Abstract
Background: The antiretroviral treatment (ART) scale-up service has been a recent development in Ethiopia, but its impact on mortality has not been well investigated. The aim of this study was to assess the early survival outcome of the scale-up service by utilizing routine hospital data.
Methods: All adult HIV/AIDS patients who started on antiretroviral treatment in Shashemene and Assela hospitals from January 1, 2006 to May 31, 2006 were included and followed up for 2 years. Data were extracted from standard patient medical registrations. Kaplan–Meier curves were used to estimate survival probability and the Cox proportional hazard model was applied to determine predictors of mortality. Two alterative assumptions (real case and worst case) were made in determining predictors of mortality.
Results: The median age of patients was 33 years and 57% were female. Eighty-five percent had CD4 <200 cells/µL with a median CD4 count of 103 cells/µL. The median survival time was 104.4 weeks. A total of 28 (10.3%) deaths were observed during the 2-year period and 48 patients (18%) were lost to follow up. The majority of deaths occurred in the first 4 months of treatment. In multivariate analysis, 2-year survival was significantly associated with the clinical stage of the disease, baseline hemoglobin, and cotrimoxazole prophylaxis therapy (CPT) at or before ART initiation in both assumptions. The median CD4 count and body weight showed a marked improvement during the first 6 months of treatment, followed by stagnation thereafter.
Conclusion: The study has shown an overall low mortality but a high loss to follow-up rate of the cohort. Advanced clinical stage, anemia, low body weight, and lack of CPT initiation were independent predictors of mortality – but not gender. CPT initiation should be encouraged in routine HIV care services, and patient retention mechanisms have to be strengthened. Stagnation in immunological and weight recovery after the first 6 months should be further investigated. The utilization of routine data should be encouraged in order to facilitate appropriate decision making.
Introduction
In 2008, an estimated 33.4 million people were living with HIV/AIDS worldwide; nearly 70% of these were found in sub-Saharan Africa Citation1. Due to a lack of accessibility to treatment services, the sub-Saharan region has also taken the largest share of the mortality burden caused by the pandemic Citation2. However, the recent extensive investments in expanding HIV/AIDS treatment in low- and middle-income countries have resulted in a marked improvement in antiretroviral therapy (ART) coverage from 7% in 2003 to 42% in 2008 Citation3.
A few studies have been conducted to investigate the survival benefit of ART scale-up services in Africa. Though their findings showed a higher mortality compared to high-income countries, the survival of the patients improved and the incidence of opportunistic infections (OIs) decreased Citation4Citation5Citation6Citation7. In these studies, the degree of immunodeficiency was measured by clinical or CD4 criteria; anemia, malnutrition, and bacterial infections were all found to be important factors affecting treatment outcome and subsequent survival Citation7.
In Ethiopia, 1,037,267 people were estimated to be living with HIV in 2008. The antiretroviral scale-up service has been expanding to all hospitals and most health centers since 2005, and has achieved ART treatment provision to a total of 175,612 patients (60.6%) out of 289,734 who required it. A study done by Kloos et al. in 2006 concluded that the sharp increase in national ART utilization was the result of a combination of free and decentralized treatment services. The same study indicated a national ART dropout rate of 20% Citation8.
The available evidence in the country regarding the survival outcome in ART scale-up services is, however, limited. Two previous studies, conducted in one district hospital of Ethiopia before the national scale-up service started, showed an improved survival and decreased tuberculosis incidence in the early years of ART Citation9Citation10. But no study has focused on the survival outcome after the ART scale-up service was initiated.
This study aimed to analyze the early survival outcome of ART scale-up services and to identify predictors of mortality in HIV-infected patients starting ART in two hospitals from the Oromiyaa region, Ethiopia.
Methods
Setting
Ethiopia is a low-income country in east Africa, with a total population of approximately 73 million according to the 2007 demographic census. The 2005 Demographic and Health Survey estimated the national adult HIV prevalence to be 2.1% Citation11.
