Abstract
Human enterovirus 71 (EV71) epidemics have affected various countries in the past 40 years. EV71 commonly causes hand, foot and mouth disease (HFMD) in children, but can result in neurological and cardiorespiratory complications in severe cases. Genotypic changes of EV71 have been observed in different places over time, with the emergence of novel genotypes or subgenotypes giving rise to serious outbreaks. Since the late 1990s, intra- and inter-typic recombination events in EV71 have been increasingly reported in the Asia-Pacific region. In particular, ‘double-recombinant’ EV71 strains belonging to a novel genotype D have been predominant in mainland China and Hong Kong over the last decade, though co-circulating with a minority of other EV71 subgenotypes and coxsackie A viruses. Continuous surveillance and genome studies are important to detect potential novel mutants or recombinants in the near future. Rapid and sensitive molecular detection of EV71 is of paramount importance in anticipating and combating EV71 outbreaks.
Human enterovirus 71 (EV71) is a small, non-enveloped, icosahedral virus that belongs to the human EV species A in the genus Enterovirus within the family Picornaviridae. EV71 and coxsackievirus A16 (CVA16) are common etiological agents of hand, foot and mouth disease (HFMD) in children, but the former can cause severe complications, such as aseptic meningitis, acute flaccid paralysis (AFP), meningoencephalitis and cerebellitis, with mortality rate ranging from 10 to 25.7% (Citation1, Citation2). Another neurotropic EV, poliovirus, is nearly completely eradicated as a result of global immunization efforts (Citation3). Thus, in the absence of effective vaccines and antivirals against EV71, EV71 may become an important pathogen, replacing poliovirus, with increasing health threat to humans. Since the late 1990s, EV71 has seriously affected the Asia-Pacific region (Citation4–Citation10). In recent years, there have been an increasing number of reports of HFMD outbreaks with fatal cases due to EV71 in China (Citation11–Citation18). In 2012, EV71 was found to be associated with ‘mystery disease’ that killed most patients shortly after admission to hospital in Cambodia (Citation19). EV71 is well known to cause outbreaks, which often occur in a cyclical pattern, every 2–3 years, in various countries (Citation20).
The single-stranded positive-sense RNA genome of EV71 is around 7.4-kb long, which is flanked by 5′ and 3′ untranslated regions (UTRs). The polyprotein consists of P1, P2, and P3 regions, which encodes structural proteins, VP4, VP2, VP3, VP1, and non-structural proteins, 2A, 2B, 2C and 3A, 3B, 3C, 3D, respectively. Based on molecular typing using VP4 and VP1 gene sequences (Citation21), EV71 is classified into three genotypes, A, B (subgenotypes B1–B5), and C (subgenotypes C1–C5) (Citation21, Citation22). A separate subgenotype B0 has recently been identified in a retrospective analysis of EV71 strains in the Netherlands from 1963 to 1967 (Citation23). Two studies on complete genome analysis of EV71 strains of subgenotype C4 suggested that this subgenotype should be classified as a novel genotype D (Citation24, Citation25).
Mutation and recombination are well-known phenomena in EV evolution. The infidelity of EV 3D polymerase leads to their mutation rates of around one per genome per replication (Citation26). Mutations in various regions such as 5′UTR, VP1, VP2, 2A, 2C, and 3D of EV71 have shown to be associated with alterations of virulence in animal models and humans (Citation27–Citation33). Recombination occurs in enteroviruses as a result of template switching during negative-strand synthesis, which is thought to be mediated by a ‘copy-choice’ mechanism (Citation34, Citation35). Inter-typic and intra-typic recombination events were frequently detected in EV71 strains circulating in the Asia-Pacific region (Citation24, Citation36–Citation38). In recent years, recurring HFMD outbreaks caused by EV71 of subgenotype C4 (‘double-recombinant’ belonging to a novel genotype D) have been reported in Hong Kong and different provinces in China (Citation11–Citation16, Citation39). In this review, we provide an update on the epidemiology and genetic evolution of EV71.
Methods
Keywords including ‘EV71’, ‘EV71’, ‘EV’, ‘epidemiology’, ‘evolution’, ‘genotype’, ‘mutation’, and ‘recombination’ were used for Medline search. The search results were then manually screened for literature on the epidemiology and genetic evolution of EV71.
Epidemiology of EV71
Many studies have reported the detection of EV71 in clinical specimens of patients from various countries in Asia, Australia, Europe, and America () (Citation40–Citation47). During 1969–1972, the prototype EV71 strain BrCr and related strains were first identified from patients with neurological disease in California (Citation40). In the early 1970s, EV71 occurred in patients mainly with meningitis in USA, Sweden, and Australia (Citation41–Citation43). A large outbreak of poliomyelitis-like disease occurred in Bulgaria in 1975, during which 21% of around 700 cases showed paralytic forms and 27 were fatal as a result of EV71 infections (Citation44, Citation48) (Citation49). Three years later, EV71 was the major causative agent of meningitis and encephalitis during the severe epidemic of acute central nervous system (CNS) diseases in Hungary (Citation45). During the 1970s, Japan experienced two outbreaks of HFMD by EV71, in which a significant proportion of patients with HFMD accompanied CNS disorders and some of them died (Citation46, Citation50) (Citation51). Based on the above findings, EV71 isolates in America, Australia, and Europe were strongly associated with severe CNS complications, while those in Japan showed both dermatotropic and neurotropic features. Mortality rates due to EV71 outbreaks in the 1980s were low when compared to those occurred in Bulgaria and Hungary in the 1970s (Citation52).
Table 1 Summary of major EV71 outbreaks in different geographical regions
Since the late 1990s, recurrent EV71 epidemics of various scales have occurred in the Asia-Pacific region, including Australia, China, Malaysia, Singapore, Taiwan, Thailand, and Vietnam (Citation4–Citation10). A huge number of deaths associated with HFMD outbreaks occurred in Malaysia, Taiwan, and China. During the outbreak with 2,628 HFMD cases in Sarawak Malaysia, 29 children died due to rapidly progressive cardiorespiratory failure caused by EV71 in 1997 (Citation53). In Taiwan, 78 of 405 children with severe complications died in the large HFMD outbreak in 1998 (Citation54), followed by another outbreak in 2000 with 41 deaths among 80,677 HFMD cases (Citation8). During these two outbreaks, EV71 accounted for a major proportion of deaths in children in Taiwan. Since May 2008, HFMD has been a notifiable disease in the national surveillance system in China (Citation17). From 2008 to 2011, recurring HFMD outbreaks have occurred in various provinces in China and the number of HFMD cases increased from 488,955 with 126 deaths to 1,619,706 with 509 deaths (Citation11–Citation18, Citation55). During this period, coxsackieviruses A2, A4, A5, A6, A10, A12, A16, and EV71 were co-circulating in the outbreaks (Citation13, Citation14) (Citation56), of which EV71 was responsible for most fatal cases (Citation16).
The number of EV71-associated HFMD cases was relatively low in Europe compared to that in the Asia-Pacific region. In a prospective study from Norway (Citation57), EV71 was detected in stool specimens from asymptomatic children, and the absence of disease may be due to host factors (immune system, genetic effect, nutritional and hygiene status) and/or viral factors. Since HFMD is not a disease under surveillance in Europe and healthy individuals are usually not the subjects under surveillance, the prevalence of EV71 may be underestimated (Citation23, Citation58–Citation60).
EV71 epidemics usually occur in summer months. In studies with clinical specimens collected throughout the year, seasonal patterns of EV71 infection have been demonstrated. Several studies showed that EV71 could be detected throughout the year, but its predominance was found in different months in various regions (Citation9, Citation10) (Citation57). A higher incidence was observed in summer months in Norway (Citation57), but in the fall in Vietnam and Thailand (Citation9, Citation10). Some countries have shown two peak activities of EV71 infections during their study periods. In 1998, there were two peaks of EV71 infections (one in June and the other in October) in the large outbreak in Taiwan (Citation61–Citation63). In southern Vietnam, a smaller peak (March–May) and a higher peak (September–December) of EV71 infections were found in 2005, and these months are interim periods between the dry and wet seasons (Citation10). In Hong Kong, a higher peak (May–June) and a smaller peak (October–December) have been noted in 2008 (Citation39). In the Netherlands, a higher peak (June–July) and a smaller peak (September–October) of cases were observed during 1963–2008 (Citation23). Some studies have also reported variation of peak season between different years. In Australia, peak activity of EV71 shifted from summer in 1973 to winter in 1986 (Citation43, Citation59) (Citation64). In Japan, EV71 was detected in summer months in 1998–1999, but was only detected in the fall during 2001–2002 (Citation65). In Malaysia, EV71 predominance shifted from summer in 2000 to spring in 2003 (Citation66). The observed seasonal changes may be due to climatic factors that favor viral survival, variations in host immune response to infection and host behaviors that increase contact between individuals.
