Abstract
Human cutaneous leishmaniasis (CL) has previously been reported in West Africa, but more recently, sporadic reports of CL have increased. Leishmania major has been identified from Mauritania, Senegal, Mali, and Burkina Faso. Three zymodemes (MON-26, MON-117, and MON-74, the most frequent) have been found. The geographic range of leishmaniasis is limited by the sand fly vector, its feeding preferences, and its capacity to support internal development of specific species of Leishmania. The risk of acquiring CL has been reported to increase considerably with human activity and epidemics of CL have been associated with deforestation, road construction, wars, or other activities where humans intrude the habitat of the vector. In the Ho Municipality in the Volta Region of Ghana, a localised outbreak of skin ulcers, possibly CL, was noted in 2003 without any such documented activity. This outbreak was consistent with CL as evidenced using various methods including parasite identification, albeit, in a small number of patients with ulcers.
This paper reports the outbreak in Ghana. The report does not address a single planned study but rather a compilation of data from a number of ad-hoc investigations in response to the outbreak plus observations and findings made by the authors. It acknowledges that a number of the observations need to be further clarified. What is the detailed epidemiology of the disease? What sparked the epidemic? Can it happen again? What was the causative agent of the disease, L. major or some other Leishmania spp.? What were the main vectors and animal reservoirs? What are the consequences for surveillance of the disease and the prevention of its reoccurrence when the communities see a self-healing disease and may not think it is important?
Introduction and aims
General notes on leishmaniasis
Leishmania areFootnote1 intracellular protozoa that cause infections with diverse clinical manifestations ranging from chronic but often self-healing skin lesions, cutaneous leishmaniasis (CL), to erosive mucosal membrane destruction of the nasopharynx known as mucocutaneous leishmaniasis, and a life-threatening systemic infection with hepato-spleenomegly in visceral leishmaniasis. Leishmania are characteristically transmitted by the bite of various species of sand flies. The disease is determined by complex interactions between the infecting species of Leishmania and the immunological status of the host. Many species of sand flies are potential vectors and some 100 species of animals could serve as reservoir hosts, such as various wild or domestic animals but also human beings.
Effective prevention, control, and monitoring of leishmania transmission require an identification of the involved specific sand fly vector, a detailed knowledge of the biology of the vector species, as well as their influence on the transmission of the Leishmania. In the Old World, including West Africa, the known vectors of leishmaniasis are species belonging to the genus Phlebotomus. Although Sergentomyia species are also common in these areas, they have not been reported as vectors of leishmaniasis.
Leishmaniasis is one of the major infectious diseases affecting the poorest regions and the poorest populations of the world. The CL forms are the most common ones. They produce skin ulcers on exposed skin, causing serious disability and permanent scarring after healing. These forms have been described in Northern and Eastern Africa (refs) but are said to be rare in West Africa and had not prior to 1999 been described in Ghana. In 1999, however, chronic ulcers were reported from one village that shares a border with the Republic of Togo and investigations were done to identify the aetiology of the disease. This is the point of departure for us describing the subsequent outbreaks in the present report.
Cutaneous leishmaniasis in West Africa
Sporadic cases of canine leishmaniasis were reported from Dakar, Senegal, as early as 1915 Citation1 and human leishmaniasis from Nigeria 1944 Citation2 Citation3. The CL is found in Senegal Citation4 and L. major has been identified in a number of non-primate mammals Citation5. Case reports of CL exist from The Gambia Citation6 Citation7.
The history of CL has been presented by Boakye et al. Citation8. In their review, the authors point out that CL is proposed to be endemic in parts of West Africa that ‘may include northern Ghana.’ Human disease requires an animal reservoir (typically mammalian) and a vector capable of transmitting disease to humans - sand flies of the genera Phlebotomus in the Old World. Neither the reservoir animals nor the vectors are well described in West Africa.
An ecological study of an epidemic of CL in Senegal between 1975 and 1978 allowed, for the first time, a description of a CL focus in West Africa Citation9-Citation11. An outbreak of cases of CL was also reported in Ouagadougou, Burkina Faso in 1996; 1,845 cases were identified through retrospective and prospective studies in 1996 to 1998 Citation12. The Leishmania parasite was detected by positive smears in 28 of 52 (54%) patients tested in 1998. L. major was identified by Polymerase Chain Reaction (PCR) in one patient.
