Abstract
Background: Consumption of plant sterols has been reported to reduce low density lipoprotein (LDL) cholesterol concentrations by 5–15%. Factors that affect plant sterol efficacy are still to be determined.
Objectives: To more precisely quantify the effect of plant sterol enriched products on LDL cholesterol concentrations than what is reported previously, and to identify and quantify the effects of subjects’ characteristics, food carrier, frequency and time of intake on efficacy of plant sterols as cholesterol lowering agents.
Design: Fifty-nine eligible randomized clinical trials published from 1992 to 2006 were identified from five databases. Weighted mean effect sizes were calculated for net differences in LDL levels using a random effect model.
Results: Plant sterol containing products decreased LDL levels by 0.31 mmol/L (95% CI, –0.35 to –0.27, P= < 0.0001) compared with placebo. Between trial heterogeneity was evident (Chi-square test, P = <0.0001) indicating that the observed differences between trial results were unlikely to have been caused by chance. Reductions in LDL levels were greater in individuals with high baseline LDL levels compared with those with normal to borderline baseline LDL levels. Reductions in LDL were greater when plant sterols were incorporated into fat spreads, mayonnaise and salad dressing, milk and yoghurt comparing with other food products such as croissants and muffins, orange juice, non-fat beverages, cereal bars, and chocolate. Plant sterols consumed as a single morning dose did not have a significant effect on LDL cholesterol levels.
Conclusion: Plant sterol containing products reduced LDL concentrations but the reduction was related to individuals’ baseline LDL levels, food carrier, and frequency and time of intake.
Introduction
Dietary incorporation of plant sterols and stanols is recommended for blood cholesterol reduction Citation1Citation2. Berger et al. reviewed clinical trials on efficacy of plant sterols as cholesterol lowering agents and reported that the consumption of plant sterols/stanols have been reported to reduce low density lipoprotein (LDL) cholesterol levels by 5–15% Citation3. Reasons for such large variations need to be investigated.
Earlier studies that have tested the efficacy of plant sterols/stanols as cholesterol lowering agents incorporated plant sterols/stanols into either regular or low fat spreads Citation8Citation9Citation10Citation11Citation12Citation13. Since it appears counterintuitive to use a high fat food product to deliver a cholesterol lowering agent, clinical trials have been conducted to test the efficacy of plant sterols/stanols incorporated into low fat products Citation14. A number of clinical trials have tested the efficacy of plant sterols/stanols incorporated into low fat foods including low fat milk Citation15Citation16, low fat yoghurt Citation16Citation17Citation18Citation19Citation20, bakery products Citation21, orange juice Citation22Citation23, cereal bars Citation24 and low and non-fat beverages Citation25Citation26Citation27. However, although plant sterols/stanols that are incorporated into low fat food have been shown to reduce blood cholesterol Citation24Citation27Citation28, the same food carrier tested in different trials gave different magnitude in LDL cholesterol reduction. Plant sterol/stanol enriched yoghurt and milk drinks have resulted in LDL cholesterol reduction in the range of 5–14% in various clinical trials Citation29. The study by Clifton et al. Citation30 compared the effect of plant stanol esterified to fatty acids and incorporated in a number of food matrices including bread, breakfast cereal, milk and yoghurt on plasma lipids. Plant stanol esters in low fat milk were almost three times more effective than in bread and cereal in lowering plasma cholesterol levels. Whether all plant sterols/stanols enriched low fat food matrices are efficacious as plant sterol/stanol enriched spread carrier in lowering blood cholesterol has not been studied thoroughly. It remains to be determined which food matrices are viable carriers to deliver an effective dose of plant sterols/stanols.
The optimal number of servings per day of plant sterol/stanol containing products was addressed in only one study. Plat et al. Citation31 showed that 2.5 g of plant stanols in margarines and shortenings consumed for four weeks once per day at lunch or divided over three meals, lowered LDL cholesterol levels to a similar extent, about 10%. The intake of a single dose of plant sterol/stanol enriched products is thought to increase consumers’ compliance and adds convenience. However, further studies using a single dose of plant sterols/stanols consumed either at breakfast Citation18Citation19Citation32, or with lunch or the principal meal Citation19Citation31Citation33Citation34 yielded conflicting results. For example, when plant sterol enriched margarine was consumed with breakfast, no reduction in cholesterol levels was observed Citation32, in spite of the previously demonstrated efficacy as a single serving at lunch Citation31. In another study, intake of the single dose of plant sterols provided in yoghurt drinks with lunch resulted in a larger decrease in LDL levels than the intake of same dose of plant sterols provided 30 min before breakfast Citation19. Since plant sterol/stanol products are being marketed for consumption once a day, it remains to be investigated whether the effect of single dose of plant sterols/stanols consumed at different time of the day is comparable to that consumed as multiple dosages throughout the day.
Several potential modifiers for the effect of plant sterol/stanol supplementation on reduction of LDL levels were studied in some trials, including age and gender, baseline LDL levels, and genetic profile. Again, results from various studies are inconsistent. For example, baseline LDL levels have been shown to modify the effect of plant sterols/stanol in some Citation35Citation36, but not other studies Citation8Citation37Citation38. Furthermore, identification of effect modifiers in the cholesterol lowering action of plant sterols/stanol will help target individuals who may benefit more from such an intervention.
Accordingly, instead of conducting additional randomized clinical trials to resolve the disagreement surrounding the influence of the aforementioned factors on the cholesterol lowering action of plant sterols/stanols, it was considered that an appropriate meta-analysis could be used as an alternative novel approach. Previous meta-analyses have studied the efficacy of plant sterols/stanols as cholesterol lowering agents. The first Citation4 looked at the cholesterol lowering action of plant sterols/stanols added to fat spreads mostly in the form of margarines. Another Citation5 looked at the efficacy and safety of plant sterols/stanols as cholesterol lowering agents, but since 2003 a number of clinical trials have examined the efficacy of low fat foods containing plant sterols/stanols and observed substantially weaker effects. A recent meta-analysis Citation6 sought to investigate effects of plant sterols/stanol in lowering total and LDL cholesterol levels of familial hypercholesterolemia subjects. Two previous meta-analyses conducted on plant sterols/stanols were non-systematic reviews Citation4Citation5, which failed to describe how reviewers searched, selected and evaluated the quality of studies. Narrative reviews are qualitative summaries of a certain topic Citation7. While systematic meta-analyses include a comprehensive search of the primary studies on a specific clinical question, selection of studies by using clear eligibility criteria, critical evaluation of the quality of studies, and generating results using a pre-specified method Citation7.