Clinical impact of EV71 infection
EV71 infection usually causes HFMD or herpangina (Citation54, Citation67), but it can result in more severe illness, which is characterized by high-grade fever (body temperature above 39°C), vomiting, and cardiopulmonary or neurological complications (Citation54). In the large-scale EV71 epidemic in Taiwan in 1998, 78 patients died with severe illnesses, including AFP, aseptic meningitis, encephalitis, pulmonary edema or hemorrhage, and myocarditis, among whom around 80% had pulmonary edema or hemorrhage (Citation54). During an HFMD outbreak in Australia in 1999, there was a study showing that 14 children with EV71 infection had neurological complications including meningitis, acute cerebellar ataxia, acute transverse myelitis, Guillain–Barré syndrome, benign intracranial hypertension, febrile convulsion, and opso-myoclonus syndrome (Citation68). In a follow-up study of 142 children after EV71 infection with CNS involvement, neurological disease and cardiorespiratory failure were likely associated with neurologic sequelae (limb weakness and atrophy), delayed neurodevelopment, and reduced cognitive function (Citation69). In 2012, more than 50 children, who presented with fever, respiratory illness, and neurological complications, died within a short period of time after admission to hospital in Cambodia, where EV71 was eventually identified as a possible cause of the outbreak (Citation70).
Detection of EV71
Traditional methods of EV71 detection are virus isolation and serological tests (Citation40, Citation41) (Citation45, Citation46) (Citation48, Citation64) (Citation71, Citation72). For cell culture, human rhabdomyosarcoma (RD) and monkey kidney cell lines (e.g., Vero) are commonly used to isolate EV71 (Citation1), but this method is rather time-consuming (take days to weeks) and has poor sensitivity (Citation73). For serology, cases were reported as positive when paired sera from patients showed fourfold increase in neutralizing antibody titers against EV71 (Citation40, Citation44–Citation46, Citation48). However, acute and convalescent sera are usually taken at least 2 weeks apart (Citation74), rendering serological tests on paired sera, together with virus isolation, unsuitable for managing EV71 in outbreak situations, during which early detection of EV71 is required to allow prompt implementation of preventive and control measures. Since the late 1990s, rapid and sensitive molecular diagnostic tests such as reverse-transcriptase polymerase chain reaction (RT-PCR) have been increasingly applied for EV71 detection. In epidemiological studies during the HFMD outbreaks, 5′UTR and VP1 were the most widely used targets for EV71 detection (). 5′UTR was used because this region showed high sensitivity for the detection of EV71 (Citation61, Citation73) (Citation75). However, 5′UTR is a hot spot of recombination in enteroviruses (Citation76, Citation77), making this region inappropriate for genotyping (Citation21). In contrast, VP1 gene is most commonly used for phylogenetic analysis as it shows a high degree of genetic diversity and no homologous recombination has been reported to take place within the VP1 gene in EV71 (Citation78). To better determine the prevalence of EV71 in clinical specimens, 5′UTR should be used for detection and VP1 for genotype and subgenotype classification.
Table 2 Summary of studies on the phylogeny of EV71 strains
Genotypic changes in EV71
Based on molecular characterization using VP1 gene sequences, EV71 was classified into genotypes A, B, and C () (Citation79). The prototype strain BrCr isolated in 1970 during the epidemic in California was classified as genotype A (Citation40, Citation79) (Citation80). There was no report on the circulation of EV71 genotype A strains thereafter until 2008 when re-emergence of this genotype occurred in central China (Citation81). Genotype A might not be the first genotype detected in human population because a novel subgenotype of EV71 circulating in the Netherlands during 1963–1967 was recently identified as subgenotype B0 (Citation23), which existed earlier than genotype A. Since the 1970s, EV71 strains of genotype B (subgenotypes B1–B5) have been circulating globally. Subgenotype B1 was the major type responsible for EV71 epidemics in America (Citation79), Europe (Citation6, Citation23) (Citation82), Asia (Citation21, Citation83–Citation85) and Australia (Citation23, Citation79) in the 1970s, while subgenotype B2 became predominant in the United States (Citation79), the Netherlands (Citation23), Australia (Citation86), and Japan (Citation83) in the 1980s. In the mid-1980s, EV71 strains of subgenotype C1 emerged and have been circulating in different regions afterwards () (Citation4, Citation9) (Citation10, Citation21) (Citation23, Citation39) (Citation57–Citation60, Citation79) (Citation82–Citation92).
Fig. 1 Phylogenetic tree of the VP1 region of EV71 strains detected in various countries, showing different genotypes and subgenotypes of EV71. Eight hundred and fifty-five nucleotide positions in each VP1 region were included in the analysis. The tree was constructed by the neighbor joining method and bootstrap values calculated from 1,000 trees. The scale bar indicates the estimated number of substitutions per 100 nucleotides. EV71 strains of potential novel genotype or subgenotype were highlighted in gray. GenBank accession numbers are indicated in parentheses.
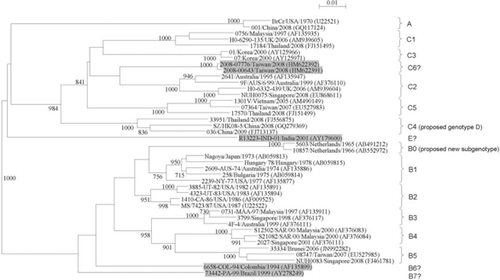
Table 3 Summary of EV71 subgenotypes in different geographical regions from 1963 to 2011
The emergence of a novel genotype or subgenotype of EV71 can lead to large outbreaks, with well-known examples in the Asia-Pacific region since 1997 (). In Malaysia, a widespread community HFMD outbreak with fatal cases occurred in 1997, during which the most prevalent subgenotype B3 was co-circulating with other subgenotypes B4, C1, and C2 (Citation6, Citation21) (Citation23, Citation53) (Citation79, Citation85) (Citation91). Subgenotypes B4 and C1 were identified as a cause of an outbreak in 2000 (Citation6, Citation91), while subgenotype B5 was noted in an outbreak in 2005 (Citation65, Citation66) (Citation85, Citation91). In Australia, co-circulation of subgenotypes B3 and C2 contributed to a large outbreak in 1999, with subgenotypic changes to B4 and C1 in 2000 (Citation4, Citation21) (Citation23, Citation85) (Citation86). In Singapore, three major outbreaks have been caused by subgenotypes B4 (2000) and B5 (2006 and 2008) (Citation4, Citation85) (Citation93, Citation94). Brunei reported its first EV71 outbreak in 2006, of which most EV71 isolates belonged to subgenotype B5 (Citation95). In Taiwan, the predominant subgenotype C2 accounted for a devastating EV71 outbreak in 1998, followed by a shift to subgenotype B4 (1999–2003), C4 (2004–2005), C5 (2006), B5 (2007–2009), and C4 (2010) (Citation8, Citation21–Citation23, Citation85) (Citation96–Citation103). Intriguingly, similar pattern of inter-genotypic change (a change from one genotype to another) of EV71 has been observed in Japan in outbreaks since 1997 (Citation65, Citation83–Citation85, Citation87) (Citation104).