This study was conducted at two district hospitals, Assela and Shashemene, located in the southern part of the Oromiyaa region, Ethiopia. These are referral hospitals in the Arsi zone with a catchment population of 2 and 1.5 million people, respectively. Both hospitals provide general outpatient and inpatient services, including surgical and obstetric emergency care. Infectious diseases account for most of the inpatient and outpatient visits. Both hospitals have been providing voluntary counseling and testing (VCT) services for more than a decade, and a free ART service was started in January 2006. The adult HIV prevalence rate of the region was 1.5% in 2008.
Patients who have tested positive for HIV at different service outlets in the hospitals, or who have been referred from elsewhere, are enrolled in HIV care. The clinical services are provided by a multi-disciplinary ART team (MDAT) comprising a doctor, a nurse, a pharmacy technician, and a data clerk. Initial evaluation includes clinical and CD4 staging, which determines whether ART should be initiated. Treatment may also be initiated with clinical parameters alone when the CD4 test is not available. In addition to the medical eligibility, patient adherence and commitment is mandatory. The medical eligibility criteria for ART in the Ethiopian context is presented in Citation12.
Table 1. Eligibility criteria for ART in adults and adolescents; adapted from the Ethiopian national ART guideline
Based on the 2007 ART guidelines of Ethiopia, the preferred first-line regimen for adults and adolescents comprises stavudine (d4T) or zidovudine (AZT), combined with lamuvidine (3TC) and either efavirenez (EFV) or nevirapine (NVP), depending on the patient's clinical condition Citation12.
Once the decision to initiate ART is made, the appropriate regimen of highly active antiretroviral therapy (HAART) will be started. Patients are advised to return after 2 weeks to determine toxicity/tolerance or adherence problems. The subsequent appointment schedule depends on the condition of the patient, but usually medication for 1 month will be dispensed and patients are advised to have a review after day 28. After 3 months of treatment, appointments are extended for patients with better adherence to treatment and an improved clinical condition. Cotrimoxazole prophylaxis therapy (CPT) is also provided at the initial stage based on the guidelines.
During every visit, the patient's counseling addresses several issues including safe sexual practice, family planning, adherence assessment, and support. A CD4 test is repeated every 6 months and patients showing signs of treatment failure or poor clinical response are referred to tertiary care for viral load tests and initiation of a second line regimen. Liver enzyme tests are also requested when necessary.
Patients who miss appointments are contacted by telephone within days, but reaching those who do not have a telephone remains a problem. Recently, a tracing mechanism started with the involvement of peer educators who often carry out planned home visits for patients lost from care or treatment. Both laboratory tests and ART drugs including CPT are provided free of charge as part of the PEPFAR and Global Fund for HIV, TB, and malaria.
Patient selection
A cohort of antiretroviral naïveFootnote1 patients, who were initiated on treatment between January 1, 2006 and May 31, 2006, from the above two hospitals, were included in the study and monitored for 2 years. The inclusion criteria were; Citation1 that they should be a HIV-infected patient >15 years of age, and Citation2 that they should have received ART on at least two clinical visits. Patients starting treatment in other places, who had previous ART history, were without baseline CD4 count and lacking basic personal information, were excluded.
Data were extracted from the available standard national medical registers, which have been adopted by the Ministry of Health (MoH). The registers include the Pre ART register (register of patients at their first visit), the ART register (registration after ART initiation), and the follow-up patient form; the latter is the form completed for all patients at each visit, and on which information regarding progressive weight change, clinical stage, drug toxicity, adherence, newly diagnosed OI, and laboratory test results is documented.
Statistical analysis
Epi-info 3.5.1 and STATA 10 were used for statistical analysis. The main outcome variables were death and the time of its occurrence during the 2-year period. The date of censoring, including the lost-to follow-up date and transferred-out date, was also used for survival time calculations. The progressive 6-monthly CD4 and weight changes were additional variables included in the analysis.