Meta-analysis is a statistical tool that generates pooled estimates of effects from the results of randomized controlled trials Citation7. It is an unbiased tool to assess an intervention and may lead to resolution of controversy. Therefore, a systematic meta-analysis could resolve the apparent controversy concerning the influences of food carrier, frequency and time of intake, as well as subjects’ baseline characteristics on cholesterol lowering action of plant sterols/stanols.
The objectives of the present meta-analysis were to more precisely quantify the effect of plant sterol/stanol enriched products on LDL cholesterol concentrations and to identify and quantify the effect of subjects’ characteristics, food carrier, frequency and time of intake.
2 Materials and methods
2.1 Literature search
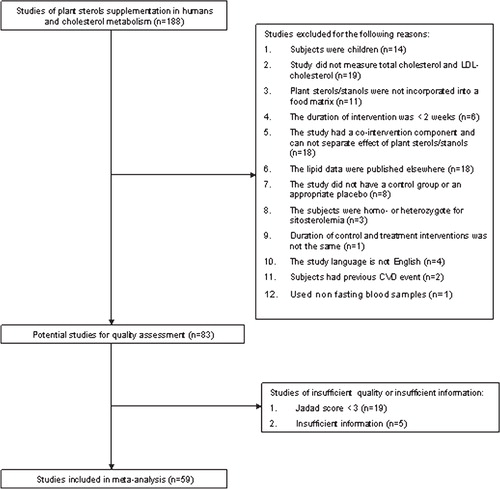
Studies that examined the efficacy of plant sterols/stanols as cholesterol lowering agents in humans were identified by searching five databases PubMed, Embase, Medline, Cochrane Library and Web of Science using the terms “plant sterol”, “plant stanol”, “phytosterol” and “phytostanol” as words in the title, abstract or keywords. When available, the search was restricted to clinical trials. In addition, a manual search using reference lists of review articles Citation3Citation4Citation5Citation39 was performed. For non-English language literature, if available, the abstract written in English was used to extract the required information; otherwise the trial was not included in the analysis. All citations were exported into reference manager software (EndNote version 8.0.2) and studies on plant sterols/stanols and cholesterol metabolism were identified. Fifty-nine eligible randomized clinical trials published from 1992 to 2006 were identified from the five databases.
2.2 Selection criteria
Randomized placebo controlled studies conducted to test the efficacy of plant sterols/stanols incorporated into food matrices on circulating cholesterol levels in adults were included in this meta-analysis. Therefore, studies were first excluded from the meta-analysis for not measuring circulating LDL levels as a primary or secondary outcome, for having duration of intervention of less than two weeks, for examining children or adults who were homo- or heterozygote for sitosterolemia or who possessed a history of cardiovascular disease. Studies were also excluded for having a co-intervention that could not be separated from plant sterol/stanol treatment, for incorporating plant sterols/stanols in the form of capsule or tablets, or for not having a control group or an appropriate control/placebo. In addition, studies were excluded if lipid profiling was done on non-fasting blood samples or if lipid profile data were published elsewhere. A total of 84 clinical trials met the first inclusion criteria and were then screened for the quality criteria ().
2.3 Quality assessment of trials
Randomized controlled studies were assessed for methodological quality with the Jadad score as described in Citation40. A Jadad score of three or above, out of a maximum of five, was used to indicate that a study is of reasonable quality to be included in the meta-analysis Citation41.
Table 1. Calculation of Jadad score to assess study quality1.
2.4 Data abstraction
All data were abstracted from the original articles. No data were directly obtained from the original authors. For studies that met the inclusion criteria and that possessed a Jadad score of equal or more than three, data were extracted for parameters related to (i) trial design; (ii) type of plant sterols/stanols; (iii) dose (g/day) and duration of plant sterol/stanol treatment; (iv) frequency and time of intake of plant sterols/stanols; (v) food carrier, to which plant sterols/stanols were incorporate; (vi) characteristics of the study population; (vii) the mean values and the standard deviations (SD) of LDL cholesterol levels; and (viii) sample size. Two reviewers (SSA and RB) independently identified articles for inclusion, assessed quality and extracted data.
2.5 Quantitative data synthesis
For studies that reported multiple time points for the same subjects, only endpoints for the longest duration of the intervention were used. For studies in which the outcomes were presented as percentage change from baseline, and no endpoint data were available Citation37Citation42Citation43, endpoint data were imputed using the baseline values and percentage change from baseline and the SD of the baseline data for the endpoint SD. Where studies reported absolute change from baseline and no endpoint data were available Citation26Citation44Citation45, we imputed endpoints using baseline plus change for the mean and using the SD of the baseline data for the endpoint SD.
The primary outcome for this meta-analysis was the difference in LDL cholesterol levels, reported in mmol/L, due to plant sterol/stanol treatment. For parallel arm designed trials, endpoint LDL cholesterol in the treatment group was subtracted from endpoint LDL cholesterol in the control group Citation46. We did not use differences in changes from baseline as the primary outcome because this would imply imputing SDs for changes from baseline for the majority of parallel studies, which is not recommended Citation46. For crossover trials, the LDL cholesterol value at the end of the treatment period was subtracted from that at the end of the control period Citation46. Within-individual changes were used when presented; otherwise, group means were used. SDs were extracted from the studies or, if not reported, derived from standard errors (SEs) of mean, confidence intervals (CIs), paired t-value or P-value as provided Citation46. If different treatments were tested within the same trial, they were evaluated as separate strata, as is described by “a, b, c and d” suffixes in Tables and Figures. To obtain the pooled treatment effect size (ES), ES estimates and SE were entered into RevMan 4.2 under the “generic inverse variance” outcome. Heterogeneity between trial results was tested for using a standard chi-squared test. A P-value < 0.1 was used to indicate that significant heterogeneity was present Citation46.
