Abstract
Background: The incidence of fragility fractures has increased during the last half of the 1900′s. One important determinant of fractures is the bone mineral content (BMC) or bone mineral density (BMD), the amount of mineralised bone. If we could increase peak bone mass (the highest value of BMC reached during life) and/or decrease the age-related bone loss, we could possibly improve the skeletal resistance to fracture.
Objective: This review evaluates the importance of exercise as a strategy to improve peak bone mass, including some aspects of nutrition.
Design: Publications within the field were searched through Medline (PubMed) using the search words: exercise, physical activity, bone mass, bone mineral content, bone mineral density, BMC, BMD, skeletal structure and nutrition. We included studies dealing with exercise during growth and young adolescence. We preferably based our inferences on randomised controlled trials (RCT), which provide the highest level of evidence.
Results: Exercise during growth increases peak bone mass. Moderate intensity exercise intervention programs are beneficial for the skeletal development during growth. Adequate nutrition must accompany the exercise to achieve the most beneficial skeletal effects by exercise.
Conclusion: Exercise during growth seems to enhance the building of a stronger skeleton through a higher peak bone mass and a larger bone size.
Introduction
One of the major medical problems in society during the latter part of the last century is the increasing incidence of fragility fractures Citation1Citation2Citation3Citation4Citation5Citation6Citation7Citation8Citation9Citation10Citation11Citation12Citation13Citation14Citation15. During their lifetime, half of all women and one-third of all men will suffer a fracture Citation1Citation3. The fractures cause increased morbidity, mortality and costs for society Citation16. The increase in fracture incidence is to some extent attributed to an increased prevalence of osteoporosis, predominantly due to an increased aging population in Citation15Citation17Citation18.
Peak bone mass is defined as the highest level of bone mineral density (BMD) or bone mineral content (BMC) or bone mass (BM) reached during life. These are all estimations of the amount of mineralised bone. BM is a more generalized term when describing the amount of mineral, BMC the amount of mineral measured within the scanned skeletal region and BMD the amount of mineral measured within the scanned skeletal region but partially adjusted for the bone size. If growing children built a skeleton with a lower peak bone mass than 50 years ago, then the fracture risk ought to have increased during the same period. It seems possible that this has occurred, as today we live a more sedentary life than some decades ago, although no long-term studies are available to support this assumption Citation4. If we could implement changes in the current lifestyle by increasing levels of physical activity, we could possibly also increase the accrual of bone mineral so that young individuals of today reach a high peak bone mass. A higher peak bone mass would then probably reduce the number of fractures, as 50% of the BMD in old age is attributed to the peak bone mass Citation19.
Epidemiological studies have convincingly shown that BMC and BMD are closely associated with the risk of sustaining a fracture Citation20. A 10% decrease in BMD (corresponding to one standard deviation; SD) is associated with a doubled fracture risk Citation20. However, even if BMD seems to be an excellent tool when evaluating fracture risk at a group level, it is a less reliable predictor when evaluating the individual fracture risk. Many other risk factors influence the risk of suffering a fracture Citation3Citation17Citation20Citation21. Bone structure is a trait that, independently of BMD, influences the bone's resistance to trauma Citation22. Although recent studies have suggested to shift the focus from BMD to interventions that reduce the number of falls as a more efficient fracture prophylactic tool approach Citation23, this review focuses on data that support or oppose the view that exercise during growth may influence the accrual of bone mineral and gain in bone structure. Some aspects of nutrition are covered as well.
2 Method
The search for papers to be included in the review was done in Medline (PubMed). The search words: exercise, physical activity, bone mass, bone mineral content, BMC, bone mineral density, BMD, skeletal structure and nutrition were used. Only papers or abstracts published in the English language and studies that evaluate exercise and the skeleton during growth and adolescence were included in the review. No restriction in time period was used. From the relevant papers included in the Medline search, a further search was undertaken by choosing the connection ‘related manuscripts’. Preferably, prospective, randomised controlled trials (RCT) were then included in this overview, as this is the highest ranked study design in evidence-based systems Citation24. All published RCTs in pre-pubertal (Tanner stage I), early pubertal (Tanner stage II and III) and pubertal (Tanner stage IV and V) children were evaluated. If no RCTs were found, the next level of evidence in the evidence-based hierarchy was scrutinised, i.e. non-randomised controlled studies, then retrospective and prospective observation cohort studies, and finally case-control studies. As there exists an enormous amount of publications with these study designs, we aimed to include those with the largest sample size and the longest follow-up period. But, it must be emphasised that this is not a systematic review with pre-specified inclusion criteria or a meta-analysis. Neither did we intend to include all papers published within this topic since Nilsson et al. first wrote their article in 1970 ‘bone density in athletes’ Citation25. Instead, we tried to interpret the enormous amount of data within the field in order to summarize the current view within this topic, to evaluate if exercise and nutrition during growth are of biological significance for the skeleton.