The survival time was calculated in weeks using the time interval between the date of ART initiation and Citation1 the date of event (death), Citation2 the date transferred out (TO), Citation3 the date of the first missed appointment for lost cases, and Citation4 the date on which the patient completed the 24 months of follow up. The Kaplan–Meier model was used to estimate the survival probability after ART initiation, and p values were used to compare survival curves. The Cox proportional hazard model was used to assess the relationship between baseline variables and mortality. The predictor variables used in the analysis were age, sex, type of facility, WHO clinical stage, baseline CD4 count, baseline body weight, hemoglobin at cut-off point of 10 g/dL, and CPT initiation. Variables that were statistically significant (p<0.05) in the univariate analysis were subsequently fitted into the multivariate analysis. Analyses of two scenarios, a real-case assumption (confirmed dead cases were used as events) and a worst-case assumption (lost cases were also considered as events), were conducted separately.
Ethical considerations
Permission was granted from Oromiyaa Regional Health Office and hospitals. Data were kept confidential and anonymous. Results of the study were summarized and communicated to the authorities in the region.
Results
Baseline characteristics
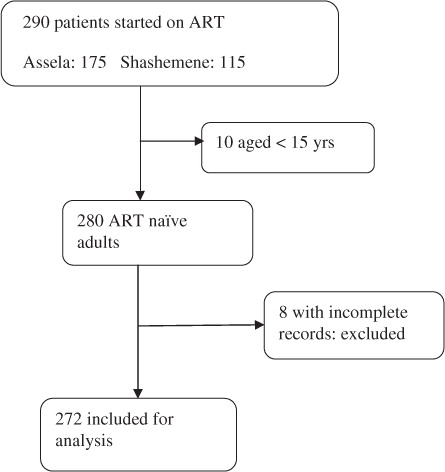
A total of 290 patients were initiated with ART in both facilities during the study period. Data available for analysis included 272 ART patients (). The cohort contributed to a total of 409.9 person-years of follow up. The mean duration of follow up was 104.4 weeks.
The demographic characteristics of the cohort are summarized in . Seven percent of patients were under the age of 24, 47.4% were aged 25–34, 28.7% were aged 35–44, and 16.9% were 45 or older at the time of the treatment initiation. The median age of patients at the start of the treatment was 33 years (range 18–68). The majority of the patients were women (57%). Sixty percent of the patients started treatment in Assela hospital. Nearly 82% of the patients had at least primary school education or more. Forty-three percent of patients were married, 15.4% single, 22.4% divorced, and 18.4% were widows or widowers at the time of the treatment initiation. Of the total, 59% of the patients had a baseline weight less than 50 kg, although the median baseline weight differed significantly between the sexes: 46 kg (range 24–91) for females and 52 kg (range 14–79) for males (p<0.01).
Table 2. Socio-demographic characteristics of the study participants
The clinical parameters of the patients are summarized in . Seventy-one percent of patients were staged clinically as III/IV. By exclusive CD4 criteria, 85% of patients had a CD4 count of <200 cells/µL, indicating that the majority had advanced HIV disease at the initiation of ART. The median CD4 count of the patients at the time of the treatment initiation was 103 cells/µL (range 1–423). Eighty-two percent of the patients started ART based on the CD4 <200 cells/µL criterion, and the rest for clinical or combined reasons. Twenty percent had one or more major OIs other than TB, and 23.3% had TB a year before initiation of ART in both facilities. Nearly 40% of patients had a baseline hemoglobin level of <10 g/dL.
