Abstract
Background: As HIV infection continues to devastate low-income countries, efforts to search for an effective HIV vaccine are crucial. Therefore, participation in HIV vaccine trials will be useful for the development of a preventive vaccine that will work and thus reduce the global HIV epidemic.
Objective: The objective of this study was to analyse the willingness to volunteer (WTV) in a Phase I/II HIV vaccine trial among police officers in Dar es Salaam, Tanzania.
Design: We included a convenience sample of 329 participants (79% males) from sensitisation workshops that were held once at each of the 32 police stations. Participants were recruited from 23 stations which were included according to availability. Data about personal characteristics, general HIV and AIDS knowledge and sexual behaviour, attitudes towards vaccines and willingness to participate in the HIV vaccine trial were obtained through an interview-administered questionnaire with both closed and open-ended questions.
Results: Overall, 61% of the participants expressed WTV in HIV vaccine trials. WTV was significantly associated with: positive attitude towards use of effective vaccine, Odds ratio (OR), 36.48 (95% CI: 15.07–88.28); the intention to tell others about one's decision to participate in the trial, OR, 6.61 (95% CI: 3.89–11.24); Tanzania becoming a partner in developing the vaccine, OR, 4.28 (95% CI: 2.28–8.03); having an extra sexual partner, OR, 3.05 (95% CI: 1.63–5.69); perceived higher risk of getting HIV infection, OR, 2.11 (95% CI: 1.34–3.33); and high knowledge about HIV and AIDS, OR, 1.92 (95% CI: 1.22–3.01).
Conclusion: The results indicated that a majority of police officers in this study were willing to participate in HIV vaccine trials. However, there is a need to provide the respondents with precise information about the purpose of a Phase I/II HIV vaccine trial and the fact that it does not protect against HIV infection, in order to avoid increasing risky behaviour.
Introduction
In common with many other sub-Saharan countries, Tanzania is facing challenges in controlling HIV transmission, highlighting the inadequacy of current HIV prevention efforts in Africa Citation1. The HIV and AIDS epidemic in Tanzania appears to be relatively stable at 6.2% in the population segment aged 15–49 years old Citation2. The epidemic shows strong regional variations Citation3Citation4 and urban residents have considerably higher infection levels at 10.9% compared with rural residents 5.2% Citation5. In Tanzania, the primary mechanism for HIV transmission is unprotected heterosexual intercourse, and the most important factor that fuels the HIV epidemic is unprotected sex with multiple concurrent partners Citation6. The current national response is directed towards increasing individual and community awareness of the risk of HIV and its serious implications for the individual, the family and the community Citation4. Anti-retroviral therapy is currently available, but only 20% of all adults and children with advanced HIV infection are able to access it Citation7. Despite all these efforts, new infections continue to occur due to sexual practices with concurrent sexual partners without protection Citation6Citation7Citation8Citation9Citation10Citation11.
The availability of a safe, effective and accessible preventive vaccine may represent a long-term hope for controlling the HIV pandemic, especially in low-income countries Citation12. Development of an HIV vaccine is difficult. With the existing different HIV subtypes, multiple trials are needed from different geographical sites. A large number of HIV vaccine trials have been done in United States (US) and Europe but only a few in low-income countries, where the HIV infection rates and the burden of disease is the highest in the world Citation13. However, injectable drug users, gay and bisexual men, men who have sex with men and women vary greatly in their willingness to participate in vaccine trials according to a systematic review of HIV vaccine preparedness studies in the Organization for Economic Co-operation and Development (OECD) countries Citation14. Results from such contexts may not be generalised to countries such as Tanzania where those at risk are generally people aged 15–49 years old, and the major HIV transmission route is heterosexual contact Citation6.
Tanzania is one of the low-income countries that has met WHO eligibility criteria for conducting Phase I/II HIV vaccine trials. Although the main focus of a Phase I/II HIV vaccine trial is to evaluate the safety and immunogenic effect of the vaccine Citation13, understanding the potential participants’ willingness to take part in the trial is crucial for the development of future vaccines. In earlier studies, willingness to take part in HIV vaccine trials was associated with the perception of one's own risk of getting HIV infection Citation15Citation16Citation17Citation18 and the desire to help the community Citation15Citation19Citation20. In the Ugandan military, willingness to take part in the HIV vaccine trial was associated with sexual risk-taking and unrealistic expectations of the vaccine's capacity to protect against infection Citation21. Very little is known about willingness to participate in the Tanzanian context. In the present study, we describe willingness and associated factors to join in a Phase I/II HIV vaccine trial among members of the police force in Dar es Salaam, Tanzania.
