental degradation on disease emergence are still not fully understood, increased funding of research in this field is also sorely needed.
Conclusions
The international attention to newly emerged or discovered bat-associated EIDs has increased dramatically during recent decades. The above brief review of recent history highlights that pathogen discovery has been accomplished in very different scenarios, from accidental detection of ‘orphan’ viruses to the confirmation of bat origin of known diseases using targeted surveillance. Moreover, although this brief review has focused upon the relationship of bats and emerging viruses, a number of other diverse agents are also associated with bats including bacteria such as Bartonella Citation156, and long-standing relationships with certain fungal diseases such as histoplasmosis Citation157. It is anticipated that, with the advance of modern molecular tools and increased scientific activities in this field, additional bat EIDs with public health, veterinary, and conservation implications will be uncovered and better understood with practical effective prevention and control modalities necessary for application in the near future.
Disclaimer
Use of trade names and commercial sources are for identification only and do not imply endorsement by the US Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of their institutions.
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study.
References
- Woolhouse ME, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis. 2005; 11: 1842–47.
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL. Global trends in emerging infectious diseases. Nature. 2008; 451: 990–93. 10.1038/nature06536.
- Maudlin I, Eisler MC, Welburn SC. Neglected and endemic zoonoses. Philos Trans R Soc Lond B Biol Sci. 2009; 364: 2777–87. 10.1098/rstb.2009.0067.
- Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: Important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006; 19: 531–45. 10.1128/CMR.00017-06.
- Kuzmin IV, Mayer AE, Niezgoda M, Markotter W, Agwanda B, Breiman RF. Shimoni bat virus, a new representative of the Lyssavirus genus. Virus Res. 2010; 149: 197–210. 10.1016/j.virusres.2010.01.018.
- Nel LH, Rupprecht CE. Emergence of lyssaviruses in the Old World: The case of Africa. Curr Top Microbiol Immunol. 2007; 315: 161–93.
- Kuzmin IV, Rupprecht CE. Bat rabies Rabies. 2nd ed, Jackson AC, Wunner WHAcademic Press/Elsevier: London, 2007; 259–307.
- Pal SR, Arora BM, Chuttani PN, Broer S, Choudhury S, Joshi RM. Rabies virus infection of a Flying fox bat, Pteropus poliocephalus, in Chandigarh, Northern India. Trop Geogr Med. 1980; 32: 265–67.
- Smith PC, Lawhaswasdi K, Vick WE, Stanton JS. Isolation of Rabies virus from fruit bats in Thailand. Nature. 1968; 216: 384.10.1038/216384a0.
- Arguin PM, Murray-Lillibridge K, Miranda ME, Smith JS, Calaor AB, Rupprecht CE. Serologic evidence of lyssavirus infections among bats, the Philippines. Emerg Infect Dis. 2002; 8: 258–62. 10.3201/eid0803.010330.
- Reynes JM, Molia S, Audry L, Hout S, Ngin S, Walston J. Serologic evidence of lyssavirus infection in bats, Cambodia. Emerg Infect Dis. 2004; 10: 2231–34.
- Lumlertdacha B, Boongird K, Wanghongsa S. Survey for bat lyssaviruses, Thailand. Emerg Infect Dis. 2005; 11: 232–36.
- Carini A. Sur une grande epizootie de rage [On a large outbreak of rabies]. Ann Institute Pasteur. 1911; 25: 843–46.
- Constantine DG. Transmission of pathogenic organisms by vampire batsNatural history of vampire bats. Greenhall AM, Schmidt UCRC Press: Boca Raton FL, 1988; 167–89.
- Baer GM, Smith JS. Rabies in nonhemathophagous batsThe natural history of rabies. 2nd ed, Baer GMCRC Press: Boca Raton FL, 1991; 341–66.
- Pawan JL. Rabies in the vampire bat of Trinidad, with special reference to the clinical course and the latency of infection. Ann Trop Med Parasitol. 1936; 30: 401–22.
- Moreno JA, Baer GM. Experimental rabies in the vampire bat. Am J Trop Med Hyg. 1980; 29: 254–59.
- Aguilar-Setien A, Loza-Rubio E, Salas-Rojas M. Salivary excretion of Rabies virus by healthy vampire bats. Epidemiol Infect. 2005; 133: 517–22. 10.1017/S0950268805003705.
- Blanton JD, Palmer D, Rupprecht CE. Rabies surveillance in the United States during 2009. J Am Vet Med Assoc. 2010; 237: 646–57.