Table 3. Clinical characteristics of the study participants on initiation of ART
The regimen d4T + 3TC + NVP was the most frequently prescribed (36% of patients) followed by the regimen d4T + 3TC + EFV (28%). The initial ART regimen was changed for 32 (12%) of the patients during the 2-year follow-up period. The main reasons for regimen change were ‘toxicity’ in 55% of the cases, followed by ‘new TB diagnoses’ in 29% of the cases for which NVP-based regimens were switched to EFV-based regimens. The AZT + 3TC + NVP regimen was significantly more associated with subsequent regimen change compared to other regimens, for toxicity reasons (p=0.029). The main adverse effect was anemia due to AZT. CPT was initiated at or before ART initiation for 88.6% of the patients ().
Survival analysis
A total of 28 (10.3%) patients were reported dead in the 2-year period after initiation of ART, and all of the deaths except two occurred in the first year of follow up. The median survival time for event (dead) cases was 13.4 weeks (range 1.7–105), indicating that the majority of the deaths occurred before the fourth month of treatment. The rate of occurrence of death during the follow-up period was 7/100 person-years. There was no information available regarding the cause of death. The remaining patients were censored for different reasons: 48 (18%) were lost to follow up, 19 (7%) TO to other facilities, and 176 (64.7%) were alive at the end of the 24-month follow-up period. The median baseline CD4 count for lost to follow-up cases was 79 cells/µL, and was significantly different from the survivors and TO groups, which were 104 and 108 cells/µL, respectively. The median follow-up time for lost cases was around 8.6 weeks.
The baseline variables clinical stage, hemoglobin, and CPT initiation were associated with progression to death in the univariate analysis using the real-case assumption, but no association was found for gender, CD4 count, literacy, marital status, type of ART regimen, and facility of care. In the multivariable analysis, hemoglobin ≤10 g/dL, clinical stage IV, and non-CPT initiation at or before the start of the treatment, were significant predictors of mortality (). The probability of 2 years’ survival was significantly different for groups with a hemoglobin level >10 g/dL compared to low hemoglobin groups, p=0.0079 (b). Similarly, the 2 years’ survival probability was significantly different between the groups with and without CPT, p=0.000 (a). A statistically significant difference in survival probability was observed among WHO clinical stages, ranging from 0.96 for clinical stage I/II to 0.74 for stage IV, p=0.012 (c).
Fig. 2. Kaplan–Meier survival curves according to: (a) CPT prophylaxis; (b) baseline hemoglobin; (c) clinical stage.
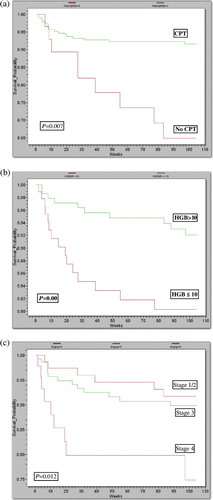
Table 4. Hazard ratios of mortality according to baseline variables in HIV patients starting ART in Ethiopia (real-case assumption)
In the worst-case assumption, advanced clinical stage, hemoglobin ≤10 g/dL, non-CPT initiation, and low baseline body weight were associated with poor survival in univariate analysis. All the above baseline variables, except baseline body weight, were found to be significantly associated with mortality in the multivariate analysis ().
Table 5. Hazard ratios of mortality according to baseline variables in HIV patients starting ART in Ethiopia (worst-case assumption)
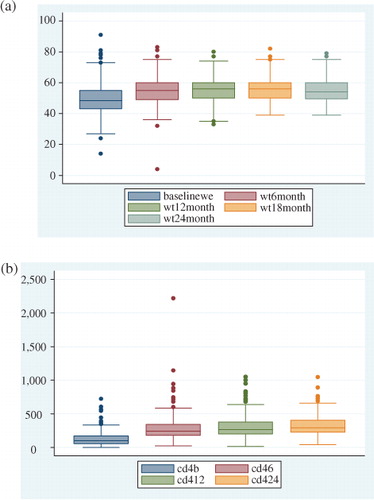
The indicators progressive CD4 change and weight increment were used for measuring overall treatment outcome. The median baseline CD4 count increased from 103 cells/µL at baseline to 242 cells/µL at 6 months, 265 cells/µL at 12 months, and 292 cells/µL at 24 months. The median body weight increased by 4 kg at 6 months, but showed no progressive improvement thereafter ().