The reservoir hosts identified from Senegal and The Gambia are the rodents Mastomys erythroleucus, Tatera gambiana, Arvicanthis niloticus, and Mastomys erythroleucus plus a dog in The Gambia Citation13. In Nigeria, Mastomys natalensis and Tatera gambiana were identified as probable reservoirs Citation14. No evidence of reservoir hosts has been published from Ghana.
Anthropophilic West African Phlebotomus sand flies identified from Ghana include P. dubscqi and P. Rhodaini. Sand fly species collected in 1997 and 2002 from Navrongo in the Upper East Region of Ghana showed the presence of 14 different species, 13 of which belonged to the genus Sergentomyia. The only Phlebotomus species was P. duboscqi, a known vector of CL in West Africa.
Aims of this report
The aim of this report is to collate the various reports of an outbreak of ulcerative skin disease in Ghana and to present additional data, to facilitate further studies and surveillance of what appears to be an intriguing form of a newly emerged CL in the country.
Description of the outbreak and results of investigations
Outbreak area
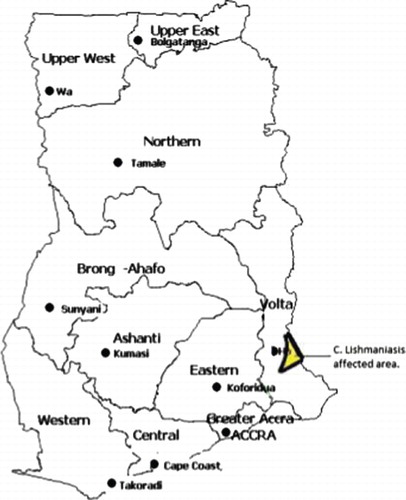
The suspected CL cases were noted for the first time in the Ho Municipality, Volta Region of Eastern Ghana, in 1999 (Ghana Ministry of Health, Annual Report 2004). This moist semi-deciduous forest ecosystem is not typically associated with leishmaniasis. Although there has not been any cases reported in Ghana before 1999, the West African CL belt cuts across the country's northern, arid, Sahel savannah regions that borders Burkina Faso.
The Ho Municipality, which is one of the 138 administrative municipalities/districts in Ghana, is located in the middle zone of the Volta Region (). It is bordered in the North by the Hohoe Municipality, in the East by the Ghana-Togo border, in the West by the Kpando district, and in the South by the North-Tongu and Akatsi Districts. It covers an area of about 2,564 square kilometres and has an estimated population of 249,000 (projection from the 2000 population census) with an annual growth rate of 1.8%. Ho town doubles as the capital of the Municipality and the Regional Capital (of the Volta Region). For the purpose of health care delivery, the Municipality is divided into six sub-districts: Tsito, Abutia, Adaklu, Kpetoe, Kpedze-Vane, and Ho-Shia.
The northern and eastern zones of the Municipality are mountainous and are covered with forest while the southern zone, on the other hand, is of mixed savannah and grassland with some marshy areas. The vegetation of the Municipality is mainly forest and forest savannah. There are two main seasons: wet and dry. The wet season encompasses the major rains from May to August and the minor from October to November. The rest of the year is relatively dry. Malaria and onchocerciasis are the main diseases transmitted throughout the year. The main economic activities are farming and trading. There are also minor economic activities like pottery, woodcarving, kente weaving, and cattle rearing in the southern part of the Municipality.
Case detection
Patients reporting to health facilities
Ulcer biopsies were taken at the Volta Regional Hospital (VRH) from 15 patients from villages in two sub-districts (Ho-shia and Kpedze-Vane) with ulcers who agreed verbally to go to the hospital for a biopsy. Initially the patients had visited their respective health facilities in November 2002 with ulcers, which were not responding to antibiotic treatment. Apart from the ulcers, the patients did not have any other complaints. The biopsies were sent to the pathology laboratory at Korle-Bu Teaching Hospital in Accra. The presence of what the pathologist identified as L-Dbodies (intracellular kinetoplastic organisms also called Leishman-donovan bodies) was consistent with the diagnosis of CL that prompted further investigations. The background characteristics and microscopic result of the 15 patients is shown in .
Table 1. Background characteristics of the patients who volunteered for ulcer biopsies and microscopy report
Nine of the 15 patients were females. The median age was 33 years, ranging from 12-71 years. Their main occupations were farming (n = 6), trading (n = 2), and studying (n = 5). One was a teacher and another self-employed. The average length of stay in the community was 22 years, ranging from 6 months to 71 years. The majority of the patients (12/15) had lived in the community for 12 years or more. The average duration of the ulcers before investigation was 6 weeks (range 3-12).
