Abstract
Dengue fever is a mosquito-borne viral disease estimated to cause about 230 million infections worldwide every year, of which 25,000 are fatal. Global incidence has risen rapidly in recent decades with some 3.6 billion people, over half of the world's population, now at risk, mainly in urban centres of the tropics and subtropics. Demographic and societal changes, in particular urbanization, globalization, and increased international travel, are major contributors to the rise in incidence and geographic expansion of dengue infections. Major research gaps continue to hamper the control of dengue. The European Commission launched a call under the 7th Framework Programme with the title of ‘Comprehensive control of Dengue fever under changing climatic conditions’. Fourteen partners from several countries in Europe, Asia, and South America formed a consortium named ‘DengueTools’ to respond to the call to achieve better diagnosis, surveillance, prevention, and predictive models and improve our understanding of the spread of dengue to previously uninfected regions (including Europe) in the context of globalization and climate change.
The consortium comprises 12 work packages to address a set of research questions in three areas:
Research area 1
Develop a comprehensive early warning and surveillance system that has predictive capability for epidemic dengue and benefits from novel tools for laboratory diagnosis and vector monitoring.
Research area 2
Develop novel strategies to prevent dengue in children.
Research area 3
Understand and predict the risk of global spread of dengue, in particular the risk of introduction and establishment in Europe, within the context of parameters of vectorial capacity, global mobility, and climate change.
In this paper, we report on the rationale and specific study objectives of ‘DengueTools’. DengueTools is funded under the Health theme of the Seventh Framework Programme of the European Community, Grant Agreement Number: 282589 Dengue Tools.
Dengue is the most important arboviral disease of humans Citation1. Global incidence has risen rapidly in recent decades. Some 3.6 billion people, half of the world's population, are now at risk, mainly in the urban centers of the tropics and subtropics Citation1 Citation2. Global reports of dengue hemorrhagic fever (DHF) have increased by fivefold in the past 20 years. The World Health Organization (WHO) estimates that there are 50–100 million infections worldwide every year, of which 21,000 are fatal Citation3. However, these numbers are gross underestimates since recent studies suggest that the figures are much higher, with as many as 230 million infections, tens of millions of cases of dengue fever (DF), and millions of cases of DHF Citation2 Citation4 Citation5 Citation6 . The disease is now endemic in more than 100 countries in Asia, Africa, and the Americas. The Asia-Pacific region is the most seriously affected, with increasing number and magnitude of dengue epidemics and expanding geographical distribution Citation7. Dengue inflicts a significant health, economic, and social burden on the populations of endemic areas. The number of disability-adjusted life years (DALYs) worldwide is estimated to range between 528 and 621 per million population Citation8. There is no specific treatment for dengue, but appropriate medical care can save the lives of patients with DHF.
There are four antigenically distinct dengue viruses belonging to the genus Flavivirus of the family Flaviviridae. Infection with one serotype confers long-term immunity to that serotype but not to the others. The strain of virus, secondary infection, age, and host genetics are the most important risk factors for severe disease, although the pathogenetic mechanisms remain poorly understood Citation8.
Dengue viruses are transmitted by mosquitoes of the genus Aedes, subgenus Stegomyia. The principal vector, Aedes Stegomyia aegypti, is now well established in much of the tropical and subtropical world, particularly in urban areas. It is a domestic species, highly susceptible to dengue virus infection, feeding preferentially on human blood during the daytime often taking multiple blood meals during a single gonotrophic cycle Citation8. It typically breeds in clean stagnant water in artificial containers and is, therefore, well adapted to urban life. A second species, Aedes Stegoymyia albopictus, is generally considered a less-effective epidemic vector because, unlike A. aegypti, it feeds on many animals other than humans and is less tied to the domestic environment Citation9. Nevertheless, this species has transmitted small outbreaks in some regions, and in recent years, its importance has increased because of its rapidly expanding range due to global commerce in used tyres and other containers Citation10.
Current surveillance systems and control strategies are clearly insufficient to prevent epidemic dengue in endemic countries or to prevent its introduction into uninfested regions (including Europe) Citation11. For this reason, in 2010, the European Commission announced a funding call for the 7th Framework (Box 1). Within this call, we have focused on better diagnostics, surveillance, and prediction of dengue introduction into currently uninfected regions.