Discussion
This 2-year retrospective cohort study of HIV/AIDS patients on ART gives an insight into survival and its determinants in a hospital setting in Ethiopia. In this cohort, 10.3% of the patients died, most of the deaths occurring within the first 4 months. The overall mortality rate was lower compared to other studies in Africa, in which it was shown that in Tanzania and Cameroon, 29.7 and 23%, respectively, of the patients died during the first 12 months of ART Citation5Citation7. The high mortality in the first few months of therapy was similar to other studies from different African countries, including Ethiopia Citation4Citation5Citation7Citation9Citation13. The reason is probably that most of the patients (85.5%) had advanced disease as evidenced by a baseline of CD4 <200 cells/µL. Prior lack of access to free ART services, stigma related to HIV, and limited availability of VCT services in most areas, might have played a role in delaying diagnosis. Lack of proper screening of latent OI and limited availability of prophylaxis and diagnostic facilities for OIs may have also contributed to the early mortality. The high mortality in the first few months indicates the need to initiate ART early, which would require early diagnosis of HIV. This could be addressed by improving counseling and testing services at health facilities, in addition to strong community education and mobilization. The presence of advanced disease at baseline in most cases requires closer attention and frequent follow up by clinicians in order to prevent and treat conditions in early phases.
The cause of death was not possible to investigate in the current study. Studies from other African countries have indicated TB, acute bacterial infection, flare up of latent OI, and immune reconstitution syndromes as the main causes of death Citation13Citation14Citation15.
The proportion of lost to follow up was 18%, which was much higher than other studies from African settings such as in Cameroon (5%) and Tanzania (9.7%) Citation5Citation7. The inverse relationship between overall low mortality and high lost to follow-up rate found in our study has also been observed in a systematic review of ART clinical outcomes in 13 African cohorts by Rosen et al. in 2007 Citation16. Higher attrition is expected in programs of shorter duration. Possible explanations include a lack of resources and technical efficiency, which could affect patient retention and the ability to monitor long-term outcomes. In our context, where the majority of the patients were in advanced clinical stage evidenced by a median CD4 count of 79 cells/µL, most of the lost cases were probably unrecorded deaths rather than living patients. In handling this issue, a worst-case assumption was also applied during the analysis.
In both assumptions, clinical stage at baseline, which is an established predictor of survival in most studies, was significantly associated with 2 years’ survival Citation10Citation17Citation18. Hemoglobin ≤10 g/dL at baseline was also strongly associated with mortality in both assumptions. Several previous studies revealed anemia as an independent predictor of mortality in HIV patients Citation5Citation17Citation19. The anemia could be explained as part of the advanced clinical stage, especially secondary to latent OIs, but the exact cause of anemia was impossible to determine. The cause of anemia in HIV patients is likely to be multifactorial (factors such as bleeding, nutritional deficiency, hemolytic anemia, bone marrow suppression, etc.). Considering the availability and affordability of hemoglobin tests, anemia evaluation should be incorporated into the routine care of HIV patients.
Similar to other studies, baseline CPT was strongly related to survival in both assumptions, where patients starting on CPT had a significantly lower risk of death Citation20Citation21. According to the national treatment guidelines, CPT has to be started for all patients who are eligible for ART, unless clinical contraindications exist. In our study, several patients without contraindications were not started on CPT – this might indicate a lack of quality in the clinical care. Thus, the routine use of CPT has to be strengthened in this setting Citation20Citation21. There was no significant difference in survival rates between the sexes, unlike in other studies where men had a comparably higher mortality than women Citation7Citation22. In contrast to many studies in which the CD4 count is an established predictor of mortality, it was not found to be associated with survival in our study. This may partly be explained by the fact that the majority of patients (82%) had a CD4 <200 cells/µL, which could have made the comparison with higher CD4 counts statistically unstable Citation23Citation24.
