Abstract
Background
Rift Valley fever (RVF) is a mosquito-borne viral zoonosis that primarily affects ruminants but also has the capacity to infect humans.
Objective
To determine the abundance and distribution of mosquito vectors in relation to their potential role in the virus transmission and maintenance in disease epidemic areas of Ngorongoro district in northern Tanzania.
Methods
A cross-sectional entomological investigation was carried out before the suspected RVF outbreak in October 2012. Mosquitoes were sampled both outdoors and indoors using the Centre for Disease Control (CDC) light traps and Mosquito Magnets baited with attractants. Outdoor traps were placed in proximity with breeding sites and under canopy in banana plantations close to the sleeping places of animals.
Results
A total of 1,823 mosquitoes were collected, of which 87% (N=1,588) were Culex pipiens complex, 12% (N=226) Aedes aegypti, and 0.5% (N=9) Anopheles species. About two-thirds (67%; N=1,095) of C. pipiens complex and nearly 100% (N=225) of A. aegypti were trapped outdoors using Mosquito Magnets. All Anopheles species were trapped indoors using CDC light traps. There were variations in abundance of C. pipiens complex and A. aegypti among different ecological and vegetation habitats. Over three quarters (78%) of C. pipiens complex and most (85%) of the A. aegypti were trapped in banana and maize farms. Both C. pipiens complex and A. aegypti were more abundant in proximity with cattle and in semi-arid thorn bushes and lower Afro-montane. The highest number of mosquitoes was recorded in villages that were most affected during the RVF epidemic of 2007. Of the tested 150 pools of C. pipiens complex and 45 pools of A. aegypti, none was infected with RVF virus.
Conclusions
These results provide insights into unique habitat characterisation relating to mosquito abundances and distribution in RVF epidemic-prone areas of Ngorongoro district in northern Tanzania.
Rift Valley fever (RVF) is a mosquito-borne viral infection of both ruminants and humans (Citation1, Citation2). The disease occurs as intermittent epidemics with intervals of 10–15 years, mainly after periods of exceptionally heavy rainfall (Citation3). In Tanzania, major outbreaks were reported in Ngorongoro district in 1997–1998 and 2006–2007 causing massive abortions and deaths in livestock. The 2006–2007 outbreak was reported in 25 districts in Arusha, Manyara, Kilimanjaro, Tanga, Dodoma, Iringa, and Morogoro regions of the country causing 144 human deaths (Citation4, Citation5). Previous studies in other RVF outbreak areas indicated that RVF epidemics are associated with the distribution and increased population of mosquito vectors Citation6–(Citation10) .
Several mosquito species are able to act as vectors for transmission of the RVF virus Citation11–(Citation17) . However, the dominant vector species varies between different regions, and different species can play different roles in sustaining the transmission of the virus. Studies of mosquito vectors in north-eastern Kenya have shown that Anopheles squamosus, Aedes ochraceus, and Aedes mcintoshi play major roles as local important vectors Citation10–(Citation12, Citation18). Many mosquito species have demonstrated the capability to transmit the virus to animals (Citation6, Citation7) (Citation19, Citation20). Culex pipiens complex and Aedes aegypti are the major RVF mosquito vectors located in many disease endemic areas Citation21–(Citation27) . The RVF virus is spread primarily by the bite of infected mosquitoes, mainly the C. pipiens complex and Aedes species, which can acquire the virus from feeding on infected animals. The Aedes female mosquito is also capable of transmitting the virus trans-ovarially to her offspring via eggs leading to new generations of infected mosquitoes hatching from eggs. The RVF virus persists trans-ovarially within mosquito eggs that can survive for several years in dry conditions (Citation18).
Attempts to implement early-warning systems and effective surveillance strategies for epidemics require an understanding of the abundance and distribution of mosquito vectors with transmission patterns of the disease. However, this has often been hindered by a lack of reliable information on mosquito vectors responsible for the occurrence and persistence of the disease during epidemic and inter-epidemic periods (IEP). In Tanzania, like in many parts of Africa, little is known about RVF mosquito vectors abundance and transmission intensities (Citation7). It was the objective of this study to determine the abundance and distribution of mosquito vectors in relation to their potential role in the RVF virus transmission and maintenance in disease epidemic areas of Ngorongoro district in northern Tanzania.