- De Serres G, Dallaire F, Côte M, Skowronski DM. Bat rabies in the United States and Canada from 1950 through 2007: Human cases with and without bat contact. Clin Infect Dis. 2008; 46: 1329–37.
- Messenger SL, Smith JS, Rupprecht CE. Emerging epidemiology of bat-associated cryptic cases of rabies in humans in the United States. Clin Infec Dis. 2002; 35: 738–47. 10.1086/342387.
- Gibbons RV, Holman RC, Mosberg SR, Rupprecht CE. Knowledge of bat rabies and human exposure among United States cavers. Emerg Infect Dis. 2002; 8: 532–34.
- Srinivasan A, Burton EC, Kuehnert MJ, Rupprecht C, Sutker WL, Ksiazek TG. Rabies in transplant recipients investigation team. Transmission of Rabies virus from an organ donor to four transplant recipients. N Engl J Med. 2005; 352: 1103–11. 10.1056/NEJMoa043018.
- Morimoto K, Patel M, Corisdeo S, Hooper DC, Fu ZF, Rupprecht CE. Characterization of a unique variant of bat Rabies virus responsible for newly emerging human cases in North America. Proc Natl Acad Sci U S A. 1996; 93: 5653–58. 10.1073/pnas.93.11.5653.
- Botvinkin AD, Poleschuk EM, Kuzmin IV, Borisova TI, Gazaryan SV, Yager P. Novel lyssavirus isolated from bats in Russia. Emerg Infect Dis. 2003; 9: 1623–25.
- Leonova GN, Belikov SI, Kondratov IG, Krylova NV, Pavlenko EV, Romanova EV. A fatal case of bat lyssavirus infection in Primorye Territory of the Russian Far East. Rabies Bull Europe. 2010; 33(4): 5–8.
- Botvinkin AD, Selnikova OP, Antonova LA, Moiseeva AB, Nesterenko EY. Human rabies case caused from a bat bite in the Ukraine. Rabies Bull Europe. 2006; 29(3): 5–7.
- Kuzmin IV, Botvinkin AD, Poleschuk EM, Orciari LA, Rupprecht CE. Bat rabies surveillance in the former Soviet Union. Dev Biol (Basel). 2006; 125: 273–82.
- Tang X, Luo M, Zhang S, Fooks AR, Hu R, Tu C. Pivotal role of dogs in rabies transmission, China. Emerg Infect Dis. 2005; 11: 1970–72.
- Dacheux L, Larrous F, Mailles A, Boisseleau D, Delmas O, Biron C. European bat lyssavirus transmission among cats, Europe. Emerg Infect Dis. 2009; 15: 280–84. 10.3201/eid1502.080637.
- Fekadu M, Shaddock JH, Sanderlin DW, Smith JS. Efficacy of rabies vaccines against Duvenhage virus isolated from European house bats (Eptesicus serotinus), classic rabies and rabies-related viruses. Vaccine. 1988; 6: 533–39. 10.1016/0264-410X(88)90107-7.
- Hanlon CA, Kuzmin IV, Blanton JD, Weldon WC, Manangan JS, Rupprecht CE. Efficacy of rabies biologics against new lyssaviruses from Eurasia. Virus Res. 2005; 111: 44–54. 10.1016/j.virusres.2005.03.009.
- Kuzmin IV, Franka R, Rupprecht CE. Experimental infection of big brown bats (Eptesicus fuscus) with West Caucasian bat virus (WCBV). Dev Biol (Basel). 2008; 131: 327–37.
- Boulger LR, Porterfield JS. Isolation of a virus from Nigerian fruit bats. Trans R Soc Trop Med Hyg. 1958; 52: 421–24. 10.1016/0035-9203(58)90127-5.
- Kuzmin IV, Niezgoda M, Franka R, Agwanda B, Markotter W, Beagley JC. Lagos bat virus in Kenya. J Clin Microbiol. 2008; 46: 1451–61. 10.1128/JCM.00016-08.
- Aubert MFA. Rabies in individual countries, France. Rabies Bull Europe. 1999; 23(2): 6.
- Markotter W, Kuzmin I, Rupprecht CE, Randles J, Sabeta CT, Wandeler AI. Isolation of Lagos bat virus from water mongoose. Emerg Infect Dis. 2006; 12: 1913–18.
- Markotter W, Kuzmin I, Rupprecht CE, Nel LH. Phylogeny of Lagos bat virus: Challenges for lyssavirus taxonomy. Virus Res. 2008; 135: 10–21. 10.1016/j.virusres.2008.02.001.
