Abstract
Historically, food fortification has served as a tool to address population-wide nutrient deficiencies such as rickets by vitamin D fortified milk. This article discusses the different policy strategies to be used today. Mandatory or voluntary fortification and fortified foods, which the consumer needs, also have to comply with nutritional, regulatory, food safety and technical issues. The ‘worldwide map of vitamin fortification’ is analysed, including differences between develop and developing countries. The vitamins, folate and vitamin D, are taken as practical examples in the review of the beneficial effect of different strategies on public health. The importance of the risk–benefit aspect, as well as how to identify the risk groups, and the food vehicles for fortification is discussed.
In the past, food fortification along with nutritional education and the decrease in food costs relative to income have been of great success in eliminating the common nutritional deficiencies. These deficiencies, such as goiter, rickets, beriberi and pellagra, have since been replaced with another set of ‘emergent deficiencies’ that were not previously considered a problem (e.g. folate and neural tube defects, zinc and child growth and selenium and cancer). In addition, the different nutrition surveys in the so-called affluent countries have identified ‘shortfall’ nutrients specific to various age and/or physiological status. Complex, multiple lifestyle diseases, such as atherosclerosis, diabetes, cancer and obesity, have emerged. It is widely known that these are not simply deficiency diseases, but rather conditions that are present in a relatively well-nourished society. Food fortification was proven an effective tool for tackling nutritional deficiencies among population; but today, a more reasonable approach is to use food fortification as a mean to support but not replace dietary improvement strategies (i.e. nutritional education campaigns) Citation1.
The main goal of the present article is to review the past record and its effect on public health including a look into the future.
An example of success in food fortification programmes can be found in the United States, where great efforts have been made within public health officials and educators, the private industry and epidemiological evaluation Citation2. In the early 1920s, medical researchers announced that iodine could prevent goiter that was widespread at that time Citation2. Through this was a successful fortification programme. Thereafter in 1932, milk was fortified with vitamin D, and again, this was heavily supported by the medical community because of the prevalence of rickets in children Citation2. This was followed by the 1941 fortification of flour and bread with the B vitamins, which was ‘presented as insurance against nutritional deficiencies’, when B vitamin deficiencies were prevalent in the United States and most of Europe Citation3. Cooperation between the private and public sectors was essential to address public health needs. That said, are the products that are currently on the market serving the nutritional needs of the population? It is clear that nowadays is a different world. What began in the 1920s of the last century as a response to a public health need has escalated into an industry-driven fortification with frequent conflicts of interests with the public health campaign. One of the main issues today is that consumers are seeking foods with health benefits, in an era of complex diseases. Currently, international organisations and the public sector are actively working on this field Citation4.
Food fortification in today's world
Historically, food fortification, such as iodised salt or vitamin D-fortified milk, served as a public health measure to address population-wide nutrient deficiencies. By means of restoring nutrients removed during food processing or replacing nutrients in substitute foods, today's food fortification comprises several initiatives, where foods must meet not only consumer needs and preferences but also comply with nutritional, regulatory, food safety and technical constraints. Previous fortification policies were great successes, but authorities are facing other problems in developed countries that seem to be inhabited by well-nourished people.
More than 67 countries require fortification of certain staple foods. The main examples are folic acid fortified wheat or maize flour to reduce the risk of neural tube birth defects (NTD) and limitation of fortification of foods with certain nutrients such as vitamin D. The first question to consider is whether these approaches are aligned with nutritional needs, as nutritional deficiencies are often limited to a subset of the population Citation5. And secondly, if all food ingredients are safe at the level of addition when used as intended, or in other words: which is the right dose of the fortificant so that it is effective but not toxic? Citation5.
Internationally, the Codex Alimentarius of the Food and Agriculture Organization (FAO) and the World Health Organization (WHO) have established general principles for the addition of vitamins and minerals to foods. For example, the guidelines on food fortification with micronutrients published in 2006 Citation6 outline FAO/WHO initiatives for folic acid. However, each country determines its own policy or regulations, and fortification approaches can vary widely throughout the world.
In the past few years, food industry made calcium and vitamin D-fortified juices, breads fortified with omega-3 fatty acids and vegetable oil spreads with plant sterols available for consumers searching for foods with additional health benefits Citation7. This is the basis of voluntary fortification, the practice by which different concentrations of vitamins, mineral and other nutrients are added to processed foods, and decisions about which products and how to fortify them are made by food manufacturers. Harmonisation of this practice is essential as it is common in many countries. Therefore, it is expected that Europe will be under a unique regulation in the near future. This regulation will control the addition of nutrients to foods and the nutritional claims stated on their labels (Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20December 2006 on nutrition and health claims made on foods).