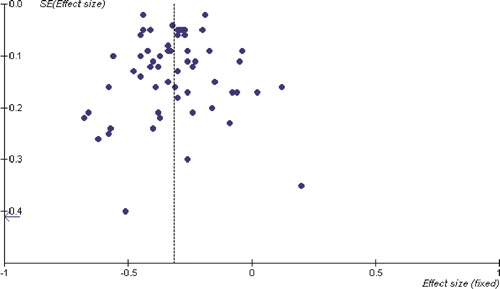
Calculations used in this meta-analysis are presented in the Appendix. Estimates of the pooled treatment ES of plant sterol/stanol containing food on LDL cholesterol levels and 95% CIs were calculated by using both fixed effect and random effect models. If the test for heterogeneity was significant, we presented the results of the random effect models. Otherwise, estimated results based on a fixed effect model are presented. We presented the ES as mmol/L, and not as percentage difference, as most of the studies did not report the SD of the percentage difference in LDL values between the control and the treatment group or phases. The presence of publication bias was examined for using a funnel plot, in which the SEs of the studies were plotted against their corresponding ESs.
3 Results
Fifty-nine studies comprising 95 relevant strata were assessed as eligible for meta-analysis with >4500 subjects. A summary of trial design and characteristics is shown in and . Twenty-nine studies utilized a crossover design while 30 used a parallel design. Sample sizes ranged from 8 to 185 subjects.
Table 2. Design and subject characteristics of randomized controlled studies of plant sterols/stanols.
Table 3. Features of plant sterol intervention of randomized controlled studies of plant sterols/stanols.
Individual trial results and the pooled ES for all trials are shown in . In the overall pooled estimate, plant sterol/stanol consumption decreased LDL levels by 0.31 mmol/L (95% CI, –0.35 to –0.27, P= < 0.0001) compared with placebo. Between trial heterogeneity was evident (Chi-square test, P = <0.0001; I2=65%). It was estimated that as 65% of the variability in the ES is due to heterogeneity between the trials (clinical and methodological diversity) rather than chance. Thus, we performed a subgroup analysis according to predefined criteria by subjects’ characteristics and study design features as summarized in . Initial serum LDL cholesterol levels had a powerful effect on changes in lipid concentrations. Therefore, subjects were grouped into two groups, one included subjects with high baseline levels of LDL and the other group included subjects with low baseline levels of LDL, as defined according to ATPIII Citation85. A greater decrease in LDL levels was observed in subjects with optimal to borderline high levels of baseline LDL. The LDL cholesterol levels of the former decreased by 0.37 mmol/L (95% CI: –0.42, –0.31) and those of the latter decreased by 0.28 mmol/L (95% CI: –0.31, –0.25). The placebo adjusted reduction in LDL levels produced by consumption of plant sterols was the same across all age groups.
Figure 2. Effect size and 95% CI in LDL cholesterol levels associated with consumption of plant sterol/stanol containing food products.
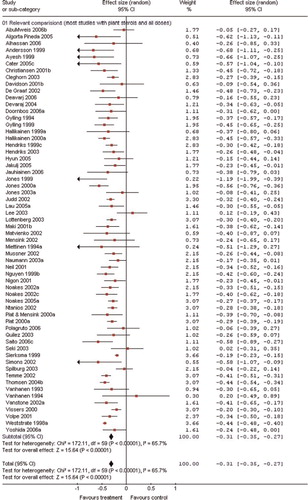
Table 4. Pooled estimates of treatment effect on LDL cholesterol in subgroups of trials defined by subject characteristics and study design features
There was evidence of a dose response effect. The minimum (–0.25 mmol/L; 95% CI: –0.32, –0.18) and the maximum (–0.42 mmol/L; 95% CI: –0.46, –0.39) reductions in LDL cholesterol levels were achieved by the intake of <1.5g/day and >2.5 g/day of sterols/stanols, respectively. The reductions in LDL were –0.29 mmol/L (95% CI: –0.34, –0.24) and –0.32mmol/L (95% CI: –0.36, –0.28) for intakes of 1.5–2.0 g/d and 2.1–2.5 g/d, respectively.
The effect of plantsterols//stanols on LDL cholesterol is influenced by the food carrier to which plant sterols/stanols are incorporated. We predefined the food product groups according to their fat content, i.e. low fat products contain 3 g or less fat per serving, as well as their physical form, i.e. liquid versus solid. Therefore, we ended up with four groups, i.e. fat spreads, mayonnaise and salad dressing, milk and yoghurt, and other food group. Other food products subgroup included studies testing the efficacy of plant sterols/stanols incorporated in chocolate, cereal bars, beverages, juices, meat, and croissants and muffins. All these were included in one subgroup and not further analyzed because of an insufficient number of clinical trials.
Plant sterols/stanols incorporated into fat spreads, mayonnaise and salad dressing or milk and yoghurt reduced LDL cholesterol levels to a greater extent than plant sterols/stanols incorporated into other food products. Compared to control, LDL levels were reduced by 0.33 (95% CI, –0.38 to –0.28), 0.32 (95% CI, –0.40 to –0.25), –0.34 (95% CI, –0.40 to –0.28) and 0.20 (95% CI, –0.28 to –0.11) mmol/L in the fat spreads, mayonnaise and salad dressing, milk and yoghurt, and other food products, respectively. Other food product subgroups included studies testing the efficacy of plant sterols/stanols incorporated in chocolate Citation47Citation48, orange juice Citation22Citation23, cheese Citation49, non-fat beverage Citation26Citation27, meat Citation33, croissants and muffins Citation21, oil in bread Citation43, and cereal bars Citation24.
The favorable effect of plant sterols/stanols on LDL cholesterol levels was also shown to be influenced by the frequency and time of intake of plant sterols. For instance, plant sterols/stanols consumed 2–3 times/day reduced LDL cholesterol levels by 0.34 mmol/L (95% CI: –0.38, –0.18) while plant sterols/stanols consumed once per day in the morning did not result in a significant reduction in LDL levels. On the other hand, plant sterols/stanols consumed once/day with lunch or the principal meal reduced LDL levels by 0.30 mmol/L (95%: –0.39, –0.21).
