Background
Studies investigating the effect of power frequency (50–60 Hz) electromagnetic fields (EMF) on melatonin synthesis in rats have been inconsistent with several showing suppression of melatonin synthesis, others showing no effect and a few actually demonstrating small increases. Scant research has focused on the ensuing sleep patterns of EMF exposed rats. The present study was designed to examine the effects of extremely low power frequency electromagnetic fields (EMF) on the production of melatonin and the subsequent sleep structure in rats.
Methods
Eighteen male Sprague-Dawley rats were exposed to a 1000 milligauss (mG) magnetic field for 1 month. Urine was collected for the final 3 days of the exposure period for analysis of 6-sulphatoxymelatonin, the major catabolic product of melatonin found in urine. Subsequent sleep was analyzed over a 24-hour period.
Results
Melatonin production was mildly increased in exposed animals. Although there were no statistically significant changes in sleep structure, exposed animals showed slight decreases in REM (rapid eye movement) sleep as compared to sham (non-exposed) animals.
Conclusions
Power frequency magnetic fields induced a marginally statistically significant increase in melatonin levels in exposed rats compared to control. Subsequent sleep analysis indicated little effect on the sleep architecture of rats, at least not within the first day after 1 month's continuous exposure. Varying results in the literature are discussed and future research suggested.
In recent decades there has been rapid growth of extremely low-frequency (50–60 Hz) artificial electromagnetic fields, (EMFs) due to continued industrial urbanization. The literature is replete with epidemiological studies that have demonstrated a small but consistent correlation between increasing time spent near electromagnetic generating sources and certain forms of cancer, specifically childhood leukemia and hormone dependent cancers such as breast and prostate cancers Citation1–Citation3. Some in vitro studies have verified possible potentiation of cancer cell growth Citation4 even though some experimental evidence has found that EMF exposure may actually be beneficial to specific prostate cancer cell lines Citation5. Still, health concerns seem to proliferate in numerous fronts. For example, new concerns have surfaced recently such as potential decreases in reproduction after acute EMF exposure in a rodent model Citation6. Behavioral conditions have also been added to the list of concerns, such as depression Citation7 Citation8, learning disruptions Citation9–Citation12, slowed reaction times Citation13 Citation14, and sleep disturbances Citation15 Citation16.
Research indicates that EMFs could contribute to some or all of these ailments by interfering with the action of melatonin. In past studies, the use of electric blankets in the home, exposure to EMFs in the laboratory setting, and exposure to medical imaging devices such as a nuclear magnetic resonance (NMR) have all lowered melatonin levels in some people Citation17–Citation19. As such, a melatonin hypothesis has been proposed as a link for EMF and cancer Citation20 Citation21. According to this view, EMF exposure affects the pineal gland, resulting in a suppression of melatonin production. Melatonin has been shown to have anticarcinogenic Citation22 and free radical scavenging properties Citation24 Citation25, the reduction of which may lead to increased cancer risk. Moreover, there is recent research that the melatonin receptor may be disrupted by EMFs Citation23. This connection, however, is far from proven and remains controversial. Overall, when suppression of melatonin is found in animal exposure studies, it is typically in the order of a 25–30% reduction Citation26. Also, after chronic exposure, levels of melatonin seem to return to normal after 3 days Citation27 Citation28. Thus, even if melatonin production is suppressed by EMF, the effects may not be long-lasting.
In addition to numerous studies finding decreases in melatonin production, null results have also been found. In fact, several studies have found no changes in circulatory melatonin after exposure to EMFs Citation29–Citation35. Finally, there have been a few studies where measurable albeit small increases in melatonin either during or after EMF exposure have been reported Citation36 Citation50.
Melatonin, in addition to having possible anti-cancer properties, has been well established as a modulator of normal sleep in most animals Citation61 Citation62. If magnetic fields have a modulating effect on melatonin production, a similar effect might be expected on normal sleep patterns. However, few studies have explicitly and systematically examined the sleep structure of an exposed group to power frequency fields. Graham and Cook Citation33 found that reductions in total sleep time, sleep efficiency, and REM sleep, and increased time spent in Stage 2 sleep, were associated with intermittent (vs. continuous or sham) exposure to a 60 Hz magnetic field in 24 males. In another study of 18 males exposed overnight to a 50 Hz, 10 milligauss (mG) magnetic field, the exposed group was associated with a reduced slow wave sleep, total sleep time, and slow wave activity as compared to a sham exposed group Citation15. Circulating melatonin, growth hormone, prolactin, testosterone, and cortisol were not affected. Hong et al. Citation63 examined nine males after 16 weeks of pre-exposure and night exposure using electric sheets as bedding using 7–80 mG field strengths. No changes were found in melatonin or circadian functioning.