Inter-genotypic change of EV71 has also been detected in Europe. In the Netherlands, genotype B (subgenotypes B0, B1, and B2) from 1963 to 1986 was changed to genotype C (subgenotypes C1 and C2) since 1987 (Citation23). In Denmark, only subgenotype C2 was identified in 2005–2006, with a shift to predominant subgenotype B5 in 2007 (Citation59). In Germany, a shift from subgenotype B2 (1997–1998) to subgenotype C1 (2000–2003) has been noted, followed by intra-genotypic change (a change from one subgenotype to another of the same genotype) to C4 (2004) and C2 (2006–2007) (58). Intra-genotypic change of EV71 has been found in some other European and Asian countries. Two studies demonstrated that there was a shift from subgenotype C1 (2001–2003 in Austria; 2000 in Hungary) to C4 (2004 in Austria; 2004–2005 in Hungary) (Citation82, Citation89). During 2000–2009, all EV71 strains belonged to genotype C in France, in which C1 strains predominated from 2000 to 2005, but C2 strains became predominant since 2007 (Citation60, Citation105). In Korea, subgenotype C3 was identified in an EV71 epidemic in 2000, followed by the detection of subgenotype C4 in 2003 and 2009 (Citation21, Citation85) (Citation106–Citation108).
Circulation of a single EV71 subgenotype was reported in several places. In Iran, only EV71 of subgenotype C1 was detected from a child with AFP in 2005 (Citation90). In Greece, all EV71 strains detected from children with HFMD, febrile illness, or maculopapular rash belonged to subgenotype C2 in 2009–2010 (Citation109). Coexistence of more than one subgenotype was observed in some other regions (). During 1998–2006, subgenotype C1 predominated in the United Kingdom, with the existence of subgenotype C2 in 1999 and 2006 (Citation88). The first report of subgenotype C5 in Vietnam showed that this predominant subgenotype was co-circulating with subgenotypes C1 and C4 in 2005 (Citation10). In Thailand, subgenotypes C1 and C4 were identified in 2008, followed by the detection of subgenotypes C1, C2, C4, and B5 in 2009 (Citation9, Citation84) (Citation92, Citation110). In China, the major subgenotype C4, which was further divided into C4a (2002–2011) and C4b (1998–2004), co-circulated with subgenotype C2 (Citation5, Citation12–Citation17, Citation84) (Citation110–Citation112). Similar subgenotype predominance was noted in Hong Kong (situated on China's south coast), where the main subgenotype of EV71 belonged to C4, with co-detection of a minority of subgenotypes B3, B4, B5, C1, and C2 strains during 1998–2008 (Citation39).
Identification of EV71 genotype or subgenotype has not been standardized yet, although several studies have proposed certain criteria for typing. Distinct clusters with high bootstrap supports in phylogenetic trees constructed by using VP1 gene indicated the presence of a novel genotype or subgenotype of EV71 (Citation4, Citation79). Furthermore, cutoff values for genotyping and subgenotyping of EV71 using VP1 have been proposed, with a nucleotide sequence divergence of 15–20% between genotypes and of 4–14% between subgenotypes (Citation25, Citation79) (Citation101). According to these criteria, previously untyped EV71 isolates may belong to a novel genotype or subgenotype. Two studies demonstrated that an EV71 strain 6658-COL-94 from Columbia and a strain 73442-PA-99 from Brazil were closely related to genotype B isolates (Citation79, Citation113). Based on phylogenetic analysis using VP1 nucleotide sequences of EV71 (), the two strains formed branches separate from other genotype B strains. Moreover, VP1 sequence divergence between the Colombian strain and other genotype B strains was 6.1–12% and between the Brazilian strain and other genotype B strains was 8.2–12%, suggesting that they should belong to novel subgenotypes B6 and B7, respectively. As shown in , EV71 strains 2008-00643 and 2008-07776 from Taiwan (Citation100) formed a cluster distinct from subgenotype C2 isolates. In addition, the VP1 sequence divergence between theses Taiwanese strains and other genotype C strains is 6.7–13.6%. These indicated that the two Taiwanese strains should be regarded as a new subgenotype C6. Phylogenetic analysis of 5′UTR, VP4 and VP1 sequences of an EV71 strain R13223-IND-01 isolated from a case of AFP in India in 2001 showed that it may belong to a novel genotype (Citation114). As shown in , this isolate formed a branch distinct from other EV71 strains. Furthermore, the VP1 sequence divergence between this Indian strain and other strains of EV71 genotypes A, B, and C was 14.9–18.5%. These revealed that the strain R13223-IND-01 should be designated as a new genotype E. Recently, Chan et al. proposed new cutoff values for genotyping based on complete genome sequences of EV71, with a nucleotide divergence of 17–22% between genotypes and of 10–14% between subgenotypes (Citation25). Complete genome sequencing and further sequence analysis are required to better determine the genotype or subgenotype of EV71.
Relationship between EV71 genotypes and disease severity
Several research groups have examined the relationship between EV71 genotypes and outcomes of EV71 infections. In a study from Australia, EV71 of subgenotype C2 was strongly linked to severe neurological disease in 1999 (Citation4). Another study from Malaysia revealed that children infected with EV71 of subgenotype B4 were less likely to have CNS complications than those infected with other subgenotypes (Citation66). However, a recent study from the Netherlands demonstrated that children with genotype B virus were more likely to have neurological complications than those with genotype C virus (Citation23). In Taiwan, EV71 of genotypes B and C could be isolated from both fatal and mild HFMD cases from 1998 to 2000 (Citation8, Citation97). Furthermore, VP1 sequences of EV71 isolated from patients with mild HFMD and fatal encephalitis in Malaysia in 1997 were almost identical (Citation79). Hence, more careful and systematic analysis will be required to delineate the correlation between the EV71 genotypes and disease severity.
Recombination events in EV71
Under selective pressures from hosts and environment, recombination possibly takes place when at least two viruses infect the same cell, resulting in the production of progenies with new genomic combinations that may favor viral survival during evolution (Citation115). As recombination in RNA viruses might enhance their host range (Citation116) and pathogenicity (Citation117), and confer antiviral resistance (Citation118), it is important to assess the impact of recombination in EV71. Recombination is a common phenomenon in human enteroviruses, with preferential recombination sites in non-structural protein coding regions P2 and P3 (), where a high nucleotide sequence identity between two parental strains may favor homologous recombination by a ‘copy-choice’ mechanism (Citation34, Citation35). Breakpoints are frequently detected at 5′UTR, P2 and P3 by recombination analysis using sequencing of different gene regions or complete genomes (Citation24, Citation37) (Citation38, Citation76) (Citation77, Citation119–Citation123). In a recent study using VP1 and 3D for recombination analysis on 308 EV71 isolates collected from 19 countries over a 40-year period, 11 3D clades were identified, each specific to EV71 and associated with specific subgenotypes but interspersed phylogenetically with clades of CVA16 and other HEV-A serotypes (Citation124). Sporadic recombination events were detected within genotypes but no evidence for inter-typic recombination as described in other studies (Citation24, Citation36) (Citation37, Citation125). Since recombination breakpoints can occur at 5′UTR and different gene regions of EV71, amplification of two gene regions (VP1 and 3D) may not be sufficient to reveal the actual recombination events.
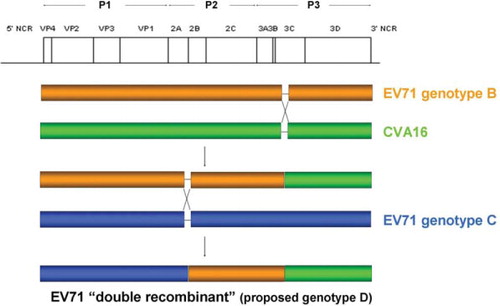
Fig. 2 Schematic diagram showing intra- and inter-typic recombination events occurred in EV71 strains SZ/HK08-5 and SZ/HK08-6 of subgenotype C4 (proposed genotype D).
Recent studies on EV71 recombination usually involve complete genome sequence analysis. During the HFMD outbreaks in Malaysia in 1997, EV71 subgenotype B3 was predominant, which was also found in other areas in the Asia-Pacific region including Japan, Singapore, Australia, and Hong Kong (Citation4, Citation21) (Citation23, Citation36) (Citation39, Citation83) (Citation85) () but disappeared after 1999. Complete genome analysis of two strains SHA63 and SHA66 showed that they were most closely related to EV71 genotype B strains for 5′UTR, P1 and P2 regions, but to CVA16 for the P3 and 3′UTR (Citation36). This was the first to demonstrate inter-typic recombination (between different types of human enteroviruses, e.g., EV71 and CVA16). Since 1998, emergence of EV71 subgenotype C4 has been responsible for the HFMD outbreaks in China. Phylogenetic and bootscan analyses on two complete genomes of EV71 C4 strains SHZH98 and SHZH03 from Shenzhen revealed that they were most closely related to EV71 subgenotype C2 strain for P1 region, but to CVA16 for P3 region (Citation37). In the same study, a CVA16 strain Tainan5079 was closely related to CVA16 strain G-10 for P1 region, but to EV71 strain BrCr of genotype A for P2 and P3 regions. These indicated that inter-typic recombination events have occurred between EV71 subgenotype C2 and CVA16 strain G-10, and between CVA16 strain G-10 and EV71 genotype A, both with a breakpoint at 2A region, a recombination hot spot in enteroviruses (Citation1, Citation120) (Citation121).