We found no evidence of publication bias in this meta-analysis, as indicated by the funnel plot symmetry ().
4 Discussion
The present meta-analysis is the first systematic quantitative review of randomized clinical trials yielding information on factors that might affect efficacy of plant sterols/stanols as cholesterol lowering agents. Since the meta-analyses of Law Citation4 and Katan et al. Citation5 examining plant sterol/stanol effects on circulating cholesterol levels, several studies have been conducted examining the action of various plant sterol/stanol containing products on blood cholesterol levels using different study designs. The present work shows that the intake of plant sterol/stanol containing food products was associated with a significant decrease in LDL cholesterol (–0.31 mmol/L). However, the substantial heterogeneity among individual trials indicates that the effects of plant sterols/stanols on LDL cholesterol levels are not uniform.
A larger reduction in LDL cholesterol levels was observed in subjects with a high to very high baseline levels of LDL, compared to those with optimal to borderline high baseline levels. Some previous Citation35Citation36, but not other studies Citation8Citation37Citation38, have reported that the higher the baseline levels of LDL-cholesterol the more the reduction in LDL due to plant sterols consumption. The present meta-analysis has confirmed that baseline LDL cholesterol levels affect magnitude of reduction in LDL after plant sterol/stanol consumption which could explain the wide variation in responsiveness seen in previous studies. Nevertheless, plant sterols/stanols do reduce LDL levels in individuals with normal to high baseline LDL levels as well as in adults across different age groups. Therefore, everyone, excluding individuals with β-sitosterolemia and heterozygote for the disease, can reduce his/her blood cholesterol levels by consuming plant sterols/stanols.
A positive dose response relationship was apparent with the greatest reduction in LDL levels obtained with intakes of 2.5 g/day of plant sterols/stanols. The meta-analysis by Katan et al. Citation5 showed that there is little additional effect of plant sterols/stanols at doses higher than 2.5 g/day. It should be noted that studies included in the subgroups with intakes ≥2.1 g/day incorporated plant sterols/stanols mainly in fat spreads, while the other subgroups included a variety of food products, which could explain why heterogeneity was absent with intakes of ≥2.1g/day.
Plant sterols/stanols reduce LDL cholesterol through interfering with cholesterol absorption Citation9Citation50Citation51Citation52. Because of their inert crystalline structure, pure plant sterols/stanols are not consistently effective in lowering cholesterol absorption. Thus, plant sterols/stanols should be adequately formulated before use. The most accepted method used to optimize the effect of plant sterols/stanols on cholesterol absorption is esterification to fatty acids and dissolving plant sterols/stanols within food fats Citation53. Some studies have shown that free plant sterols/stanols when mixed with fat spread are also effective in reducing LDL cholesterol levels Citation51Citation54. Later on, plant sterols/stanols were added to low and non-fat food products. The results presented here show that compared with plant sterol/stanol containing fat spreads, mayonnaise and salad dressing, and milk and yoghurt, other plant sterol/stanol containing food products, including chocolate Citation47Citation48, orange juice Citation22Citation23, cheese Citation49, non-fat beverage Citation26Citation27, meat Citation33, croissants and muffins Citation21, oil in bread Citation43, and cereal bars Citation24 demonstrated less of a LDL-reduction efficacy. This finding highlights the importance of food carrier and proper formulation of plant sterols/stanols. Although milk and yoghurt drinks contain much less fat than fat spreads and mayonnaise, milk and yoghurt drinks demonstrated similar efficacy as of products with higher fat content. Thus, the food carrier to which plant sterols/stanols are added does not have to contain a high fat content to be an effective means of release of plant sterols/stanols to compete with cholesterol absorption, given that a proper plant sterol formulation is provided. Unfortunately, exact methods used to formulate plant sterols/stanols in the milk and yoghurt studies are not described in adequate detail. Studies were reported only if they used free Citation15Citation17 or esterified Citation16Citation18Citation19Citation20Citation34 sterols or stanols. It is also possible that plant sterols/stanols in milk may be more readily incorporated into milk globule membranes, thus more readily compete with cholesterol for transfer into the micelles, while in the other low fat foods plant sterols/stanols may be trapped in the center of the lipid droplets and not be available until the fat is digested Citation30. Future work is needed to identify proper formulation of plant sterols/stanols to improve their efficacy in food products other than those with high fat contents, i.e. vegetable and dairy spreads and mayonnaise, or milk and yoghurts.
In a previous study from our group, consumption of a single dose of different preparations of plant sterols in the morning failed to lower LDL levels Citation32. Some studies have shown that consumption of single dose of plant sterols/stanols with lunch lowered LDL levels Citation31Citation33. One study has tested the efficacy of plant stanol consumed at different frequencies. In the study by Plat et al. Citation31 subjects consumed the plant stanol enriched margarine at breakfast and at lunch and ate a cake or cookie containing plant stanol-enriched shortening within one hour after supper. The higher portion of plant stanol during the 3 times/day phase was given using a different food carrier and was consumed without a meal in comparison to the single dose phase, thus, multiple factors might contribute to the differences in results obtained between the study phases. Additionally, the availability of plant stanol in the cakes and cookies might be affected by baking conditions. To what extent this affected the cholesterol lowering action of 3 times/day phase of plant stanol intake is unknown. To examine that question, we conducted a subgroup analysis looking at frequency and time of intake of plant sterols/stanols. The results of this meta-analysis show that the time of intake of a single dose of plant sterols/stanols may affect their cholesterol-lowering action as consumption of single dose with lunch or main meal, but not before or with breakfast, lowered LDL levels. The results of the subgroup analyses examining time of intake of plant sterols/stanols should be interpreted with caution, however. The number of subjects included in the individual subgroups was small and many of the included studies did not report data on time of intake, resulting in the potential to be misled by bias. The exact mechanisms responsible for the effects of plant sterols/stanols on LDL levels are still being investigated. Based on current knowledge, plant sterols/stanols reduce solubilization of cholesterol in micelles and also may affects the site of absorption and intra-cellular trafficking of cholesterol Citation55.