The proportion of ART regimen change during the 2-year period was comparable or even lower than that observed in other cohorts Citation6Citation25. This supports the view that with proper and standardized clinical care, ART can be safely used in resource-limited settings. The main reason for regimen change was toxicity, followed by new TB diagnosis. The NVP-containing regimen was switched to an EFV-based regimen in TB patients, due to feared pharmacological interaction of rifampicin and NVP. Unlike findings in the developed world, regimen change due to treatment failure was not observed in this particular study. Similar findings were observed in one hospital-based study from Arbaminch, Ethiopia, where a regimen change due to drug side effects (but not due to treatment failure) was observed Citation9Citation25.
As the ART scaling-up service in Ethiopia was only started a few years ago, treatment failure may not yet be a common occurrence. The lack of availability of viral load and ART drug resistance testing in the facilities might have limited the capacity of diagnosing treatment failure, even though clinical and immunologic clues of failure (9 cases of TB and 22 cases of other OIs) were evident.
The progressive change in the weight and median CD4 count of the cohort was used as an indicator of treatment outcome. The initial increase in the median value of both measurements, pointing out an early immune recovery, is comparable to other studies in Africa Citation26Citation27. The reasons for the subsequent stagnation, however, are not clear, so long-term adherence problems should be taken into consideration in future studies.
The short observation period of the cohort, together with a high rate of censoring and lost to follow up with untraced outcome, might have biased the study. Most of the lost to follow-up cases had evidence of unfavorable outcome (advanced clinical stage and low CD4 count), so considering them as censored might have resulted in differential misclassification, i.e. patients with advanced disease and poor survival likelihood would be considered as still being alive. To address this limitation, the worst-case scenario assumption was made. The similar findings in both assumptions strengthen our results. Other types of bias may have resulted from the quality of the data, but it was assumed to be non-differential.
Conclusion
The study has shown a high mortality of the cohort in the first 4 months. An overall lower mortality, but higher lost to follow-up rate, compared to other African studies, was observed. Clinical stage IV, hemoglobin ≤10 g/dL, and CPT initiation (but not gender) were strongly related to mortality. The high early mortality has to be addressed by increasing the availability of early HIV diagnosis and treatment services, and by strengthening the quality of existing ones. Proper nutritional assessment, including anemia evaluation and treatment, has to be part of routine care. The patient retention mechanisms must also be strengthened to address the higher lost to follow-up rate. The initiative of tracing lost cases through house visits using peer educators requires special support and monitoring. Stagnation in immunological and clinical recovery after the first 6 months of treatment should be investigated for possible treatment failure and drug resistance.
Even though the current ART scale-up service has included more than a hundred thousand patients, this study could help as baseline data in showing the survival experience of the earlier cohort. The utilization of routine data should be encouraged in the Ethiopian setting for the improvement of patient outcomes.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Acknowledgements
We would like to thank Dr. Hassan Nuru, former head of Oromiyaa regional Health office, Dr. Desta and Dr. Melese, medical directors of Assela and Shashemene hospitals, respectively, for their cooperation during data collection. Data clerks of both hospitals, especially Awol and Sewasew, were particularly helpful. Our gratitude also goes to Dr. Nigusse Deyessa for his support.
Notes
1ART Naïve: an individual who has never been on antiretroviral therapy for the treatment of HIV infection.
References
- UNAIDS. AIDS epidemic update 2009. UNAIDS., Geneva, 2009.
- CDC. The Global HIV/AIDS pandemic. MMWR 2006; Sect. 841-4. Available from: http://http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5531a1.htm [cited: January, 2009]. .