The present study attempts to advance the knowledge base on the understanding of chronic EMF exposure. Specifically, we proposed to better comprehend the effects of extremely low frequency electromagnetic radiation (EMF) at 1000 mG on melatonin production and sleep behavior in rodents. We use 1000 mG as it is a common strength in the literature, and is approximately equivalent to exposure while using a standard blow dryer at roughly 1 ft away from the head Citation64. Although some industrial settings may see EMFs at this strength, 1000 mG is still higher than most epidemiology studies Citation21. To our knowledge, no systematic study has been performed using sleep as an end point to be evaluated in the rat. We hypothesize a change in nighttime melatonin in exposed animals as compared to sham exposed rats. Additionally, if melatonin levels are indeed altered, changes in the normal sleep architecture are also expected.
Method
Subjects
Male Sprague-Dawley rats (N=18: exposed n=9; sham exposed n=9) were individually housed before and during experimental protocols. All protocols were approved by the Saint Louis University Animal Care and Use Committee. During the experiment, animals were between the ages of 4 and 7 months, age matched between groups. The rats were exposed at a stable temperature (23–24°C) and were maintained in a 12 h light/dark cycle; lights on at 7 a.m. Food and water were available ad lib. Animals were fed every day and cages cleaned.
Equipment
Two identical exposure structures were used (one charged, the other not) located in a 10×4 m room with masonry walls and 4 m high ceiling. The large room allowed for the exposure and sham exposure group to be in the same room. The exposure apparatus was designed and constructed with the assistance from the Parks College of Engineering at Saint Louis University.
Two Sonotube reinforced cardboard tubes 8 ft long and 2 ft in diameter served as framework for the exposure structures. One tube was wrapped with 18 gauge insulated copper wire; the other was not wound and served as the sham exposure environment. The exposure apparatus was energized by a standard 110 V, 60 Hz outlet and a step-down transformer (a variac) providing the necessary voltage of 8 V that excited the wire from a three-phase, four-wire supply to create a horizontal, linearly polarized 1000 milligauss (or 1 Gauss or 1.0×10–4 T) magnetic field within the exposure volume. The magnetic field in the sham exposure Sonotube was less than 1 mG and was 7 m away from the exposure. The magnetic field was measured by a Bell Technology model 4090 3-axis EMF meter.
The animals were singly housed with the cages being constructed of translucent polypropylene 12″×9″×8″, fitted with 2″ high PVC filters as covers. Two 12 in. high wooden cradle horses supported each Sonotube. Water was contained in a glass bottle and accessed via a glass dipper. Food was placed on the plastic, grated floor of the cages.
Design and procedure
Before exposure, five electrodes for sleep recording were implanted according to standard procedures Citation38. These five placements, in a pin-plug assembly, permitted the fastening of stainless steel jeweler screws (0–80 by 1/8 in.) that allowed for ECoG recordings from the parietal-frontal and the parietal-occipital regions of the brain. The pin plug was then permanently attached to the skull using dental cement, forming a mound that permanently seals the skull, electrodes, and plug as a single unit. Post operatively, the animal received post-operative injections of 0.1 mg/kg buprenorphine twice daily for 3 days.
After surgery, the animals were allowed to recover for 1 week at which time two animals were placed in the sleep lab for another week for acclimation. The animals were continually maintained on a 12–12 hours schedule throughout the experiment and were staggered in twos as they entered the exposure room. At the end of acclimation, the animals were placed into the exposure apparatus, which involved being placed into the plastic cages described above with one rat in the energized tube, and the other in the sham non-energized tube. Animals remained in the tubes for 30 days. Exposure was 24 hours a day with the exception of approximately 10 min each day for handling and care of the animals when the coils were deactivated. Animals were staggered into the acclimation room and exposure facilities two at a time each week until no more than four animals were in each tube at once. Field measurements ensured a uniform exposure for all rats in the energized tube.
On the final 3 days of exposure (day 28, 29, 30), urine was collected every 6 hours from the basin of the cage at the end and middle of the dark period and at the end and middle of the light period. Urine collection was from the same plastic cages with a slight modification. Standard metallic metabolic cages could not be used due to the configuration of the apparatus and due to possible eddy currents being generated by the materials of typical metabolic cages. To collect the urine during the 3 days of collection, the cages were fitted with a porous plastic grid affixed approximately one inch from the basin of the cage, this allowed for urine to pass freely to the basin while the grid contained any expelled feces. The collection period for 3 days was done to minimize variance for melatonin analysis. The urine, after collection, was centrifuged and the supernatant stored at -80°C until all animals’ urine had been collected. Subsequently, all samples were pooled so each animal had a ‘dark’ urine sample and a ‘light’ urine sample. Upon completion of all urine collection and storage, the urine was thawed overnight in a laboratory refrigerator at 4°C. Separately, the dark and light samples for each rat were then pooled and volume measured.