The efficacy of plant sterols/stanols as a cholesterol-lowering agent may demonstrate a time-of-day variation, possibly coinciding with the diurnal rhythm of cholesterol metabolism. Diurnal rhythm in cholesterol synthesis has been shown in humans Citation56Citation57Citation58, where cholesterol fractional synthetic rate values peaked at 6:00 h and were lowest during the daytime period. Moreover, bile acid synthesis in humans has also a diurnal rhythm that is opposite from the diurnal rhythm of cholesterol synthesis Citation59. Therefore, until mechanisms have been elucidated by which plant sterols/stanols and in particular single dose of plant sterols/stanols reduce LDL levels, and until there are more studies on consumption of plant sterols/stanols as single dose; plant sterols should be consumed in two to three portions per day.
In conclusion, plant sterol/stanol containing products significantly reduced LDL concentrations but the reduction was related to individuals’ baseline LDL levels, food carrier, frequency and time of intake.
5 Conflict of interest and funding
The contribution of the authors were as follow: SSA designed and implemented the search strategy, assessed study quality, extracted data, performed statistical analysis, interpreted the results and wrote and edited the manuscript. RIB was involved in literature search, study selection and quality assessment, and data extraction. PJHJ provided guidance and critical revision of the manuscript. We would like to thank Mrs Mary Cheang, a statistical consultant at the Department of Community Health Sciences, University of Manitoba, for reviewing the method section and providing statistical advice. SSA and RIB have no conflict of interest. PJHJ is a consultant for Danone, Unilever, Forbes Meditech, Whitewave and Enymotec Inc.
References
- National Cholesterol Education Program Expert Panel Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. 2002; 106: 3143-421
- Fletcher B, Berra K, Ades P, Braun LT, Burke LE, Durstine JL, Fair JM, Fletcher GF, Goff Det al.Managing abnormal blood lipids – A collaborative approach. 2005; 112: 3184-209
- Berger A, Jones PJ, Abumweis SS. Plant sterols: factors affecting their efficacy and safety as functional food ingredients. 2004; 3: 5.
- Law M. Plant sterol and stanol margarines and health. 2000; 320: 861-4
- Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. 2003; 78: 965-78
- Moruisi KG, Oosthuizen W, Opperman AM. Phytosterols/stanols lower cholesterol concentrations in familial hypercholesterolemic subjects: a systematic review with meta-analysis. 2006; 25: 41-8
- Pai M, McCulloch M, Gorman JD, Pai N, Enanoria W, Kennedy G, Tharyan P, Colford JM. Systematic reviews and meta-analyses: an illustrated, step-by-step guide. 2004; 17: 86-95
- Weststrate JA, Meijer GW. Plant sterol-enriched margarines and reduction of plasma total- and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. 1998; 52: 334-43
- Gylling H, Radhakrishnan R, Miettinen TA. Reduction of serum cholesterol in postmenopausal women with previous myocardial infarction and cholesterol malabsorption induced by dietary sitostanol ester margarine-Women and dietary sitostanol. 1997; 96: 4226-31
- Hallikainen MA, Uusitupa MIJ. Effects of 2 low-fat stanol ester-containing margarines on serum cholesterol concentrations as part of a low-fat diet in hypercholesterolemic subjects. 1999; 69: 403-10
- Miettinen TA, Puska P, Gylling H, Vanhanen H, Vartiainen E. Reduction of serum-cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. 1995; 333: 1308-12
- Hendriks HFJ, Weststrate JA, van Vliet T, Meijer GW. Spreads enriched with three different levels of vegetable oil sterols and the degree of cholesterol lowering in normocholesterolaemic and mildly hypercholesterolaemic subjects. 1999; 53: 319-27
- Andersson A, Karlstrom B, Mohsen R, Vessby B. Cholesterol-lowering effects of a stanol ester-containing low-fat margarine used in conjunction with a strict lipid-lowering diet. 1999; 1: S80-S90
- St-Onge MP, Jones PJH. Phytosterols and human lipid metabolism: efficacy, safety, and novel foods. 2003; 38: 367-75
- Thomsen AB, Hansen HB, Christiansen C, Green H, Berger A. Effect of free plant sterols in low-fat milk on serum lipid profile in hypercholesterolemic subjects. 2004; 58: 860-70
- Noakes M, Clifton PM, Doornbos AME, Trautwein EA. Plant sterol ester-enriched milk and yoghurt effectively reduce serum cholesterol in modestly hypercholesterolemic subjects. 2005; 44: 214-22
- Volpe R, Niittynen L, Korpela R, Sirtori C, Bucci A, Fraone N, Pazzucconi F. Effects of yoghurt enriched with plant sterols on serum lipids in patients with moderate hypercholesterolaemia. 2001; 86: 233-9
- Hyun YJ, Kim OY, Kang JB, Lee JH, Jang Y, Liponkoski L, Salo P. Plant stanol esters in low-fat yogurt reduces total and low-density lipoprotein cholesterol and low-density lipoprotein oxidation in normocholesterolemic and mildly hypercholesterolemic subjects. 2005; 25: 743-53
- Doornbos AME, Meynen EM, Duchateau G, van der Knaap HCM, Trautwein EA. Intake occasion affects the serum cholesterol lowering of a plant sterol-enriched single-dose yoghurt drink in mildly hypercholesterolaemic subjects. 2006; 60: 325-33
- Mensink RP, Ebbing S, Lindhout M, Plat J, van Heugten MMA. Effects of plant stanol esters supplied in low-fat yoghurt on serum lipids and lipoproteins, non-cholesterol sterols and fat soluble antioxidant concentrations. 2002; 160: 205-13