3 Results and discussion
3.1 Physical activity on competitive level and the skeleton
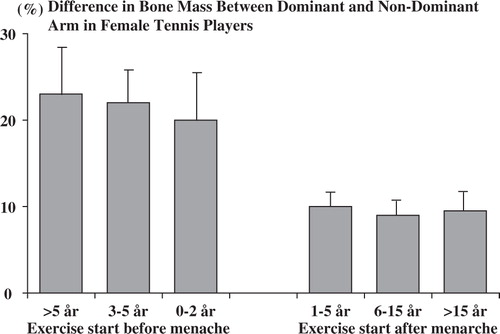
Today, there is compelling evidence indicating that physical activity affects the skeleton and the BMC and BMD in an anabolic way Citation26. The first study that addressed this hypothesis showed that athletes subjected to high load activities had 10–20% higher BMD compared to the controls Citation25. Further cross-sectional studies supported this view when comparing the dominant and non-dominant arm in racket players, a study design that controlled for the genetic regulation of the BMC. The BMC was 25–35% higher among professional tennis players in the dominant arm compared to the non-dominant arm Citation27. Furthermore, life-long tennis players aged 70–84 years had 4–7% higher BMC in the dominant compared to the non-dominant forearm Citation28. Later studies have verified these findings Citation29 and also defined at what age period physical activity has the most pronounced anabolic effects Citation30. After adjustment for different training history, the dominant and non-dominant arm difference were two to four times higher if the training was started before than after menarche Citation30 ().
Figure 1. The mean playing-to-non-playing arm difference in the bone mineral content of the humeral shaft (percentage difference of bone mineral content) according to the biological age at which training was started, that is, according to the starting age of playing relative to the age at menarche. Bars represent 95% CIs. Adapted from Kannus et al. Citation30.
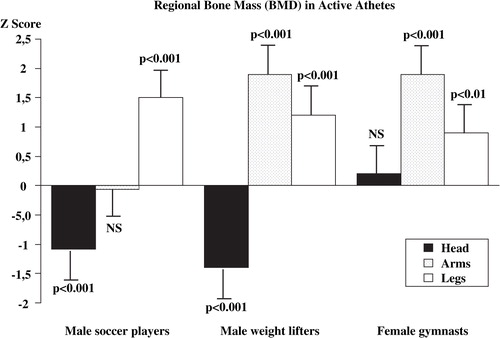
If exercise is performed at a high level of activity, as in competitive athletes, a 10–20% gain in BMC can be expected Citation31Citation32Citation33Citation34Citation35 (). Both male and female gymnasts, soccer players, weight-lifters and ballet dancers are reported to have a 10–25% higher BMC compared to non-exercising controls Citation31Citation32Citation33Citation34Citation35Citation36Citation37Citation38Citation39. It is important to emphasise that the increase in BMC is only found in loaded skeletal parts and not in unloaded parts (). Thus, male weight lifters had a 10–20% higher BMC compared to the controls in the arms, which are highly loaded skeletal regions, during weight lifting Citation31Citation40. On the contrary, male soccer players had no different BMC in the arms compared to the controls Citation34Citation35, but in the lower legs BMC was higher in the soccer players at the same magnitude as in the arm of the male weight lifters, i.e. 10–20% higher than in the controls Citation32Citation34Citation35 (). Other athletes, training endurance exercise do not have higher BMC than controls Citation25. Moreover, exercise, on both more moderate and elite level confers increased BMC. But, this type of exercise also induces benefits in bone size and skeletal structure that increase bone strength independently of the BMC Citation22Citation29Citation41Citation42.
Figure 2. Bone mineral density (BMD) of the head, the arms and the legs, in active male soccer players, male weight lifters and female gymnasts expressed as Z scores (number of standard deviations (SD) above or below age predicted mean). Adapted from Karlsson et al. Citation31, Citation34 and Bass et al. Citation39.
3.2 Which types of exercise provide skeletal benefits?
Exercise studies have also shown which type of exercise confers maximal anabolic effects on the skeleton. Skeletal load that includes a dynamic load, a load with a high magnitude, a high frequency load, a fast load and a load with unusually distributed strains provide the most pronounced osteogenic stimuli Citation43Citation44Citation45Citation46. The required mechanical load necessary to stimulate osteogenesis decreases as the strain magnitude and frequency increases Citation47Citation48. A most important fact is that the osteogenic response to high magnitude loading becomes saturated after a few loading cycles Citation44, whereafter additional loading has limited benefits Citation49. That is, the duration of exercise is of much less importance because a short duration of load or a small number of repetitions is enough to achieve the maximal anabolic effect Citation43Citation44Citation45Citation46Citation50. Bone cell mechano sensitivity seems to recover following a period without loading. Thus, separating loading into short bouts with periods of rest optimises the osteogenic response to loading Citation51Citation52Citation53, even to low magnitude stimuli Citation54Citation55. In animal studies, 4 h of rest doubled the response and the sensitivity was almost completely restored after 8 h of recovery Citation51.
With this knowledge, it seems as if high intensity sports like squash, tennis, soccer, ice-hockey, badminton, volleyball and weight-lifting performed on several different occasions during the week are most effective if the aim is to improve the skeletal strength Citation25Citation30Citation31Citation32Citation33Citation34Citation35Citation39 (). In contrast, endurance exercise is less beneficial for the skeleton. Long-distance running, not a high impact activity but at least promoting weight-bearing activity, produces minor skeletal benefits Citation25Citation56, while non-weight-bearing activities, such as cycling and swimming, do not seem to produce any skeletal benefits of biological significance Citation24Citation25.