Analysis of the urine involved an ELISA kit (ALPCO, Dunham, NH) for 6-sulphatoxymelatonin (aMT6), the main urine metabolite of plasma melatonin. This metabolite is considered to be a reliable measure of circulating melatonin Citation39. Urine creatinine was also measured using the Jaffe method Citation40. While the measurement of aMT6 is an adequate alternative to plasma melatonin measurements, and less invasive, the only disadvantage to the procedure is the uncertainty of whether the entire urine fraction has been collected Citation41. When animals drink greater than average volume, the amount of fluid filtered increases in the kidneys and the amount of urine concentration decreases. In this study, the interest is the amount of circulating melatonin in the blood, as measured indirectly by a urine metabolite. In the case of an animal that drinks more than average, the aMT6 per ml of urine will appear low due to the large dilution. Creatinine is diluted the same way but is known to be excreted at a constant rate, filtered and excreted in the urine with no diurnal rhythm Citation41. Therefore, it is possible to relate the concentration of aMT6 to the concentration of a known, evenly excreted substance and report the melatonin in units of creatinine, as done here.
After urine was collected from each pair (one each from exposed and sham conditions), the animals were placed into the sleep facility for subsequent analysis of sleep architecture. The Physiological Psychology Laboratory, which houses the sleep research center, has facilities to analyze sleep on two animals at a time. The group of animals exposed (n=9) was similar to that of the sham exposed (n=8). Unequal numbers resulted from a sham exposed animal that could not be recorded due to the head-piece coming dislodged from the skull. The rats were placed in the animal sleep room immediately following removal from exposure where total sleep time during night and day, waking time, low-voltage sleep, high-voltage sleep, and paradoxical sleep (REM sleep) were analyzed over a 24-hours period. Sleep tracings were amplified by a Grass polysomnograph model 78D and interfaced with a Power Mac G4. Electroencephalogical tracings were scored by the first author according to the method described by Rechtschaffen Citation38. Rats were numerically coded by another experimenter so sleep data could be analyzed blindly. The code was broken only after all data had been scored. The design of the experiment is outlined below:
Surgically implant cortical electrodes in 18 animals. | |||||
One week recovery time. | |||||
One week adaptation time in sleep chamber. | |||||
One month continuous exposure at 1000 mG in exposure facility; half the animals sham exposed. | |||||
Urine collection for last 3 days of exposure. | |||||
Sleep analysis in sleep chamber upon removal from exposure apparatus. | |||||
Results
Independent t-tests were performed to analyze differences among the two groups of animals on the dependent variable of 6-sulphatoxymelatonin per unit of creatinine. All creatinine levels were converted to mg/ml and divided by the concentration of aMT6s that was expressed in ng/ml. The end results were expressed as 6-sulphatoxymelatonin per mg of creatinine. Total sleep time during dark, total sleep time during light, low-voltage sleep, high-voltage sleep, and paradoxical sleep were all analyzed by Mann-Whitney U non-parametric test since the Levine's test for homogeneity of variance demonstrated a non-normal distribution of percentages within each variable.
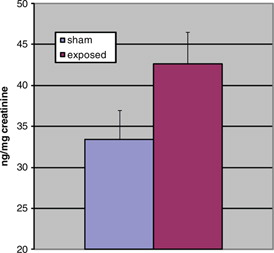
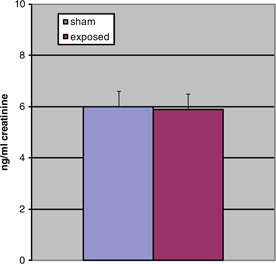
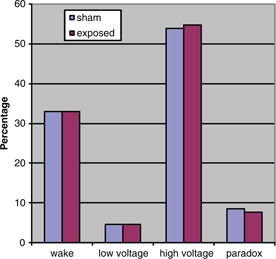
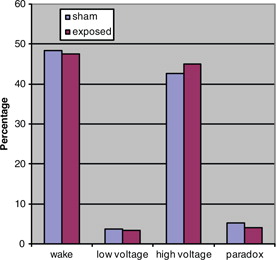
The aMT6s production of rats during the dark period was marginally significantly increased (t=1.75, p=0.05 [one-tailed]) in the exposed animals as compared to the sham exposed. shows the production of animals in both groups during the dark. Light-time levels of both groups did not demonstrate a significant difference as shown in . Sleep architecture during the dark was not significantly altered from control animals in all stages examined (). Paradoxical sleep was slightly decreased in the exposed animal but was not statistically significant (U=24.5, p=0.18). Sleep during light-time hours showed similar features as dark-time (). There were continued slight paradoxical sleep differences during sleep in the dark. No between group differences were found on total sleep time.
Discussion
The primary goal of this project was to examine the effects of continuous 60 Hz magnetic fields on the production of melatonin and on the sleep architecture of rats. The results here do not support the notion that magnetic fields reduce melatonin production as suggested by the melatonin hypothesis. Further, this study demonstrated a magnetic field that induced a marginally statistically significant increase in dark 6-sulphatoxymelatonin (aMT6s) levels. Subsequent sleep analysis indicates there is little effect of a 1000 mG magnetic field on the sleep architecture of rats, at least not within the first day after 1 month's continuous exposure.