- Quilez J, Rafecas M, Brufau G, Garcia-Lorda P, Megias I, Bullo M, Ruiz JA, Salas-Salvado J. Bakery products enriched with phytosterol esters, alpha-tocopherol and beta-carotene decrease plasma LDL-cholesterol and maintain plasma beta-carotene concentrations in normocholesterolemic men and women. 2003; 133: 3103-9
- Devaraj S, Autret BC, Jialal I. Reduced-calorie orange juice beverage with plant sterols lowers C-reactive protein concentrations and improves the lipid profile in human volunteers. 2006; 84: 756-61
- Devaraj S, Jialal I, Vega-Lopez S. Plant sterol-fortified orange juice effectively lowers cholesterol levels in mildly hypercholesterolemic healthy individuals. 2004; 24: E25-E8
- Yoshida M, Vanstone CA, Parsons WA, Zawistowski J, Jones PJH. Effect of plant sterols and glucomannan on lipids in individuals with and without type II diabetes. 2006; 60: 529-37
- Shin MJ, Lee JH, Jang Y, Lee-Kim YC, Park E, Kim KM, Chung BC, Chung N. Micellar phytosterols effectively reduce cholesterol absorption at low doses. 2005; 49: 346-51
- Spilburg CA, Goldberg AC, McGill JB, Stenson WF, Racette SB, Bateman J, McPherson T, Ostlund RE. Fat-free foods supplemented with soy stanol-lecithin powder reduce cholesterol absorption and LDL cholesterol. 2003; 103: 577-81
- Jones PJH, Vanstone CA, Raeini-Sarjaz M, St-Onge MP. Phytosterols in low- and nonfat beverages as part of a controlled diet fail to lower plasma lipid levels. 2003; 44: 1713-9
- Davidson MH, Maki KC, Umporowicz DM, Ingram KA, Dicklin MR, Schaefer E, Lane RW, McNamara JR, Ribaya-Mercado JDet al.Safety and tolerability of esterified phytosterols administered in reduced-fat spread and salad dressing to healthy adult men and women. 2001; 20: 307-19
- AbuMweis SS, Nicolle C, Jones PJ. Cholesterol-lowering action of plant sterol-enriched products. 2006; 2: 101-10
- Clifton PM, Noakes M, Sullivan D, Erichsen N, Ross D, Annison G, Fassoulakis A, Cehun M, Nestel P. Cholesterol-lowering effects of plant sterol esters differ in milk, yoghurt, bread and cereal. 2004; 58: 503-9
- Plat J, van Onselen ENM, van Heugten MMA, Mensink RP. Effects on serum lipids, lipoproteins and fat soluble antioxidant concentrations of consumption frequency of margarines and shortenings enriched with plant stanol esters. 2000; 54: 671-7
- AbuMweis SS, Vanstone CA, Ebine N, Kassis A, Ausman LM, Jones PJH, Lichtenstein AH. Intake of a single morning dose of standard and novel plant sterol preparations for 4 weeks does not dramatically affect plasma lipid concentrations in humans. 2006; 136: 1012-6
- Matvienko OA, Lewis DS, Swanson M, Arndt B, Rainwater DL, Stewart J, Alekel DL. A single daily dose of soybean phytosterols in ground beef decreases serum total cholesterol and LDL cholesterol in young, mildly hypercholesterolemic men. 2002; 76: 57-64
- Pineda JA, Ranedo MJC, Anda JA, Terreros SF. Hypocholesteremic effectiveness of a yogurt containing plant stanol esters. 2005; 205: 63-6
- Mussner MJ, Parhofer KG, von Bergmann K, Schwandt P, Broedl U, Otto C. Effects of phytosterol ester-enriched margarine on plasma lipoproteins in mild to moderate hypercholesterolemia are related to basal cholesterol and fat intake. 2002; 51: 189-94
- Naumann E, Plat J, Mensink RP. Changes in serum concentrations of noncholesterol sterols and lipoproteins in healthy subjects do not depend on the ratio of plant sterols to stanols in the diet. 2003; 133: 2741-7
- Maki KC, Davidson MH, Umporowicz DM, Schaefer EJ, Dicklin MR, Ingram KA, Chen S, McNamara JR, Gebhart BWet al.Lipid responses to plant-sterol-enriched reduced-fat spreads incorporated into a National Cholesterol Education Program Step I diet. 2001; 74: 33-43
- Ntanios FY, Homma Y, Ushiro S. A spread enriched with plant sterol-esters lowers blood cholesterol and lipoproteins without affecting vitamins A and E in normal and hypercholesterolemic Japanese men and women. 2002; 132: 3650-5
- Jones PJH, MacDougall DE, Ntanios F, Vanstone CA. Dietary phytosterols as cholesterol-lowering agents in humans. 1997; 75: 217-27
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. 1996; 17: 1-12
- Whelan AM, Jurgens TM, Bowles SK. Natural health products in the prevention and treatment of osteoporosis: systematic review of randomized controlled trials. 2006; 40: 836-49
- Simons LA. Additive effect of plant sterol-ester margarine and cerivastatin in lowering low-density lipoprotein cholesterol in primary hypercholesterolemia. 2002; 90: 737-40
- Seki S, Hidaka I, Kojima K, Yoshino H, Aoyama T, Okazaki M, Kondo K. Effects of phytosterol ester-enriched vegetable oil on plasma lipoproteins in healthy men. 2003; 12: 282-91
- Homma Y, Ikeda I, Ishikawa T, Tateno M, Sugano M, Nakamura H. Decrease in plasma low-density lipoprotein cholesterol, apolipoprotein B, cholesteryl ester transfer protein, and oxidized low-density lipoprotein by plant stanol ester-containing spread: a randomized, placebo-controlled trial. 2003; 19: 369-74
- Miettinen TA, Vanhanen H. Dietary sitostanol related to absorption, synthesis and serum level of cholesterol in different apolipoprotein-E phenotypes. 1994; 105: 217-26
- Deeks JJ, Higgins JPT, Altman DG. Analysing and presenting results. In: Higgins JPT, Greens S. Cochrane handbook for systematic reviews of interventions 425 [updated May 2005]; Section 8 http://wwwcochraneorg/resources/handbook/hbookhtm; 2005. Accessed March 5, 2007.