3.3 Physical activity and the accrual of bone mineral during growth
Randomised and non-randomised prospective controlled exercise intervention studies with 6–24 months’ duration infer that moderate physical activity in the pre- and peri-pubertal period could enhance BMC accrual with a magnitude that, if retained into old ages, would actually reduce the number of fragility fractures Citation42Citation57Citation58Citation59Citation60Citation61Citation62Citation63Citation64Citation65Citation66Citation67Citation68Citation69Citation70Citation71Citation72Citation73Citation74Citation75Citation76 (). Few of these trials, however, follow the children with intervention beyond 1 year Citation66Citation67Citation77Citation78. MacKelvie et al. Citation65Citation66Citation67Citation68 followed pre-pubertal girls in Tanner stage I (classification of pubertal stage) for 20 months and reported that an exercise intervention programme including specific osteogenic activities for 12 min, three times per week in 10-year-old girls led to 4% higher gain in femoral neck and lumbar spine BMC in the intervention group (n=32) than in the control group (n=43) Citation65. In 64 boys involved in the same programme (31 in the intervention group and 33 in the control group), there was a difference in the BMC of the femoral neck only, but of the same magnitude as in the girls Citation68.
Table 1. The skeletal response to exercise seen in randomized and non-randomized prospective controlled exercise intervention studies in pre- and peri-pubertal children and in post-pubertal girls
The other long-term trial, the prospective Paediatric Osteoporosis Prevention (POP) study, included a moderate intense school-based exercise intervention program comprising 40 min of general physical activity per school day (200 min per week), while the controls ware subjected to the general Swedish school curriculum of 60 min per week Citation77Citation78. Eighty boys aged 7–9 years were included in the intervention program with 57 age-matched boys as controls. The mean annual BMC gain in the third lumbar vertebra was 3% (p<0.01) and in L3 width 1.3% (p<0.01) greater in the intervention than in the control group. The weekly duration of exercise estimated through the questionnaire correlated with a gain in BMC in the third lumbar vertebra (r=0.25, p=0.005) and was also related to vertebra width (r=0.20, p=0.02) Citation78. Forty-nine girls aged 7–9 years were included in the intervention group while 50 served as controls Citation77. All girls were pre-menarchal and in Tanner stage I during the study. The annual gain in BMC was greater in the intervention group than in the controls, in the second to fourth lumbar vertebrae (L2–L4) mean 3.8% (p=0.007), in the L3 vertebra mean 7.2% (p<0.001) and the legs mean 3% (p=0.07). There was also a greater mean annual gain in bone size, in the L3 vertebra mean 1.8% (p<0.001) and in the femoral neck mean 0.3% (p=0.02) in the intervention group Citation77. Three- and four-year data have also been presented from the POP study in abstract form, showing that the benefits remain with 3 and 4 years of extra school training at a similar magnitude as after 2 years’ intervention Citation79Citation80.
The rest of the cited RCTs in this review have followed the children for a shorter follow-up period than 16 months. Most of the studies include children that on a voluntary basis wanted to participate in an exercise study, a fact that increases the risk of selection bias. One study reported that exercises including jumping up and down a small step 30 min per day, three times per week, increased BMD in the greater trochanter by 1.4% over a period of 8 months Citation70. Most of the studies in pre- and peri-pubertal children have used specifically designed osteogenic intervention programmes, such as jumping up and down a small height, or high intense short-term programs and provide similar data Citation57Citation59Citation61Citation62Citation70Citation71Citation81Citation82 (). These repetitive types of exercise have been shown to be effective in the short-term perspective, but involve the risk of boring the children, leading to high dropout frequencies Citation83. There are fewer RCTs in post-pubertal children (). No increase in BMC or BMD could be demonstrated after a 6–9 months’ intervention period in these girls Citation58Citation62Citation76. On the other hand, two 15-month trials in post-pubertal girls indicate that physical training may insert skeletal effects also during this period Citation72Citation74. However, these data should be interpreted with caution as there were only 17 girls in one study Citation72 and in the second, additional calcium support of 1000 mg per day was given Citation74.
In summary, available prospective controlled exercise intervention studies support the hypothesis forwarded by Kannus et al. more than 10 years ago Citation30, that training in the late pre- and early pubertal period seems to be more effective when trying to enhance the BMD, than providing the same type of training after puberty (, ).
3.4 Effects of physical activity on skeletal structure during growth
In addition to the increased accrual of bone mineral, exercise during growth is important because of the associated changes in bone geometry that translate to greater increases in bone strength than provided by an increase in BMC alone Citation22. Bone size was approximately 10% larger when comparing the upper limbs of young pre-pubertal gymnasts and normally active children Citation84Citation85, or the playing and non-playing arms of young pre-pubertal tennis players Citation29Citation86. This beneficial effect seems to have occurred due to an exercise-induced periosteal expansion. Alternatively, bone mineral may be deposited on the endosteal surface, producing a thicker cortical shell without a wider bone. For example, cortical cross-sectional area was 5–12% greater in the lower limbs of young runners or young gymnasts compared to controls Citation84Citation87Citation88. The enlargement of bone cross section in response to loading has been reported to increase from pre- to peri-puberty in male but not in female tennis players Citation29Citation86. Apposition of bone on the periosteal surface of cortical bone is a more effective means of increasing the bending and torsion strength of bone than acquisition of bone on the endosteal surface Citation89.