- Delmas O, Holmes EC, Talbi C, Larrous F, Dacheux L, Bouchier C. Genomic diversity and evolution of the lyssaviruses. PLoS One. 2008; 3: e2057.10.1371/journal.pone.0002057.
- Markotter W, Kuzmin IV, Rupprecht CE, Nel LH. Lagos bat virus virulence in mice inoculated by the peripheral route. Epidemiol Infect. 2009; 137: 1155–62. 10.1017/S0950268808001945.
- Badrane H, Bahloul C, Perrin P, Tordo N. Evidence of two Lyssavirus phylogroups with distinct pathogenicity and immunogenicity. J Virol. 2001; 75: 3268–76. 10.1128/JVI.75.7.3268-3276.2001.
- Kuzmin IV, Niezgoda M, Franka R, Agwanda B, Markotter W, Beagley JC. Possible emergence of West Caucasian bat virus in Africa. Emerg Infect Dis. 2008; 14: 1887–89. 10.3201/eid1412.080750.
- Markotter W, Van Eeden C, Kuzmin IV, Rupprecht CE, Paweska JT, Swanepoel R. Epidemiology and pathogenicity of African bat lyssaviruses. Dev Biol (Basel). 2008; 131: 317–25.
- van Thiel PP, de Bie RM, Eftimov F, Tepaske R, Zaaijer HL, van Doornum GJ. Fatal human rabies due to Duvenhage virus from a bat in Kenya: Failure of treatment with coma-induction, ketamine, and antiviral drugs. PLoS Negl Trop Dis. 2009; 3: e428.10.1371/journal.pntd.0000428.
- Fraser GC, Hooper PT, Lunt RA, Gould AR, Gleeson LJ, Hayatt AD. Encephalitis caused by a lyssavirus in fruit bats in Australia. Emerg Infect Dis. 1996; 2: 327–31. 10.3201/eid0204.960408.
- Gould AR, Kattenbelt JA, Gumley SG, Lunt RA. Characterisation of an Australian bat lyssavirus variant isolated from an insectivorous bat. Virus Res. 2002; 89: 1–28. 10.1016/S0168-1702(02)00056-4.
- Allworth A, Murray K, Morgan J. A human case of encephalitis due to a lyssavirus, recently identified in fruit bats. Commun Dis Intell. 1996; 20: 504.
- Hanna JN, Carney IK, Smith GA, Tannenberg AE, Deverill JE, Botha JA. Australian bat lyssavirus infection: A second human case, with a long incubation period. Med J Australia. 2000; 172: 597–99.
- Warrilow D, Smith IL, Harrower B, Smith GA. Sequence analysis of an isolate from a fatal human infection of Australian bat lyssavirus. Virology. 2002; 297: 109–19. 10.1006/viro.2002.1417.
- Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P. Fruit bats as reservoirs of Ebola virus. Nature. 2005; 438: 575–76. 10.1038/438575a.
- Swanepoel R, Smit SB, Rollin PE, Formenty P, Leman PA, Kemp A. International Scientific and Technical Committee for Marburg Hemorrhagic Fever Control in the Democratic Republic of Congo. Studies of reservoir hosts for Marburg virus. Emerg Infect Dis. 2007; 13: 1847–51.
- Towner JS, Pourrut X, Albariño CG, Nkogue CN, Bird BH, Grard G. Marburg virus infection detected in a common African bat. PLoS One. 2007; 2(1): 764.10.1371/journal.pone.0000764.
- Martini GA. Marburg agent disease: In man. Trans R Soc Trop Med Hyg. 1969; 63: 295–302. 10.1016/0035-9203(69)90001-7.
- Leroy EM, Epelboin A, Mondonge V, Pourrut X, Gonzalez JP, Muyembe-Tamfum JJ. Human ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2009. Vector Borne Zoonotic Dis. 2007; 9: 723–28.
- Timen A, Koopmans M. Marburg hemorrhagic fever—The Netherlands ex Uganda. ProMed [cited 2008 July 11]. Available from: http://www.promedmail.org, archive no. 20080711.2115.
- Centers for Disease Control and Prevention. 2008: Marburg hemorrhagic fever, imported case—United States. [cited 2009 Feb 3]. Available from: http://www.cdc.gov/ncidod/dvrd/spb/outbreaks/index.htm#USA.
- Towner JS, Amman BR, Sealy TK, Carroll SA, Comer JA, Kemp A. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 2009; 5(7): e1000536.10.1371/journal.ppat.1000536.
- Kuzmin IV, Niezgoda M, Franka R, Agwanda B, Markotter W, Breiman RF. Marburg virus in fruit bat, Kenya. Emerg Infect Dis. 2010; 16: 352–54.