- de Graaf J, Nolting P, van Dam M, Belsey EM, Kastelein JJP, Pritchard PH, Stalenhoef AFH. Consumption of tall oil-derived phytosterols in a chocolate matrix significantly decreases plasma total and low-density lipoprotein-cholesterol levels. 2002; 88: 479-88
- Polagruto JA, Wang-Polagruto JF, Braun MM, Lee L, Kwik-Uribe C, Keen CL. Cocoa flavanol-enriched snack bars containing phytosterols effectively lower total and low-density lipoprotein cholesterol levels. 2006; 106: 1804-13
- Jauhiainen T, Salo P, Niittynen L, Poussa T, Korpela R. Effects of low-fat hard cheese enriched with plant stanol esters on serum lipids and apolipoprotein B in mildly hypercholesterolaemic subjects. 2006; 60: 1253-7
- Jones PJ, Raeini-Sarjaz M, Ntanios FY, Vanstone CA, Feng JY, Parsons WE. Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. 2000; 41: 697-705
- Vanstone CA, Raeini-Sarjaz M, Parsons WE, Jones PJ. Unesterified plant sterols and stanols lower LDL-cholesterol concentrations equivalently in hypercholesterolemic persons. 2002; 76: 1272-8
- Normen L, Dutta P, Lia A, Andersson H. Soy sterol esters and beta-sitostanol ester as inhibitors of cholesterol absorption in human small bowel. 2000; 71: 908-13
- Ostlund RE. Phytosterols and cholesterol metabolism. 2004; 15: 37-41
- Jones PJH, Ntanios FY, Raeini-Sarjaz M, Vanstone CA. Cholesterol-lowering efficacy of a sitostanol-containing phytosterol mixture with a prudent diet in hyperlipidemic men. 1999; 69: 1144-50
- Rozner S, Garti N. The activity and absorption relationship of cholesterol and phytosterols. 2006; 282: 435-56
- Cella LK, Vancauter E, Schoeller DA. Effect of meal timing on diurnal rhythm of human cholesterol synthesis. 1995; 32: E878-E83
- Jones PJH, Pappu AS, Illingworth DR, Leitch CA. Correspondence between plasma mevalonic acid levels and deuterium uptake in measuring human cholesterol synthesis. 1992; 22: 609-13
- Jones PJH, Schoeller DA. Evidence for diurnal periodicity in human cholesterol-synthesis. 1990; 31: 667-73
- Galman C, Angelin B, Rudling M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. 2005; 129: 1445-53
- Alhassan S, Reese KA, Mahurin J, Plaisance EP, Hilson BD, Garner JC, Wee SO, Grandjean PW. Blood lipid responses to plant stanol ester supplementation and aerobic exercise training. 2006; 55: 541-9
- Ayesh R, Weststrate JA, Drewitt PN, Hepburn PA. Safety evaluation of phytosterol esters. Part 5. Faecal short-chain fatty acid and microflora content, faecal bacterial enzyme activity and serum female sex hormones in healthy normolipidaemic volunteers consuming a controlled diet either with or without a phytosterol ester-enriched margarine. 1999; 37: 1127-38
- Cater NB, Garcia-Garcia AB, Vega GL, Grundy SM. Responsiveness of plasma lipids and lipoproteins to plant stanol esters. 2005; 96: 23D-8
- Christiansen LI, Lahteenmaki PLA, Mannelin MR, Seppanen-Laakso TE, Hiltunen RVK, Yliruusi JK. Cholesterol-lowering effect of spreads enriched with microcrystal line plant sterols in hypercholesterolemic subjects. 2001; 40: 66-73
- Cleghorn CL, Skeaff CM, Mann J, Chisholm A. Plant sterol-enriched spread enhances the cholesterol-lowering potential of a fat-reduced diet. 2003; 57: 170-6
- Gylling H, Miettinen TA. Serum-cholesterol and cholesterol and cipoprotein metabolism in hypercholesterolemic NIDDM patients before and during sitostanol ester-margarine treatment. 1994; 37: 773-80
- Gylling H, Miettinen TA. Cholesterol reduction by different plant stanol mixtures and with variable fat intake. 1999; 48: 575-80
- Hallikainen MA, Sarkkinen ES, Gylling H, Erkkila AT, Uusitupa MIJ. Comparison of the effects of plant sterol ester and plant stanol ester-enriched margarines in lowering serum cholesterol concentrations in hypercholesterolaemic subjects on a low-fat diet. 2000; 54: 715-25
- Hendriks HFJ, Brink EJ, Meijer GW, Princen HMG, Ntanios FY. Safety of long-term consumption of plant sterol esters-enriched spread. 2003; 57: 681-92
- Jakulj L, Trip MD, Sudhop T, von Bergmann K, Kastelein JJP, Vissers MN. Inhibition of cholesterol absorption by the combination of dietary plant sterols and ezetimibe: effects on plasma lipid levels. 2005; 46: 2692-8
- Judd JT, Baer DJ, Chen SC, Clevidence BA, Muesing RA, Kramer M, Meijer GW. Plant sterol esters lower plasma lipids and most carotenoids in mildly hypercholesterolemic adults. 2002; 37: 33-42
- Lau VWY, Journoud M, Jones PJH. Plant sterols are efficacious in lowering plasma LDL and non-HDL cholesterol in hypercholesterolemic type 2 diabetic and nondiabetic persons. 2005; 81: 1351-8
- Lee YM, Haastert B, Scherbaum W, Hauner H. A phytosterol-enriched spread improves the lipid profile of subjects with type 2 diabetes mellitus-A randomized controlled trial under free-living conditions. 2003; 42: 111-7
- Lottenberg AM, Nunes VS, Nakandakare ER, Neves M, Bernik M, Lagrost L, dos Santos JE, Quintao E. The human cholesteryl ester transfer protein I405V polymorphism is associated with plasma cholesterol concentration and its reduction by dietary phytosterol esters. 2003; 133: 1800-5
- Neil HAW, Meijer GW, Roe LS. Randomised controlled trial of use by hypercholesterolaemic patients of a vegetable oil sterol-enriched fat spread. 2001; 156: 329-37
- Nguyen TT, Dale LC, von Bergmann K, Croghan IT. Cholesterol-lowering effect of stanol ester in a US population of mildly hypercholesterolemic men and women: a randomized controlled trial. 1999; 74: 1198-206
- Nigon F, Serfaty-Lacrosniere C, Beucler I, Chauvois D, Neveu C, Giral P, Chapman MJ, Bruckert E. Plant sterol-enriched margarine lowers plasma LDL in hyperlipidemic subjects with low cholesterol intake: effect of fibrate treatment. 2001; 39: 634-40
- Noakes M, Clifton P, Ntanios F, Shrapnel W, Record I, McInerney J. An increase in dietary carotenoids when consuming plant sterols or stanols is effective in maintaining plasma carotenoid concentrations. 2002; 75: 79-86