2 Methods
This study is part of a larger HIV and AIDS study in Tanzania that includes studies of HIV incidence; studies of laboratory reference values as well as willingness to participate in an HIV vaccine trial, leading up to a subsequent vaccine trial in the police cohort. Thus, the police force is a cohort with data collection at different stages. This study was carried out in 23 out of 32 police stations, which were purposively included according to availability of the study participants. The 32 stations serve both urban and peri-urban areas of Dar es Salaam region and they are scattered in the three municipalities: Temeke, Kinondoni and Ilala. About 3,000 police officers, of whom 20% are women, make up the police force in Dar es Salaam. In the present study, 21% were female. Police officers were chosen for the study because they are well educated in the sense that the majority have attained four years of secondary education, come from an established organisation and are easy to access. A previous study had proven that they could make independent informed decisions to take part in HIV incidence studies which had gained the support of higher police authorities Citation22.
Between August 2005 and November 2006, we recruited a sample of 594 police officers through sensitisation workshops to assess the existing socio-behavioural attitudes in preparation of the Phase I/II HIV vaccine trial. These workshops were held in the selected police stations, and all the staff were invited. The workshops were organised by a field team (doctors, nurses and counsellors from Muhimbili University and national referral hospital together with collaborators from the police force, a medical doctor and a nurse from the Health Unit in the police force) to update the police officers with basic information about HIV infection at national and individual level. The workshops were designed to inform the police officers about the nature of the HIV incidence study and the HIV vaccine trial project. An information booklet describing the study more in detail was made available to participating police officers in the national language, Kiswahili. Thus, all participants received general information about the nature of the project, what was expected of the participating officers, potential benefits, impact on the police force at large, study duration and study objectives.
Forty-six visits were made for data collection, i.e. two visits within each of the selected stations. During the first visit, written consent was only obtained from those who were interested to participate in the studies after the workshop. Respondents’ characteristics were recorded in the enrolment document. A pre-tested, interviewer-administered questionnaire with both closed and open-ended questions (first questionnaire) regarding knowledge, attitudes about HIV and AIDS and sexual practices was given. Pre-test counselling was provided before HIV testing. During the second visit, seven days later, post-test counselling and HIV results were given and a second written consent was obtained before filling out the questionnaire (second questionnaire) on vaccine knowledge, attitudes to vaccine studies and willingness, which was administered regardless of the HIV status of the participant. All questions were posed in the national language. Trained counsellors and researchers completed the questionnaires. During both contacts, participants were encouraged to ask for clarifications on any issue concerning the study individually. Questionnaires were double-checked by two people the next day, before entry into the database.
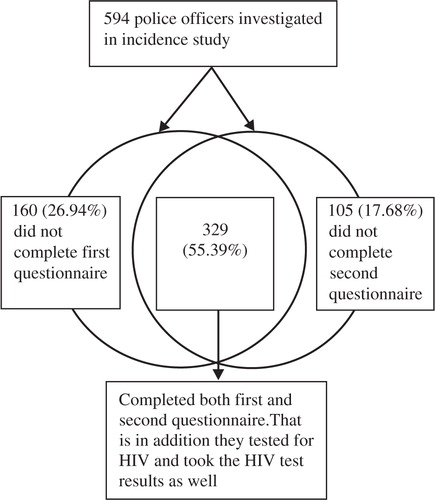
One hundred and five interviewees (17.7%) did not complete a second questionnaire. The main reason was that respondents did not come for HIV test results at the second study visit where the second questionnaire was completed. Of these 105, 13 (12%) came but did not want to complete the second questionnaire after testing HIV positive. The counsellors adhered strictly to rules of confidentiality during the second visit by only providing the first author (EAMT) with the total number of individuals declining and the reasons for not completing the questionnaire. Also, during the second visit, 160 (26.9%) of respondents bypassed the first questionnaire, allegedly because of time constraints. They requested just taking the HIV test. The two questionnaires were finally combined using proxies of the individuals’ unique identities to associate the selected variables. Thus, we enrolled a convenience sample of 329 (55.39%) police officers who completed both questionnaires ().
2.1 Data analysis
Data analysis was carried out in three stages.