Various recombinant forms of EV71 accounted for outbreaks in Taiwan since 1998. Inter-typic recombination between EV71 and coxsackievirus A8 (CVA8) was detected in EV71 subgenotype C2 isolates that were responsible for the large outbreak associated with severe encephalitis in 1998 (Citation96), which was in line with a previous study (Citation126). During 2000–2001, outbreaks in Taiwan were caused by EV71 subgenotype B4, which was possibly evolved from genotypes B3 and B2 (Citation96). From 2004 to 2005, the predominant EV71 subgenotype C4 emerged and was shown to be a recombinant resulting from intra-typic recombination (between the same type of human EV, e.g., EV71) between genotype C and genotype B (Citation96), consistent with a previous study (Citation38).
In China, a dramatic increase in the number of HFMD cases from 2007 to 2008 suggested EV71 and CVA16 might have undergone recombination (Citation24). Complete genome analysis of two EV71 strains (SZ/HK08-5 and SZ/HK08-6) and two CVA16 strains (SZ/HK08-3 and SZ/HK08-7) from Shenzhen revealed inter-typic recombination between CVA16 strain G-10 and EV71 genotype A at the 2A–2B junction the two CVA16 strains (Citation24), in line with previous results on CVA16 strain Tainan5079 (Citation37). For the two EV71 strains, intra-typic recombination between EV71 genotypes C and B at 2A–2B junction and inter-typic recombination between EV71 genotype B and CVA16 strain G-10 in 3C region were observed (). These ‘double-recombinant’ EV71 strains circulating in China and other EV71 subgenotype C4 strains were proposed to be a novel genotype D (Citation24). The proposal of this new genotype was also supported by another study conducted by Chan et al. (Citation25). In this study, EV71 subgenotype C4 was shown to have a nucleotide sequence divergence of 18.1% (17–20%), which exceeded the average threshold divergence of 14.95% for EV71 subgenotyping when comparing with EV71 subgenotypes C1–C5. Based on the evidence from the two studies (Citation24, Citation25), EV71 subgenotype C4 should be redesignated as the novel genotype D. Since 2008, there has been an increase in the number of studies on recombination in EV71 during HFMD outbreaks in China, including the ‘double-recombinant’ subgenotype C4 strains probably belonging to the proposed genotype D (Citation11, Citation125) (Citation127). Although the correlation between natural recombination in EV71 and pathogenicity remains uncertain, an in vitro study demonstrated that a chimeric recombinant virus with improved growth and larger plaque phenotypes could be artificially constructed by replacing the structural region of a slow-growth EV71 strain with the region of a rapid-growth EV71 strain (Citation128). Thus, it is possible to generate a highly pathogenic EV71 strain when a less virulent strain can acquire an antigenically distinct capsid region or non-structural regions from a more virulent strain via natural recombination.
Over the past decade, EV71 of predominant subgenotype C4 has been co-circulating with some other subgenotypes in mainland China and Hong Kong (Citation5, Citation17) (Citation23, Citation39) (Citation129), which may increase the chance of recombination. In addition, densely populated areas with poor hygiene, sanitation, and healthcare infrastructure may further hasten not only recombination, but also viral mutation. As Hong Kong is a gateway to China with extensive passenger movements and global transport networks, it may be a place for the spread of novel EV71 mutants or recombinants to other cities, posing pandemic threats as in the case of SARS.
Antiviral strategies against EV71
Despite the occurrence of recurrent EV71 outbreaks with severe complications and fatal cases in the past few decades, effective antivirals against EV71 are still not available (Citation130). Intravenous immunoglobulin (IVIG) has been used in patients with complicated EV71 infections, which may help suppress viral replication and limit organ damage through anti-inflammatory activities (Citation66). In vitro and in vivo studies demonstrated that ribavirin and type I interferons exhibited protective effects on EV71 (Citation131, Citation132). Pleconaril has demonstrated antiviral activity against a broad spectrum of EV serotypes in vitro and in vivo (Citation133), but it cannot inhibit the cytopathic effect induced by EV71 (Citation134). In a study by Shih et al., mutation in VP1 of EV71 was shown to confer resistance to the inhibitory effects of pyridyl imidazolidinone (Citation135). EV71 mutants resistant to inhibitors of 2C protein of EV71, including metrifudil, N(6)-benzyladenosine and NF449, have also been identified (Citation136). In another study, Chen et al. demonstrated that EV71 displayed resistance to an antiviral agent DTriP-22 after an arginine-to-lysine substitution (R163K) in 3D polymerase (Citation137). So far, none of these antivirals possessed efficacy high enough for clinical use.
Due to the high frequency of mutations and recombination in EV71, viral factors may not be suitable targets for drug design. In contrast, targeting cellular factors temporarily dispensable for the host but essential for viral replication may prevent viral escape. RNA interference (RNAi) screening has been increasingly used to search for cellular factors required for viral infections (Citation138–Citation147) and this strategy holds a potential for antiviral development (Citation148). Further investigations to identify host factors important for EV71 replication will help explore the mechanisms of EV71 pathogenesis.
Concluding remarks
Over the past few decades, EV71 epidemics have occurred in various countries and caused a significant proportion of severe complications and deaths in children, particularly in the Asia-Pacific region. Mutation and recombination are the major evolutionary forces leading to emergence of genetically diverse EV71 variants that have accounted for the recurrent HFMD outbreaks. Despite recent findings of intra-typic and inter-typic recombination, the correlation between recombination and virulence in EV71 remains unclear. Owing to the common occurrence of recombination in EV71, sequencing of more than one region (e.g., VP1 and 3D) would allow the rapid and accurate genotyping of EV71 in clinical settings. To date, the majority of HFMD cases due to EV71 have been noted in China, which is the most populous country in the world. Since Hong Kong is well connected to China with international travel networks, the former may be a hub that facilitates the global dissemination of novel mutants or recombinants of EV71, posing pandemic threats in the near future. Continuous genomic studies on the evolution of EV71 in Hong Kong and other Asia-Pacific regions are important to detect new mutants or recombinants with epidemic potential. Frequent genetic variations in EV71 have hampered the development of drugs targeting to viral proteins and this obstacle could theoretically be overcome by targeting host factors that are inessential for humans but important for virus propagation. Genome-wide RNAi screening technology has successfully been applied for the identification of cellular factors crucial for replication of emerging viruses, such as HIV and influenza viruses. We foresee that this screening strategy will help unravel EV71–host interactions and provide insight into the discovery of novel antivirals to combat future EV71 epidemics.
Conflict of interest and funding
The authors declare no conflict of interest. This work was partly supported by the University Development Fund, The University of Hong Kong; HKSAR Research Fund for the Control of Infectious Diseases of the Food and Health Bureau; and Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Disease of the HKSAR Department of Health.
References
- Wong SS, Yip CC, Lau SK, Yuen KY. Human enterovirus 71 and hand, foot and mouth disease. Epidemiol Infect. 2010; 138: 1071–89.
- Ho M. Enterovirus 71: the virus, its infections and outbreaks. J Microbiol Immunol Infect. 2000; 33: 205–16.
- Centers for Disease Control and Prevention. Progress toward eradication of polio – worldwide, January 2011–March 2013. MMWR Morb Mortal Wkly Rep. 2013; 62: 335–38.
- McMinn P, Lindsay K, Perera D, Chan HM, Chan KP, Cardosa MJ. Phylogenetic analysis of enterovirus 71 strains isolated during linked epidemics in Malaysia, Singapore, and Western Australia. J Virol. 2001; 75: 7732–8.