The animal literature is inconsistent with respect to the effects of magnetic fields on melatonin levels. Inhibitory effects of magnetic fields on melatonin production covering a variety of exposure paradigms have been found Citation19 Citation42–Citation47. There is also a preponderance of negative results Citation29 Citation31 Citation32 Citation35 Citation44 Citation48 Citation49. This report demonstrated a mild stimulatory effect, which has also been reported Citation36 Citation50. Jentsch et al. Citation36 administered a 300 mG field as an independent variable to test extinction of a conditioned reaction in rats. They found that in the presence of the field, the animals had an increased retention of conditioned reactions and, more relevant to this study, an increase in serum melatonin. The findings were significant only during illumination as the differences at night, while still in the direction of increased melatonin in exposed rats, were not significant. Interestingly, the effects on the conditioned reactions also disappeared in the dark. The experimenters did not speculate as to why the melatonin results were different from the melatonin hypothesis.
Another study that reported an increase in melatonin from magnetic fields was after a 24 hours exposure to a 1000 mG field Citation50. This experiment demonstrated no differences in 6-sulphatoxymelatonin during the period of exposure but did show a statistically significant increase in the melatonin metabolite the day following exposure as compared to a baseline measurement. The authors suggested perhaps a rebound phenomenon was the reason for the results. Similarly, an epidemiological study found railway workers had no differences in 6-hydroxymelatonin sulfate (another metabolite found in urine) during exposure, but levels increased briefly after the work ceased Citation51.
A study performed on a non-mammal also found increases in melatonin after exposure to magnetic fields Citation52. Using a teleost fish (brook trout), a pulsing 400 mG field surrounded a couple of tanks for 45 min. Results demonstrated a more than two-fold increase in serum melatonin in the exposed fish. Two explanations were offered. First, the pineal glands may have been directly affected by an increase in calcium efflux into pineal photoreceptors, thereby increasing pinealocyte production of melatonin. Or, results may be due to the well documented sensitivity of fish to electric fields that may have caused a stress response in the animals leading to the melatonin increase.
A well-controlled laboratory study using a longitudinal design and using very high field strengths (52 G) also provides evidence for a possible compensatory response. Using adult male rats, animals were exposed for 1, 3, 7, 15, or 21 days for 30 min each day. Using blood melatonin as an end point, a depressed blood melatonin was found after 15 days, but the difference disappeared after 21 days Citation53.
In another study that lasted 60 days examined electric blanket use of men and women, melatonin levels were measured indirectly using 6-hydroxymelatonin sulfate. About one-quarter of subjects in this experiment demonstrated lowered melatonin levels when blanket use first began and again at the completion of the study, but not when urine was sampled during the middle (30 days into experiment) of the study Citation18. This may again provide further evidence to an adaptive response in at least some individuals after continuous exposure protocols but possible dysfunction in pineal gland activity in an unpredictably altered magnetic field environment, either during onset or offset of exposure.
Interestingly, upon further inspection of the numerous articles where no statistically significant effect was reported on melatonin production, at least one study found melatonin levels that varied not only as a function of how long exposure lasted but also as a function of time of collection Citation31. Female Sprague-Dawley rats were exposed to 1000 mG for 2, 4, 8, and 13 weeks of exposure. Animals were tested for serum melatonin at 4 and 5 hours after lights out. After 2 weeks exposure, animals had an increase by 20% in melatonin 5 hours after lights out but a 40% decrease 6 hours after lights out, a significant difference. Subsequent measurements at 4, 8, and 13 weeks of continuous exposure found no significant differences between sham exposed and exposed animals’ blood melatonin levels at either 5 or 6 hours after darkness. However, as time passed, there seemed to be a trend toward an increase in melatonin in the exposed group, albeit a statistically non-significant difference. Overall, Loscher et al. Citation31 determined that a 1000 mG field had no suppressive effect on the production of melatonin. This was stated even though at 2 weeks a significant decrease in melatonin was found 6 hours into the dark cycle. The authors suggest the effect did not last into the latter portions of the experiment due to an adaptation to the presence of the field.
When attempting to replicate the findings at 2 weeks and compare to the effects of 1 week exposure and a 1 day exposure, Loscher et al. Citation31 were unable to reproduce the original significant results. In fact, they found moderate increases 6 hours into the dark cycle in melatonin in the exposed animals after 1 and 2 weeks. In addition to a possible adaptation or compensation phenomenon, Loscher's lab indicated a possible phase shift of melatonin determined by assaying samples collected at different times during the nocturnal cycle. In our laboratory, no hourly measures were made of the aMT6s but urine samples were instead pooled for a single night and day measure. The urine of our rats was collected four times a day (in the middle and end of light and dark periods) because we supposed that from a pathophysiological view, the total nocturnal melatonin production is more significant than the phase shift or peak amplitude of the nocturnal melatonin profile. An hourly collection may have provided additional insights as to whether our results reflect an adaptation phenomenon or a phase-shift in the normal melatonin rhythm. Our melatonin data seems to indicate that at our field strength, 30 days of exposure may slightly increase the pineal production of melatonin as measured by urine collected over the entire dark period in nine rats as compared to nine sham exposed animals.