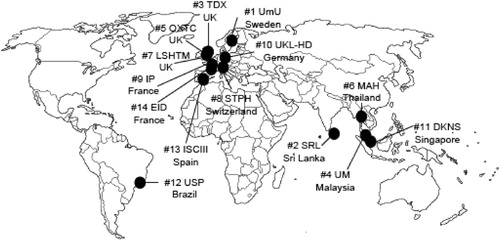
We formed a consortium of 14 partners based in South East Asia, South Asia, South America, and various centers in Europe (). Led and hosted by the University of Umeå in Sweden, the consortium includes partners from academic institutions, the Ministry of Health in Sri Lanka, a research-intensive small to middle size research enterprise (SME), and two research companies. Experts in this consortium include physicians, epidemiologists, mathematical modellers, climate experts, environmental scientists, laboratories, ecologists, chemical ecologists, entomologists, and a health economist (). The strength of the consortium lies in the breadth of expertise and the international network of partners from which it draws to address the challenging tasks of the 7th European Union Framework. The acronym of our 4-year project is ‘DengueTools’.
Table 1. Integrated expertise of members the consortium
Rationale and research objectives
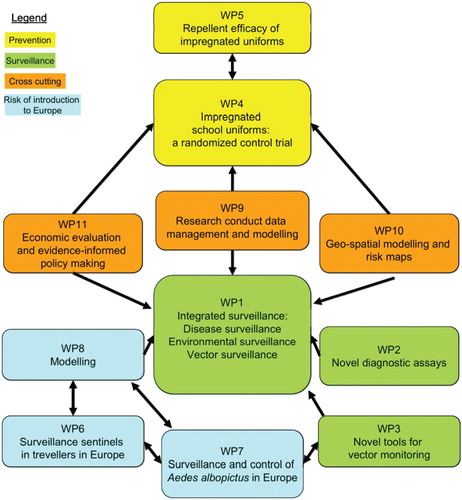
DengueTools created 12 work packages () to address gaps related to Citation1 surveillance, Citation2 control, and Citation3 risk of introduction of dengue to uninfected areas. Research gaps identified in these three research areas are summarized in Box 2.
Fig. 2. Twelve work packages (WP) and their interconnectedness around the three research areas. Work package 12 handles the management and dissemination of all work packages (not included in this figure).
Research area 1
Main research objective
Develop a comprehensive early warning surveillance system that has predictive capability for epidemic dengue using novel tools for laboratory diagnosis and vector monitoring.
Rationale
The dramatic global spread and increased frequency and magnitude of epidemic DF/DHF in the past 40 years underscore the critical need for more effective surveillance, prevention, and control of this disease. Most endemic countries do not have adequate surveillance to monitor dengue disease. The main purpose of surveillance is to provide information on risk assessment, epidemic response, and programme evaluation and to allow timely action to prevent or control dengue epidemic.
The risk of epidemic dengue transmission is determined by a combination of factors that include the level of herd immunity to virus serotypes; virulence characteristics of the virus strain; survival, feeding behavior, and abundance of Aedes mosquitoes; weather; and socioeconomic factors and human population density, distribution, and movement Citation12. Several potential predictive indicators for outbreaks have been described but require further study Citation13 Citation14.
The appearance of a new dengue serotype can be used as an early warning sign, although it does not always predict the occurrence of an immediate epidemic Citation15. The association between the introduction of a new serotype and outbreak occurrence remain unknown but the time lag may vary from 1 to 2 months to years.
The evolution of dengue viruses may have a major impact on their epidemic potential, virulence for humans, and geographic spread around the world Citation16. Currently, dengue viruses can be classified as being of epidemiologically low, medium, or high impact; i.e. some viruses may remain in sylvatic cycles with little or no transmissibility to humans; others are associated with DF only, and some strains have the potential to cause major epidemics and more severe DHF and DSS Citation16 Citation17 Citation18 . Phylogenetic and epidemiological analyses suggest that the genotypes and subtypes with greater epidemic potential regularly displace those that have lower epidemic potential. Nevertheless, a more complete understanding of the evolution and epidemiology of dengue viruses, particularly with respect to predicting epidemics of more severe disease, requires large-scale prospective studies that compare phenotypic expression with genetic changes in the viruses Citation18 Citation19.