The complexity of the skeletal response to loading is also illustrated by the heterogeneity of the geometrical adaptations along the length of a bone Citation52Citation53. For instance, in young tennis players, loading has been shown to induce endosteal apposition at the distal humerus but not at the mid humerus. Periosteal expansion has been reported to occur differently in the proximal, mid-diaphyseal and distal parts as well as in the medio-lateral and anterior-posterior directions within the same bone Citation27Citation90.
Some but not all of the cited RCTs in pre-pubertal children have also inferred that bone structure, evaluated as bone area or bone size, in both boys and girls may be influenced in a beneficial way even by moderate intense intervention programs Citation38Citation57Citation61Citation77Citation82Citation91 (). As already mentioned, the osteogenic benefits achieved by exercise during growth are also maturity- and sex-dependent Citation92, with exercise interventions being most effective for both BMC and bone structure when initiated during pre- or early puberty. It is thought that exercise may preferentially affect the surface of bone that is undergoing apposition during growth Citation93. Accordingly, the pre-pubertal skeleton demonstrates the capacity to respond to loading by adding more bone on the periosteal surface than would normally occur through growth-induced periosteal apposition Citation29Citation85Citation86. Several studies also mentioned exercise-induced endosteal apposition in pre-pubertal boys Citation84Citation86Citation94, whereas such a response is not seen in pre-pubertal girls observed under the same conditions Citation29Citation84. Further studies are needed to determine the sex- and maturity-dependent osteogenic response to loading.
3.5 Adverse effects of exercise
Most skeletal changes associated with physical activity are beneficial and there are only sparse descriptions of adverse effects. If the physical level is increased, normally the skeleton responds with an increased BMC Citation31Citation32Citation33Citation34Citation35Citation36Citation37Citation38Citation39. However, in some individuals the BMC remains unchanged or even decreases, something that has been seen as a factor behind both stress fracture and shin splints Citation95. But the most serious adverse skeletal side effect from exercise is seen in individuals with a very high intensity activity. Menstrual and hormonal alterations are frequently connected with dieting, low body mass and eating disorders – in addition to strenuous training. A long duration of hard training can lead to decreased oestrogen levels and a training-induced amenorrhoea, often accompanied by reduced BMC Citation96. Several studies in females within different sports have verified that this menstrual dysfunction leads to lowered BMC, even lower than in controls Citation96. If the menstrual dysfunction is normalised, the BMC slowly increases but is not fully restored Citation97. Interestingly, a similar negative metabolic effect after very hard exercise has also been seen in men Citation98.
3.6 Effect of nutrition on exercise-induced skeletal benefits
There are few studies that specifically evaluate how nutritional parameters may modify exercise-induced skeletal effects. Observational studies infer that energy and protein malnourished children have reduced bone size and BMC Citation99. Furthermore, prospective studies suggest that there is a positive association between dietary protein intake in children over 4 years and bone size and BMC Citation100.
Several studies have shown that the beneficial exercise-induced skeletal effects are seen only if the calcium intake is high, around 1300 mg per day in growing individuals and 1000 mg per day in adults Citation101Citation102Citation103. Prospective controlled exercise intervention trials also infer that extra calcium supplement interacts with increased exercise, resulting in a higher BMC And more advantageous skeletal structure Citation42. However, after cessation of the trial, all BMC benefits were lost after 12 months while the structural benefits remained Citation104. The exercise–nutrition interaction has been verified in several other trials Citation42Citation60Citation63Citation74Citation101Citation103Citation105. The underlying mechanisms for the exercise and nutritional interaction are poorly understood. Even fewer studies have evaluated the importance of Vitamin D, protein intake and energy intake. In summary, even if the literature is sparse, available data support that optimal beneficial skeletal effects are dependent on adequate nutrition.
4 Conclusions
Activity at a level that most individuals can perform increases the accrual of bone mineral during growth. Adequate nutrition must accompany the exercise to achieve the strongest possible skeleton. These data support the importance of community and health care interventions towards the young generation. A physically active lifestyle that includes skeletal mechanical load and an adequate nutrition seems to increase peak bone mass and in a longer perspective, possibly reduce the numbers of fragility fractures.
5 Recommendations
Based on current scientific knowledge, we should recommend a physically active lifestyle and an adequate nutritional intake for growing children, as one prevention strategy to reduce the current high incidence of fractures.
6 Conflict of interest
No conflicts of interest exist.
Acknowledgements
Financial support was obtained from the University Hospital Foundations, Centrum for Sports Medical Research (CIF), the Swedish Society of Medicine, and the Swedish Society of Medical Research.