An explanation for result discrepancy may lie at the cellular level. There are studies examining intracellular free calcium (Ca2+) as a function of magnetic field exposure. For example, Blackman et al. Citation54 used chicken brains to demonstrate an enhancement in calcium ion efflux. Conversely, Bawin and Adey Citation55 found a reduction in efflux. This discrepancy may explain why some studies find an increase in serum melatonin and some a decrease. If Ca2+ efflux is reduced at the level of the pineal, it seems reasonable that the subsequent increase in intracellular calcium could increase melatonin production. Using the same logic, if there is less free calcium in the pinealocyte, less melatonin would be released.
No study has been found that examined the sleep in the rat after continuous exposure to a magnetic field. Akerstedt et al. Citation15 examined human sleep after an acute exposure of a relatively weak field (10 mG). In their experiment, human subjects were exposed for one night and sleep was analyzed during the exposure period. They found significant decreases in slow-wave sleep, paradoxical sleep, and subjective measures of sleep quality as compared to non-exposed controls. In our study, animals were not exposed per se during the sleep analysis. We removed them after 30 days of exposure to a much higher field strength (1000 mG) and immediately placed them in the sleep room for analysis of sleep for 24 hours. Notwithstanding a measurable increase in melatonin in exposed rats, no significant differences were found in any portion of the animal sleep structure as compared to sham exposed. This is compatible with results found in the literature. Melatonin has been established to have soporific actions in humans Citation62, but the evidence in the nocturnal rodent is less clear. In the rat, both an increase in sleep Citation56 and a decrease Citation57 have been reported. In one study, rats were injected with a moderate dose (3 mg/kg) of melatonin at dark onset and found that melatonin briefly inhibited paradoxical sleep, but found no overall hypnotic effects of the melatonin group Citation58. It should be noted that in our study, we also found slight decreases in paradoxical sleep in the exposed animals with increased melatonin (p=0.18). Overall, in a nocturnal animal, melatonin levels are likely to induce changes that are typical for the dark period of the species. In the nocturnal rat, it should not be surprising that we found a slight decrease in paradoxical sleep in the exposed animals when melatonin levels were higher.
We did find mild, non-significant changes in slow wave and in paradoxical sleep in the exposed animals. But, overall, we can conclude that a 1-month exposure to a field strength of 1000 mG at 60 Hz does not seem to significantly affect the sleep of rats upon removal from the exposure.
There are other frequencies of magnetic fields upon which similar measures have been made. While we intended to focus on the power frequency spectrum (e.g., 60 Hz), it is worth noting that there have been experiments utilizing frequencies such as in the cellular phone range that have also impacted serum melatonin levels in rats. Interestingly, these data are also in conflict with each other. For example, Köylü et al. Citation59 found that a 900 MHz microwave level field induced oxidative changes in the hippocampus which was interestingly modulated by melatonin administration. Meanwhile, Koyu et al. Citation60 demonstrated no effects on serum melatonin levels from either a 900 MHz or 1800 MHz field.
Concerns about magnetic fields across many frequencies are a confusing biomedical and public health controversy. The research is far from being unequivocal in the search for a true melatonin theory of EMF exposure. Many studies performed in this area are of sound scientific methodology, but when all literature is examined, there is considerable inconsistency and even abject contradiction. The problem is that we need to determine what biologically significant exposure really is, and the identification of a true possible mechanism of action would be useful. In the laboratory, more replication of interesting results and confirmations of possible associations that have been found in field studies need to be pursued further. Even if the epidemiological findings in this area that spawn laboratory hypotheses are deemed spurious, further low-cost exposure studies may have an important impact on our society as additional magnetic fields are likely to be encountered in the environment.
Conflict of interest and funding
This project was funded by an internal grant from the Graduate School of Saint Louis University.
Acknowledgements
We are grateful for the Parks College of Engineering, Aviation and Technology at Saint Louis University for all their support and assistance with construction and maintenance of materials. We also benefitted from Dr. Michael Morrissey of the Washington University School of Medicine for his support and skill in the surgical procedures and for Dr. Christopher M. Bloom of Providence College for his review and suggestions for the manuscript.
References
- Charles LE, Loomis D, Shy CM, Newman B, Millikan R, Nylander-French LA. Electromagnetic fields, polychlorinated biphenyls, and prostate cancer mortality in electric utility workers. Am J Epidemiol. 2003; 157: 683–91. 10.1093/aje/kwg044.
- Stevens RG. Biologically based epidemiological studies of electric power and cancer. Environ Health Perspect. 1993; 101: 93–100.
- Wartenberg D. Residential EMF exposure and childhood leukemia: meta-analysis and population attributable risk. Bioelectromagnetics. 2001;Suppl 5:S86–104.