Remarkably, the indiscriminate addition of nutrients to foods and the fortification of fresh products are not allowed by the US Government, and fortification of unprocessed foods is prohibited in European countries. Furthermore, fortification of certain types of foods, such as sugars and some snack foods (e.g. candies and carbonated beverages), is discouraged. Importantly, food has to taste good and be appealing to consumers, so fortification is often self-limiting.
Who are the usual consumers of supplements and fortified foods?
Fortified foods and multivitamin supplements are frequently included in the daily diets of many populations from western developed countries. Amongst frequent users are women and their relatives, the elderly and chronic patients but especially people who are well educated or from a high socio-economic background Citation8. This group are considered the ‘healthy and active people’, who paradoxically may not necessarily present any nutritional deficiency. Recent publications from the US National Health and Nutrition Examination Survey (NHANES) reported a high proportion of seniors Citation9 Citation10 and also of children 1–13 years Citation11 who were frequent consumers of these products. A new era in nutrition has clearly evolved very recently with unknown expected results at population level in the future.
Vitamin content and stability in processed and ready-to-eat foods
Vitamin content of foods is susceptible to losses and/or can be destroyed during technological/cooking processes induced by a number of factors. Most losses are because of their solubility in water that is dependent on the cooking method used. However, some vitamins are subject to additional losses: B vitamins are more labile to temperature and light, whereas fat-soluble vitamins are labile to oxygen Citation12 Citation13. Losses depend on the type of food and its process: for example, thiamine, natural folates and vitamin C can reach up to 100% loss when different concomitant cooking and processing conditions are given Citation12 Citation14.
Although the availability of processed foods containing added vitamins and other nutrients is increasing, food tables and databases show a lack of accurate and updated data on their composition. This is the case for not only critical nutrients such as folic acid and iron but also for many other nutrients and non-nutritive compounds Citation15 Citation16 Citation17 . To overcome these difficulties, the EuroFIR project is making a great contribution in unifying and harmonising food composition databases across Europe Citation18.
Is it possible to follow the Mediterranean diet adherence in a ‘fortified world’?
The Mediterranean diet model is based on a high consumption of ‘fresh’ products, including vegetables, fruits, legumes and fish, with olive oil as the main source of fat. Recent publications underline the concern that Mediterranean countries are drifting away from this healthy model Citation19 Citation20, moving towards a higher intake of meat, processed, high-fat, high-salt and sugary foods Citation21. These ‘new’ patterns affect the nutritional status of different population groups living in these countries, particularly as vitamin recommended intakes are not met by a high percentage of the population Citation22.
In addition, serving/portion size is an untargeted issue in fortification of staples. Recommended and/or standard serving sizes are the basis for intake assessments, but it should be taken into account that they will vary depending on the population–age–gender group: they each have specific requirements and recommended intakes. Manufacturers normally include fixed serving sizes in the nutrition label of their products, but how realistic are these? In a pilot study, Whittaker et al. Citation16 found that regular adult breakfast cereal consumers ate nearly twice the recommended serving found on the product's label, meaning that they also consumed twice the intended vitamin/mineral content per serving of cereal. This fact is of great importance for monitoring and assessing nutrient intakes, especially for children and adolescents. Moreover, nutrient contents/levels in fortified food labels do not always match label information. Different studies, including those from our group, found overages in fortified products, showing that manufacturers may add higher quantities of the nutrient to ensure its presence at declared levels throughout shelf life Citation16 Citation23 Citation24.
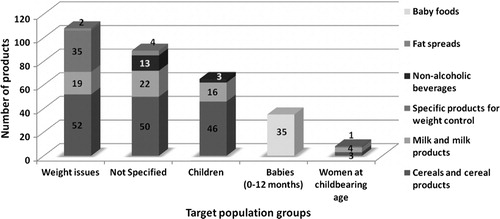
In the Spanish market, we found a wide choice of fortified food groups that may contain different nutrient levels depending on product group (dairy, cereals, etc.), brand name and target population (i.e. children). However, there is a high proportion of products that present no specific target population for consumption () Citation25.
Fig. 1. Distribution and main target consumption population groups of folic acid-fortified foods available in the Spanish market.Note: Adapted from Samaniego-Vaesken et al. Citation25.