- Murray K, Selleck P, Hooper P, Hyatt A, Gould A, Gleeson L. A morbillivirus that caused fatal disease in horses and humans. Science. 1995; 268: 94–97. 10.1126/science.7701348.
- Selvey LA, Wells RM, McCormack JG, Ansford AJ, Murray K, Rogers RJ. Infection of humans and horses by a newly described morbillivirus. Med J Aust. 1995; 162: 642–45.
- Hooper PT, Gould AR, Russell GM, Kattenbelt JA, Mitchell G. The retrospective diagnosis of a second outbreak of equine morbillivirus infection. Aust Vet J. 1996; 74: 244–45. 10.1111/j.1751-0813.1996.tb15414.x.
- Rogers RJ, Douglas IC, Baldock FC, Glanville RJ, Seppanen KT, Gleeson LJ. Investigation of a second focus of equine morbillivirus infection in coastal Queensland. Aust Vet J. 1996; 74: 243–44. 10.1111/j.1751-0813.1996.tb15413.x.
- Young PL, Halpin K, Selleck PW, Field H, Gravel JL, Kelly MA. Serologic evidence for the presence in Pteropus bats of a paramyxovirus related to equine morbillivirus. Emerg Infect Dis. 1996; 2: 239–40. 10.3201/eid0203.960315.
- Mackenzie JS. Emerging viral diseases: An Australian perspective. Emerg Infect Dis. 1999; 5: 1–5. 10.3201/eid0501.990101.
- Williamson MM, Hooper PT, Selleck PW, Gleeson LJ, Daniels PW, Westbury HA. Transmission studies of Hendra virus (equine morbillivirus) in fruit bats, horses and cats. Aust Vet J. 1998; 76: 813–18. 10.1111/j.1751-0813.1998.tb12335.x.
- Williamson MM, Hooper PT, Selleck PW, Westbury HA, Slocombe RF. Experimental Hendra virus infection in pregnant guinea-pigs and fruit bats (Pteropus poliocephalus). J Comp Pathol. 2000; 122: 201–7. 10.1053/jcpa.1999.0364.
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK. Nipah virus: A recently emergent deadly paramyxovirus. Science. 2000; 288: 1432–35. 10.1126/science.288.5470.1432.
- Chua KB. Nipah virus outbreak in Malaysia. J Clin Virol. 2003; 26: 265–75. 10.1016/S1386-6532(02)00268-8.
- Wang LF, Yu M, Hansson E, Pritchard LI, Shiell B, Michalski WP. The exceptionally large genome of Hendra virus: Support for creation of a new genus within the family paramyxoviridae. J Virol. 2000; 74: 9972–79. 10.1128/JVI.74.21.9972-9979.2000.
- Yob JM, Field H, Rashdi AM, Morrissy C, van der Heide B, Rota P. Nipah virus infection in bats (order chiroptera) in peninsular Malaysia. Emerg Infect Dis. 2001; 7: 439–41.
- Chua KB, Koh CL, Hooi PS, Wee KF, Khong JH, Chua BH. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect. 2002; 4: 145–51. 10.1016/S1286-4579(01)01522-2.
- Reynes JM, Counor D, Ong S, Faure C, Seng V, Molia S. Nipah virus in Lyle's flying foxes, Cambodia. Emerg Infect Dis. 2005; 11: 1042–47.
- Wacharapluesadee S, Lumlertdacha B, Boongird K, Wanghongsa S, Chanhome L, Rollin P. Bat Nipah virus, Thailand. Emerg Infect Dis. 2005; 11: 1949–51.
- Hsu VP, Hossain MJ, Parashar UD, Ali MM, Ksiazek TG, Kuzmin I. Nipah virus encephalitis reemergence, Bangladesh. Emerg Infect Dis. 2004; 10: 2082–87.
- Li Y, Wang J, Hickey AC, Zhang Y, Li Y, Wu Y. Antibodies to Nipah or Nipah-like viruses in bats, China. Emerg Infect Dis. 2008; 14: 1974–75. 10.3201/eid1412.080359.
- Luby SP, Rahman M, Hossain MJ, Blum LS, Husain MM, Gurley E. Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis. 2006; 12: 1888–94.
- Montgomery JM, Hossain MJ, Gurley E, Carroll DG, Croisier A, Bertherat E. Risk factors for Nipah virus encephalitis in Bangladesh. Emerg Infect Dis. 2008; 14: 1526–32. 10.3201/eid1410.060507.