Materials and methods
Study area
This study was carried out in Ngorongoro district (2°S45′50.4″, 35°E34′04.8″) in northern Tanzania ( and ). The district is within the Serengeti–Masai Mara ecosystem defined by the limits of the annual wildlife migration. It represents a unique interaction between livestock, wildlife, and humans while involving animal migration from neighbouring Kenya, which has experienced similar RVF outbreaks since the 1930s. The district has been described as the main RVF hotspot area during 2006–2007 (Citation5).
Fig. 1 Map of Ngorongoro district indicating sites where mosquito collections were done and habitat characteristics such as vegetation features.
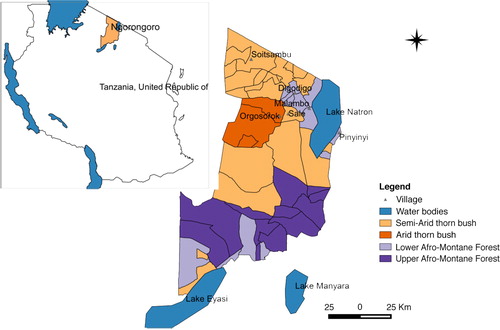
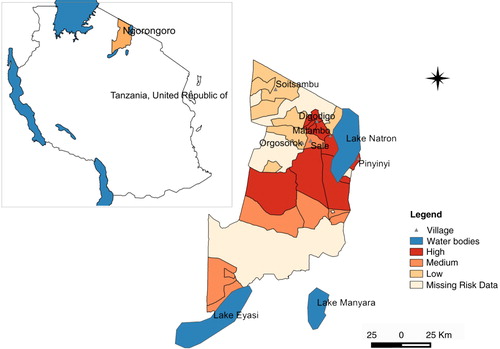
Fig. 2 Map of Ngorongoro district indicating level of impact due to RVF epidemic in 2006–2007 as distributed in three levels of risk, high, medium, and low.
The study area falls under a semi-arid rangeland area in the Rift Valley having bimodal rainfall with a long rainy season in March–May and a short rainy season (October–December). The total amount of annual rainfall ranges from 700 to 1,800 mm with a mean monthly temperature of 19°C. The district has extreme habitat diversity comprising areas of subalpine pasture at 4,000 m, montane evergreen forest, arid thorn bush, and treeless short grass plains at 1,800 m. The vegetation mainly consists of various shrubs and acacia bushes. Livestock species kept are primarily cattle, goats, sheep, and donkeys. This study involved mosquito collection in six villages namely Orgosorok, Soitsambu, Digodigo, Malambo, Sale, and Pinyinyi.
Mosquito collection
Mosquito collection was carried out in the dry season from September to October 2012 before the suspected RVF outbreak (Citation28). All outbreaks in East Africa have been reported to occur following periods of abnormal drought, followed by abnormal heavy rains and the consequent emergence of large numbers of Aedes and Culex mosquitoes (Citation18, Citation29–Citation31) . Three Mosquito Magnets and three Centre for Disease Control (CDC) light traps were set for three consecutive days both outdoors and indoors. Outdoor mosquito collections were made using either unbaited or Octenol-baited Mosquito Magnets (Cordless LibertyPlus) and Carbon dioxide-baited CDC light traps (John W. Hock Company, Gainesville, FL, USA). Indoor mosquito collections were made using unbaited CDC light traps. Traps were set using the procedure previously described in other studies Citation32–(Citation34) . Outdoor traps were placed in proximity with potential breeding sites and under canopy in banana plantations and in proximity to animal shelters.
Habitat characteristics data such as topography and vegetation types were recorded and observed during the study period for each village surveyed.
Mosquito identification
Using a microscope, mosquitoes were identified to genus or species level using morphological keys (Citation35, Citation36). Due to intraspecific variability of morphometrical characteristics, inconsistencies in original descriptions of morphological characters, the small size of the structures being observed under the light microscope that would require identification using molecular techniques, all Culex species were grouped together as C. pipiens complex to possibly include C. pipiens pipiens, C. pipiens quinquefasciatus, C. pipiens torrentium, C. pipiens molestus, and so on. Mosquitoes that could not be identified morphologically directly in the field due to minor specimen damage such as a missing leg or wing were sent to the Amani Research Centre of the National Institute for Medical Research (NIMR) for further identification assistance. Mosquitoes were then killed and kept on ice during transportation to the laboratory.