The ‘big two’ in vitamin fortification today: folic acid and vitamin D
Folic acid
Folate is a general denomination that includes all vitamers with the activity of the B vitamin pteroylglutamic acid. Folic acid is a monoglutamate synthetic compound added to food for fortification; it is a more chemically stable form than the natural vitamers Citation26. Several studies have found a higher bioavailability in men for folic acid, Citation27 and the result of these findings was the establishment and use of the dietary folate equivalents (DFE). The term DFE accounts for the higher bioavailability of the synthetic form versus the natural folate vitamers, where DFE equals the micrograms of food folate plus 1.7 times the micrograms of added folic acid. Then use of DFE was recommended by the US Food and Nutrition Board of the Institute of Medicine for planning and evaluating folate intakes Citation28.
Folic acid is an essential micronutrient involved in the prevention of macrocytic anaemia that acts as a co-factor in cellular development and homeostasis throughout all life stages Citation29. It has been identified as a potential factor in the aetiology of vascular disease Citation30, certain types of cancer Citation31 Citation32, cognitive impairment in the elderly Citation33 and prevention of congenital abnormalities known as NTD Citation34 Citation35. As scientific evidence is still inconclusive Citation36 Citation37, the only established recommendations at present are aimed at women of childbearing age for reducing their risk of having a NTD affected pregnancy.
Strategies to improve folate status in the target population
The evidence linking folic acid to NTD prevention led to the introduction of three potential strategies to improve folate status among target populations: pharmacological supplementation, mandatory or voluntary fortification of staple foods with folic acid and the advice to increase intakes of natural folate food sources. The potential strengths and weaknesses of these approaches, summarised in , have been widely evaluated, but questions remain regarding their effectiveness.
Table 1. Comparative analysis of strategies to improve folate intake
The main public health measure for primary prevention of NTD in many countries is the recommendation of folic acid supplements taken periconceptionally amongst women who are planning a pregnancy. Increasing awareness and knowledge of the role of periconceptional folate in women of childbearing age by educational campaigns is difficult, as women do not generally plan their pregnancies, and those at higher risk of NTD affected pregnancies (lower socio-economic, education status) are not always reached by health promotion messages and are less likely to be supplement takers Citation9.
In 1998, the US Food and Nutrition Board advised that all women capable of becoming pregnant should consume a daily dose of 400 µg of synthetic folic acid, either in the form of fortified foods or supplements, in addition to naturally occurring folates from food Citation28.
With regard to the advice to increase dietary intake of naturally occurring folate, efforts, such as health promotion campaigns to achieve dietary changes on a population basis, have been proven to have limited success Citation21 Citation44. Not only does it require a behavioural change, but changes also need to be accessible, affordable and sustainable Citation21. In addition, some countries introduced mandatory fortification of a staple food and/or allowed manufacturers to voluntarily fortify certain foodstuffs Citation17.
Voluntary fortification requires women to know about and choose fortified foods, or that commonly and regularly eaten staple foods are fortified (e.g. milk) and affordable. In other words, most women must consume the fortified foods whether or not they are aware of their folic acid content. In addition, variations in folic acid amounts added by different manufacturers to different foodstuffs Citation25, or higher than expected content ‘overages’ Citation16 Citation24, add difficulty to monitoring folate intakes from these foods by the population.
Mandatory fortification seems to be the key to overcome many of the drawbacks described above. Most women will consume fortified food regardless of socio-economic disparities or family planning. Many countries adopted this measure starting more than 10 years ago in the United States and Canada Citation28. So far, about 67 fortify either wheat or maize flour with folic acid Citation45. Conversely, other countries are still reluctant to implement this population-wide strategy, as is the case in European countries that only allow addition of this vitamin on a voluntary basis Citation45.
In a recent report on folic acid-preventable spina bifida and anencephaly, Bell and Oakley Citation41 estimated that 27% of the world's population now has access to folic acid-fortified flour. However, after 18 years of knowledge, only 10% of preventable birth defects are actually prevented by current fortification programmes. The main lack is in developing countries Citation41.
Examination of selected national policies towards mandatory folic acid fortification
Mandatory folic acid fortification raises a number of scientific, ethical and technical challenges Citation46. Lawrence et al. Citation43 summarised the implications of different folic acid fortification policies worldwide to assess their nature and rationale, as well as the lessons learned from their implementation. All of the reviewed countries have identified the folic acid/NTD relationship as important and recommend the consumption of folic acid supplements (400 µg/day) and food folate for women of childbearing age. Despite having access to the same epidemiological evidence, the selected countries have diverse policy positions on mandatory folic acid fortification, which reflect different interpretations of the potential risks and benefits. An example of two countries that apply different fortification policies are China and Finland. Differences are, for instance, the high NTD rates in China in contrast to those in Finland Citation43.