- Chadha MS, Comer JA, Lowe L, Rota PA, Rollin PE, Bellini WJ. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis. 2006; 12: 235–40.
- Iehlè C, Razafitrimo G, Razainirina J, Andriaholinirina N, Goodman SM, Faure C. Henipavirus and Tioman virus antibodies in pteropodid bats, Madagascar. Emerg Infect Dis. 2007; 13: 159–61. 10.3201/eid1301.060791.
- Hayman DT, Suu-Ire R, Breed AC, McEachern JA, Wang L, Wood JL. Evidence of henipavirus infection in West African fruit bats. PLoS One. 2008; 3: e2739.10.1371/journal.pone.0002739.
- Drexler JF, Corman VM, Gloza-Rausch F, Seebens A, Annan A, Ipsen A. Henipavirus RNA in African bats. PLoS One. 2009; 4: e6367.10.1371/journal.pone.0006367.
- Thomas DW. The annual migrations of three species of West African fruit bats (chiroptera: pteropodidae). Can J Zool. 1983; 61: 2266–72. 10.1139/z83-299.
- Chua KB, Wang LF, Lam SK, Crameri G, Yu M, Wise T. Tioman virus, a novel paramyxovirus isolated from fruit bats in Malaysia. Virology. 2001; 283: 215–29. 10.1006/viro.2000.0882.
- Pritchard LI, Chua KB, Cummins D, Hyatt A, Crameri G, Eaton BT. Pulau virus; a new member of the Nelson Bay orthoreovirus species isolated from fruit bats in Malaysia. Arch Virol. 2006; 151: 229–39. 10.1007/s00705-005-0644-4.
- Philbey AW, Kirkland PD, Ross AD, Davis RJ, Gleeson AB, Love RJ. An apparently new virus (family paramyxoviridae) infectious for pigs, humans, and fruit bats. Emerg Infect Dis. 1998; 4: 269–71. 10.3201/eid0402.980214.
- Chant K, Chan R, Smith M, Dwyer DE, Kirkland P. Probable human infection with a newly described virus in the family paramyxoviridae. The NSW Expert Group. Emerg Infect Dis. 1998; 4: 273–75. 10.3201/eid0402.980215.
- Philbey AW, Kirkland PD, Ross AD, Field HE, Srivastava M, Davis RJ. Infection with Menangle virus in flying foxes (pteropus spp.) in Australia. Aust Vet J. 2008; 86: 449–54. 10.1111/j.1751-0813.2008.00361.x.
- Bowden TR, Westenberg M, Wang LF, Eaton BT, Boyle DB. Molecular characterization of Menangle virus, a novel paramyxovirus which infects pigs, fruit bats, and humans. Virology. 2001; 283: 358–73. 10.1006/viro.2001.0893.
- Yaiw KC, Bingham J, Crameri G, Mungall B, Hyatt A, Yu M. Tioman virus, a paramyxovirus of bat origin, causes mild disease in pigs and has a predilection for lymphoid tissues. J Virol. 2008; 82: 565–68. 10.1128/JVI.01660-07.
- Yaiw KC, Crameri G, Wang L, Chong HT, Chua KB, Tan CT. Serological evidence of possible human infection with Tioman virus, a newly described paramyxovirus of bat origin. J Infect Dis. 2007; 196: 884–86. 10.1086/520817.
- Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003; 362: 263–70. 10.1016/S0140-6736(03)13967-0.
- Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003; 302: 276–78. 10.1126/science.1087139.
- Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005; 310: 676–79. 10.1126/science.1118391.
- Lau SK, Woo PC, Li KS, Huang Y, Tsoi HW, Wong BH. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci U S A. 2005; 102: 14040–45. 10.1073/pnas.0506735102.
- Cui J, Han N, Streicker D, Li G, Tang X, Shi Z. Evolutionary relationships between bat coronaviruses and their hosts. Emerg Infect Dis. 2007; 13: 1526–32.
- Tang XC, Zhang JX, Zhang SY, Wang P, Fan XH, Li LF. Prevalence and genetic diversity of coronaviruses in bats from China. J Virol. 2006; 80: 7481–90. 10.1128/JVI.00697-06.
- Poon LL, Chu DK, Chan KH, Wong OK, Ellis TM, Leung YH. Identification of a novel coronavirus in bats. J Virol. 2005; 79: 2001–9. 10.1128/JVI.79.4.2001-2009.2005.
- Lau SK, Li KS, Huang Y, Shek CT, Tse H, Wang M. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J Virol. 2010; 84: 2808–19. 10.1128/JVI.02219-09.