The major components of dengue surveillance include monitoring the disease, mosquito vectors, and environmental and social risks Citation3. A Cochrane review of the application of tools for dengue outbreak prediction/detection and trend monitoring in surveillance systems highlighted the lack of evidence on the most feasible and sustainable surveillance approach Citation14. Prospective studies are needed to better define the most appropriate and cost-effective dengue surveillance system and to identify triggers for dengue emergency response. For surveillance to effectively provide early warning for epidemic transmission, it must be active, laboratory based, and comprehensive in its coverage of the spectrum of clinical illness, the catchment area, and the factors that influence transmission dynamics. We propose to study individual and combined indicators for outbreak detection and prediction to assess the relative value of each. Particular attention will be given to the role of viral factors. These data will be used to develop models capable of predicting epidemics, thereby providing more precise information on risk areas to target control activities.
Finally, given the limited resources in most dengue endemic countries, it is crucial to identify the most sensitive, specific, and cost-effective signal or combination of signals for an early warning system that would allow timely institution of control measures. Our consortium will address the affordability and sustainability of an active sentinel surveillance system, an important issue for policy makers.
Diagnostic assays
The lack of laboratory capacity and capability and the availability of affordable, sensitive, and specific diagnostics contribute to the poor surveillance in resource-limited countries. Accurate laboratory diagnosis of dengue patients is important to the individual patient and for surveillance and control efforts. An accurate point-of-care diagnosis can assist in patient management, prevent unnecessary and possibly expensive antibiotic usage, facilitate triage of febrile patients into appropriate clinics, and reduce health care utilization costs. We propose to develop two potential diagnostic assays, by using the recombinase polymerase amplification technology and the loop-mediated isothermal amplification approach.
Furthermore, new rapid, inexpensive, and operationally amenable methodologies are needed for vector surveillance. We propose to develop novel tools for vector monitoring, including the use of attractants to increase trap efficacy in the field. Available adult traps vary in cost, species-specificity, and capture rate. For example, BG-Sentinel traps are expensive and require electrical power, which restrict the number of such traps than can be deployed. Different applications may have different requirements in terms of preferred specificity for different mosquito species, or even sex or age of mosquitoes within a given species; so, there may not be one optimal trap for all purposes. We will investigate both host kairomones Citation20 Citation21 and oviposition attractants Citation22 Citation23 Citation24 Citation25 Citation26 in different trap formats. We will test putative attractants by using laboratory-based behavioral bioassays to evaluate and shortlist candidate compounds for subsequent field evaluation of efficacy and persistence in both adult and ovitraps.
Deliverables for research area 1
Develop a comprehensive, early warning, laboratory-based sentinel disease surveillance system and determine the most useful and cost-effective predictive factors for epidemic dengue;
Develop predictive models in particular signature forecasting and a flagging system;
Study how viral evolution influences the virulence and epidemic potential of dengue viruses;
Develop and validate novel diagnostic assays for point-of-care use;
Develop improved field devices and attractants for vector monitoring;
Develop novel assays for virus detection and characterization in Aedes mosquitoes;
Study entomological and environmental indicators that may influence dengue transmission dynamics;
Develop geospatial modeling and risk maps;
Evaluate the predictive capability of climate variables (weather variability) and their operational utility for surveillance;
Relate the costs of dengue surveillance and prevention to their effect on the burden of the disease;
Engage policy makers on the sustainability of an integrated surveillance system.
Research area 2
Main research objective
Develop novel strategies to prevent dengue in children.
Rationale
In the absence of specific antiviral therapy and vaccination, other prevention strategies need to be developed. Vector control in itself has had poor results to date. Insecticide-treated fabrics have emerged as a key component of arboviral disease control efforts. Insecticide-treated bed nets (ITNs) have been shown to be a successful intervention for the control of malaria Citation27. The efficacy of ITNs in reducing malaria transmission is related to the night biting activities of anopheline mosquitoes, but their use may have little or no impact on dengue incidence because Aedes species have daytime feeding patterns. However, window curtains and domestic water container covers treated with insecticide can reduce densities of dengue vectors and potentially affect dengue transmission Citation28.
Additional novel approaches using insecticide-treated materials need to be evaluated for the control of dengue. Personal clothing would potentially be an excellent target because clothes are worn during day times. There have been a number of programs that have applied insecticides to personal clothing, but this has been limited to military and recreational markets.