- Li L, He Y, Yang H, Zhu J, Xu X, Dong J. Genetic characteristics of human enterovirus 71 and coxsackievirus A16 circulating from 1999 to 2004 in Shenzhen, People's Republic of China. J Clin Microbiol. 2005; 43: 3835–9.
- Herrero LJ, Lee CS, Hurrelbrink RJ, Chua BH, Chua KB, McMinn PC. Molecular epidemiology of enterovirus 71 in peninsular Malaysia, 1997–2000. Arch Virol. 2003; 148: 1369–5.
- Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis. 2003; 9: 78–85.
- Wang JR, Tuan YC, Tsai HP, Yan JJ, Liu CC, Su IJ. Change of major genotype of enterovirus 71 in outbreaks of hand–foot-and-mouth disease in Taiwan between 1998 and 2000. J Clin Microbiol. 2002; 40: 10–5.
- Chatproedprai S, Theanboonlers A, Korkong S, Thongmee C, Wananukul S, Poovorawan Y. Clinical and molecular characterization of hand–foot-and-mouth disease in Thailand, 2008–2009. Jpn J Infect Dis. 2010; 63: 229–33.
- Tu PV, Thao NT, Perera D, Huu TK, Tien NT, Thuong TC. Epidemiologic and virologic investigation of hand, foot, and mouth disease, southern Vietnam, 2005. Emerg Infect Dis. 2007; 13: 1733–41.
- Zhang Y, Zhu Z, Yang WZ, Ren J, Tan XJ, Wang Y. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J. 2010; 7: 94.
- Sun LM, Zheng HY, Zheng HZ, Guo X, He JF, Guan DW. An enterovirus 71 epidemic in Guangdong Province of China, 2008: epidemiological, clinical, and virogenic manifestations. Jpn J Infect Dis. 2011; 64: 13–8.
- Yang F, Zhang T, Hu Y, Wang X, Du J, Li Y. Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virol J. 2011; 8: 508.
- Yan XF, Gao S, Xia JF, Ye R, Yu H, Long JE. Epidemic characteristics of hand, foot, and mouth disease in Shanghai from 2009 to 2010: enterovirus 71 subgenotype C4 as the primary causative agent and a high incidence of mixed infections with coxsackievirus A16. Scand J Infect Dis. 2012; 44: 297–305.
- Liu MY, Liu W, Luo J, Liu Y, Zhu Y, Berman H. Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010. PLoS One. 2011; 6: e25287.
- Ni H, Yi B, Yin J, Fang T, He T, Du Y. Epidemiology and etiological characteristics of hand, foot, and mouth disease in Ningbo, China, 2008–2011. J Clin Virol. 2012; 54: 342–8.
- Tan X, Huang X, Zhu S, Chen H, Yu Q, Wang H. The persistent circulation of enterovirus 71 in People's Republic of China: causing emerging nationwide epidemics since 2008. PLoS One. 2011; 6: e25662.
- Wang Q, Wang Z. Epidemiology of hand, foot and mouth disease in China, 2008. Disease Surveillance. 2010; 25: 181–4.
- Seiff A. Cambodia unravels cause of mystery illness. Lancet. 2012; 380: 206.
- Tee KK, Lam TT, Chan YF, Bible JM, Kamarulzaman A, Tong CY. Evolutionary genetics of human enterovirus 71: origin, population dynamics, natural selection, and seasonal periodicity of the VP1 gene. J Virol. 2010; 84: 3339–50.
- Cardosa MJ, Perera D, Brown BA, Cheon D, Chan HM, Chan KP. Molecular epidemiology of human enterovirus 71 strains and recent outbreaks in the Asia-Pacific region: comparative analysis of the VP1 and VP4 genes. Emerg Infect Dis. 2003; 9: 461–8.
- Huang YP, Lin TL, Kuo CY, Lin MW, Yao CY, Liao HW. The circulation of subgenogroups B5 and C5 of enterovirus 71 in Taiwan from 2006 to 2007. Virus Res. 2008; 137: 206–12.
- van der Sanden S, Koopmans M, Uslu G, van der Avoort H, Dutch Working Group for Clinical Virology. Epidemiology of enterovirus 71 in the Netherlands, 1963 to 2008. J Clin Microbiol. 2009; 47: 2826–33.
- Yip CC, Lau SK, Zhou B, Zhang MX, Tsoi HW, Chan KH. Emergence of enterovirus 71 “double-recombinant” strains belonging to a novel genotype D originating from southern China: first evidence for combination of intratypic and intertypic recombination events in EV71. Arch Virol. 2010; 155: 1413–24.
- Chan YF, Sam IC, AbuBakar S. Phylogenetic designation of enterovirus 71 genotypes and subgenotypes using complete genome sequences. Infect Genet Evol. 2010; 10: 404–12.
- Drake JW. Rates of spontaneous mutation among RNA viruses. Proc Natl Acad Sci U S A. 1993; 90: 4171–5.
- Tong CY, Bible JM, Platt C. A fatal case of enterovirus 71 infection with a single nucleotide variation in domain V of the 5’ untranslated region. Pediatr Infect Dis J. 2011; 30: 1013–4.
- Yeh MT, Wang SW, Yu CK, Lin KH, Lei HY, Su IJ. A single nucleotide in stem loop II of 5'-untranslated region contributes to virulence of enterovirus 71 in mice. PLoS One. 2011; 6: e27082.
- Huang SW, Wang YF, Yu CK, Su IJ, Wang JR. Mutations in VP2 and VP1 capsid proteins increase infectivity and mouse lethality of enterovirus 71 by virus binding and RNA accumulation enhancement. Virology. 2012; 422: 132–43.
- Wang W, Duo J, Liu J, Ma C, Zhang L, Wei Q. A mouse muscle-adapted enterovirus 71 strain with increased virulence in mice. Microbes Infect. 2011; 13: 862–70.
- Chang GH, Lin L, Luo YJ, Cai LJ, Wu XY, Xu HM. Sequence analysis of six enterovirus 71 strains with different virulences in humans. Virus Res. 2010; 151: 66–73.
- Wang YF, Chou CT, Lei HY, Liu CC, Wang SM, Yan JJ. A mouse-adapted enterovirus 71 strain causes neurological disease in mice after oral infection. J Virol. 2004; 78: 7916–24.
- Li R, Zou Q, Chen L, Zhang H, Wang Y. Molecular analysis of virulent determinants of enterovirus 71. PLoS One. 2011; 6: e26237.
- Javis TC, Kirkegaard K. Poliovirus RNA recombination: mechanistic studies in the absence of selection. EMBO J. 1992; 11: 3135–45.
- Kirkegaard K, Baltimore D. The mechanism of RNA recombination in poliovirus. Cell. 1986; 47: 433–43.
- Chan YF, AbuBakar S. Recombinant human enterovirus 71 in hand, foot and mouth disease patients. Emerg Infect Dis. 2004; 10: 1468–70.
- Chan YF, AbuBakar S. Phylogenetic evidence for inter-typic recombination in the emergence of human enterovirus 71 subgenotypes. BMC Microbiol. 2006; 6: 74.
- Huang SC, Hsu YW, Wang HC, Huang SW, Kiang D, Tsai HP. Appearance of intratypic recombination of enterovirus 71 in Taiwan from 2002 to 2005. Virus Res. 2008; 131: 250–9.
- Ma E, Chan KC, Cheng P, Wong C, Chuang SK. The enterovirus 71 epidemic in 2008—public health implications for Hong Kong. Int J Infect Dis. 2010; 14: e775–80.
- Schmidt NJ, Lennette EH, Ho HH. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis. 1974; 129: 304–9.
- Deibel R, Gross LL, Collins DN. Isolation of a new enterovirus (38506). Proc Soc Exp Biol Med. 1975; 148: 203–7.
- Blomberg J, Lycke E, Ahlfors K, Johnsson T, Wolontis S, von Zeipel G. New enterovirus type associated with epidemic of aseptic meningitis and/or hand, foot, and mouth disease. Lancet. 1974; 2: 112.
- Kennett ML, Birch CJ, Lewis FA, Yung AP, Locarnini SA, Gust ID. Enterovirus type 71 in Melbourne. Bull World Health Organ. 1974; 51: 609–15.