- Gloza-Rausch F, Ipsen A, Seebens A, Göttsche M, Panning M, Felix Drexler J. Detection and prevalence patterns of group I coronaviruses in bats, northern Germany. Emerg Infect Dis. 2008; 14: 626–31. 10.3201/eid1404.071439.
- Dominguez SR, O'Shea TJ, Oko LM, Holmes KV. Detection of group 1 coronaviruses in bats in North America. Emerg Infect Dis. 2007; 13: 1295–1300.
- Tong S, Conrardy C, Ruone S, Kuzmin IV, Guo X, Tao Y. Detection of novel SARS-like and other coronaviruses in bats from Kenya. Emerg Infect Dis. 2009; 15: 482–85. 10.3201/eid1503.081013.
- Turmelle AS, Olival KJ. Correlates of viral richness in bats (order chiroptera). Ecohealth. 2009; 6: 522–39. 10.1007/s10393-009-0263-8.
- Woo PC, Lau SK, Huang Y, Yuen KY. Coronavirus diversity, phylogeny and interspecies jumping. Exp Biol Med. 2009; 234: 1117–27. 10.3181/0903-MR-94.
- Dobson AP. Virology. What links bats to emerging infectious diseases?. Science. 2005; 310: 628–29. 10.1126/science.1120872.
- Nadin-Davis SA, Huang W, Armstrong J, Casey GA, Bahloul C, Tordo N. Antigenic and genetic divergence of Rabies viruses from bat species indigenous to Canada. Virus Res. 2001; 74: 139–56. 10.1016/S0168-1702(00)00259-8.
- Middleton DJ, Morrissy CJ, van der Heide BM, Russell GM, Braun MA, Westbury HA. Experimental Nipah virus infection in pteropid bats (pteropus poliocephalus). J Comp Pathol. 2007; 136: 266–72. 10.1016/j.jcpa.2007.03.002.
- Wong S, Lau S, Woo P, Yuen KY. Bats as a continuing source of emerging infections in humans. Rev Med Virol. 2007; 17: 67–91. 10.1002/rmv.520.
- Brunet-Rossinni AK, Austad SN. Ageing studies on bats: A review. Biogerontology. 2004; 5: 211–22. 10.1023/B:BGEN.0000038022.65024.d8.
- Chua KB, Crameri G, Hyatt A, Yu M, Tompang MR, Rosli J. A previously unknown reovirus of bat origin is associated with an acute respiratory disease in humans. Proc Natl Acad Sci U S A. 2007; 104: 11424–29. 10.1073/pnas.0701372104.
- Wibbelt G, Moore MS, Schountz T, Voigt CC. Emerging diseases in chiroptera: Why bats?. Biol Lett. 2010; 6: 438–40. 10.1098/rsbl.2010.0267.
- Chakravarty AK, Sarkar SK. Immunofluorescence analysis of immunoglobulin bearing lymphocytes in the Indian fruit bat: Pteropus giganteus. Lymphology. 1994; 27: 97–104.
- Sarkar SK, Chakravarty AK. Analysis of immunocompetent cells in the bat, pteropus giganteus: Isolation and scanning electron microscopic characterization. Dev Comp Immunol. 1991; 15: 423–30. 10.1016/0145-305X(91)90034-V.
- Turmelle AS, Ellison JA, Mendonca MT, McCracken GF. Histological assessment of cellular immune response to the phytohemagglutinin skin test in Brazilian free-tailed bats (tadarida brasiliensis). J Comp Physiol B. 2010; 180: 1155–64. 10.1007/s00360-010-0486-6.
- Omatsu T, Bak EJ, Ishii Y, Kyuwa S, Tohya Y, Akashi H. Induction and sequencing of Rousette bat interferon alpha and beta genes. Vet Immunol Immunopathol. 2008; 124: 169–76. 10.1016/j.vetimm.2008.03.004.
- Kepler TB, Sample C, Hudak K, Roach J, Haines A, Walsh A. Chiropteran types I and II interferon genes inferred from genome sequencing traces by a statistical gene-family assembler. BMC Genomics. 2010; 11: 444.10.1186/1471-2164-11-444.
- He G, He B, Racey PA, Cui J. Positive selection of the bat interferon alpha gene family. Biochem Genet. 2010; 48: 840–46. 10.1007/s10528-010-9365-9.
- Bouma HR, Carey HV, Kroese FG. Hibernation: The immune system at rest?. J Leukoc Biol. 2010; 88: 619–24. 10.1189/jlb.0310174.