There is an urgent need for integrated and complementary population-based strategies to protect vulnerable children. Children carry the main burden of morbidity and mortality caused by dengue Citation3 Citation29. Dengue is often more severe in children, and children have a higher risk for developing DHF Citation3 Citation30. Infection in children causes large disruptions in schooling and parental wage earning that in turn has direct impacts on nutrition and overall family health Citation31. For example, in Northern Thailand, it has been estimated that dengue contributes 465.3 DALYs per million population per year among school-aged children, which accounts for 15% of all DALYs loss from febrile illness in this age group Citation32. Over the past 40 years, 65% of reported cases in Thailand were confined to the 5–14 years age group. Because children of this age group spend a considerable amount of their day at school, it is likely that schools are primary sites for exposure to the daytime biting Aedes mosquitoes. Schools should therefore be a key target for control. From a pragmatic point of view, schools would be an easier target in comparison to dispersed populations. School uniforms are a cultural norm in most developing countries and are worn throughout the day on an almost daily basis. We hypothesize that impregnated school uniforms may reduce the incidence of dengue in school-aged children Citation33. To test our hypothesis, we will conduct a community-based randomized controlled trial of the efficacy of impregnated school uniforms on the incidence of dengue in school-aged children. Furthermore, we will test the repellency effect of school uniforms in different sizes and forms and with different impregnation methods in a laboratory setting.
Deliverables for research area 2
Determine the efficacy of impregnated school uniforms in reducing the incidence of dengue in school-aged children;
Determine the protection efficacy of school uniforms impregnated with repellent under different laboratory scenarios;
Develop a cost-effectiveness framework and undertake an economic evaluation of the school-based preventive intervention;
Produce policy briefs and engage with policy makers to ensure the scalability of such school-based intervention programs, should the trial show efficacy.
Research area 3
Main research objective
Contribute to the understanding and predicting the risk of spread of dengue, in particular the risk of introduction and establishment in Europe, within the context vector competence, human mobility, and climate change.
Rationale
Dengue is the second most common cause of fever in returning international travellers, and the frequency of importation of dengue into currently non-endemic areas via travellers is increasing Citation34. There have been small outbreaks of dengue in Texas, USA, since 1980, all associated with increased transmission and importation from Mexico. In 2001, a small outbreak transmitted by A. albopictus, the first in 56 years, occurred in Hawaii. In 2009 and 2010, small outbreaks transmitted by A. aegypti occurred in Key West, Florida. Also, in 2010, the first authochthonous transmission of dengue occurred in Europe since before World War II: two cases in France and one in Croatia (www.eurosurveillance.org). A. albopictus is the only vector present in Europe with a wide and increasing distribution in many countries.
It is important to determine the risk of dengue becoming endemic in Europe. A. albopictus, although typically not an effective epidemic vector of dengue Citation9, is capable of transmitting dengue, and it is further spreading northward from countries bordering the Mediterranean. The risk of infection depends on vectorial capacity and viral replication rate in a temperate climate. The key question, therefore, is whether the presence of this vector is conducive to epidemic transmission in the event of importations into Europe.
Deliverables for research area 3
Monitor dengue importation into Europe;
Produce phylogenetic trees of imported dengue viruses to Europe using Bayesian modeling;
Compare the clinical manifestations and usefulness of the 2009 WHO dengue classification schemes in travellers (non-endemic populations) versus endemic populations (Sri Lanka);
Describe the breeding sites for A. albopictus in Southern France;
Determine the vectorial capacity of A. albopictus in a temperate climate;
Assess the impact of ultralow volume (ULV) spacing spraying on populations of adult A. albopictus in southern France;
Develop predictive models that integrate vector competence, vectorial capacity, and force of infection with global human mobility to assess the risk of epidemic transmission following the introduction of dengue viruses;
Produce interactive web-based tools on the risk of global spread of dengue, particularly to Europe.
Conclusions
Dengue fever is within the scope of the global health priorities. DengueTools is designed to address this priority. This cooperative and multidisciplinary project is also designed to improve the potential for new knowledge generation by integrating research groups with different scientific backgrounds, research centers from different European countries, and fostering research links between the North and South.