- Watson JM, Parrish EA, Rinehart CA. Selective potentiation of gynecologic cancer cell growth in vitro by electromagnetic fields. Gynecol Oncol. 1998; 71: 64–7. 10.1006/gyno.1998.5114.
- Koh EK, Ryu BK, Jeong DY, Bang IS, Nam MH, Chae KS. A 60-Hz sinusoidal magnetic field induces apoptosis of prostate cancer cells through reactive oxygen species. Int J Radiat Biol. 2008; 84: 945–55. 10.1080/09553000802460206.
- Tenorio BM, Jimenez GC, Morais RN, Peixoto CA, Albuquerque Nogueira R, Silva Junior VA. Testicular development evaluation in rats exposed to 60 Hz and 1 mT electromagnetic field. J Appl Toxicol. 2011; 31: 223–30. 10.1002/jat.1584.
- Wartenberg D. EMFs: cutting through the controversy. Pub Health Rep. 1996; 111: 204–17.
- Van Wijngaarden E, Savitz DA, Kleckner RC, Cai J, Loomis D. Exposure to electromagnetic fields and suicide among electric utility workers: a nested case-control study. Occup Environ Med. 2000; 57: 258–63. 10.1136/oem.57.4.258.
- Lai H. Spatial learning deficit in the rat after exposure to a 60 Hz magnetic field. Bioelectromagnetics. 1996; 17: 494–6.
- Mostafa RM, Mostafa YM, Ennaceur A. Effects of exposure to extremely low-frequency magnetic field of 2 G intensity on memory and corticosterone level in rats. Physiol Behav. 2002; 76: 589–95. 10.1016/S0031-9384(02)00730-8.
- Thomas JR, Schrot J, Liboff AR. Low intensity magnetic fields alter operant behavior in rats. Bioelectromagnetics. 1986; 7: 349–57. 10.1002/bem.2250070402.
- Sun H, Che Y, Liu X, Zhou D, Miao Y, Ma Y. Effects of prenatal exposure to a 50-Hz magnetic field on one-trial passive avoidance learning in 1-day-old chicks. Bioelectromagnetics. 2010; 31: 150–5. 10.1002/bem.20596.
- Cook CM, Saucier DM, Thomas AW, Prato FS. Exposure to ELF magnetic and ELF-modulated radiofrequency fields: the time course of pshysiological and cognitive effects observed in recent studies. Bioelectromagnetics. 2006; 27: 613–27. 10.1002/bem.20247.
- Graham C, Cohen, HD, Cook MR. Immunological and biochemical effects of 60-Hz electric and magneticfields in humans. MRI Project No. RA-338-C. Kansas City: Midwest Research Institute, 1990.
- Akerstadt T, Arnetz B, Ficca G, Paulsson LE, Kallner A. A 50-Hz electromagnetic field impairs sleep. J Sleep Res. 1999; 8: 77–81. 10.1046/j.1365-2869.1999.00100.x.
- El-Helaly M, Abu-Hashem E. Oxidative stress, melatonin level, and sleep insufficiency among electronic equipment repairers. Indian J Occup Environ Med. 2010; 14: 66–70. 10.4103/0019-5278.75692.
- Wilson BW, Stevens RG, Anderson LE. Neuroendocrine mediated effects of electromagnetic field exposure: possible role of the pineal gland. Life Sci. 1989; 45: 1319–32. 10.1016/0024-3205(89)90018-0.
- Wilson BW, Wright CW, Morris RE, Buschbom DP, Brown DP, Miller DL. Evidence for an effect of ELF electromagnetic fields on human pineal gland function. J Pineal Res. 1990; 9: 113–26. 10.1111/j.1600-079X.1990.tb00901.x.
- Davis S, Mirick DK, Chen C, Stanczyk FZ. Effects of 60-Hz magnetic field exposure on nocturnal 6-sulfatoxymelatonin, estrogens, luteinizing hormone, and follicle-stimulating hormone in healthy reproductive-age women: results of a crossover trial. Ann Epidemiol. 2006; 16: 622–31. 10.1016/j.annepidem.2005.11.005.
- Henshaw DL, Reiter RJ. Do magnetic fields cause increased risk of childhood leukemia via melatonin disruption?. Bioelectromagnetics. 2005;Suppl 7:S86–97.
- Stevens RG. The melatonin hypothesis: electric power and breast cancer. Environ Health Perspect. 1996; 104: 135–40.
- Lissoni P. Modulation of anticancer cytokines IL-2 and IL-12 by melatonin and the other pineal indoles 5-methoxytryptamine and 5-methoxytryptophol in the treatment of human neoplasms. Ann N Y Acad Sci. 2000; 917: 560–7. 10.1111/j.1749-6632.2000.tb05421.x.
- Girgert R, Hanf V, Emons G, Gründker C. Signal transduction of the melatonin receptor MT1 is disrupted in breast cancer cells by electromagnetic fields. Bioelectromagnetics. 2010; 31: 237–45.