References
- Cooper C, Campion G, Melton LJ, 3rd. Hip fractures in the elderly: a world-wide projection. 1992; 2: 6: 285-9. 10.3402/fnr.v52i0.1871
- Cooper C, Melton LJ3rd. Magnitude and impact of osteoporosis and fractures. Osteoporosis, Marcus R, Feldman D, Kelsey J. Academic Press: San Diego CA; 1996, 419-34
- Johnell O, Gullberg B, Kanis JA, Allander E, Elffors L, Dequeker Jet al.Risk factors for hip fracture in European women: the MEDOS Study. Mediterranean Osteoporosis Study. 1995; 10: 11: 1802-15
- Jonsson B.Life style and fracture risk. ThesisUniversity of Lund: Sweden; 1993
- Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. 2001; 12: 5: 417-27. 10.3402/fnr.v52i0.1871
- Kannus P, Niemi S, Parkkari J, Palvanen M, Vuori I, Jarvinen M. Hip fractures in Finland between 1970 and 1997 and predictions for the future. 1999; 353: 9155: 802-5. 10.3402/fnr.v52i0.1871
- Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. 1996; 18: 1 Suppl: 57S-63S. 10.3402/fnr.v52i0.1871
- Melton LJ, 3rd, Atkinson EJ, Madhok R. Downturn in hip fracture incidence. 1996; 111: 2: 146-50; discussion 151
- Melton LJ, 3rd, Lane AW, Cooper C, Eastell R, O'Fallon WM, Riggs BL. Prevalence and incidence of vertebral deformities. 1993; 3: 3: 113-9. 10.3402/fnr.v52i0.1871
- Bengner U.Epidemiological changes over 30 years in an urban population. ThesisUniversity of Lund: Sweden; 1987
- Gullberg B, Duppe H, Nilsson B, Redlund-Johnell I, Sernbo I, Obrant Ket al.Incidence of hip fractures in Malmo, Sweden (1950–1991). 1993; 14: Suppl 1: S23-9. 10.3402/fnr.v52i0.1871
- Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. 1997; 7: 5: 407-13. 10.3402/fnr.v52i0.1871
- Cummings SR, Kelsey JL, Nevitt MC, O'Dowd KJ. Epidemiology of osteoporosis and osteoporotic fractures. 1985; 7: 178-208
- Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. 2002; 359: 9319: 1761-7. 10.3402/fnr.v52i0.1871
- Obrant KJ, Bengner U, Johnell O, Nilsson BE, Sernbo I. Increasing age-adjusted risk of fragility fractures: a sign of increasing osteoporosis in successive generations?. 1989; 44: 3: 157-67. 10.3402/fnr.v52i0.1871
- Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM, Melton LJ, 3rd. Population-based study of survival after osteoporotic fractures. 1993; 137: 9: 1001-5
- Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown Jet al.The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. 2007; 18: 8: 1033-46. 10.3402/fnr.v52i0.1871
- Ahlborg H, Johnell O, Järvinen T, Karlsson MK. Prevalence of low bone mass in women – secular trend over 30 years. 2004; 19: Supp1: S49.
- Hui SL, Slemenda CW, Johnston CCJr The contribution of bone loss to postmenopausal osteoporosis. 1990; 1: 1: 30-4. 10.3402/fnr.v52i0.1871
- Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KEet al.Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. 1995; 332: 12: 767-73. 10.3402/fnr.v52i0.1871
- Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. 1996; 312: 7041: 1254-9
- Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. 2003; 349: 4: 327-34. 10.3402/fnr.v52i0.1871
- Jarvinen TL, Sievanen H, Khan KM, Heinonen A, Kannus P. Shifting the focus in fracture prevention from osteoporosis to falls. 2008; 336: 7636: 124-6. 10.3402/fnr.v52i0.1871
- Karlsson M, Bass S, Seeman E. The evidence that exercise during growth or adulthood reduces the risk of fragility fractures is weak. 2001; 15: 3: 429-50. 10.3402/fnr.v52i0.1871
- Nilsson BE, Westlin NE. Bone density in athletes. 1971; 77: 179-82
- Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. 2007; 40: 1: 14-27. 10.3402/fnr.v52i0.1871
- Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. 1977; 59: 2: 204-8
- Huddleston AL, Rockwell D, Kulund DN, Harrison RB. Bone mass in lifetime tennis athletes. 1980; 244: 10: 1107-9. 10.3402/fnr.v52i0.1871
- Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman Eet al.The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. 2002; 17: 12: 2274-80. 10.3402/fnr.v52i0.1871
- Kannus P, Haapasalo H, Sankelo M, Sievanen H, Pasanen M, Heinonen Aet al.Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. 1995; 123: 1: 27-31
- Karlsson MK, Hasserius R, Obrant KJ. Bone mineral density in athletes during and after career: a comparison between loaded and unloaded skeletal regions. 1996; 59: 4: 245-8. 10.3402/fnr.v52i0.1871
- Karlsson MK, Johnell O, Obrant KJ. Bone mineral density in weight lifters. 1993; 52: 3: 212-5. 10.3402/fnr.v52i0.1871