In China, the recommendation states that all women of childbearing age obtain health information and advice on folic acid supplementation of 400 µg/day, for 3 months before and after conception. This advice is given pre-maritally and at their periconceptional checkup that is conducted through the maternal and infant health care system. In 2001, it was agreed to initiate a flour fortification pilot programme at 2 mg folic acid/kg of flour. The pilot trial of fortified flour consumption was conducted during 2003–2006. In 2004, fortified flour was introduced into the marketplace. Although certain local government regions encouraged implementation of folic acid fortification of flour, in 2006, the State Grain Bureau drafted a national standard for fortified flour, which is still awaiting government authorisation. The main lesson learned concerns the fact that China has a diverse consumption pattern of food staples and so it is difficult to assess the best food candidate for fortification. In addition, the NTD incidence is highest in remote and less developed regions of China. Generally, in these regions, people consume homemade flour rather than commercial flour that has been fortified with folic acid Citation43.
In contrast, Finland does not allow mandatory fortification of staple foods with folic acid, and there are no policy discussions at the moment. A balanced diet, rich in folate, is recommended for all women planning a pregnancy or in early pregnancy, to obtain at least 400 µg of folate daily. In addition, a daily supplement of 400 µg folic acid is recommended for all women planning a pregnancy or in the early stages of pregnancy. Nevertheless, voluntary fortification of certain food products is allowed. In this country, between 37 and 86% of pregnancies are planned, but the first pre-natal visit is far too late for NTD prevention. The major limitation for risk evaluation and monitoring of any fortification programme to be implemented is the need for updated data on folate intake of the target group and the population in general. This can be applied to most countries whether they adopt mandatory fortification or not. In summary, policies should be determined and assessed on a ‘fit for purpose’ basis meaning each country has to address the potential risks and benefits taking into consideration its unique circumstances and possibilities.
Concerns related to folic acid fortification today
Increased folic acid intakes in some population groups have raised special concerns about potential adverse effects, as scientific evidence has still not clarified if the benefits outweigh the risks, mainly in children and the elderly.
Because high doses of folic acid are able to correct the anaemia associated with vitamin B12 deficiency, high intakes could delay the diagnosis of the latter vitamin by masking its deficiency, which could lead to irreversible neurological damage/higher risk of memory impairment Citation47. Scientific data are still inconclusive as to whether folic acid supplementation accelerates or delays age-related cognitive decline Citation48.
Of further and more recent concern is the association of folic acid with a potential increase in the risk of cancer, particularly colorectal Citation49. Folic acid has a role as a co-factor in nucleotide synthesis, and its availability can promote proliferation of rapidly dividing malignant cells. Observations from both animal and human studies have suggested the possibility of a ‘dual effect’ of this vitamin in cancer development, depending on the timing and dose of the intervention Citation49. High intakes may suppress the development of early lesions in normal tissues, probably by maintaining genetic stability but may in turn increase the progression of neoplasic cells. This complex relationship that was referred to as the ‘double-edged sword’ by Kim Citation50 is exerting a necessary halt/delay in the widespread folic acid fortification in many countries, although the safety of this policy is ensured for all population groups Citation51. Moreover, adequacy of other vitamin status, such as B2, B6 and B12, has to be taken into account, as they are interconnected and serve as co-factors for crucial enzymes of the one-carbon metabolism.
As previously described, recently published results from the NHANES study in the United States estimated that in 2003–2006, 53% of the population used dietary supplements, and from these, 34.5% used dietary supplements that contained folic acid. Total folate intake (in DFE) was higher for men than for women and higher for non-Hispanic whites than for Mexican Americans and non-Hispanic blacks. Total folate and folic acid intakes were highest for those aged 50 years, and 5% exceeded the tolerable upper intake level. Improved total folate intakes were observed in targeted populations Citation10. However, it is noteworthy that the authors specify that estimates of folic acid intakes from food sources and dietary supplements rely on the product's label not analytical values and that those amounts could be higher than declared Citation23 Citation52 as is the case for fortified foods.
To quantify the health and economic outcomes of mandatory folic acid fortification in the United States, Bentley et al. Citation53 conducted a cost-effectiveness analysis considering different health outcomes in four projected scenarios (including ‘no fortification’) and taking the adult population with folate intake distributions from the NHANES (1988–1992 and 1999–2000) as reference. The greatest benefits from fortification implementation were predicted for myocardial infarction prevention. All post-fortification strategies provided quality-adjusted life years gains and cost savings for all sub-groups, much higher for the so-called 700 µg/100 g ‘strategy’. It was concluded that health and economic gains could outweigh the losses for the US population as a whole.