- Qi W, Reiter RJ, Tan DX, Manchester LC, Calvo JR. Melatonin prevents delta-aminolevulinic acid-induced oxidative DNA damage in the presence of Fe2+. Mol Cell Biochem. 2001; 218: 87–92. 10.1023/A:1007225809674.
- Reiter RJ, Tan DX, Kim SJ, Manchester LC, Qi W, Garcia JJ. Augmentation of indices of oxidative damage in life-long melatonin-deficient rats. Mech Ageing Dev. 1999; 110: 157–73. 10.1016/S0047-6374(99)00058-5.
- Graham C, Cook MR, Riffle DW. Nocturnal melatonin levels in human volunteers exposed to intermittent 60 Hz magnetic fields. Bioelectromagnetics. 1996; 17: 263–73.
- Wilson BW. 60-Hz electric field effects on pineal melatonin rhythms. Bioelectromagnetics. 1986; 7: 239–42. 10.1002/bem.2250070213.
- Reiter RJ. Pineal gland, cellular proliferation and neoplastic growth: an historical accountThe pineal gland and cancer. Gupta G, Attanasio A, Reiter RJBrain research promotion: London, 1988; 41–64.
- Reuss S, Olcese J, Vollrath L, Skaley M, Meves M. Lack of effects of NMR strength magnetic fields on rat pineal melatonin synthesis. IRCS Med Sci. 1985; 13: 471.
- Kato M, Honma K, Shigemitsu Shiga Y. Effects of exposure to a circularly polarized 50 Hz magnetic field on plasma and pineal melatonin levels in rats. Bioelectromagnetics. 1993; 14: 97–106. 10.1002/bem.2250140203.
- Loscher W, Mevissen M, Lerchl A. Exposure of female rats to a 100-microT 50 Hz magnetic field does not induce consistent changes in nocturnal levels of melatonin. Radiat Res. 1998; 150: 557–67. 10.2307/3579873.
- Yellon SM, Truong HN. Melatonin rhythm onset in the adult siberian hamster: influence of photoperiod but not 60 Hz magnetic field exposure on melatonin content in the pineal gland and in circulation. J Bio Rhythms. 1998; 13: 52–9. 10.1177/074873098128999916.
- Graham C, Cook MR. Human sleep in 60 Hz magnetic fields. Bioelectromagnetics. 1999; 20: 277–83.
- Graham C, Cook MR, Gerkovich MM, Sastre A. Examination of the melatonin hypothesis in women exposed at night to EMF or bright light. Environ Health Perspect. 2001; 109: 501–7. 10.1289/ehp.01109501.
- Gobba F, Bravo G, Scaringi M, Roccatto L. No association between occupational exposure to ELF magnetic field and urinary 6-sulfatoximelatonin in workers. Bioelectromagnetics. 2006; 27: 667–73. 10.1002/bem.20254.
- Jentsch A., Lehmann M, Schon E, Thoss F, Zimmerman G. Weak magnetic fields change extinction of a conditioned reaction and daytime melatonin levels in the rat. Neurosci Lett. 1993; 157: 79–82. 10.1016/0304-3940(93)90647-4.
- Truong H, Yellon SM. Effects of various acute 60 Hz magnetic field exposures on the nocturnal melatonin rise in the Djungarian hamster. J Pineal Res. 1997; 22: 177–183. 10.1111/j.1600-079X.1997.tb00321.x.
- Rechtscheffan A. Standardization of sleep architecture in the rat. Sleep. 1960; 9: 20–34.
- Brown GM, Bar-Or A, Grossi D, Kashur S, Johansson SM, Yie SM. Urinary 6-sulphatoxymelatonin, an index of pineal function in the rat. J Pineal Res. 1991; 10: 141–7. 10.1111/j.1600-079X.1991.tb00831.x.
- Jaffe RA. Chronic exposure to a 60-Hz electric field: effects on neuromuscular function in the rat. Bioelectromagnetics. 1980; 2: 227–39. 10.1002/bem.2250020304.
- Klante G, Brinsscwitz T, Secci K, Wollnik F, Steinlechner S. Creatinine is an appropriate reference for urinary melatonin of laboratory animals and humans. J Pineal Res. 1997; 23: 191–7. 10.1111/j.1600-079X.1997.tb00354.x.
- Semm P. Neurobiological investigations on the magnetic sensitivity of the pineal gland in rodents and pigeons. Comp Biochem Physiol. 1983; 76A: 683–9. 10.1016/0300-9629(83)90129-9.
- Kato M, Honma K, Shigemitsu Shiga Y. Horizontal or vertical 50 Hz, 1-microT magnetic fields have no effect on pineal gland or plasma melatonin concentration of albino rats. Neurosci Lett. 1994; 168: 205–8. 10.1016/0304-3940(94)90451-0.
- Welker HA, Semm P, Willig RP, Commentz JC, Wiltschko W, Vollrath L. Effects of artificial magnetic field on serotonin N-acetyltransferase and melatonin content of the rat pineal gland. Exp Brain Res. 1983; 50: 426–32. 10.1007/BF00239209.