Survey at selected village schools
A survey was then made to three schools from where some of the cases examined at the health centres came to determine if other students had similar ulcers. This was done by the director of the municipal health services (Medical Officer) and the municipal disease control officer. Based on the clinical examination by the Medical Officer, 124 cases were found among the examined 675 students ().
Table 2. Result of the follow-up to the schools from which initial cases were reported
Passive case search in sub-districts of the Ho Municipality
A list of patients with active lesions, who spontaneously sought help for skin ulcers at the health centres in July and August 2003, was compiled by trained health workers. The patients came from 42 different villages. A total of 790 cases were recorded out of which 471/790 (59.6%) were females and 319/790 (40.4%) males. It was observed that, some lesions were single, others multiple, although this information was not consistently recorded to assess the proportions. The disease was seen in all age groups and lesions were mostly located on the head, neck, and the limbs. The lesions healed spontaneously within 3-8 months.
Active case search in sub-districts of the Ho Municipality
An active search of cases was carried out in November 2003 during the first round of National Immunisation Days (NID) for poliomyelitis in the Kpando district, Ho, and Hohoe municipalities.
A team trained to administer poliomyelitis vaccine was also trained to search for lesions using laminated pictures of various forms of lesions. They visited every house in every community to carry out both activities. They asked if anybody had lesions similar to those in the pictures or ‘Agbamekanu’ (the local name meaning; gift from somebody who has returned from a journey) in 2002 (a year before) or in 2003 (the year of the case search).
As a result of this survey, a total of 8,876 possible cases that occurred between 2002 and 2003, were identified in three municipalities/districts in the Volta Region with 8,533 cases coming from Ho Municipality alone (). None of these areas had, prior to 1999, reported cases of such ulcers. In the Ho municipality 6,185 cases occurred in 2003 compared to 2,348 in 2002.
Table 3. Active case search in three municipalities/districts in the Volta Region
A breakdown of the results into sub-districts of the Ho municipality () showed that out of a total of the 2,348 cases that occurred in year 2002 within the Ho municipality, Ho-Shia sub-district, where the disease apparently started, recorded the highest numbers (1,536). This was followed by the adjoining sub-district Kpedze-Vane (770). The third highest sub-district was Tsito, which shares boundary with Kpedze-vane (42). It was also shown that more cases occurred within these three sub-districts in year 2003 compared to 2002 (2,725 vs 770; 2,854 vs 1,536; 556 vs 42, respectively).
Table 4. A breakdown of the cumulative results of the active case search for leishmaniasis by November 2003 within the Ho municipality by sub-District
Interviews of respondents from the three sub-districts where no cases occurred prior to 2003 (Kpetoe/Ziope, Adaklu, and Abutia) revealed that the cases in these three sub-districts had a link with the endemic communities (those where cases occurred prior to 2003). Some of the cases were residing in the endemic communities and had been transferred to new work stations. Others were diagnosed while visiting neighbouring districts from the endemic communities or had returned home from visits to endemic communities. This provides further evidence of an actual increase in disease incidence in 2003, although an increased awareness of health care providers cannot be excluded.
Monitoring and understanding the outbreak
As of 2003, the disease had spread to all villages in the two biggest sub-districts (Kpedze/Vane and Ho/Shia) in the Ho Municipality as shown in the active case search and people who visited those communities were also affected. The disease affected all age groups. Based on these observations, the disease epidemiology appears to be more complicated than initially thought. However, no systematic case detection follow-up was done after this period. Cases continued to be detected in 2004, 2005, and 2006, but this was not based on a systematic data collection but a sample collection for parasite identification. In 2007 a small active case detection study was carried out in the Ho Municipality as part of a Master of Science thesis study.