- Li Y, Ge X, Zhang H, Zhou P, Zhu Y, Zhang Y. Host range, prevalence, and genetic diversity of adenoviruses in bats. J Virol. 2010; 84: 3889–97. 10.1128/JVI.02497-09.
- Bennett M. Bats and human emerging diseases. Epidemiol Infect. 2006; 134: 905–7. 10.1017/S0950268806006674.
- Turmelle AS, Jackson FR, Green D, McCracken GF, Rupprecht CE. Host immunity to repeated Rabies virus infection in big brown bats. J Gen Virol. 2010; 91: 2360–66. 10.1099/vir.0.020073-0.
- Amengual B, Bourhy H, Lopez-Roig M, Serra-Cobo J. Temporal dynamics of European bat lyssavirus type 1 and survival of myotis myotis bats in natural colonies. PLoS One. 2007; 2: e566.10.1371/journal.pone.0000566.
- Steece R, Altenbach JS. Prevalence of rabies specific antibodies in the Mexican free-tailed bat (tadarida brasiliensis mexicana) at Lava Cave, New Mexico. J Wildl Dis. 1989; 25: 490–96.
- Dimitrov DT, Hallam TG, Rupprecht CE, McCracken GF. Adaptive modeling of viral diseases in bats with a focus on rabies. J Theor Biol. 2008; 255: 69–80. 10.1016/j.jtbi.2008.08.007.
- Shaw ML, Cardenas WB, Zamarin D, Palese P, Basler CF. Nuclear localization of the Nipah virus W protein allows for inhibition of both virus- and toll-like receptor 3-triggered signaling pathways. J Virol. 2005; 79: 6078–88. 10.1128/JVI.79.10.6078-6088.2005.
- Shaw ML, García-Sastre A, Palese P, Basler CF. Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively. J Virol. 2004; 78: 5633–41. 10.1128/JVI.78.11.5633-5641.2004.
- Hagmaier K, Stock N, Goodbourn S, Wang LF, Randall R. A single amino acid substitution in the V protein of Nipah virus alters its ability to block interferon signalling in cells from different species. J Gen Virol. 2006; 87: 3649–53. 10.1099/vir.0.82261-0.
- Wacharapluesadee S, Boongird K, Wanghongsa S, Ratanasetyuth N, Supavonwong P, Saengsen D. A longitudinal study of the prevalence of Nipah virus in pteropus lylei bats in Thailand: Evidence for seasonal preference in disease transmission. Vector Borne Zoonotic Dis. 2010; 10: 183–90. 10.1089/vbz.2008.0105.
- Plowright RK, Field HE, Smith C, Divljan A, Palmer C, Tabor G. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (pteropus scapulatus). Proc Biol Sci. 2008; 275: 861–69. 10.1098/rspb.2007.1260.
- Field HE, Mackenzie JS, Daszak P. Henipaviruses: Emerging paramyxoviruses associated with fruit bats. Curr Top Microbiol Immunol. 2007; 315: 133–59.
- Halpin K, Young PL, Field HE, Mackenzie JS. Isolation of Hendra virus from pteropid bats: A natural reservoir of Hendra virus. J Gen Virol. 2000; 81: 1927–32.
- Watanabe S, Masangkay JS, Nagata N, Morikawa S, Mizutani T, Fukushi S. Bat coronaviruses and experimental infection of bats, the Philippines. Emerg Infect Dis. 2010; 16: 1217–23. 10.3201/eid1608.100208.
- Leroy EM, Rouquet P, Formenty P, Souquiere S, Kilbourne A, Froment JM. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science. 2004; 303: 387–90. 10.1126/science.1092528.
- Lahm SA, Kombila M, Swanepoel R, Barnes RF. Morbidity and mortality of wild animals in relation to outbreaks of Ebola haemorrhagic fever in Gabon, 1994–2003. Trans R Soc Trop Med Hyg. 2007; 101: 64–78.
- Johnson BK, Wambui C, Ocheng D, Gichogo A, Oogo S, Libondo D. Seasonal variation in antibodies against Ebola virus in Kenyan fever patients. Lancet. 1986; 1(8490): 1160.10.1016/S0140-6736(86)91876-3.
- White LJT. Patterns of fruit-fall phenology in the Lopè Reserve, Gabon. J Trop Ecol. 1994; 10: 289–308. 10.1017/S0266467400007975.
- Puechmaille SJ, Verdeyroux P, Fuller H, Gouilh MA, Bekaert M, Teeling EC. White-nose syndrome fungus (geomyces destructans) in bat, France. Emerg Infect Dis. 2010; 16: 290–93.