- Karlsson MK, Johnell O, Obrant KJ. Bone mineral density in professional ballet dancers. 1993; 21: 3: 163-9. 10.3402/fnr.v52i0.1871
- Karlsson MK, Linden C, Karlsson C, Johnell O, Obrant K, Seeman E. Exercise during growth and bone mineral density and fractures in old age. 2000; 355: 9202: 469-70. 10.3402/fnr.v52i0.1871
- Karlsson MK, Magnusson H, Karlsson C, Seeman E. The duration of exercise as a regulator of bone mass. 2001; 28: 1: 128-32. 10.3402/fnr.v52i0.1871
- Duppe H, Gardsell P, Johnell O, Ornstein E. Bone mineral density in female junior, senior and former football players. 1996; 6: 6: 437-41. 10.3402/fnr.v52i0.1871
- Valdimarsson O, Alborg HG, Duppe H, Nyquist F, Karlsson M. Reduced training is associated with increased loss of BMD. 2005; 20: 6: 906-12. 10.3402/fnr.v52i0.1871
- Valdimarsson O, Sigurdsson G, Steingrimsdottir L, Karlsson MK. Physical activity in the post-pubertal period is associated with maintenance of pre-pubertal high bone density – a 5-year follow-up. 2005; 15: 5: 280-6. 10.3402/fnr.v52i0.1871
- Bass S, Pearce G, Bradney M, Hendrich E, Delmas PD, Harding Aet al.Exercise before puberty may confer residual benefits in bone density in adulthood: studies in active prepubertal and retired female gymnasts. 1998; 13: 3: 500-7. 10.3402/fnr.v52i0.1871
- Karlsson MK, Vergnaud P, Delmas PD, Obrant KJ. Indicators of bone formation in weight lifters. 1995; 56: 3: 177-80. 10.3402/fnr.v52i0.1871
- Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. 2002; 17: 12: 2281-9. 10.3402/fnr.v52i0.1871
- Specker B, Binkley T. Randomized trial of physical activity and calcium supplementation on bone mineral content in 3- to 5-year-old children. 2003; 18: 5: 885-92. 10.3402/fnr.v52i0.1871
- Lanyon LE. Control of bone architecture by functional load bearing. 1992; 7: Suppl 2: S369-75
- Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. 1984; 66: 3: 397-402
- Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. 1985; 37: 4: 411-7. 10.3402/fnr.v52i0.1871
- Turner CH, Woltman TA, Belongia DA. Structural changes in rat bone subjected to long-term, in vivo mechanical loading. 1992; 13: 6: 417-22. 10.3402/fnr.v52i0.1871
- Cullen DM, Smith RT, Akhter MP. Bone-loading response varies with strain magnitude and cycle number. 2001; 91: 5: 1971-6
- Hsieh YF, Turner CH. Effects of loading frequency on mechanically induced bone formation. 2001; 16: 5: 918-24. 10.3402/fnr.v52i0.1871
- Umemura Y, Ishiko T, Yamauchi T, Kurono M, Mashiko S. Five jumps per day increase bone mass and breaking force in rats. 1997; 12: 9: 1480-5. 10.3402/fnr.v52i0.1871
- Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. 1984; 17: 12: 897-905. 10.3402/fnr.v52i0.1871
- Robling AG, Burr DB, Turner CH. Recovery periods restore mechanosensitivity to dynamically loaded bone. 2001; 204: Pt 19: 3389-99
- Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. 2002; 17: 8: 1545-54. 10.3402/fnr.v52i0.1871
- Robling AG, Hinant FM, Burr DB, Turner CH. Shorter, more frequent mechanical loading sessions enhance bone mass. 2002; 34: 2: 196-202. 10.3402/fnr.v52i0.1871
- Srinivasan S, Agans SC, King KA, Moy NY, Poliachik SL, Gross TS. Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. 2003; 33: 6: 946-55. 10.3402/fnr.v52i0.1871
- Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. 2002; 17: 9: 1613-20. 10.3402/fnr.v52i0.1871
- Michel BA, Lane NE, Bjorkengren A, Bloch DA, Fries JF. Impact of running on lumbar bone density: a 5-year longitudinal study. 1992; 19: 11: 1759-63
- Alwis G, Linden C, Dencker M, Stenevi-Lundgren S, Gardsell P, Karlsson MK. Bone mineral accrual and gain in skeletal width in pre-pubertal school children is independent of the mode of school transportation-one-year data from the prospective observational pediatric osteoporosis prevention (POP) study. 2007; 8: 6610.3402/fnr.v52i0.1871
- Blimkie CJ, Rice S, Webber CE, Martin J, Levy D, Gordon CL. Effects of resistance training on bone mineral content and density in adolescent females. 1996; 74: 9: 1025-33. 10.3402/fnr.v52i0.1871
- Bradney M, Pearce G, Naughton G, Sullivan C, Bass S, Beck Tet al.Moderate exercise during growth in prepubertal boys: changes in bone mass, size, volumetric density, and bone strength: a controlled prospective study [see comments]. 1998; 13: 12: 1814-21. 10.3402/fnr.v52i0.1871
- Courteix D, Jaffre C, Lespessailles E, Benhamou L. Cumulative effects of calcium supplementation and physical activity on bone accretion in premenarchal children: a double-blind randomised placebo-controlled trial. 2005; 26: 5: 332-8. 10.3402/fnr.v52i0.1871
- Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. 2001; 16: 1: 148-56. 10.3402/fnr.v52i0.1871
- Heinonen A, Sievanen H, Kannus P, Oja P, Pasanen M, Vuori I. High-impact exercise and bones of growing girls: a 9-month controlled trial. 2000; 11: 12: 1010-7. 10.3402/fnr.v52i0.1871
- Iuliano-Burns S, Saxon L, Naughton G, Gibbons K, Bass SL. Regional specificity of exercise and calcium during skeletal growth in girls: a randomized controlled trial. 2003; 18: 1: 156-62. 10.3402/fnr.v52i0.1871
- Laing EM, Wilson AR, Modlesky CM, O'Connor PJ, Hall DB, Lewis RD. Initial years of recreational artistic gymnastics training improves lumbar spine bone mineral accrual in 4- to 8-year-old females. 2005; 20: 3: 509-19. 10.3402/fnr.v52i0.1871
- MacKelvie KJ, Khan KM, Petit MA, Janssen PA, McKay HA. A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trial in girls. 2003; 112: 6 Pt : e1: 44710.3402/fnr.v52i0.1871
- Mackelvie KJ, McKay HA, Khan KM, Crocker PR. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. 2001; 139: 4: 501-8. 10.3402/fnr.v52i0.1871
- MacKelvie KJ, McKay HA, Petit MA, Moran O, Khan KM. Bone mineral response to a 7-month randomized controlled, school-based jumping intervention in 121 prepubertal boys: associations with ethnicity and body mass index. 2002; 17: 5: 834-44. 10.3402/fnr.v52i0.1871
- MacKelvie KJ, Petit MA, Khan KM, Beck TJ, McKay HA. Bone mass and structure are enhanced following a 2-year randomized controlled trial of exercise in prepubertal boys. 2004; 34: 4: 755-64. 10.3402/fnr.v52i0.1871
- McKay HA, MacLean L, Petit M, MacKelvie-O'Brien K, Janssen P, Beck Tet al.Bounce at the Bell’: a novel program of short bouts of exercise improves proximal femur bone mass in early pubertal children. 2005; 39: 8: 521-6. 10.3402/fnr.v52i0.1871
- McKay HA, Petit MA, Schutz RW, Prior JC, Barr SI, Khan KM. Augmented trochanteric bone mineral density after modified physical education classes: a randomized school-based exercise intervention study in prepubescent and early pubescent children [see comments]. 2000; 136: 2: 156-62. 10.3402/fnr.v52i0.1871
- Morris FL, Naughton GA, Gibbs JL, Carlson JS, Wark JD. Prospective ten-month exercise intervention in premenarcheal girls: positive effects on bone and lean mass. 1997; 12: 9: 1453-62. 10.3402/fnr.v52i0.1871
- Nichols DL, Sanborn CF, Love AM. Resistance training and bone mineral density in adolescent females. 2001; 139: 4: 494-500. 10.3402/fnr.v52i0.1871
- Petit MA, McKay HA, MacKelvie KJ, Heinonen A, Khan KM, Beck TJ. A randomized school-based jumping intervention confers site and maturity-specific benefits on bone structural properties in girls: a hip structural analysis study. 2002; 17: 3: 363-72. 10.3402/fnr.v52i0.1871
- Stear SJ, Prentice A, Jones SC, Cole TJ. Effect of a calcium and exercise intervention on the bone mineral status of 16-18-y-old adolescent girls. 2003; 77: 4: 985-92
- Van Langendonck L, Claessens AL, Vlietinck R, Derom C, Beunen G. Influence of weight-bearing exercises on bone acquisition in prepubertal monozygotic female twins: a randomized controlled prospective study. 2003; 72: 6: 666-74. 10.3402/fnr.v52i0.1871
- Witzke KA, Snow CM. Effects of plyometric jump training on bone mass in adolescent girls. 2000; 32: 6: 1051-7. 10.3402/fnr.v52i0.1871
- Linden C, Ahlborg HG, Besjakov J, Gardsell P, Karlsson MK. A school curriculum-based exercise program increases bone mineral accrual and bone size in prepubertal girls: two-year data from the Pediatric Osteoporosis Prevention (POP) study. 2006; 21: 6: 829-35. 10.3402/fnr.v52i0.1871
- Alwis G, Linden C, Ahlborg H, Dencker M, Gardsell P, Karlsson M. A school-curriculum-based exercise program in pre-pubertal boys for two years induces skeletal benefits in lumbar spine – data from the Pediatric Osteoporosis Prevention (POP) study. Acta Paediatr 2008, Jul 30 [Epub ahead of print].
- Linden C, Gärdsell P, Ahlborg H, Karlsson M. A school curriculum based exercise program increase bone mineral accrual in boys and girls during early adolescence – four year data from the POP study (Pediatric Preventive Osteoporotic study). 2005; 20: 1: 75.
- Linden C, Gärdsell P, Johnell O, Karlsson M. A school curriculum based exercise program increase bone mineral accrual in boys and girls during early adolescence – three year data from the POP study (Pediatric Preventive Osteoporotic study) – a prospective controlled intervention study in 223 children. 2004; 19: Supp 1: S38.