- Shindarov LM, Chumakov MP, Voroshilova MK, Bojinov S, Vasilenko SM, Iordanov I. Epidemiological, clinical, and pathomorphological characteristics of epidemic poliomyelitis-like disease caused by enterovirus 71. J Hyg Epidemiol Microbiol Immunol. 1979; 23: 284–95.
- Nagy G, Takatsy S, Kukan E, Mihaly I, Domok I. Virological diagnosis of enterovirus type 71 infections: experiences gained during an epidemic of acute CNS diseases in Hungary in 1978. Arch Virol. 1982; 71: 217–27.
- Ishimaru Y, Nakano S, Yamaoka K, Takami S. Outbreaks of hand, foot, and mouth disease by enterovirus 71. High incidence of complication disorders of central nervous system. Arch Dis Child. 1980; 55: 583–8.
- Samuda GM, Chang WK, Yeung CY, Tang PS. Monoplegia caused by enterovirus 71: an outbreak in Hong Kong. Pediatr Infect Dis J. 1987; 6: 206–8.
- Chumakov M, Voroshilova M, Shindarov L, Lavrova I, Gracheva L, Koroleva G. Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch Virol. 1979; 60: 329–40.
- Melnick JL, Schmidt NJ, Mirkovic RR, Chumakov M, Lavrova I, Voroshilova M. Identification of Bulgarian strain 258 of enterovirus 71. Intervirology. 1980; 12: 297–302.
- Hagiwara A, Tagaya I, Yoneyama T. Epidemic of hand, foot and mouth disease associated with enterovirus 71 infection. Intervirology. 1978; 9: 60–3.
- Tagaya I, Takayama R, Hagiwara A. A large-scale epidemic of hand, foot and mouth disease associated with enterovirus 71 infection in Japan in 1978. Jpn J Med Sci Biol. 1981; 34: 191–6.
- Landry ML, Fonseca SN, Cohen S, Bogue CW. Fatal enterovirus 71 infection: rapid detection and diagnostic pitfalls. Pediatr Infect Dis J. 1995; 14: 1095–100.
- Chan LG, Parashar UD, Lye MS, Ong FG, Zaki SR, Alexander JP. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristics of the disease. Clin Infect Dis. 2000; 31: 678–83.
- Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF. An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med. 1999; 341: 929–35.
- Ministry of Health, People's Republic of China. [updated 2012; cited 2012 Sep 10]. Available from: http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohjbyfkzj/s3578/201202/54106.htm.
- Hu YF, Yang F, Du J, Dong J, Zhang T, Wu ZQ. Complete genome analysis of coxsackievirus A2, A4, A5, and A10 strains isolated from hand, foot, and mouth disease patients in China revealing frequent recombination of human enterovirus A. J Clin Microbiol. 2011; 49: 2426–34.
- Witsø E, Palacios G, Rønningen KS, Cinek O, Janowitz D, Rewers M. Asymptomatic circulation of HEV71 in Norway. Virus Res. 2007; 123: 19–29.
- Diedrich S, Weinbrecht A, Schreier E. Seroprevalence and molecular epidemiology of enterovirus 71 in Germany. Arch Virol. 2009; 154: 1139–42.
- Badran SA, Midgley S, Andersen P, Bottiger B. Clinical and virological features of enterovirus 71 infections in Denmark, 2005 to 2008. Scand J Infect Dis. 2011; 43: 642–8.
- Schuffenecker I, Mirand A, Antona D, Henquell C, Chomel JJ, Archimbaud C. Epidemiology of human enterovirus 71 infections in France, 2000–2009. J Clin Virol. 2011; 50: 50–6.
- Wang JR, Tsai HP, Chen PF, Lai YJ, Yan JJ, Kiang D. An outbreak of enterovirus 71 infection in Taiwan, 1998. II. Laboratory diagnosis and genetic analysis. J Clin Virol. 2000; 17: 91–9.
- Wang SM, Liu CC, Tseng HW, Wang JR, Huang CC, Chen YJ. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin Infect Dis. 1999; 29: 184–90.
- Liu CC, Tseng HW, Wang SM, Wang JR, Su IJ. An outbreak of enterovirus 71 infection in Taiwan, 1998: epidemiologic and clinical manifestations. J Clin Virol. 2000; 17: 23–30.
- Gilbert GL, Dickson KE, Waters MJ, Kennett ML, Land SA, Sneddon M. Outbreak of enterovirus 71 in Victoria, Australia, with a high incidence of neurological involvement. Pediatr Infect Dis J. 1988; 7: 484–8.
- Mizuta K, Abiko C, Murata T, Matsuzaki Y, Itagaki T, Sanjoh K. Frequent importation of enterovirus 71 from surrounding countries into the local community of Yamagata, Japan, between 1998 and 2003. J Clin Microbiol. 2005; 43: 6171–5.
- Ooi MH, Wong SC, Podin Y, Akin W, del Sel S, Mohan A. Human enterovirus 71 disease in Sarawak, Malaysia: a prospective clinical, virological, and molecular epidemiological study. Clin Infect Dis. 2007; 44: 646–56.
- Honkanen H, Oikarinen S, Pakkanen O, Ruokoranta T, Pulkki MM, Laitinen OH. Human enterovirus 71 strains in the background population and in hospital patients in Finland. J Clin Virol. 2013; 56: 348–53.
- McMinn P, Stratov I, Nagarajan L, Davis S. Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot, and mouth disease in Western Australia. Clin Infect Dis. 2001; 32: 236–42.
- Chang LY, Huang LM, Gau SS, Wu YY, Hsia SH, Fan TY. Neurodevelopment and cognition in children after enterovirus 71 infection. N Engl J Med. 2007; 356: 1226–34.
- Biswas T. Enterovirus 71 causes hand, foot and mouth disease outbreak in Cambodia. Natl Med J India. 2012; 25: 316.
- Chonmaitree T, Menegus MA, Schervish-Swierkosz EM, Schwalenstocker E. Enterovirus 71 infection: report of an outbreak with two cases of paralysis and a review of the literature. Pediatrics. 1981; 67: 489–93.
- Lin Y, Wen K, Pan Y, Wang Y, Che X, Wang B. Cross-reactivity of anti-EV71 IgM and neutralizing antibody in series sera of patients infected with Enterovirus 71 and Coxsackievirus A 16. J Immunoassay Immunochem. 2011; 32: 233–43.
- Singh S, Chow VT, Phoon MC, Chan KP, Poh CL. Direct detection of enterovirus 71 (EV71) in clinical specimens from a hand, foot, and mouth disease outbreak in Singapore by reverse transcription-PCR with universal enterovirus and EV71-specific primers. J Clin Microbiol. 2002; 40: 2823–7.
- Fact sheet on Infection Control for Enteroviral Infection in Hospitals. [updated 2010; cited 2012 Sep 10]. Available from: http://www.chp.gov.hk/files/pdf/fact_sheet_on_enteroviral_infection_for_hospitals.pdf.
- AbuBakar S, Chee HY, Al-Kobaisi MF, Xiaoshan J, Chua KB, Lam SK. Identification of enterovirus 71 isolates from an outbreak of hand, foot and mouth disease (HFMD) with fatal cases of encephalomyelitis in Malaysia. Virus Res. 1999; 61: 1–9.
- van der Sanden S, van Eek J, Martin DP, van der Avoort H, Vennema H, Koopmans M. Detection of recombination breakpoints in the genomes of human enterovirus 71 strains isolated in the Netherlands in epidemic and non-epidemics years, 1963–2010. Infect Genet Evol. 2011; 11: 886–94.
- Santti J, Hyypia T, Kinnunen L, Salminen M. Evidence of recombination among enteroviruses. J Virol. 1999; 73: 8741–9.
- Oberste MS, Maher K, Kilpatrick DR, Pallansch MA. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol. 1999; 73: 1941–8.
- Brown BA, Oberste MS, Alexander JP, Kennett ML, Pallansch MA. Molecular epidemiology and evolution of enterovirus 71 strains isolated from 1970 to 1998. J Virol. 1999; 73: 9969–75.
- Brown BA, Pallansch MA. Complete nucleotide sequence of enterovirus 71 is distinct from poliovirus. Virus Res. 1995; 39: 195–205.