- UNAIDS/WHO. Towards universal access, scaling up priority HIV/AIDS interventions in the health sector. Geneva: UNAIDS/WHO. 2009.
- Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al.. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006; 296: 782–93.
- Johannessen A, Naman E, Ngowi BJ, Sandvik L, Matee MI, Aglen HE, et al.. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008; 8: 52.
- Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al.. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004; 18: 887–95.
- Sieleunou I, Souleymanou M, Schonenberger AM, Menten J, Boelaert M. Determinants of survival in AIDS patients on antiretroviral therapy in a rural centre in the Far-North Province, Cameroon. Trop Med Int Health. 2009; 14: 36–43.
- Kloos H, Assefa Y, Adugna A, Mulatu MS, Mariam DH. Utilization of antiretroviral treatment in Ethiopia between February and December 2006: spatial, temporal, and demographic patterns. Int J Health Geogr. 2007; 6: 45.
- Jerene D, Naess A, Lindtjorn B. Antiretroviral therapy at a district hospital in Ethiopia prevents death and tuberculosis in a cohort of HIV patients. AIDS Res Ther. 2006; 3: 10.
- Jerene D, Endale A, Hailu Y, Lindtjorn B. Predictors of early death in a cohort of Ethiopian patients treated with HAART. BMC Infect Dis. 2006; 6: 136.
- CSA. Ethiopia demographic and health survey 2005. Addis Ababa: Central Statistical Agency. 2006.
- HAPCO. Guidelines for management of opportunistic infections and anti retroviral treatment in adolescents and adults in Ethiopia. Addis Ababa: MoH. 2007.
- Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al.. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006; 367: 817–24.
- Brown DM, Thorne JE, Foster GL, Duncan JL, Brune LM, Munana A, et al.. Factors affecting attrition in a longitudinal study of patients with AIDS. AIDS Care. 2006; 18: 821–9.
- Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al.. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006; 367: 1335–42.
- Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007; 4: e298.
- Etard JF, Ndiaye I, Thierry-Mieg M, Gueye NF, Gueye PM, Laniece I, et al.. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006; 20: 1181–9.
- Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005; 19: 2141–8.
- Moore RD, Keruly JC, Chaisson RE. Anemia and survival in HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1998; 19: 29–33.
- Mermin J, Lule J, Ekwaru JP, Downing R, Hughes P, Bunnell R, et al.. Cotrimoxazole prophylaxis by HIV-infected persons in Uganda reduces morbidity and mortality among HIV-uninfected family members. AIDS. 2005; 19: 1035–42.
- Walker AS, Mulenga V, Ford D, Kabamba D, Sinyinza F, Kankasa C, et al.. The impact of daily cotrimoxazole prophylaxis and antiretroviral therapy on mortality and hospital admissions in HIV-infected Zambian children. Clin Infect Dis. 2007; 44: 1361–7.
- Chen SC, Yu JK, Harries AD, Bong CN, Kolola-Dzimadzi R, Tok TS, et al.. Increased mortality of male adults with AIDS related to poor compliance to antiretroviral therapy in Malawi. Trop Med Int Health. 2008; 13: 513–9.
- Molenberghs G, Williams PL, Lipsitz SR. Prediction of survival and opportunistic infections in HIV-infected patients: a comparison of imputation methods of incomplete CD4 counts. Stat Med. 2002; 21: 1387–408.
- Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al.. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002; 360: 119–29.
- d'Arminio Monforte A, Lepri AC, Rezza G, Pezzotti P, Antinori A, Phillips AN, et al.. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. I.CO.N.A. Study Group. Italian cohort of antiretroviral-naive patients. AIDS. 2000; 14: 499–507.
- Lawn SD, Myer L, Bekker LG, Wood R. CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect Dis. 2006; 6: 59.
- Bennett KK, DeGruttola VG, Marschner IC, Havlir DV, Richman DD. Baseline predictors of CD4 T-lymphocyte recovery with combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2002; 31: 20–6.