The frequency distribution of all variables was tabulated. Four statements assessed HIV and AIDS knowledge where each correct response scored 1 and each incorrect one scored 0, the median score being 4. Sixteen statements assessed attitudes towards condom use, each positive response scoring 1 and each negative one scoring 0, while the median score was 12. Scores for HIV and AIDS knowledge were dichotomised as ‘high knowledge’ for those who scored above or on a par with the median (median score ≥ 4) or ‘low knowledge’ (median score < 4). Scores for attitudes towards condom use were dichotomised as ‘positive attitude towards condom use’ (median score > 12) or ‘negative attitude towards condom use’ (median score ≤ 12). Knowledge about how a vaccine works was assessed by asking an open-ended question analysed as either ‘Right’, ‘Not right’ or ‘I do not know’. ‘Right’ means a vaccine protects one from infection and ‘Not right’ means otherwise. Attitudes towards vaccines were assessed by using ‘Yes’ or ‘No’ or ‘I don't know’ responses. The respondents were given the opportunity to justify the ‘Yes’ and ‘No’ choices. The open-ended questions gave multiple responses but we report the percentage for the main identified reason only.
We analysed the willingness and examined the association between willingness and socio-demographic characteristics, knowledge, attitudes, self-perception of risk and risk behaviours. The willingness to volunteer (WTV) for the HIV vaccine trial was assessed by ‘Yes’ and ‘No’ choices. Cross tabulation was performed between all variables.
Binary logistic regression was performed to estimate the Odds ratio (OR) and 95% CIs of factors associated with WTV for the HIV vaccine trial. Missing responses were not analysed and non-significant results are not reported. Statistical analysis was conducted using SPSS 15.0 for Windows (SPSS, Inc Chicago, IL, USA).
2.2 Ethical issues
The project was reviewed and approved by the Institutional Review Board at Muhimbili University of Health and Allied Sciences (MUHAS), formally Muhimbili University College of Health Sciences (MUCHS). Each police officer provided written consent after reading and allegedly understanding all the details about the study. The police higher authority was informed about the study both orally and in writing. They received a written information sheet followed by a check list before commencement of the study to ensure their understanding of the principal study concepts.
3 Results
3.1 Socio-demographic and behavioural characteristics of the study population
The median age of the 329 participants interviewed was 37 years (range 19–61 years) and 79% were male. Whereas females dominated in the youngest age group,≤24 years, there was a majority of males in the oldest age group, 45+ years. A higher proportion of males and females were married (68%) and single (62%), respectively. Most were Christians, educated to four years in secondary school with a median of three children. Females were less likely to have had four or more lifetime partners. Condom use was not favoured by about half and not used even in extra-marital sexual encounters, whereas 60% considered themselves at risk ().
Table 1. Socio-demographic and behavioural characteristics of the participants
3.2 Knowledge about vaccines and experiences
Almost all (95% of 260 males and 90% of 69 females) knew in principle how vaccines work, that they protect from infections, and had also been vaccinated at some time (95% males and 94% females). However, a majority (70% males and 55% females) were not able to recall their vaccination experiences.
3.3 Attitudes to HIV vaccines
3.3.1 Use of an effective HIV vaccine
Most respondents (79% of 260 males and 71% of 69 females) would use an effective vaccine against HIV, if it were made available. The main reason for not using it was the fear of vaccine side effects. Six percentage were not sure.
3.3.2 Tanzania becoming a partner in developing an HIV vaccine
Almost all (85% of 259 males and 78% of 69 females) said ‘Yes’ to the question: ‘Do you think that Tanzania should be a partner in developing HIV vaccine that is especially designed for Tanzania?’ In responses to the open-ended question, the most commonly stated reason was that involving Tanzania would reassure them about the safety of the vaccine. Of those who said no (11%), the main reason was lack of trust in vaccine trials (e.g. Why should this vaccine be special for Tanzanians only?). Five percentage were not sure.
3.3.3 Sharing the intention to participate with significant others (sexual partner, parents, friends and relatives)
Most (69% of 236 males and 54% of 63 females) responded ‘Yes’ to the question: ‘If you were to participate in a HIV vaccine trial, would you tell anyone? If yes, whom would you tell?’ The most common person to share the information with would be the sexual partner and the most commonly reason stated for sharing was to take care of them if they suffered from side effects of the vaccine. The unmarried/divorced/separated/widowed stated that they would share the information with friends, family members, relatives or parents in that order. Similarly, the most common reason stated was to be taken care of if one was adversely affected by the vaccine. For those who felt no reason to tell anyone (15%), they said that the main reason was that participation would be a personal decision. Nineteen percentage were undecided.