In Spain, where currently voluntary folic acid fortification is allowed, the food industry is offering a significant and increasing number of folic acid-fortified products from different food groups aimed at a wide variety of populations () Citation25. Furthermore, overage seems to be a common practice, and there is still a lack of reliable data to assess the impact of the increasing number of folic acid-fortified foods. In a recently published study by our group, breakfast cereal products were analysed to evaluate folic fortification levels. According to laboratory values, ‘low fat’ (n=15) contained the highest total folate (445–630 µg/100 g), which represented almost twice the declared label values, but similar results were obtained for fibre, muesli and corn flake categories Citation24.
Fig. 2. Distribution of main folic acid-fortified food groups available in Spain.Note: Adapted from Samaniego-Vaesken et al. Citation25.

Vitamin D
Background: how may whole nations become vitamin D deficient?
The term vitamin D is a generic term that comprises a family of fat-soluble metabolites involved in calcium metabolism and homeostasis and thus bone formation and resorption. The physiologically active form of vitamin D is the 1,25-dihydroxyvitamin D3. Without an adequate status of this metabolite, the small intestine cannot absorb more than 10%–15% of dietary calcium Citation54. Sun exposure (ultraviolet B radiation) is the main source of vitamin D3 to humans as it can be synthesised in the skin. In fact, our ape ancestors probably had adequate vitamin D status unlike the modern human: we cover about 95% of our skin surface, spend less time in the sun, wear sunscreen and are subjected to geographical differences that account for less sun hours per day. Awareness of the role excessive exposure plays in increasing risk of skin cancer has risen. In addition, nutritional sources of vitamin D are limited: oily fish is considered to be one of the best and its recommended intakes are of at least 3–4 times per week Citation55. But a question that has evolved recently is: ‘are there enough fish in the sea?’ it seems not Citation55. Foods such as milk, orange juice, cereals and some breads are fortified with vitamin D, but contribution from diets is very variable Citation54. Human's requirements of vitamin D are met mostly by casual exposure to sun. During winter, storage of body fat can be utilised as the skin has a large synthesis capacity Citation54.
Epidemiological evidence shows that there is a high prevalence of vitamin D deficiency especially among elderly population groups. It is a known fact that vitamin D production in the skin decreases about four times with age Citation55; in addition, this group is prone to avoid sun exposure and limit their outdoor activities. The SENECA study revealed that there is scarce cutaneous vitamin D production from November to May in most of European seniors. This was primarily a consequence of avoiding the sun, and surprisingly, lowest levels were observed in the Mediterranean seniors Citation56 Citation57.
How many are deficient? The SENECA study: vitamin D status in elderly population
Van der Wielen et al. Citation56 measured 25-hydroxyvitamin D in serum (25(OH)-D), in adult population across 11 European countries during winter and found that free-living elderly, regardless of geographical location, were at high risk of inadequate vitamin D status. Thirty-six percent of men and 47% of women had 25(OH)-D concentrations below 30 nmol/L; threshold with an increased risk of osteomalacia (bone disease produced by severe vitamin D deficiency i.e. 25(OH)-D ≤ 25 nmol/L). Authors’ recommended dietary enrichment or supplementation with vitamin D should be seriously considered during this season. In the studied population, major food sources of vitamin D were fish (62%), eggs (20%) and dairy products (8%) and intakes (µg/day),as well as serum 25(OH)-D levels (nmol/L) were lower for women. Assessment of overall vitamin D status was regarded as marginal in 62% of seniors.
In the Optimal Fortification with Vitamin D (OPTIFORD) European project, relative contribution of both sun exposure during summer and diet to vitamin D status were analysed across five European countries, comparing adolescents versus elderly women. When dietary intake of vitamin D was assessed, authors found that elderly females consumed 3.9±5.0 µg/day, whereas adolescent girls consumed 2.8±2.7 µg/day with fish and eggs as the main food sources. However, sun exposure, measured with an adhesive skin dosimeter (J/m2), revealed that Spanish elderly women received less than half the sun exposure of their adolescent counterparts Citation58. This situation was not observed in the rest of the participating countries (i.e. Finland).
When vitamin D exposure and status, measured as 25(OH)-D in plasma in winter versus summer, in a Spanish elderly women population were compared, both were clearly higher in the latter. Nevertheless, vitamin D deficiency affected 28% of the women during summer time, although this percentage was doubled in winter. In addition, dietary intakes of ≥ 95% of the population sample did not reach the recommended dietary intakes for vitamin D in the summer or winter season (34.5 and 31.3%, respectively); with oily fish as the main food source Citation59.