Clinical presentation of lesions
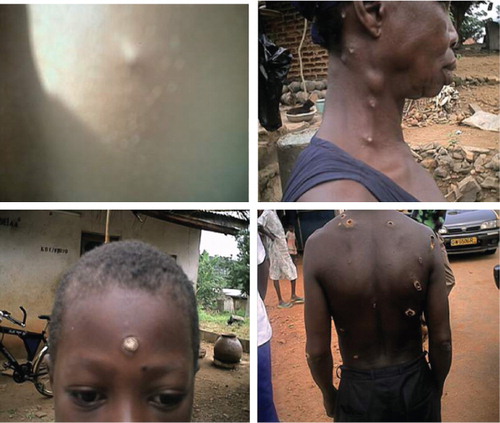
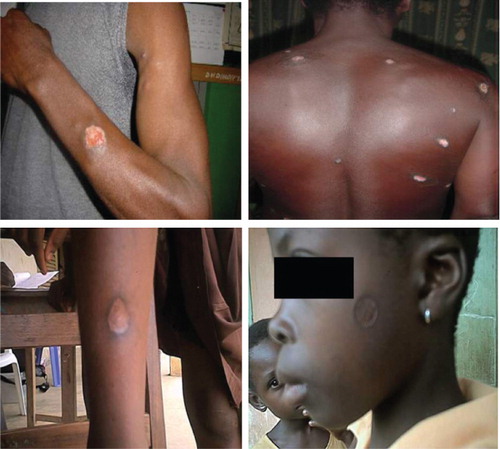
Early lesions started as pustules then became nodules or deep ulcers with flat base and raised margins (). Patients presented with both single and multiple ulcerative lesions (). Some presented with healed lesions that had left unsightly scars (). All patients photographed consented for their photographs to be taken and used for scientific purposes.
Parasite identification
Histology
summerises the cases described for clinical and parasitological identification. Ulcer biopsies were taken from 15 patients aged 12-71 years who reported to the health centres in two sub-districts of the Ho Municipality of the Volta Region with chronic ulcers in 2002 (). Microscopic examination of biopsies fixed in formalin and embedded in paraffin showed intracellular kinetoplastic organisms reminiscent of Leishmania parasites/amastigotes (so called LD bodies) in 10 out of the 15 biopsy specimens. The other five specimens showed a heavy infiltrate of histiocytes, plasma cells, lymphocytes, neutrophils, and giant cells in the ulcers, suggestive of CL. Due to lack of appropriate equipment no photographs were taken of the histological sections.
Table 5. Summary of cases used for clinical and parasite identification
Smears and culture
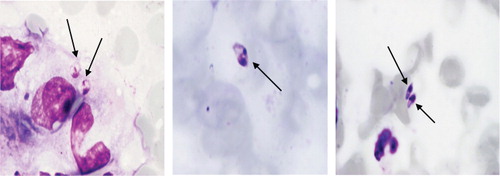
To try to more clearly identify the nature of the kinetoplastic organisms found in the biopsies in 2003, scrapings were taken from the edge of the ulcers from another four patients from the same area as the cases that underwent biopsies in 2002. At least two scraping samples were taken from each of the four patients and smears made on glass slides, dried, fixed, and stained with May Grünewald-Giemsa. The smears all showed kinetoplastic amastigotes albeit at very low numbers ().
Scrapings from the same four patients were also put onto NNN medium (standardised for use at the reference Parasitology Laboratory of the Swedish Institute for Infectious Disease Control) for Leishmania culture. None of the samples showed growth of parasites even after 8 weeks of culture despite the positive smear results.
Identification using Polymerase Chain Reaction
In 2004, skin biopsies obtained from five of the stored amastigote positive histological samples from 2002 were further examined using standard PCR used for identification of leishmaniasis in the reference laboratory in Sweden. Fifteen 10 µm thick sections per biopsy were cut and collected in the same tube and their DNA extracted as instructed by the protocol for fixed tissues found in the manual of the QIAamp DNA mini Kit (Qiagen Inc., Valencia, California). The extracted total DNA was freeze-dried and sent for PCR analysis at the Swiss Tropical Institute, Basel, Switzerland. This PCR assay is designed to target the gene for the spliced leader RNA (mini-exon) that is present as tandem repeats in the genus Leishmania and other kinetoplastida Citation15. The PCR-based analysis was performed twice on each sample but no Leishmania-specific DNA could be amplified from any of these five samples.
Skin tissue samples collected in 2004-2005 from Ho with cutaneous lesions were reported to be infected by L. Major Citation16. The exact PCR method used in this study is described in Fryauff et al. Citation16. During field investigations in January 2006 to identify the disease vector(s) and reservoir(s), nine biopsies were collected from five suspected CL cases in the village of Taviefe, 10 km north of Ho. Another sample was collected from the same village in February 2007. None of the samples taken in 2006 and 2007 showed L. major but rather a previously uncharacterised species of Leishmania spp., inferred by DNA sequence analysis of the small subunit ribosomal RNA gene and the internal transcribed spacer region (ITS1), was detected in tissue biopsies from these patients Citation17.