Effective surveillance and control for dengue is lacking. We will coordinate and facilitate one of the largest prospective integrated surveillance programs ever done in an endemic setting, in an attempt to find an answer to the most feasible strategy that will reliably predict new outbreaks and provide time-sensitive information for containment. Furthermore, we will attempt to develop novel inexpensive diagnostic tools, both for human diagnostics as well as for vector monitoring that will enhance an integrated surveillance system. Given the limited resources in many dengue endemic countries, it is of utmost importance to relate the costs of novel strategies for dengue surveillance and prevention to their effect on the burden of the disease. We will evaluate the economic value and benefit of each component of the integrated surveillance.
A second research area will focus on innovative strategies to reduce dengue infections in children by targeting schools. Reducing the incidence of dengue in school children will not only reduce morbidity and mortality but also reduce the number of school days lost, increase school performance, and reduce the economic burden on parents.
Dengue carries the risk of further geographic expansion and introduction into uninfected areas, including Europe. We will assess the risk of introduction of dengue under different future scenarios (such as climate change and/or increased global mobility over the next decades) into Europe and develop predictive models to enhance Europe's preparedness for future dengue epidemics.
During the course of these 4 years, we will evaluate several novel tools and strategies that will have the potential to be scaled-up and, therefore, would need translation into public policy. Our consortium includes a health economist and policy analyst who will translate our research findings into evidence-informed policy making. Engagement with stakeholders and policy makers will start early in the project to ensure the potential for scaling up our recommendations.
DengueTools is funded under the Health theme of the Seventh Framework Programme of the European Community, Grant Agreement Number: 282589 Dengue Tools.
Conflict of interest and funding
A Wilder-Smith was the Principal Investigator of a Sanofi Pasteur funded Phase 2b vaccine trial for dengue. She has received speaker fees from GSK, Sanofi Pasteur and Novartis. Luke Alphey has employment and equity interest in Oxitec, Ltd. Oxitec is developing novel methods for the control of pest insects including Aedes aegypti and owns intellectual property relevant to the subject matter of this paper. Duane Gubler is on the scientific advisory boards of NITD, Inviragen, DVI, and ADITEC. He has received consultant fees from NITD and royalties and stock options from Inviragen. David Brooks works for TwistDx, a company that develops diagnostic assays. All other authors have no conflict of interest.
References
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002; 33: 330–42.
- Beatty ME, Letson GW, Margolis HS. Estimating the global burden of dengue. In: Abstract Book: Dengue 2008. The Second International Conference on Dengue and Dengue Haemorrhagic Fever, Phuket, Thailand.
- WHO. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: World Health Organization. 2009.
- Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop Med Health. 2011; 39: 1–9.
- Baggett HC, Graham S, Kozarsky PE, Gallagher N, Blumensaadt S, Bateman J, et al.. Pretravel health preparation among US residents traveling to India to VFRs: importance of ethnicity in defining VFRs. J Travel Med. 2009; 16: 112–8.
- Beatty ME, Beutels P, Meltzer MI, Shepard DS, Hombach J, Hutubessy R, et al.. Health economics of dengue: a systematic literature review and expert panel's assessment. Am J Trop Med Hyg. 2011; 84: 473–88.
- Wilder-Smith A, Gubler DJ. Geographic expansion of dengue: the impact of international travel. Med Clin North Am. 2008; 92: 1377–90.
- Wilder-Smith A, Ooi EE, Vasudevan SG, Gubler DJ. Update on dengue: epidemiology, virus evolution, antiviral drugs, and vaccine development. Curr Infect Dis Rep. 2010; 12: 157–64.
- Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010;4:e656. Review.
- Reiter P. Yellow fever and dengue: a threat to Europe?. Euro Surveill. 2010; 15: 19509.
- Gubler DJ. Prevention and control of Aedes aegypti-borne diseases: lesson learned from past successes and failures. AsPac J Mol Biol Biotechnol. 2011; 19: 111–4.
- Focks DA, Daniels E, Haile DG, Keesling JE. A simulation model of the epidemiology of urban dengue fever: literature analysis, model development, preliminary validation, and samples of simulation results. Am J Trop Med Hyg. 1995; 53: 489–506.
- Leitmeyer KC, Vaughn DW, Watts DM, Salas R, Villalobos I, de C, et al.. Dengue virus structural differences that correlate with pathogenesis. J Virol. 1999; 73: 4738–47.
- Runge-Ranzinger S, Horstick O, Marx M, Kroeger A. What does dengue disease surveillance contribute to predicting and detecting outbreaks and describing trends?. Trop Med Int Health. 2008; 13: 1022–41.