Vitamin D fortification and supplementation: implications and public health potential
Available evidence found in meta-analyses of double-blinded trials indicates that supplemental vitamin D has dose-dependent beneficial effects on bone health and muscle strength. These are both related to prevention of falls and, therefore, hip and other non-vertebral fractures that are the main causes of disability in the elderly Citation60. 25(OH)-D serum concentrations of 75–110 nmol/L provide an adequate status for fall and fracture prevention Citation61.
What remains to be proven are other additional and not less important, public health benefits. In cancer prevention, vitamin D may have a role downregulating or inhibiting cell proliferation in colorectal cancer Citation62. It has also been shown to act as an important immune system regulator: vitamin D receptors are present on T-cells, B-cells, macrophages and other immune cells, providing modulatory effects that could have benefits for the occurrence of autoimmune diseases such as Type I diabetes, rheumatoid arthritis and asthma Citation63.
Based on data of a benefit–risk assessment published in 2009 by Bischoff-Ferrari et al. Citation61, it has been proposed that 800–1,000 IU of vitamin D per day may be needed to achieve a satisfactory status in all adults of 75–100 nmol/L of 25(OH)-D. However, as in the case of folic acid, authors agree that larger trials are needed to confirm benefits and safety of these new and promising approaches.
Vitamin D supplements use and adherence
In a recent study that estimated the prevalence and patterns of use of over-the-counter medications and dietary supplements in a sample of adults aged 57–85 years (n = 3,005), findings showed that 49% (95% CI, 46.2%–52.7%) used a dietary supplement Citation64. Amongst the most popular supplements were multivitamins (28%), calcium (17%) and vitamin C (9%). Only 4.5% of the users declared a daily consumption of Vitamin D supplements.
Another study conducted in Italy investigated the global adherence to osteoporosis treatment in a nationwide survey carried out in post-menopausal women (n= 9,851) Citation65. They were referred to centres for osteoporosis management for a follow-up assessment, at least 1 year after having been prescribed either a treatment with calcium + vitamin D alone or other drugs (hormone replacement therapy, etc.). Results showed that 19.1% of the patients discontinued the prescribed drug before attending the bone mass re-evaluations. Most frequent reasons for discontinuation were drug-related side effects, insufficient motivation to treatment and fear of side effects. Lack of motivation was the most common cause for poor compliance in the case of calcium + vitamin D supplements, whereas best treatment adherence was observed in patients with severe osteoporosis Citation65.
Fortification tomorrow
At present, it seems clear that ‘optimal nutrition’ remains a moving target in the food fortification field: to appropriately design a fortification programme, nutritional status of many micronutrients has to be assessed. Furthermore, as nutrigenomic studies identify individuals with higher, or at least different nutrient requirements, nutrient fortification may help optimise an individual's nutritional needs. The scientific evidence base continues to evolve, and discovery of new compounds with health-promoting effects will call for new strategies involving both nutrition guidance and fortification with a focus on benefit–risk assessment.
In summary, questions and issues to be fully addressed regarding whether we are targeting the most appropriate food vehicles with nutrients and the levels needed by those who actually consume them are currently still under discussion. Further studies comprising the monitoring of population intakes in countries, where fortification is implemented at different levels will grant greater insight into improved policies. What is clear is that harmonising food fortification policies, within the scope of optimal and safe levels, will enable suitable competition in a global food supply with no trade barriers and possibly achieve the necessary right balance for vitamin food fortification.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Tontisirin K, Nantel G, Bhattacharjee L. Food-based strategies to meet the challenges of micronutrient malnutrition in the developing world. 2002; 61: 243-50
- Backstrand JR. The history and future of food fortification in the United States: a public health perspective. 2002; 60: 15-26
- Bishai D, Nalubola R. The history of food fortification in the United States: its relevance for current fortification efforts in developing countries. 2002; 51: 37-53
- EC. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Official Journal of the European Union L 404/92006.
- Refsum H, Smith AD. Are we ready for mandatory fortification with vitamin B-12?. 2008; 88: 253-54
- Allen L, de Benoist B, Dary O, Hurrell RGuidelines on food fortification with micronutrients. Geneva: World Health Organization and Food and Agriculture Organization of the United Nations; 2006.