- Yu H, Chen W, Chang H, Tang R, Zhao J, Gan L. Genetic analysis of the VP1 region of enterovirus 71 reveals the emergence of genotype A in central China in 2008. Virus Genes. 2010; 41: 1–4.
- Kapusinszky B, Szomor KN, Farkas A, Takacs M, Berencsi G. Detection of non-polio enteroviruses in Hungary 2000–2008 and molecular epidemiology of enterovirus 71, coxsackievirus 16, and echovirus 30. Virus Genes. 2010; 40: 163–73.
- Hosoya M, Kawasaki Y, Sato M, Honzumi K, Kato A, Hiroshima T. Genetic diversity of enterovirus 71 associated with hand, foot and mouth disease epidemics in Japan from 1983 to 2003. Pediatr Infect Dis J. 2006; 25: 691–4.
- Shimizu H, Utama A, Onnimala N, Li C, Li-Bi Z, Yu-Jie M. Molecular epidemiology of enterovirus 71 infection in the Western Pacific Region. Pediatr Int. 2004; 46: 231–5.
- Lin KH, Hwang KP, Ke GM, Wang CF, Ke LY, Hsu YT. Evolution of EV71 genogroup in Taiwan from 1998 to 2005: an emerging of subgenogroup C4 of EV71. J Med Virol. 2006; 78: 254–62.
- Sanders SA, Herrero LJ, McPhie K, Chow SS, Craig ME, Dwyer DE. Molecular epidemiology of enterovirus 71 over two decades in an Australian urban community. Arch Virol. 2006; 151: 1003–13.
- Iwai M, Masaki A, Hasegawa S, Obara M, Horimoto E, Nakamura K. Genetic changes of coxsackievirus A16 and enterovirus 71 isolated from hand, foot, and mouth disease patients in Toyama, Japan between 1981 and 2007. Jpn J Infect Dis. 2009; 62: 254–9.
- Bible JM, Iturriza-Gomara M, Megson B, Brown D, Pantelidis P, Earl P. Molecular epidemiology of human enterovirus 71 in the United Kingdom from 1998 to 2006. J Clin Microbiol. 2008; 46: 3192–200.
- Ortner B, Huang CW, Schmid D, Mutz I, Wewalka G, Allerberger F. Epidemiology of enterovirus types causing neurological disease in Austria 1999–2007: detection of clusters of echovirus 30 and enterovirus 71 and analysis of prevalent genotypes. J Med Virol. 2009; 81: 317–24.
- Shahmahmoodi S, Mehrabi Z, Eshraghian MR, Azad TM, Tabatabaie H, Yousefi M. First detection of enterovirus 71 from an acute flaccid paralysis case with residual paralysis in Iran. J Clin Virol. 2008; 42: 409–11.
- Chua KB, Chua BH, Lee CS, Chem YK, Ismail N, Kiyu A. Genetic diversity of enterovirus 71 isolated from cases of hand, foot and mouth disease in the 1997, 2000 and 2005 outbreaks, Peninsular Malaysia. Malays J Pathol. 2007; 29: 69–78.
- Puenpa J, Theamboonlers A, Korkong S, Linsuwanon P, Thongmee C, Chatproedprai S. Molecular characterization and complete genome analysis of human enterovirus 71 and coxsackievirus A16 from children with hand, foot and mouth disease in Thailand during 2008–2011. Arch Virol. 2011; 156: 2007–13.
- Wu Y, Yeo A, Phoon MC, Tan EL, Poh CL, Quak SH. The largest outbreak of hand, foot, and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010; 14: e1076–81.
- Ang LW, Koh BK, Chan KP, Chua LT, James L, Goh KT. Epidemiology and control of hand, foot and mouth disease in Singapore, 2001–2007. Ann Acad Med Singap. 2009; 38: 106–12.
- AbuBakar S, Sam IC, Yusof J, Lim MK, Misbah S, MatRahim N. Enterovirus 71 outbreak, Brunei. Emerg Infect Dis. 2009; 15: 79–82.
- Huang SW, Hsu YW, Smith DJ, Kiang D, Tsai HP, Lin KH. Reemergence of enterovirus 71 in 2008 in Taiwan: dynamics of genetic and antigenic evolution from 1998 to 2008. J Clin Microbiol. 2009; 47: 3653–62.
- Shih SR, Ho MS, Lin KH, Wu SL, Chen YT, Wu CN. Genetic analysis of enterovirus 71 isolated from fatal and non-fatal cases of hand, foot and mouth disease during an epidemic in Taiwan, 1998. Virus Res. 2000; 68: 127–36.
- Chu PY, Lin KH, Hwang KP, Chou LC, Wang CF, Shih SR. Molecular epidemiology of enterovirus 71 in Taiwan. Arch Virol. 2001; 146: 589–600.
- Lee MH, Huang LM, Wong WW, Wu TZ, Chiu TF, Chang LY. Molecular diagnosis and clinical presentations of enteroviral infections in Taipei during the 2008 epidemic. J Microbiol Immunol Infect. 2011; 44: 178–83.
- Huang YP, Lin TL, Hsu LC, Chen YJ, Tseng YH, Hsu CC. Genetic diversity and C2-like subgenogroup strains of enterovirus 71, Taiwan, 2008. Virol J. 2010; 7: 277.
- Kung SH, Wang SF, Huang CW, Hsu CC, Liu HF, Yang JY. Genetic and antigenic analyses of enterovirus 71 isolates in Taiwan during 1998–2005. Clin Microbiol Infect. 2007; 13: 782–7.
- Huang SW, Kiang D, Smith DJ, Wang JR. Evolution of re-emergent virus and its impact on enterovirus 71 epidemics. Exp Biol Med (Maywood). 2011; 236: 899–908.
- Lee MS, Lin TY, Chiang PS, Li WC, Luo ST, Tsao KC. An investigation of epidemic enterovirus 71 infection in Taiwan, 2008: clinical, virologic, and serologic features. Pediatr Infect Dis J. 2010; 29: 1030–4.
- Mizuta K, Aoki Y, Suto A, Ootani K, Katsushima N, Itagaki T. Cross-antigenicity among EV71 strains from different genogroups isolated in Yamagata, Japan, between 1990 and 2007. Vaccine. 2009; 27: 3153–8.
- Vallet S, Legrand-Quillien M-C, Dailland T, Podeur G, Gouriou S, Schuffenecker I. Fatal case of enterovirus 71 infection, France, 2007. Emerg Infect Dis. 2009; 15: 1837–40.
- Jee YM, Cheon DS, Kim K, Cho JH, Chung YS, Lee J. Genetic analysis of the VP1 region of human enterovirus 71 strains isolated in Korea during 2000. Arch Virol. 2003; 148: 1735–46.
- Jeong EJ, Lee JH, Kim MS, Bae GR, Jung C, Lee K. Molecular characterization of enteroviruses detected in Gyeong-Ju and Po-Hang provinces of Korea in 2003. Arch Virol. 2010; 155: 1707–12.
- Cho HK, Lee NY, Lee H, Kim HS, Seo JW, Hong YM. Enterovirus 71-associated hand, foot and mouth diseases with neurologic symptoms, a university hospital experience in Korea, 2009. Korean J Pediatr. 2010; 53: 639–43.
- Siafakas N, Attilakos A, Vourli S, Stefos E, Meletiadis J, Nikolaidou P. Molecular detection and identification of enteroviruses in children admitted to a university hospital in Greece. Mol Cell Probes. 2011; 25: 249–54.
- Zhang Y, Tan XJ, Wang HY, Yan DM, Zhu SL, Wang DY. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J Clin Virol. 2009; 44: 262–7.
- Zhang Y, Wang J, Guo W, Wang H, Zhu S, Wang D. Emergence and transmission pathways of rapidly evolving evolutionary branch C4a strains of human enterovirus 71 in the Central Plain of China. PLoS One. 2011; 6: e27895.
- Mao LX, Wu B, Bao WX, Han FA, Xu L, Ge QJ. Epidemiology of hand, foot, and mouth disease and genotype characterization of enterovirus 71 in Jiangsu, China. J Clin Virol. 2010; 49: 100–4.