- Yellon SM. Acute 60 Hz magnetic field exposure effects on the melatonin rhythm in the pineal gland and circulation of the adult Djungarian hamster. J Pineal Res. 1994; 16: 136–44. 10.1111/j.1600-079X.1994.tb00093.x.
- Reiter RJ. Static and extremely low frequency electromagnetic field exposure: reported effects on the circadian production of melatonin. J Cell Biochem. 1993; 51: 394–403.
- Rosen LA, Barber I, Lyle DB. A 0.5 G, 60 Hz magnetic field suppresses melatonin production in pinealocytes. Bioelectromagnetics. 1998; 19: 123–7.
- Graham C, Cook MR, Cohen HD, Riffle DW, Hoffman S, Gerkovich MM. Human exposure to 60-Hz magnetic fields: neurophysiological effects. Int J Psychophys. 1999; 33: 169–75. 10.1016/S0167-8760(99)00031-8.
- Bakos J, Nagy N, Thuróczy G, Szabó LD. One week of exposure to 50 Hz, vertical magnetic field does not reduce urinary 6-sulphatoxymelatonin excretion of male wistar rats. Bioelectromagnetics. 2002; 23: 245–8. 10.1002/bem.10014.
- Bakos J, Nagy N, Thuróczy G, Szabó LD. Urinary 6-sulphatoxymelatonin excretion is increased in rats after 24 hours of exposure to vertical 50 Hz, 100 microT magnetic field. Bioelectromagnetics. 1997; 18: 190–2.
- Pfluger DH, Minder CE. Effects of exposure to 16.7 Hz magnetic fields onurinary 6-hydroxymelatonin sulfate excretion of Swiss railway workers. J Pineal Res. 1996; 21: 91–100. 10.1111/j.1600-079X.1996.tb00275.x.
- Lerchl A, Zachmann A, Ali MA, Reiter RJ. The effects of pulsing magneticfields on pineal melatonin synthesis in a teleost fish (brook trout). Neurosci Lett. 1998; 256: 171–3. 10.1016/S0304-3940(98)00778-2.
- Martinez-Soriano F, Gimenez-Gonzalez M, Armanazas E, Ruiz-Torner A. Pineal “synaptic ribbons” and serum melatonin levels in the rat following the pulse action of 52 Gs (50 Hz) magnetic fields: an evlutive analysis over 21 days. Acta Anat. 1992; 143: 289–93. 10.1159/000147264.
- Blackman CF, Benane SG, Rainowitz JR, House DE, Joines WT. A Role for the magnetic field in the radiation-induced efflux of calcium ions from brain tissue in vitro. Bioelectromagnetics. 1985; 6: 327–37. 10.1002/bem.2250060402.
- Bawin SM, Adey WR. Sensitivity of calcium binding in cerebral tissue to weak environmental electric fields oscillating at low frequency. PNAS. 1985; 73: 1999–2003. 10.1073/pnas.73.6.1999.
- Holmes SW, Sugden D. Effects of melatonin on sleep and neurochemistry in the rat. Br J Pharmacol. 1982; 76: 95–101.
- Mendelson WB, Gillin JC, Dawson SD, Lewy AJ, Wyatt RJ. Effects of melatonin and propranolol on sleep of the rat. Brain Res. 1980; 20: 240–4. 10.1016/0006-8993(80)90793-3.
- Huber R, Deboer T, Schwierin B, Tobler I. Effect of melatonin on sleep and brain temperature in the Djungarian hamster and the rat. Physiol Behav. 1998; 65: 77–82. 10.1016/S0031-9384(98)00125-5.
- Köylü H, Mollaoglu H, Ozguner F, Naziroglu M, Delibas N. Melatonin modulates 900 Mhz microwave-induced lipid peroxidation changes in rat brain. Toxicol Ind Health. 2006; 22: 211–6. 10.1191/0748233706th263oa.
- Koyu A, Ozguner F, Cesur G, Gokalp O, Mollaoglu H, Caliskan S. No effects of 900 MHz and 1800 MHz electromagnetic field emitted from cellular phone on nocturnal serum melatonin levels in rats. Toxicol Ind Health. 2005; 21: 27–31. 10.1191/0748233705th212oa.
- Cajochen C, Krauchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003; 15: 432–7. 10.1046/j.1365-2826.2003.00989.x.
- Reiter RJ. The melatonin rhythm: both a clock and a calendar. Cell Mol Life Sci. 1993; 49: 654–64. 10.1007/BF01923947.
- Hong SC, Kurokawa Y, Kabuto M, Ohtsuka R. Chronic exposure to ELFmagnetic fields during night sleep with electric sheet: effects on diurnal melatonin rhythms in men. Bioelectromagnetics. 2001; 22: 138–43.
- NRC (National Research Council). Health effects of residential electric and magnetic fields. Washington DC: National Academy Press, 1997.