- Valdimarsson O, Linden C, Johnell O, Gardsell P, Karlsson MK. Daily physical education in the school curriculum in prepubertal girls during 1 year is followed by an increase in bone mineral accrual and bone width – data from the prospective controlled Malmo pediatric osteoporosis prevention study. 2006; 78: 2: 65-71. 10.3402/fnr.v52i0.1871
- Linden C, Alwis G, Ahlborg H, Gardsell P, Valdimarsson O, Stenevi-Lundgren Set al.Exercise, bone mass and bone size in prepubertal boys: one-year data from the pediatric osteoporosis prevention study. 2007; 17: 4: 340-7
- Bass S, Saxon L, Iuliano-Burns S, Naughton G, Daly R, Nowson Cet al.Limitations of long term exercise interventions aimed at improving bone health in normally active boys. 2003; 18: 2 Suppl: M 151.
- Ward KA, Roberts SA, Adams JE, Mughal MZ. Bone geometry and density in the skeleton of pre-pubertal gymnasts and school children. 2005; 36: 6: 1012-8. 10.3402/fnr.v52i0.1871
- Dyson K, Blimkie CJ, Davison KS, Webber CE, Adachi JD. Gymnastic training and bone density in pre-adolescent females. 1997; 29: 4: 443-50
- Ducher G, Tournaire N, Meddahi-Pelle A, Benhamou CL, Courteix D. Short-term and long-term site-specific effects of tennis playing on trabecular and cortical bone at the distal radius. 2006; 24: 6: 484-90. 10.3402/fnr.v52i0.1871
- Duncan CS, Blimkie CJ, Kemp A, Higgs W, Cowell CT, Woodhead Het al.Mid-femur geometry and biomechanical properties in 15- to 18-yr-old female athletes. 2002; 34: 4: 673-81. 10.3402/fnr.v52i0.1871
- Greene DA, Naughton GA, Briody JN, Kemp A, Woodhead H, Corrigan L. Bone strength index in adolescent girls: does physical activity make a difference?. 2005; 39: 9: 622-7; discussion 627. 10.3402/fnr.v52i0.1871
- Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. 1993; 14: 4: 595-608. 10.3402/fnr.v52i0.1871
- Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. 2000; 27: 3: 351-7. 10.3402/fnr.v52i0.1871
- Macdonald HM, Kontulainen SA, Khan KM, McKay HA. Is a school-based physical activity intervention effective for increasing tibial bone strength in boys and girls?. 2007; 22: 3: 434-46. 10.3402/fnr.v52i0.1871
- Daly RM. The effect of exercise on bone mass and structural geometry during growth. Optimizing bone mass and strength. The role of physical activity and nutrition during growth, Daly RM, Petit MA. Karger: Basel; 2007; 51: 33-49
- Ruff CB, Walker A, Trinkaus E. Postcranial robusticity in Homo. III: Ontogeny. 1994; 93: 1: 35-54. 10.3402/fnr.v52i0.1871
- Brahm H, Mallmin H, Michaelsson K, Strom H, Ljunghall S. Relationships between bone mass measurements and lifetime physical activity in a Swedish population. 1998; 62: 5: 400-12. 10.3402/fnr.v52i0.1871
- Magnusson HI, Westlin NE, Nyqvist F, Gardsell P, Seeman E, Karlsson MK. Abnormally decreased regional bone density in athletes with medial tibial stress syndrome. 2001; 29: 6: 712-5
- Pearce G, Bass S, Young N, Formica C, Seeman E. Does weight-bearing exercise protect against the effects of exercise-induced oligomenorrhea on bone density?. 1996; 6: 6: 448-52. 10.3402/fnr.v52i0.1871
- Micklesfield LK, Reyneke L, Fataar A, Myburgh KH. Long-term restoration of deficits in bone mineral density is inadequate in premenopausal women with prior menstrual irregularity. 1998; 8: 3: 155-63. 10.3402/fnr.v52i0.1871
- Malm HT, Ronni-Sivula HM, Viinikka LU, Ylikorkala OR. Marathon running accompanied by transient decreases in urinary calcium and serum osteocalcin levels. 1993; 52: 3: 209-11. 10.3402/fnr.v52i0.1871
- Garn S.The earlier gain and later loss of cortical bone. Nutritional perspectivesCharles C. Thomas: Springfield IL; 1970, 3-120
- Alexy U, Remer T, Manz F, Neu CM, Schoenau E. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. 2005; 82: 5: 1107-14
- Bass SL, Naughton G, Saxon L, Iuliano-Burns S, Daly R, Briganti EMet al.Exercise and calcium combined results in a greater osteogenic effect than either factor alone: a blinded randomized placebo-controlled trial in boys. 2007; 22: 3: 458-64. 10.3402/fnr.v52i0.1871
- Jackman LA, Millane SS, Martin BR, Wood OB, McCabe GP, Peacock Met al.Calcium retention in relation to calcium intake and postmenarcheal age in adolescent females. 1997; 66: 2: 327-333
- Specker BL. Evidence for an interaction between calcium intake and physical activity on changes in bone mineral density. 1996; 11: 10: 1539-44
- Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KEet al.Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. 2004; 89: 7: 3152-7. 10.3402/fnr.v52i0.1871
- Johannsen N, Binkley T, Englert V, Neiderauer G, Specker B. Bone response to jumping is site-specific in children: a randomized trial. 2003; 33: 4: 533-9. 10.3402/fnr.v52i0.1871