- Castro CM, Cruz AC, Silva EE, Gomes Mde L. Molecular and seroepidemiologic studies of enterovirus 71 infection in the State of Para, Brazil. Rev Inst Med Trop Sao Paulo. 2005; 47: 65–71.
- Deshpande JM, Nadkarni SS, Francis PP. Enterovirus 71 isolated from a case of acute flaccid paralysis in India represents a new genotype. Curr Sci. 2003; 84: 1350–3.
- Simon-Loriere E, Holmes EC. Why do RNA viruses recombine?. Nat Rev Microbiol. 2011; 9: 617–26.
- Shukla P, Nguyen HT, Torian U, Engle RE, Faulk K, Dalton HR. Cross-species infections of cultured cells by hepatitis E virus and discovery of an infectious virus–host recombinant. Proc Natl Acad Sci U S A. 2011; 108: 2438–43.
- Khatchikian D, Orlich M, Rott R. Increased viral pathogenicity after insertion of a 28S ribosomal RNA sequence into the haemagglutinin gene of an influenza virus. Nature. 1989; 340: 156–7.
- Kemal KS, Ramirez CM, Burger H, Foley B, Mayers D, Klimkait T. Recombination between variants from genital tract and plasma: evolution of multidrug-resistant HIV type 1. AIDS Res Hum Retroviruses. 2012; 28: 1766–74.
- Oberste MS, Penaranda S, Maher K, Pallansch MA. Complete genome sequences of all members of the species human enterovirus A. J Gen Virol. 2004; 85: 1597–607.
- Lukashev AN, Lashkevich VA, Ivanova OE, Koroleva GA, Hinkkanen AE, Ilonen J. Recombination in circulating enteroviruses. J Virol. 2003; 77: 10423–31.
- Yip CC, Lau SK, Woo PC, Chan KH, Yuen KY. Complete genome sequence of a coxsackievirus A22 strain in Hong Kong reveals a natural intratypic recombination event. J Virol. 2011; 85: 12098–9.
- Cuervo NS, Guillot S, Romanenkova N, Combiescu M, Aubert-Combiescu A, Seghier M. Genomic features of intertypic recombinant sabin poliovirus strains excreted by primary vaccines. J Virol. 2001; 75: 5740–51.
- Lindberg AM, Andersson P, Savolainen C, Mulders MN, Hovi T. Evolution of the genome of human enterovirus B: incongruence between phylogenies of the VP1 and 3CD regions indicates frequent recombination within the species. J Gen Virol. 2003; 84: 1223–35.
- McWilliam Leitch EC, Cabrerizo M, Cardosa J, Harvala H, Ivanova OE, Koike S. The association of recombination events in the founding and emergence of subgenogroup evolutionary lineages of human enterovirus 71. J Virol. 2012; 86: 2676–85.
- Wei HY, Xu YL, Huang XY, Ma H, Chen HM, Xu BL. Genomic characteristics and recombination of enterovirus 71 strains isolated in Henan Province between 2008 and 2010. Bing Du Xue Bao. 2011; 27: 433–7.
- Wang HY, Tsao KC, Hsieh CH, Huang LM, Lin TY, Chen GW. Inferring nonneutral evolution from contrasting patterns of polymorphisms and divergences on different protein coding regions of enterovirus 71 circulating in Taiwan during 1998–2003. BMC Evol Biol. 2010; 10: 294.
- Ding NZ, Wang XM, Sun SW, Song Q, Li SN, He CQ. Appearance of mosaic enterovirus 71 in the 2008 outbreak of China. Virus Res. 2009; 145: 157–61.
- Phuektes P, Chua BH, Snaders S, Bek EJ, Kok CC, McMinn PC. Mapping genetic determinants of the cell-culture growth phenotype of enterovirus 71. J Gen Virol. 2011; 92: 1380–90.
- Tao Z, Wang H, Xu A. Identification of a C2 subgenogroup strain of enterovirus 71 in a retrospective study in Shangdong Province, China, from 1990 to 2010. J Clin Microbiol. 2012; 50: 1823–4.
- Zhang D, Lu J, Lu J. Enterovirus 71 vaccine: close but still far. Int J Infect Dis. 2010; 14: e739–43.
- Li ZH, Li CM, Ling P, Shen FH, Chen SH, Liu CC. Ribavirin reduces mortality in enterovirus 71-infected mice by decreasing viral replication. J Infect Dis. 2008; 197: 854–7.
- Liu ML, Lee YP, Wang YF, Lei HY, Liu CC, Wang SM. Type I interferons protect mice against enterovirus 71 infection. J Gen Virol. 2005; 86: 3263–9.
- Pevear DC, Tull TM, Seipel ME, Groarke JM. Activity of pleconaril against enteroviruses. Antimicrob Agents Chemother. 1999; 43: 2109–15.
- Chen TC, Weng KF, Chang SC, Lin JY, Huang PN, Shih SR. Development of antiviral agents for enteroviruses. J Antimicrob Chemother. 2008; 62: 1169–73.
- Shih SR, Tsai MC, Tseng SN, Won KF, Shia KS, Li WT. Mutation in enterovirus 71 capsid protein VP1 confers resistance to the inhibitory effects of pyridyl imidazolidinone. Antimicrob Agents Chemother. 2004; 48: 3523–9.
- Arita M, Wakita T, Shimizu H. Characterization of pharmacologically active compounds that inhibit poliovirus and enterovirus 71 infectivity. J Gen Virol. 2008; 89: 2518–30.
- Chen TC, Chang HY, Lin PF, Chern JH, Hsu JT, Chang CY. Novel antiviral agent DTriP-22 targets RNA-dependent RNA polymerase of enterovirus 71. Antimicrob Agents Chemother. 2009; 53: 2740–7.
- Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008; 319: 921–6.
- Tai AW, Benita Y, Peng LF, Kim SS, Sakamoto N, Xavier RJ. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe. 2009; 5: 298–307.
- Brass AL, Huang IC, Benita Y, John SP, Krishnan MN, Feeley EM. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell. 2009; 139: 1243–54.
- Karlas A, Machuy N, Shin Y, Pleissner KP, Artarini A, Heuer D. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature. 2010; 463: 818–22.
- Zhou H, Xu M, Huang Q, Gates AT, Zhang XD, Castle JC. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe. 2008; 4: 495–504.
- König R, Zhou Y, Elleder D, Diamond TL, Bonamy GM, Irelan JT. Global analysis of host–pathogen interactions that regulate early-stage HIV-1 replication. Cell. 2008; 135: 49–60.
- König R, Stertz S, Zhou Y, Inoue A, Hoffmann HH, Bhattacharyya S. Human host factors required for influenza virus replication. Nature. 2010; 463: 813–17.
- Li Q, Brass AL, Ng A, Hu Z, Xavier RJ, Liang TJ. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc Natl Acad Sci U S A. 2009; 106: 16410–5.
- Coyne CB, Bozym R, Morosky SA, Hanna SL, Mukherjee A, Tudor M. Comparative RNAi screening reveals host factors involved in enterovirus infection of polarized endothelial monolayers. Cell Host Microbe. 2011; 9: 70–82.
- Hussain KM, Leong KL, Ng MM, Chu JJ. The essential role of clathrin-mediated endocytosis in the infectious entry of human enterovirus 71. J Biol Chem. 2011; 286: 309–21.
- Houzet L, Jeang KT. Genome-wide screening using RNA interference to study host factors in viral replication and pathogenesis. Exp Biol Med (Maywood). 2011; 236: 962–7.
- Takimoto S, Wadldman EA, Moreira RC, Kok F, Pinheiro Fde P, Saes SG. Enterovirus 71 infection and acute neurological disease among children in Brazil (1998–1990). Trans R Soc Trop Med Hyg. 1998; 92: 25–8.
- Singh S, Chow VT, Chan KP, Ling AE, Poh CL. RT-PCR, nucleotide, amino acid and phylogenetic analyses of enterovirus type 71 strains from Asia. J Virol Methods. 2000; 88: 193–204.
- Shimizu H, Utama A, Yoshii K, Yoshida H, Yoneyama T, Sinniah M. Enterovirus 71 from fatal and nonfatal cases of hand, foot and mouth disease epidemics in Malaysia, Japan and Taiwan in 1997–1998. Jpn J Infect Dis. 1999; 52: 12–5.