- Dragsted L, Renwick A, Verhagen H, Flynn A, Tuijtelaars S.New horizons for the safe addition of micronutrients to food. ILSI: Europe. 2009
- Shaikh U, Byrd RS, Auinger P. Vitamin and mineral supplement use by children and adolescents in the 1999–2004 National Health and Nutrition Examination Survey: relationship with nutrition, food security, physical activity and health care access. 2009; 163: 150-7
- Yang Q, Cogswell ME, Hamner HC et al. Folic acid source, usual intake, and folate and vitamin B-12 status in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2006. 2010; 91: 64-72
- Bailey RL, Dodd KW, Gahche JJ, et al.. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. 2010: 231–7.
- Bailey RL, McDowell MA, Dodd KW, Gahche JJ, Dwyer JT, Picciano MF. Total folate and folic acid intakes from foods and dietary supplements of US children aged 1–13 years. 2010: ajcn.2010.29652353–8.
- Harris RS, Karmas E.Nutritional evaluation of food processing2nd ed. AVI Publishers: Wesport. 1975
- Priestley RJ. Vitamins. Effects of heating on foodstuffs. London: Applied Science publishers; 1979, pp. 121–56.
- McKillop DJ, Pentieva K, Daly D et al. The effect of different cooking methods on folate retention in various foods that are amongst the major contributors to folate intake in the UK diet. 2002; 88: 681-8
- Gregory III JF. Do available food composition data for folate meet current research needs? 22nd National Nutrient Data Bank Conference Proceedings. San Francisco, USA, 1998.
- Whittaker P, Tufaro PR, Rader JI. Iron and folate in fortified cereals. 2001; 20: 247-54
- Bouckaert K, Slimani N, Nicolas G, et al.. Critical evaluation of folate data in European and international databases: recommendations for standardization in international nutritional studies. 2010.
- Westenbrink S, Oseredczuk M, Castanheira I, Roe M. Food composition databases: the EuroFIR approach to develop tools to assure the quality of the data compilation process. 2009; 113: 759-67
- Cucó G, Fernández-Ballart J, Martí-Henneberg C, Arija V. Food group and macronutrient intake behavior in a Spanish Mediterranean population. 2003; 23: 857-68
- Varela-Moreiras G, Ávila J, Cuadrado C, del Pozo de la Calle S, Moreno E, Moreiras O. Valoración de la Dieta Española de acuerdo al Panel de Consumo Alimentario. Fundación Española de la Nutrición (FEN), Ministerio de Medio Ambiente y Medio Rural y Marino (MARM), 2008.
- Wolf A, Elmadfa I, Ronald RW, Victor RP. Fruit and vegetable intake of mothers in Europe: risks/benefits. Bioactive foods in promoting health. San Diego: Academic Press; 2010, pp. 161–172.
- Elmadfa I, Freisling H. Nutritional status in Europe: methods and results. 2009; 67: S130-S134
- Rader JI, Weaver CM, Angyal G. Total folate in enriched cereal-grain products in the United States following fortification. 2000; 70: 275-89
- Samaniego-Vaesken ML, Alonso-Aperte E, Varela-Moreiras G. Analysis and evaluation of voluntary folic acid fortification of breakfast cereals in the Spanish market. 2010: In Press.
- Samaniego-Vaesken ML, Alonso-Aperte E, Varela-Moreiras G. Folic acid fortified foods available in Spain: type of products, level of fortification and target population groups. 2009; 24: 459-66
- Blakley R.The biochemistry of folic acid and related pteridines. North-Holland Publishing: Amsterdam. 1969
- Gregory III JF.The bioavailability of folateFolate in Health and DiseaseBailey LBM. Dekker: New York. 1995195-235
- IOM. Panel on Folate, other B vitamins and Choline. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline.. WashingtonDC: National Academy Press, National Academy of Sciences, Institute of Medicine, Food and Nutrition Board; 1998.
- Wills L. Treatment of pernicious anaemia of pregnancy and tropical anaemia with special reference to yeast extract as a curative agent. 1931; 7: 323-27
- Bazzano LA. Folic acid supplementation and cardiovascular disease: the state of the art. 2009; 338: 48-49
- Hirsch S, Sanchez H, Albala C, et al.. Colon cancer in Chile before and after the start of the flour fortification program with folic acid. 2009; 21: 436–39. 10.3402/fnr.v56i0.5459.
- Kim Y-I. Will mandatory folic acid fortification prevent or promote cancer?. 2004; 80: 1123-8
- Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. 2007; 85: 193-200
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. 1992; 327: 1832-5
- MRC. Medical Research Council Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. 1991; 338: 131–7.
- Wald DS, Wald NJ, Morris JK, Law M. Folic acid, homocysteine, and cardiovascular disease: judging causality in the face of inconclusive trial evidence. 2006; 333: 1114-7
- Rader JI. Folic acid fortification, folate status and plasma homocysteine. 2002; 132: S2466-S70
- Hung J, Yang TL, Urrutia TF et al. Additional food folate derived exclusively from natural sources improves folate status in young women with the MTHFR 677 CC or TT genotype. 2006; 17: 728-34
- Gregory III JF, Quinlivan EP, Davis SR. Integrating the issues of folate bioavailability, intake and metabolism in the era of fortification. 2005; 16: 229-40
- Caudill MA. Folate bioavailability: implications for establishing dietary recommendations and optimizing status. 2010: ajcn.2010.28674E.
- Bell KN, Oakley J, GP. Update on prevention of folic acid-preventable spina bifida and anencephaly. 2009; 85: 102-07
- Czeizel AE, Puhó EH, Langmar Z, Ács N, Bánhidy F. Possible association of folic acid supplementation during pregnancy with reduction of preterm birth: a population-based study. 2010; In Press.
- Lawrence MA, Weizhong C, Raija K, Irwin HR, John S, Alison T. Examination of selected national policies towards mandatory folic acid fortification. 2009; 67: S73-8
- Öhrvik VE, Olsson JC, Sundberg BE, Witthoft CM. Effect of 2 pieces of nutritional advice on folate status in Swedish women: a randomized controlled trial. 2009: ajcn.2008.27192
- Maberly G, Grummer-Strawn L, Jefferds M. Trends in wheat-flour fortification with folic acid and iron – worldwide, 2004 and 2007. 2008; 57: 8-10
- Smith AD. Folic acid fortification: the good, the bad, and the puzzle of vitamin B-12. 2007; 85: 3-5
- Morris MS, Jacques PF, Rosenberg IH, Selhub J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. 2010: ajcn.2009.28671.
- EFSA. Folic acid: an update on scientific developments. Meeting summary report. Uppsala: European Food Safety Authority; 2009.
- Smith AD, Kim Y-I, Refsum H. Is folic acid good for everyone?. 2008; 87: 517-33
- Kim YI. Folate: a magic bullet or a double edged sword for colorectal cancer prevention?. 2006; 55: 1387-9
- Mason JB. Folate, cancer risk, and the Greek god, Proteus: a tale of two chameleons. 2009; 67: 206-12
- Dwyer J, Holden J, Andrews K et al. Measuring vitamins and minerals in dietary supplements for nutrition studies in the USA. 2007; 389: 37-46
- Bentley TGK, Weinstein MC, Willett WC, Kuntz KM. A cost-effectiveness analysis of folic acid fortification policy in the United States. 2009; 12: 455-67
- Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. 2004; 79: 362-71
- Chen TC, Chimeh F, Lu Z et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. 2007; 460: 213-17
- Van der Wielen RPJ, De Groot LCPGM, Van Staveren WA et al. Serum vitamin D concentrations among elderly people in Europe. 1995; 346: 207-10
- Moreiras O, Carbajal A, Perea I, Varela-Moreiras G. The influence of dietary intake and sunlight exposure on the vitamin D status in an elderly Spanish group. 1992; 62: 303-7
- Rodriguez-Sangrador M.Influencia de la exposición solar y la dieta en el estatus nutricional de vitamina D en mujeres adolescentes y de edad avanzada. Estudio OPTIFORD-Unión Europea. Departamento de Nutrición. Madrid: Universidad Complutense; 2006.
- Rodríguez-Sangrador M, Beltrán de Miguel B, Quintanilla Murillas L, Cuadrado Vives C, Moreiras Tuny O. The contribution of diet and sun exposure to the nutritional status of vitamin D in elderly Spanish women: the five countries study (OPTIFORD Project). 2008; 23: 567-76
- Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. 2009; 339: b3692
- Bischoff-Ferrari H, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E, Willett W. Benefit–risk assessment of vitamin D supplementation. 2009.
- Gorham ED, Garland CF, Garland FC et al. Optimal Vitamin D status for colorectal cancer prevention: a quantitative meta-analysis. 2007; 32: 210-16
- Roger B, Heike B-F, Walter W. Vitamin D and Health: perspectives from mice and man. 2008; 23: 974-9
- Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. 2008; 300: 2867-78
- Rossini M, Bianchi G, Di Munno O et al. Determinants of adherence to osteoporosis treatment in clinical practice. 2006; 17: 914-21