Entomological investigations
Studies done since 2004 in the outbreak area have concentrated on determining the possible vector species. Sand flies have been collected using various trapping methods that include CDC light traps with or without baits (dry ice), malaise traps, and sticky paper traps. The sampling have mostly been done in three communities, Klefe (06°37′N-00°26′E), Taviefe (06°66′N-00°47′E), Hlefi (06°42′N-00°22′E) where cases had been reported. Sand fly species collected were identified morphologically, using the taxonomic keys of Abonnenc Citation18. In all, 20 species of sand flies were recorded from the outbreak area. Eighteen of these were Sergentomyia species with only two Phlebotomus species namely P. duboscqi and P. rhodaini. Although P. duboscqi has been reported from most West African countries as the vector of CL in the region, its importance in the Ho outbreak area is uncertain due to the low numbers collected in previous surveys. For example in a sample of 251 sand flies collected from October to December 2006, only one was P. duboscqi while none was identified from 1,918 sand flies collected from January to December 2007 on sticky traps. The 18 Sergentomyia species identified include species that have been reported to feed on man although these have not been found to be vectors of human leishmaniasis Citation8.
In 2008, collections were made from human dwellings in a bid to determine the human biting sand flies in the outbreak areas. A total of 295 blood-fed sand flies characterised yielded only Sergentomyia species; S. simillima (38.2%), S. ingrami (34.5%), S. africana africana (26.9%), and S. antennata (0.4%) (Desewu, unpublished observation from 2008). The blood-meal analysis showed that with the exception of S. antenata, the others had mixed blood meals that included human blood. Other blood sources were from chicken (bird) and goat. Unfortunately the tests did not include rodent blood. Nevertheless, the presence of mixed-blood meals indicates the possibility of supporting the transmission of Leishmania parasites from an animal host to man but it is yet to be studied whether any of these species harbours the parasite in nature.
Discussion
The outbreak of skin ulcers in the Volta region of Ghana caused national anxiety when many patients were reporting to the hospital and health centres on seeing strange ulcers on their body.
Results of the active case search in the Ho Municipality shown in seem to show a dramatic increase in the number of cases in 2003, though recall bias makes definitive conclusions impossible. Boakye et al Citation8 state that CL in West African countries seems to occur in epidemics characterised by an increase in frequency over a few years followed by a drastic reduction in incidence. A follow-up survey would help determine whether this pattern occurred in this outbreak, or whether there continues to be a significant number of unreported cases.
Nonetheless, the disease appears to have become endemic since cases were detected in 2004, 2005, and 2006 in this area. In 2007 a small active case detection study in the Ho Municipality, cases clinically compatible with leishmaniasis were identified (Godwin Kwakye Nuako unpublished data), all of whom were identified outside of the health system. It would appear that the affected communities have noted that the lesions tend to self-heal, thus making the likelihood of them choosing to visit the health facilities slim. Despite the fact that CL is a self-healing disease, there are many signs that this particular disease in Ghana is not identical to simple L. major infections previously identified in the region. The lesions in the outbreak area were often multiple, distributed all over the body including the face, the infecting organism seems difficult to identify by routine methods.
The cumulative findings using microscopic and molecular methods are consistent with the disease being CL. However, despite the identification of L. major by PCR in one study, other studies present initial intriguing results that implicate an as yet unidentified Leishmania species. The finding of few amastigotes in the small sample of skin scrapings, which did not grow in standard Leishmania culture medium, is unexplained as are the negative PCR results in LD positive biopsies. The sand fly vector implicated in the outbreak also remains unknown and interestingly, of the 20 species identified only two were Phlebotomus species with the bulk being Sergentomyia, a number of which may be man biting species.
The results suggest that this self-healing cutaneous ulcer disease is associated with Leishmania, but that the main causative agent is not the same as that described in other parts of West Africa. It is thus important to further study this disease more closely while it remains focal in nature, since this may give the opportunity to contain a possible future outbreak.
Such studies would need to involve active case detection in a broader area, surveillance within the health system and mapping, using improved parasite identification methods, PCR identification of organisms from samples from more patients. Entomological surveillance should be continued and important studies on identification of the host in domestic animals, rodents, game, and wildlife including birds should also be intensified.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Notes
1Present address: Onchocercisis Chemotherapy Research Centre, Municipal hospital, Ghana Health Service, Hohoe, Ghana.
References
- Lafont A, Heckenroth F. Un cas de leishmaniose canine a Dakar [A case of canine leishmaniasis in Dakar]. Bulletin de la Société de Pathologie Exotique [Bulle soc Trop Med]. 1915; 8: 162-4.
- Elmes GT, Hall RN. Cutaneous leishmaniasis in Nigeria. Trans R Soc Trop Med Hyg. 1944; 37: 437-9. 10.3402/gha.v4i0.5527.
- Jelliffe RS. Cutaneous leishmaniasis in Nigeria and the Western Sudan. Wes Afri Med J. 1955; 4: 92-4.
- Ranque P. Les leishmanioses au Sénégal (étude épidémiologique et écologique) [Leishmaniasis in Senegal (Epidemiological and ecological studies)]. Médecine tropicale [Trop Med]. 1978; 38: 413-7.
- Dedet JP, Derouin F, Hubert B, Schnur L, Chance ML. Isolation of Leishmania major from Mastomys erythroleucus and Tatera gambiana in Senegal (West Africa). Ann Trop Med Para. 1979; 73: 433-7.
- Conteh S, Desjeux P. Leishmaniasis in The Gambia. I. A case of cutaneous leishmaniasis and a case of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1983; 77: 298-302. 10.3402/gha.v4i0.5527.
- Greenwood BM, Ajdukiewicz AB, Conteh S, Hagan P, Mabey DC, Panton LJ. Leishmaniasis in The Gambia. 3. Is its incidence increasing?. Trans R Soc Trop Med Hyg. 1984; 78: 407-9. 10.3402/gha.v4i0.5527.
- Boakye D, Wilson M, Kweku M. A review of Leishmaniasis in West Africa. Ghana Med J. 2005; 39: 94-7.
- Dedet JP, Derouin F, Hubert B. Ecology of a cutaneous leishmaniasis focus in the Thies region (Senegal, western Africa). I. Recall of the cutaneous leishmaniasis status in Senegal and presentation of the studied area. Bull Soc Pathol Exot Filiales. 1979; 72: 124-31.
- Dedet JP, Pradeau F, De Lauture H, Philippe G, Sankalé M. Ecology of a focus of cutaneous leishmaniasis in the Thiès region (Senegal, West Africa). 3. Evaluation of the endemicity in the human population. Bull Soc Pathol Exot Filiales. 1982; 75: 577-87.
- Dedet JP, Hubert B, Desjeux P, Derouin F. Ecology of a focus of cutaneous leishmaniasis in the Thiès region (Senegal, West Africa). 5. Spontaneous infestation and the role of the reservoir of various species of wild rodents. Bull Soc Pathol Exot Filiales. 1982; 75: 599-605.
- Traoré KS, Sawadogo NO, Traoré A, Ouedraogo JB, Traoré KL, Guiguemdé TR. Preliminary study of cutaneous leishmaniasis in the town of Ouagadougou from 1996-1998]. Bull Soc Pathol Exot. 2001; 94: 52-5.
- Desjeux P, Bryan JH, Martin-Saxton P. Leishmaniasis in The Gambia. 2. A study of possible vectors and animal reservoirs, with the first report of a case of canine leishmaniasis in The Gambia. Trans R Soc Trop Med Hyg. 1983; 77: 143-8. 10.3402/gha.v4i0.5527.
- Ikeh EI, Ajayi JA, Nwana EJ. Mastomys natalensis and Tatera gambiana as probable reservoirs of human cutaneous leishmaniasis in Nigeria. Trans R Soc Trop Med Hyg. 1995; 89: 25-6. 10.3402/gha.v4i0.5527.
- Marfurt J, Niederwieser I, Makia ND, Beck HP, Felger I. Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diag Micro Infect Dis. 2003; 46: 115-24. 10.3402/gha.v4i0.5527.
- Fryauff DJ, Hanafi HA, Klena JD, Hoel DF, Appawu M, Rogers W, et al.. Short report: ITS-1 DNA sequence confirmation of Leishmania major as a cause of cutaneous leishmaniasis from an outbreak focus in the Ho district, southeastern Ghana. Am J Trop Med Hyg. 2006; 75: 502-4.
- Villinski JT, Klena JD, Abbassy M, Hoel DF, Puplampu N, Mechta S, et al.. Evidence for a new species of Leishmania associated with a focal disease outbreak in Ghana. Diag Microbiol Infect Dis. 2008; 60: 323-7. 10.3402/gha.v4i0.5527.
- Abonnenc E, Pastre J. Capture of Phlebotomus in the Republic of South Africa, with a description of P. macintoshi, n. sp. (Diptera, Psychodidae). Bull Soc Pathol Exot Filiales. 1972; 65: 721-5.