3.4 Willingness to volunteer (WTV) for an HIV vaccine trial
Sixty-one percentage (64% of the 260 males and 54% of the 69 females) would volunteer for an early vaccine trial (Phase I or II) that is designed to determine its safety. The most commonly stated reason was altruism, i.e. to save the nation. Thirty-nine percentage were not ready to volunteer because of the fear of vaccine side effects.
3.5 Factors associated with willingness to volunteer (WTV) for the HIV vaccine trial
The associations were examined between WTV and socio-demographic variables, knowledge of HIV and AIDS, attitudes towards the use of the effective vaccine, risk-related variables and perceived risks in a multivariate logistic regression model (). WTV was positively associated with: positive attitude towards using an effective HIV vaccine if available (OR, 36.48; 95% CI: 15.07–88.28); Tanzania becoming a partner in development of HIV vaccine (OR, 4.28; 95% CI: 2.28–8.03) and the intention to tell others about one's decision to participate in the trial (OR, 6.61; 95% CI: 3.89–11.24). Other factors were having a regular sexual partner other than one's wife or husband (OR, 3.05; 95% CI: 1.63–5.69); perceived risk of getting infected with HIV (OR, 2.11; 95% CI: 1.34–3.33) and high knowledge about HIV and AIDS (OR, 1.92; 95% CI: 1.22–3.01).
Table 2. Factors associated with willingness to volunteer (WTV) in HIV vaccine trial in a multivariate analysis
4 Discussion
Overall, our data illustrated that police officers would be willing to take part in an HIV vaccine trial and that willingness was more positively associated with an intention to use than with an intention not to use an effective vaccine, if one were made available. This may explain the burden that HIV infection has imposed on people's lives, not least among police officers. In contrast to the results of a study in the US among homosexual men, African–American women and persons who abuse substances, the intent to be vaccinated if a FDA-approved vaccine became available was low, only 30 and 25.3% were extremely likely and likely, respectively, to accept such a vaccine Citation23. In addition, the higher willingness associated with Tanzania becoming a partner in the development of a vaccine that is particularly designed for Tanzania, is encouraging and provides a promising basis for police officers possibly volunteering for the HIV vaccine trials.
The increased willingness was associated with having additional sexual partners, apart from a wife or husband. Such risky behaviour is unsurprising, both among police officers Citation22, and in a Tanzanian context. Previous studies from Tanzania have documented the increase of HIV transmission among those with multiple concurrent partners Citation6Citation8Citation10Citation11. Consequently, the increased willingness to participate in the vaccine trial may be associated with higher risky behaviour identified among some respondents. Respondents might have been willing to volunteer for personal protection against HIV infection as reported elsewhere Citation20Citation21Citation23Citation24. The high interest among police officers for taking part in a vaccine trial might also have been influenced by unrealistic expectations of continuing to have multiple sexual partners as noted among the Ugandan military men Citation21. In our study, over half of the respondents considered themselves at risk. Our findings are supported by other studies Citation16Citation17Citation18 where willingness was influenced by perceived self-risk for HIV infection.
The influence of sexual partners/spouses was important in this study. The majority (66%) intended to share their intention to take part in the HIV vaccine trial with a ‘significant other’, mainly the steady sexual partner. The most common reason given was to have somebody who would take care of them in case they experienced adverse effects of the vaccine. Evidently, the possibility of an adverse event associated with a vaccine is recognised, but would not deter police officers from volunteering for the trial. The greater willingness noted among those who would tell others implies that significant others have an important role in influencing volunteers to participate in the HIV vaccine trial. In the previous studies, significant others were found to be a potential source of stigma towards those who would participate in HIV vaccine trial Citation25Citation26Citation27Citation28Citation29. Therefore, the role of the support of these significant others should be recognised in the recruitment and retention of volunteers.
The limitation of our data is based on methodological challenges such as the recruited sample. Although police officers are well organised, well educated and informed in terms of HIV and AIDS-related issues, it was challenging to engage them in responding to a long questionnaire, due to unexpected emergency duties. The nature of the group, however, facilitated access and follow up. They were recruited to the study on the basis of specific health-related information delivered during the meetings. Those who participated in the meetings may have been particularly interested in health-related matters. Therefore, we can only generalise our results to the studied participants and not to other populations.