- De Simone TS, Nogueira RM, Araujo ES, Guimaraes FR, Santos FB, Schatzmayr HG, et al.. Dengue virus surveillance: the co-circulation of DENV-1, DENV-2 and DENV-3 in the State of Rio de Janeiro, Brazil. Trans R Soc Trop Med Hyg. 2004; 98: 553–62.
- Rico-Hesse R. Microevolution and virulence of dengue viruses. Adv Virus Res. 2003; 59: 315–41.
- Rosen L. The Emperor's New Clothes revisited, or reflections on the pathogenesis of dengue hemorrhagic fever. Am J Trop Med Hyg. 1977; 26: 337–43.
- Gubler DJ, Suharyono W, Lubis I, Eram S, Gunarso S. Epidemic dengue 3 in central Java, associated with low viremia in man. Am J Trop Med Hyg. 1981; 30: 1094–9.
- Holmes EC, Twiddy SS. The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol. 2003; 3: 19–28.
- Knols BGJ, van Loon JJA, Cork A, Robinson RD, Adam W, Meijerink J, et al.. Behavioural and electrophysiological responses of the female malaria mosquito Anopheles gambiae (Diptera: Culicidae) to Limburger cheese volatiles. Bull Entomol Res. 1997; 87: 151–9.
- Okumu FO, Killeen GF, Ogoma S, Biswaro L, Smallegange RC, Mbeyela E, et al.. Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PLoS One. 2010; 5: e8951.
- Reiter P. A portable, battery-powered trap for collecting gravid Culex mosquitoes. Mosq News. 1983; 4: 496–8.
- Jackson BT, Paulson SL, Youngman RR, Scheffel SL, Hawkins B. Oviposition preferences of Culex restuans and Culex pipiens (Diptera: Culicidae) for selected infusions in oviposition traps and gravid traps. J Am Mosq Control Assoc. 2005; 21: 360–5.
- Sant'ana AL, Roque RA, Eiras AE. Characteristics of grass infusions as oviposition attractants to Aedes (Stegomyia) (Diptera: Culicidae). J Med Entomol. 2006; 43: 214–20.
- Ganesan K, Mendki MJ, Suryanarayana MVS, Prakash S, Malhotra RC. Studies of Aedes aegypti (Diptera: Culicidae) ovipositional responses to newly identified semiochemicals from conspecific eggs. Aust J Entomol. 2006; 45: 75–80.
- Sharma K, Seenivasagan T, Rao A, Ganesan K, Agarwal O, Malhotra R, et al.. Oviposition responses of Aedes aegypti and Aedes albopictus to certain fatty acid esters. Parasitol Res. 2008; 103: 1065–73.
- Muller O, Traore C, Kouyate B, Ye Y, Frey C, Coulibaly B, et al.. Effects of insecticide-treated bednets during early infancy in an African area of intense malaria transmission: a randomized controlled trial. Bull World Health Organ. 2006; 84: 120–6.
- Kroeger A, Lenhart A, Ochoa M, Villegas E, Levy M, Alexander N, et al.. Effective control of dengue vectors with curtains and water container covers treated with insecticide in Mexico and Venezuela: cluster randomised trials. BMJ. 2006; 332: 1247–52.
- Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002; 2: 33–42.
- Hammond SN, Balmaseda A, Perez L, Tellez Y, Saborio SI, Mercado JC, et al.. Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg. 2005; 73: 1063–70.
- Clark DV, Mammen MP Jr, Nisalak A, Puthimethee V, Endy TP. Economic impact of dengue fever/dengue hemorrhagic fever in Thailand at the family and population levels. Am J Trop Med Hyg. 2005; 72: 786–91.
- Anderson KB, Chunsuttiwat S, Nisalak A, Mammen MP, Libraty DH, Rothman AL, et al.. Burden of symptomatic dengue infection in children at primary schools in Thailand: a prospective study. Lancet. 2007; 369: 1452–9.
- Wilder-Smith A, Lover A, Kittayapong P, Burnham G. Hypothesis: impregnated school uniforms reduce the incidence of dengue infections in school children. Med Hypotheses. 2011; 76: 861–2.
- Wilder-Smith A, Schwartz E. Dengue in travelers. N Engl J Med. 2005; 353: 924–32.