- Plat J, Mensink RP. Vegetable oil based versus wood based stanol ester mixtures: effects on serum lipids and hemostatic factors in non-hypercholesterolemic subjects. 2000; 148: 101-12
- Saito S, Takeshita M, Tomonobu K, Kudo N, Shiiba D, Hase T, Tokimitsu I, Yasukawa T. Dose-dependent cholesterol-lowering effect of a mayonnaise-type product with a main component of diacylglycerol-containing plant sterol esters. 2006; 22: 174-8
- Sierksma A, Weststrate JA, Meijer GW. Spreads enriched with plant sterols, either esterified 4,4-dimethylsterols or free 4-desmethylsterols, and plasma total- and LDL-cholesterol concentrations. 1999; 82: 273-82
- Temme EHM, Van Hoydonck PGA, Schouten EG, Kesteloot H. Effects of a plant sterol-enriched spread on serum lipids and lipoproteins in mildly hypercholesterolaemic subjects. 2002; 57: 111-5
- Vanhanen HT, Blomqvist S, Ehnholm C, Hyvonen M, Jauhiainen M, Torstila I, Miettinen TA. Serum-cholesterol, cholesterol precursors, and plant sterols in hypercholesterolemic subjects with different apoe phenotypes during dietary sitostanol ester treatment. 1993; 34: 1535-44
- Vanhanen H. Cholesterol malabsorption caused by sitostanol Ester feeding and neomycin in pravastatin-treated hypercholesterolemic patients. 1994; 47: 169-76
- Vissers MN, Zock PL, Meijer GW, Katan MB. Effect of plant sterols from rice bran oil and triterpene alcohols from sheanut oil on serum lipoprotein concentrations in humans. 2000; 72: 1510-5
- Cleeman JI, Grundy SM, Becker D, Clark LT, Cooper RS, Denke MA, Howard WJ, Hunninghake DB, Illingworth DRet al.Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). 2001; 285: 2486-97
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. 2002; 31: 140-9
Appendix A: Appendix: calculations used in the meta-analysis of plant sterols and LDL cholesterol levels
Appendix B: Effect size (ES)
For parallel trials, endpoint LDL cholesterol in the treatment group was subtracted from endpoint LDL cholesterol in the control group Citation46. For crossover trials, LDL cholesterol values at the end of the treatment period were subtracted from LDL cholesterol values at the end of the control period Citation46. Within-individual changes were used when presented; otherwise, group means were used. In symbols, the estimates of ES are:
– For parallel trials: ESII=Tf–Cf
where
ESII=the effect size of a parallel design trial,
Tf=final LDL cholesterol mean in the treatment group
Cf=final LDL cholesterol mean in the control group
– For crossover trials: ESx=T–C
where
ESx=the effect size of a crossover design trial
T = LDL cholesterol mean at the end of the treatment period
C = LDL cholesterol mean at the end of the control period
Appendix C: Standard error (SE) of effect size (ES)
– For parallel trials:
The SE of ES for a parallel study was calculated as followsWhere
SEII=standard error of effect size for a parallel study
SDT=standard deviation of LDL-cholesterol endpoints in the treatment group
SDC=standard deviation of LDL-cholesterol endpoints in the control
nT=sample size of the treatment group
nC=sample size of the control group
SDs were extracted from the studies or, if not reported, derived from SE of mean or CI for group mean, Citation46 as follows:
– From SE:
Standard error of group mean = SD/√N
– From CI for group mean:
SD = √N x (upper limit-lower limit)/2* t (1-confidence level, degree of freedom)
where
t (1-confidence level, degree of freedom) is the t-value associate with study confidence level, usually 95%, and sample size of group
– For crossover trials Citation46Citation86:
The SE of ES for a crossover study was calculated as follows
where
SEx=standard error of effect size for a crossover study
SD(diff)=standard deviation of difference between the treatment period and the control period
n=sample size
or
SEx was extracted from reported statistical values in the trial, paired t-value or P-value, CI from a paired analysis or imputed from a number of studies as follows:
– From t or P-value:
t=diff/SEx
where
diff is mean of difference between control period and treatment period
If only the exact P-value or the upper bounded P-value of the paired t-test was reported, then the correspondent t-value for that P-value was calculated by entering the P-value and the degree of freedom into a spreadsheet as follows:=tinv(P-value, degree of freedom)
– From CI:
SEx=(upper limit–lower limit)/2* t (1-confidence level, degree of freedom)
where
t (1-confidence level, degree of freedom) is the t-value associate with study confidence level, usually 95%, and degree of freedom
– From imputed SD(diff):Where
SD(diff)=standard deviation of difference between the treatment period and the control period
SD2T=LDL-cholesterol variance at the end of the treatment period
SD2C=LDL-cholesterol variance at the end of the control period
R=0.81 which is within-individual correlation between the treatment and control periods that was calculated from a number of studies.
Appendix D: Pooled effect size (ES) estimate
Treatment ES and its SE were calculated for every trial as described above. To obtain the pooled treatment ES, the ES estimates and SE were entered into RevMan 4.2 under the “Generic inverse variance” outcome. In the inverse variance method the weight given to each study is chosen to be the inverse of the variance of the effect estimate.
A fixed effect meta-analysis using the inverse variance method calculates a weighted average as follows:
Generic inverse variance weighted average = ∑(ESi/SEi2)
∑(1/SEi2)
where
ESi is the effect size in study i, SEi is the standard error of that estimate and the summation is across all studies.
Calculation of within-individual correlation between the treatment and control periods for crossover studies
WhereR=within-individual correlation between the treatment and control periods and was calculated as follows: