Abstract
Background
Nutritional supplements based on the amino acid L-arginine have been hypothesized to improve exercise performance by increasing levels of insulin and growth hormone (GH). Changes of these parameters in response to L-arginine supplementation may clarify the mechanisms underlying its putative physiological effects on physical performance.
Objective
The aim of the study was to evaluate the effect of L-arginine supplementation on serum insulin, GH, Growth Factor Insulin-like (IGF-1), and cortisol in response to exercise. Exercise performance was also evaluated.
Design
Fifteen trained runners were divided into groups supplemented with 6 g of L-arginine (ARG) or placebo (PLA). Blood samples were collected before supplementation (T0), immediately after the first exercise session (T1), after the second exercise session (T2), and after 20 min of rest (T3). The exercise consisted of two bouts of 5 km time-trial running test.
Results
There was a significant increase in serum GH (T0: 3.28±0.95 vs. 3.21±0.5 ng/mL; T1: 4.35±0.23 vs. 4.17±0.13 ng/mL; T2: 4.22±0.25 vs. 4.17±0.09 ng/mL; T3: 4.14±0.29 vs. 4.13±0.18 ng/mL) and cortisol (T0: 198.71±53.77 vs. 207.57±69.51 nmol/L; T1: 458.16±116.12 vs. 433.26±101.77 nmol/L; T2: 454.61±125.21 vs. 431.88±74.82 nmol/L; T3: 311.14±102.91 vs. 362.26±110.42 nmol/L) after T1, T2, and T3, with no significant difference between the ARG and PLA groups, respectively. There was also no significant difference observed in the variables of IGF-1, insulin, and total running time between the ARG and PLA groups.
Conclusions
The supplementation of L-arginine did not appear to stimulate the production of insulin, GH, and IGF-1 and, thus, provided no benefit in hormonal response or exercise performance in trained runners.
Nutritional supplements based on the amino acid L-arginine have been marketed to promote vasodilation due to a supposed increase in nitric oxide (NO) production (Citation1). However, some studies have shown that supplementation of L-arginine does not increase NO synthesis in healthy subjects at rest (Citation2) or in response to exercise (Citation3). Moreover, there is evidence demonstrating a significant increase in muscle blood volume after supplementation with 6 g of L-arginine orally (Citation3). These results suggest that supplementation with L-arginine may promote vasodilatation via NO-independent mechanisms.
Giugliano et al. (Citation4) demonstrated that intravenous infusion of L-arginine (1 g min−1 for 30 min) in healthy subjects increased blood flow and inhibited platelet aggregation. According to the authors, these effects were partially mediated by an increase in endogenous insulin (stimulated by L-arginine).
In addition to the alleged effect of increased NO synthase, L-arginine has also been studied as a possible stimulator of GH secretion. GH stimulates the production of insulin-like growth factor 1(IGF-1), which acts as an anabolic hormone by increasing protein synthesis. Colombani et al. (Citation5) supplemented fourteen trained males with 15 g/day arginine aspartate, or placebo for 14 days before a marathon. On the day of the marathon, blood samples were collected before and after the 31 km race, and 2 h post marathon race. The authors observed a significant increase in plasma GH only in the group supplemented with L-arginine. Moreover, Fayh et al. (Citation6) supplemented 17 subjects with 7 g of L-arginine orally for 7 days and observed no significant change in the levels of GH and IGF-1 at the end of the supplementation period.
Considering the increasing demand among physically active individuals (i.e. runners) for nutritional ergogenic aids to enhance physical performance, as well as the inconsistent data on the effectiveness of L-arginine-based nutritional supplements, the present study aimed to evaluate the effect of L-arginine supplementation on the production of insulin, GH, IGF-1, and cortisol in trained runners. Moreover, the possible effect of supplementation on exercise performance as measured by the total running time (TRT) was also investigated.
It was hypothesized that L-arginine supplementation will promote changes in the hormonal (increases in insulin, GH and IGF-1 and decreases in cortisol) parameters in response to running exercise, resulting in improved exercise performance.
Materials and methods
Sample
Fifteen physically active and healthy volunteers (four women), with at least 1 year's experience in running exercise, participated in this study. The volunteers, who were all at the same level of training, were instructed to maintain their regular exercise program until the 24 h period prior to the study, when they were instructed not to perform physical exercise. All experimental procedures were performed in accordance with the ethical standards of the Declaration of Helsinki and were approved by the Institutional Ethics Committee.
Experimental design
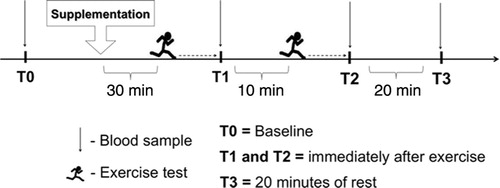
The volunteers were divided into two groups: one supplemented with L-arginine (ARG) and the other with placebo (PLA) by draw of lots (simple randomization) by a person outside the group of researchers participating in the study. On the day of the study, upon arriving at the training center, the volunteers remained at rest for 10 min. Blood samples were then collected for measuring the baseline (T0). Once the blood samples were collected, volunteers were immediately given a supplement that consisted of a single oral dose of 12 capsules containing 500 mg of L-arginine hydrochloride or corn starch (as placebo) each (total 6 g) in 500 mL of deionized water. Thirty minutes after supplementation, the volunteers underwent two running exercise tests that consisted of 5 km time trial (5K-TT), meaning that the volunteers were instructed to run 5 km in the shortest time possible. The recovery period between tests was 10 min and exercise performance was evaluated by measuring the TRT. In addition, blood samples were collected immediately after the first 5K-TT (T1), immediately after the second 5K-TT (T2), and after 20 min of rest (T3) (see ). The experimental procedures were performed in the period from 06:00 to 09:00, and the volunteers were advised fast for at least 8 h before each visit. The volunteers were not advised to control the ingestion of macronutrients (i.e. protein, lipids, and carbohydrates) from their diet on the day before the experimental procedures. However, during this period, the volunteers did not consume any food, except water.
Analysis of blood samples
Blood samples were collected in vacuum tubes containing separator gel and clot activator and centrifuged immediately at 3,000 g for 10 min at 4°C to separate serum and were stored at −80°C for later analysis. The serum concentrations of insulin, GH, IGF-1, and cortisol were analyzed by enzyme immunoassay using commercially available kits (Abcan Inc., Cambridge, UK). These analyses were also performed according to the manufacturer's instructions. For all analyses, the reading was performed using a microplate reader (VICTOR™ X4 Multilabel Plate Reader, Perkin Elmer, CA, USA). The coefficient values of variation (n=6) to insulin, GH, IGF-1, and cortisol were 4.9%, 3.7%, 4.4%, 7.5% for intra-assay and 7.2%, 8.4%, 9.1%, 12.8% for inter-assay, respectively.
Statistical analysis
Two-way analysis of variance with repeated measures on two factors (2×4; group×time) was used to identify differences in variables, such as insulin, GH, IGF-1, and cortisol between the groups ARG and PLA and between time periods (T0, T1, T2, and T3). When a significant F was found, additional post hoc tests with Bonferroni adjustment were performed. Data at a confidence level of p<0.05 were considered statistically significant. Furthermore, the statistical power (1-β err prob) was performed in order to detect the probability of type II error occurring with regard to insulin, GH, IGF-1, cortisol, and TRT in both 5K-TT tests. All analyses were performed using a commercially available statistical package (IBM SPSS® Statistics version 20 for Windows, Chicago, IL, USA), and the results were expressed as means±SD.
Results
Baseline characteristics of volunteers
At baseline, no significant differences were observed with respect to age (ARG: 36.8±7.1 years vs. PLA: 30.6±9.5 years), height (ARG: 172.3±10.6 cm vs. 175.0±7.5 cm), body mass (66.7±14.4 kg vs. 68.1±10.6 kg), and body mass index (22.2±2.6 kg/m2 vs. 22.1±2.2 kg/m2).
Hormonal parameters
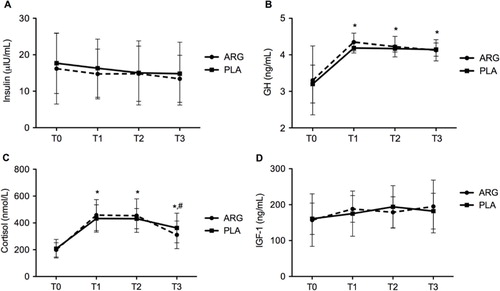
The serum concentrations of insulin, GH, IGF-1, and cortisol during the study period are shown in . Both ARG and PLA groups showed a significant increase (p <0.05) in serum GH at all times (T1, T2, and T3), when compared to pre-supplementation (T0) (T0: 3.28±0.95 ng/mL vs. 3.21±0.5 ng/mL; T1: 4.35±0.23 ng/mL vs. 4.17±0.13 ng/mL; T2: 4.22±0.25 ng/mL vs. 4.17±0.09 ng/mL; T3: 4.14±0.29 ng/mL vs. 4.13±0.18 ng/mL). However, there were no significant differences among the conditions of supplementation (ARG vs. PLA). There was no significant differences between ARG and PLA in the serum concentrations of insulin (T0: 16.19±9.7 µIU/mL vs. 17.68±8.28 µIU/mL; T1: 14.73±6.84 µIU/mL vs. 16.31±7.98 µIU/mL; T2: 14.82±7.57 µIU/mL vs. 15.03±8.78 µIU/mL; T3: 13.41±6.45 µIU/mL vs. 14.82±8.61 µIU/mL) and IGF-1 (T0: 157.11±73.32 ng/mL vs. 160.8±43.77 ng/mL; T1: 188.2±37.16 ng/mL vs. 175.14±62.94 ng/mL; T2: 178.85±43.16 ng/mL vs. 194.08±59.13 ng/mL; T3: 195.22±73.53 ng/mL vs. 182.11±49.69 ng/mL) over the time periods of the study. Serum cortisol showed no significant changes between the two groups (ARG vs. PLA). However, the PLA group had a significant increase in the level of this hormone at T1, T2, and T3 compared to T0, just as the concentration of cortisol in the ARG group increased significantly at T1 and T2 compared to baseline measurement (T0), which was then followed by a significant reduction at T3 (T0: 198.71±53.77 nmol/L vs. 207.57±69.51 nmol/L; T1: 458.16±116.12 nmol/L vs. 433.26±101.77 nmol/L; T2: 454.61±125.21 nmol/L vs. 431.88±74.82 nmol/L; T3: 311.14±102.91 nmol/L vs. 362.26±110.42 nmol/L). It is important to point out that the relevant statistical test in the present study had a virtually small power to detect the size differences between the ARG and PLA groups regarding: insulin (1-β err prob=0.072), GH (1-β err prob=0.212), IGF-1 (1-β err prob=0.367), and cortisol (1-β err prob=0.150).
Fig. 2 Serum concentrations of insulin, growth hormone (GH), insulin-like growth hormone 1 (IGF-1), and cortisol before and after supplementation with L-arginine (ARG) and placebo (PLA). T0=baseline; T1=immediately after the first exercise test; T2=immediately after the second exercise test; T3=after 20 min of rest; (A) Serum concentration of insulin (µIU/mL); (B) Serum concentration of GH (ng/mL); (C) Serum concentration of cortisol (nmol/L); (D) Serum concentration of IGF-1 (ng/mL). * denotes significantly different from T0 (p<0.05) and # denotes significantly different from T1 and T2 (p<0.05).
Physical performance
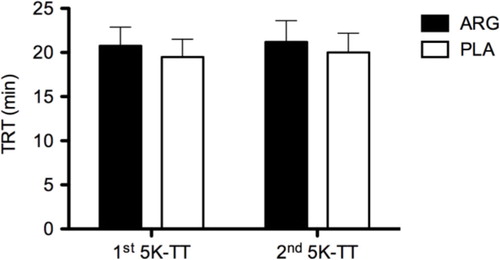
The TRT in the two sessions of 5K-TT in the ARG and PLA are depicted in . There was no significant difference in TRT between the two tests, in both ARG and PLA (first 5K-TT: 21:01:23±2:04:19 vs. 19:28:00±2:00:30; second 5K-TT: 21:24:07±2:28:28 vs. 20:01:30±2:23:32). The relevant statistical test in the present study had virtually a small power to detect a size difference between the ARG and PLA groups regarding TRT in the two sessions of 5K-TT (1-β err prob=0.059).
Discussion
This study evaluated the effects of 6 g of L-arginine supplementation on serum insulin, GH, IGF-1, and cortisol in response to running exercise in physically active individuals, as well as their performance during the running tests.
Muscle glycogen synthesis can be optimized from increased skeletal muscle glucose uptake, which is enhanced by translocation of the GLUT-4 glucose transporters from intracellular vesicles to the cell membrane in response to insulin (Citation7). Giugliano et al. (Citation4) demonstrated that systemic infusion of L-arginine (1 g min−1 during 30 min) in healthy subjects increased leg blood flow, which was associated with an increase in endogenous insulin. However, during the present study, L-arginine supplementation did not affect insulin levels when compared to the placebo group over the time period of the study. The supplementation dosage and the route of administration may have influenced the lack of significant changes in insulin levels after L-arginine supplementation. Previous studies have suggested that L-arginine administered orally does not reach systemic levels because almost 40% is catabolized in the small intestine (Citation8, Citation9), which may be a possible explanation as to why some studies involving oral arginine supplementation have failed to show a significant change in the secretion of this hormone (Citation5, Citation8).
There is evidence reporting exercise as a potent stimulator of GH secretion and explaining the mechanisms involved in this process (Citation10–(Citation13)). Furthermore, some studies have suggested that the amino acid L-arginine stimulates the secretion of GH by suppressing the secretion of somatostatin, a hormone that inhibits GH release (Citation14–(Citation17)). These studies reported that L-arginine supplementation affects the exercise-induced GH response; however, such results are controversial (Citation5, Citation6) (Citation17, Citation18).
The present study showed a significant increase in GH concentration in both ARG and PLA groups after running exercise, with no significant difference between the groups. It appears that the response in GH release was mediated only by exercise, and that L-arginine supplementation induced no additional changes in this hormone when associated with running exercise. This result is consistent with a study by Zajac et al. (Citation19) who observed no significant increase in GH concentration after L-arginine and L-ornithine supplementation in nine trained athletes submitted to resistance training.
Abel et al. (Citation17) found no difference in plasma GH concentration after supplementation with arginine aspartate (arg-asp) in eight volunteers for 4 weeks with a low (5 g total – 2.8 g of L-arginine and 2.2 g of aspartate) and high (14.4 g total – 5.7 g of L-arginine and 8.7 g of aspartate) dose of these amino acids. Similarly, Forbes et al. (Citation9) supplemented 14 physically active males with a low (0.075 g kg−1) and high (0.15 g kg−1) dose of orally administered L-arginine and found no significant change in serum GH.
In the present study, no significant changes were observed in serum concentration of IGF-1 after acute L-arginine supplementation. Forbes et al., (Citation8) supplemented physically active men with different doses of L-arginine (0.075 g kg−1 or 0.15 g kg−1) orally and observed no significant changes in serum IGF-1 after 30, 60, 90, 120, and 180 min after supplementation. Other authors also found no significant changes in the serum concentration of IGF-1 after oral L-arginine supplementation (Citation6, Citation20).
Cortisol is a catabolic hormone that increases during fasting or stress situations (such as intense training) (Citation21–(Citation24)). We hypothesized that supplementation with L-arginine could reduce the catabolic effects of cortisol, since the secretion of anabolic hormones such as insulin and GH could act to offset the state of catabolism induced by cortisol.
In the present study, there was a significant increase in serum cortisol immediately after two running exercise tests, when compared to presupplementation time (T0) in both PLA and ARG groups. However, no significant differences were observed between groups. Therefore, it may be suggested that the response of cortisol secretion observed in the present study was only influenced by exercise.
The alleged effects of L-arginine supplementation on the release of anabolic hormones has stimulated athletes to use this amino acid for promoting greater gains in muscle mass, strength, and performance (Citation12). In the present study, there was no significant difference between groups (ARG and PLA) in the TRT in the two sessions of 5K-TT. Since we did not observe any significant effect from acute L-arginine supplementation on the hormonal parameters investigated in the present study (e.g. increases in insulin or GH concentrations), no improvement in exercise performance could have been expected. However, it is important to point out that with the very small sample size of 15 volunteers, the present study lacked sufficient power to detect any significant effect on serum concentrations of insulin, GH, IGF-1, and cortisol between the ARG and PLA groups, even if they exist in reality. Furthermore, because three of the four women who participated in the study were randomly allocated to the L-arginine group, and the other in the placebo group, no gender effect associated with the L-arginine supplementation was possible to determine.
Conclusion
In conclusion, it appears that oral L-arginine supplementation stimulates neither the production of insulin, GH, and IGF-1 nor the reduction of cortisol. Furthermore, L-arginine supplementation does not enhance exercise performance in trained runners. Based on the results of the present study, nutritional supplements based on L-arginine should not be recommended to induce hormonal changes and improve exercise performance in trained runners.
Conflict of interest and funding
The Research Foundation of the State of Rio de Janeiro – FAPERJ (grant No. E-26/100.499/2012 and E-26/100.500/2012) provided financial support for the study.
Acknowledgements
The authors thank Ricky Toledano for preparing the English version of the manuscript.
References
- Alvares TS, Meirelles CM, Bhambhani YN, Paschoalin VM, Gomes PS. L-Arginine as a potential ergogenic aid in healthy subjects. Sports Med. 2011; 41: 233–48.
- Alvares TS, Junior CA, Silva JT, Paschoalin VM. Acute L-Arginine supplementation does not increase nitric oxide production in healthy subjects. Nutr Metab. 2012; 9: 54.
- Alvares TS, Conte CA, Paschoalin VM, Silva JT, Meirelles CM, Bhambhani YN, etal. Acute l-arginine supplementation increases muscle blood volume but not strength performance. Appl Physiol Nutr Metab. 2012; 37: 115–26.
- Giugliano D, Marfella R, Verrazzo G, Acampora R, Coppola L, Cozzolino D, etal. The vascular effects of L-arginine in humans. J Clin Invest. 1997; 99: 433–8.
- Colombani PC, Bitzi R, Frey-Rindova P, Frey W, Arnold M, Langhans W, etal. Chronic arginine aspartate supplementation in runners reduces total plasma amino acid level at rest and during a marathon run. Eur J Nutr. 1999; 38: 263–70.
- Fayh AP, Friedman R, Sapata KB, Oliveira AR. Effect of L-arginine supplementation on secretion of human growth hormone and insulin-like growth factor in adults. Arq Bras Endocrinol Metab. 2007; 51: 587–92.
- Hayashi T, Wojtaszewski JF, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am J Physiol. 1997; 273: E1039–51.
- Forbes SC, Bell GJ. The acute effects of a low and high dose of oral L-arginine supplementation in young active males at rest. Appl Physiol Nutr Metab. 2011; 36: 405–11.
- Castillo L, Chapman TE, Yu YM, Ajami A, Burke JF, Young VR. Dietary arginine uptake by the splanic region in adult humans. Am J Physiol. 1993; 265: E532–9.
- Wideman L, Weltman JY, Hartman ML, Veldhuis JD, Weltman A. Growth hormone release during acute and chronic aerobic and resistance exercise: recent findings. Sports Med. 2002; 32: 987–1004.
- Wallace JD, Cuneo RC, Lundberg PA, Rosen T, Jorgensen JO, Longobardi S, etal. Responses of markers of bone and collagen turnover to exercise, growth hormone (GH) administration, and GH withdrawal in trained adults males. J Clin Endocrinol Metab. 2000; 85: 124–33.
- Chromiak JA, Antonio J. Use of amino acids as growth hormone-releasing agents by athletes. Nutrition. 2002; 18: 657–61.
- Ghigo E, Arvat E, Valente F, Nicolosi M, Boffano GM, Procopio M, etal. Arginine reinstates the somatotrope responsiveness to intermittent growth hormone releasing hormone administration in normal adults. Neuroendocrinology. 1991; 54: 291–4.
- Wideman L, Weltman JY, Patrie JT, Bowers CY, Shah N, Story S, etal. Synergy of L-arginine and growth hormone (GH)-releasing peptide-2 on GH release: influence of gender. Am J Physiol Regul Integr Comp Physiol. 2000; 279: 1467–77.
- Collier SR, Casey DP, Kanaley JA. Growth hormone responses to varying doses of oral arginine. Growth Horm IGF Res. 2005; 15: 136–9.
- Collier SR, Collins E, Kanaley JA. Oral arginine attenuates the growth hormone response to resistance exercise. J Appl Physiol. 2006; 101: 848–52.
- Abel T, Knechtle B, Perret C, Eser P, Von Arx P, Knecht H. Influence of chronic supplementation of arginine aspartate in endurance athletes on performance and substrate metabolism – a randomized, double-blind, placebo-controlled study. Int J Sports Med. 2005; 26: 344–9.
- Thorner MO, Vance ML, Laws ER, Horvath E, Kovacs K.Wilson JD, Foster DW, Kronenberg HM, Larsen RR. The anterior pituitary. Williams textbook of endocrinology. 1998; 9th ed, Philadelphia: WB Saunders. 249–340.
- Zajac A, Poprzecki S, Zebrowska A, Chalimoniuk M, Langfort J. Arginine and ornithine supplementation increases growth hormone and insulin-like growth factor-1 serum levels after heavy-resistance exercise in strength-trained athletes. J Strength Cond Res. 2010; 24: 1082–90.
- Corpas E, Blackman MR, Roberson R, Scholfield D, Harman SM. Oral arginine/lysine does not increase growth hormone and insulin-like growth factor-1 secretion in old men. J Gerontol. 1993; 48: 128–33.
- Schwarz AJ, Brasel JA, Hintz RL, Mohan S, Cooper DM. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young health men. J Clin Endocrinol Metab. 1996; 81: 3492–7.
- Michael R, Guigan MC, Alison DE, Foster C. Salivary cortisol responses and perceived exertion during high intensity and low intensity bouts of resistance exercise. J Sports Sci Med. 2004; 3: 8–15.
- Lac G, Berthon P. Changes in cortisol and testosterone levels and T/C ratio during an endurance competition and recovery. J Sports Med Phys Fitness. 2000; 40: 139–44.
- Jacks DE, Sowash J, Anning J, Gloughlin MC, Andres F. Effect of exercise at three exercise intensities on salivary cortisol. J Strength Cond Res. 2002; 16: 286–9.