The use of an interview-administered questionnaire might have caused some hesitation in terms of responding to sensitive questions about sexual practices. For instance, fewer females responded to questions concerning risk-related variables as compared to males. Some people might find it sensitive to reveal the number of lifetime sexual partners in a face-to-face interview. The counsellors who were filling out the questionnaires reported that, in many cases, the respondents just kept quiet when asked that question, and sometimes requested the interviewer to move on to another question. The interviewers of course respected the respondents’ suggestions. Thus, there might have been a higher frequency of risky behaviour than was reported. The first author (EAMT) participated in the sensitisation workshops and filling out the first questionnaire where she also noted hesitation among a few participants in disclosing the number of sexual partners.
5 Conclusion
Our results add valuable information in relation to interventions preceding HIV vaccine trials in low-income countries such as Tanzania. The information is useful for HIV vaccine development and hence contributes to the global fight against HIV/AIDS. The fact that willingness to participate in HIV vaccine trials among police officers is highly associated with risky behaviour indicates a need for an educational package on HIV prevention and counselling, as well as risk behaviour screening among those who volunteer for the trial. It is important to ensure that the participants clearly understand the fact that the trial does not offer protection from HIV transmission. The expressed willingness and underlying concerns need to be explored further, and here an explorative study may be important in order to reveal hidden concerns.
Acknowledgements
The authors are grateful to the participants for their time and their participation in this study. We appreciate the work of the field team Dr. Eric Aris, Dr. Mohamed Janabi, Dr. Robert Josiah (physicians), Mary Ngatoluwa, Tumaini Massawa, Matilda Mrina and Dafrosa Mtui (nurse counsellors). We also acknowledge the contribution of the police collaborators Dr. David Siyame, Dr. Mohamed Hussein, Dr. Layon Mwanyika, Dr. Mariam Masalu and Mrs Meres Katabalwa for their tireless organisation of the sensitisation workshops. This study was funded by a grant from Sida/SAREC, Tanzania–Sweden collaboration.
References
- Bunnell R, Mermin J, De Cock KM. HIV prevention for a threatened continent: implementing positive prevention in Africa. JAMA. 2006; 296: 855–8.
- UNAIDS. Sub-Saharan African AIDS epidemic update, regional summary. Geneva: UNAIDS and WHO. 2008.
- Msisha WM, Kapiga SH, Earls FJ, Subramanian SV. Place matters: multilevel investigation of HIV distribution in Tanzania. AIDS. 2008; 22: 741–8.
- TACAIDS. Follow-up to the declaration of commitment (UNGASS), indicators country report template: reporting period Jan 2003–Dec 2005.. Dar es Salaam, Tanzania; 2006.
- Somi GR, Matee MI, Swai RO, Lyamuya EF, Killewo J, Kwesigabo G, et al.. Estimating and projecting HIV prevalence and AIDS deaths in Tanzania using antenatal surveillance data. BMC Public Health. 2006; 6: 120–6.
- THIS 2003–04. Tanzania HIV/AIDS Indicator Survey (THIS) 2003-04. Dar es Salaam: Tanzania Commission for AIDS (TACAIDS). 2005.
- TACAIDS. UNGASS Country Progress Report, Tanzania mainland. Dar es Salaam, Tanzania; 2008.
- Mmbaga EJ, Hussain A, Leyna GH, Holm-Hansen C, Mnyika KS, Sam NE, et al.. Trends in HIV-1 prevalence and risk behaviours over 15 years in a rural population in Kilimanjaro region of Tanzania. AIDS Res Ther. 2007; 4: 23–33.
- Mmbaga EJ, Hussain A, Leyna GH, Mnyika KS, Sam NE, Klepp KI. Prevalence and risk factors for HIV-1 infection in rural Kilimanjaro region of Tanzania: implications for prevention and treatment. BMC Public Health. 2007; 7: 58–67.
- Msuya SE, Mbizvo E, Hussain A, Uriyo J, Sam NE, Stray-Pedersen B. HIV among pregnant women in Moshi Tanzania: the role of sexual behavior, male partner characteristics and sexually transmitted infections. AIDS Res Ther. 2006; 3: 27–37.