- Schneider MC, Aron J, Santos-Burgoa C, Uieda W, Ruiz-Velazco S. Common vampire bat attacks on humans in a village of the Amazon region of Brazil. Cad Saude Publica. 2001; 17: 1531–36. 10.1590/S0102-311X2001000600038.
- Schneider MC, Belotto A, Adè MP, Leanes LF, Correa E, Tamayo H. Epidemiologic situation of human rabies in Latin America in 2004. Epidemiol Bull. 2005; 26: 2–4.
- Bobashev G, Morris RJ, Goedecke DM. Sampling for global epidemic models and the topology of an international airport network. PLoS One. 2008; 3: e3154.10.1371/journal.pone.0003154.
- Hollingsworth TD, Ferguson NM, Anderson RM. Will travel restrictions control the international spread of pandemic influenza?. Nat Medicine. 2006; 5: 497–99. 10.1038/nm0506-497.
- Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003; 348: 1977–85. 10.1056/NEJMoa030666.
- Centers for Disease Control and Prevention (CDC). Cluster of severe acute respiratory syndrome cases among protected health-care workers—Toronto, Canada, April 2003. MMWR Morb Mortal Wkly Rep. 2003;52:433–36.
- Tu C, Crameri G, Kong X, Chen J, Sun Y, Yu M. Antibodies to SARS coronavirus in civets. Emerg Infect Dis. 2004; 10: 2244–48.
- Xu RH, He JF, Evans MR, Peng GW, Field HE, Yu DW. Epidemiologic clues to SARS origin in China. Emerg Infect Dis. 2004; 10: 1030–37.
- Willoughby RE Jr, Tieves KS, Hoffman GM, Ghanayem NS, Amlie-Lefond CM, Schwabe MJ. Survival after treatment of rabies with induction of coma. N Engl J Med. 2005; 352: 2508–14. 10.1056/NEJMoa050382.
- Rupprecht CE. Bats, emerging diseases, and the human interface. PLoS Negl Trop Dis. 2009; 3: e451.10.1371/journal.pntd.0000451.
- Rubin J, David D, Willoughby RE Jr, Rupprecht CE, Garcia C, Guarda DC. Applying the Milwaukee protocol to treat canine rabies in equatorial Guinea. Scand J Infect Dis. 2009; 41: 372–75. 10.1080/00365540902798333.
- Manning SE, Rupprecht CE, Fishbein D, Hanlon CA, Lumlertdacha B, Guerra M. Advisory Committee on Immunization Practices Centers for Disease Control and Prevention (CDC). Human rabies prevention—United States, 2008: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2008; 57(RR-3): 1–28.
- Dreesen DW. A global review of rabies vaccines for human use. Vaccine. 1997; 15 Suppl: S2–S6. 10.1016/S0264-410X(96)00314-3.
- Mungall BA, Schopman NC, Lambeth LS, Doran TJ. Inhibition of henipavirus infection by RNA interference. Antiviral Res. 2008; 80: 324–31. 10.1016/j.antiviral.2008.07.004.
- Jones SM, Stroher U, Fernando L, Qiu X, Alimonti J, Melito P. Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J Infect Dis. 2007; 196(Suppl 2): S404–S412. 10.1086/520591.
- NASPHV.; Centers for Disease Control and Prevention; Council of State and Territorial Epidemiologists; American Veterinary Medical Association. Compendium of measures to prevent disease associated with animals in public settings, 2009: National Association of State Public Health Veterinarians, Inc. (NASPHV). MMWR Recomm Rep. 2009;58(RR-5): 1–21.
- Rupprecht CE, Hanlon CA, Slate D. Control and prevention of rabies in animals: Paradigm shifts. Dev Biol (Basel). 2006; 125: 103–11.
- Fullard JH, Simmons JA, Saillant PA. Jamming bat echolocation: The dogbane tiger moth cycnia tenera times its clicks to the terminal attack calls of the big brown bat eptesicus fuscus. J Exp Biol. 1994; 194: 285–98.
- Corcoran AJ, Barber JR, Conner WE. Tiger moth jams bat sonar. Science. 2009; 325: 325–27. 10.1126/science.1174096.
- Kosoy M, Bai Y, Lynch T, Kuzmin IV, Niezgoda M, Franka R. Bartonella spp. in bats, Kenya. Emerg Infect Dis. 2010; 16: 1875–81.
- Hoff GL, Bigler WJ. The role of bats in the propagation and spread of histoplasmosis: A review. J Wildl Dis. 1981; 17: 191–96.