- Yahya-Malima KI, Olsen BE, Matee MI, Fylkesnes K. The silent HIV epidemic among pregnant women within rural Northern Tanzania. BMC Public Health. 2006; 6: 109–118.
- Esparza J, Bhamarapravati N. Accelerating the development and future availability of HIV-1 vaccines: why, when, where, and how?. Lancet. 2000; 355: 2061–6.
- Esparza J. An HIV vaccine: how and when?. Bull World Health Organ. 2001; 79: 1133–7.
- Dhalla S, Woods R, Strathdee SA, Patrick DM, Hogg RS. HIV vaccine preparedness studies in the Organization for Economic Co-operation and Development (OECD) countries. AIDS Care. 2007; 19: 1118–27.
- Jenkins RA, Torugsa K, Markowitz LE, Mason CJ, Jamroentana V, Brown AE, et al.. Willingness to participate in HIV-1 vaccine trials among young Thai men. Sex Transm Infect. 2000; 76: 386–92.
- Kiwanuka N, Robb M, Kigozi G, Birx D, Philips J, Wabwire-Mangen F, et al.. Knowledge about vaccines and willingness to participate in preventive HIV vaccine trials: a population-based study, Rakai, Uganda. J Acquir Immune Defic Syndr. 2004; 36: 721–5.
- Starace F, Wagner TM, Luzi AM, Cafaro L, Gallo P, Rezza G. Knowledge and attitudes regarding preventative HIV vaccine clinical trials in Italy: results of a national survey. AIDS Care. 2006; 18: 66–72.
- Van de Ven P, Mao L, Crawford J, Prestage G, Grulich A, Kaldor J, et al.. Willingness to participate in HIV vaccine trials among HIV-negative gay men in Sydney, Australia. Int J STD AIDS. 2005; 16: 314–7.
- Colfax G, Buchbinder S, Vamshidar G, Celum C, McKirnan D, Neidig J, et al.. Motivations for participating in an HIV vaccine efficacy trial. J Acquir Immune Defic Syndr. 2005; 39: 359–64.
- Suhadev M, Nyamathi AM, Swaminathan S, Venkatesan P, Raja Sakthivel M, Shenbagavalli R, et al.. A pilot study on willingness to participate in future preventive HIV vaccine trials. Indian J Med Res. 2006; 124: 631–40.
- McGrath JW, George K, Svilar G, Ihler E, Mafigiri D, Kabugo M, et al.. Knowledge about vaccine trials and willingness to participate in an HIV/AIDS vaccine study in the Ugandan military. J Acquir Immune Defic Syndr. 2001; 27: 381–8.
- Bakari M, Lyamuya E, Mugusi F, Aris E, Chale S, Magao P, et al.. The prevalence and incidence of HIV-1 infection and syphilis in a cohort of police officers in Dar es Salaam, Tanzania: a potential population for HIV vaccine trials. AIDS. 2000; 14: 313–20.
- Crosby RA, Holtgrave DR. Will sexual risk behaviour increase after being vaccinated for AIDS?. Int J STD AIDS. 2006; 17: 180–4.
- Kafaar Z, Kagee A, Lesch A, Swartz L. Is participation in HIV vaccine trials a health promoting behaviour?. AIDS Care. 2007; 19: 1307–9.
- Allen M, Israel H, Rybczyk K, Pugliese MA, Loughran K, Wagner L, et al.. Trial-related discrimination in HIV vaccine clinical trials. AIDS Res Hum Retroviruses. 2001; 17: 667–74.
- Barrington C, Moreno L, Kerrigan D. Local understanding of an HIV vaccine and its relationship with HIV-related stigma in the Dominican Republic. AIDS Care. 2007; 19: 871–7.
- Lesch A, Kafaar Z, Swartz L. Community members’ perceptions of enablers and inhibitors to participation in HIV vaccine trials. S Afr JPsychol. 2006; 36: 734–61.
- McCluskey MM, Alexander SB, Larkin BD, Murguia M, Wakefield S. An HIV vaccine: as we build it, will they come?. Health Aff (Project Hope). 2005; 24: 643–51.
- Rudy ET, Newman PA, Duan N, Kelly EM, Roberts KJ, Seiden DS. HIV vaccine acceptability among women at risk: perceived barriers and facilitators to future HIV vaccine uptake. AIDS Educ Prev. 2005; 17: 253–67.