Molecular detection of RVF virus
Collected mosquitoes were kept on ice initially and then transported to the laboratory for RNA extraction. Transportation of mosquito samples from field to laboratory was done after every 7–12 days. Pools of 5–10 mosquitoes belonging to the same species were ground before RNA was extracted using TRIzol® viral RNA/DNA kit (Invitrogen, Corp), following manufacturer's instructions. Extracted RNA kept in RNase-free water and stored at −20°C was used in real-time reverse transcription polymerase chain reaction (qRT-PCR) for RVF virus detection. The LightMix qRT-PCR kits for RVF virus and Light Cycler FastStart DNA Master HybProbe reaction mix (Roche) was used for the qRT-PCR with the Light Cycler 2.0 thermocycler (Roche) for all samples (Citation37, Citation38).
Data analysis
Data was entered and summarised in Microsoft Excel spread sheets. Mosquito abundance was calculated as the number of mosquitoes collected in each village. Mosquito abundance was compared with area, which had a high RVF risk level for the 2006–2007 epidemics. The Chi-square test was used to test significance of the differences in abundance according to the characteristics of the mosquito collection site, vegetation type in proximity, and topographical characteristics of the village with a risk level according to the 2006–2007 epidemics.
Ethics considerations
Ethical clearance to conduct this study was obtained from the Medical Research Coordinating Committee (MRCC) of the Tanzania National Institute for Medical Research. Village leaders and house residents were sensitised and asked for their permission before installation of mosquito traps in their houses or premises.
Results
Mosquito abundance
A total of 1,823 mosquitoes were collected, of these 87% (N=1,588) were C. pipiens complex, 12% (N=226) A. aegypti, and 0.5% (N=9) Anopheles species. About two-thirds (67%; N=1,095) of C. pipiens complex and nearly 100% (N=225) of A. aegypti were trapped outdoors mainly using Mosquito Magnets. A. aegypti was found in four of the study sites: Digodigo (17% of 803), Malambo (13% of 179), Pinyinyi (16% of 338), and Sale (6% of 198); however, no A. aegypti was collected from Orgosorok and Soitsambu villages (– ). In Digodigo village, C. pipiens complex comprised 82% of the 803 total mosquitoes, Malambo 87% of 179, Orgosorok 100% of 264, Pinyinyi 83% of 338, Sale 94% of 198, and in Soitsambu 100% of the 41. All Anopheles species were trapped indoor using CDC light traps in Digodigo (0.6%; N=5) and Pinyinyi (1%; N=4), respectively. No Anopheles species were found in Malambo, Orgosorok, Sale, or Soitsambu. Of the two trapping methods used in this study, the use of Mosquito Magnets (either unbaited or baited with Octenol) captured the largest proportion of C. pipiens complex and A. aegypti. Although CDC light traps set indoor showed a low efficiency in catching A. aegypti, it was more efficient in capturing C. pipiens complex.
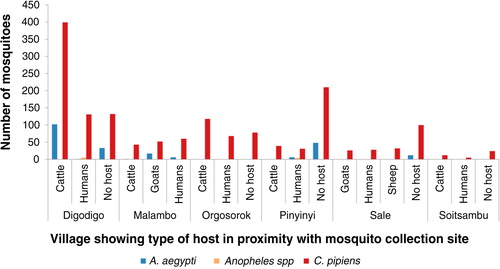
Fig. 3 Mosquito abundance in villages according to characteristics of the mosquito collection sites.
MF=maize farm; BP=banana plantation; DWS=drinking water source; NVBS=no visible breeding site; RS=river site.
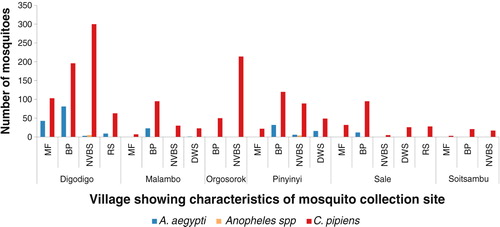
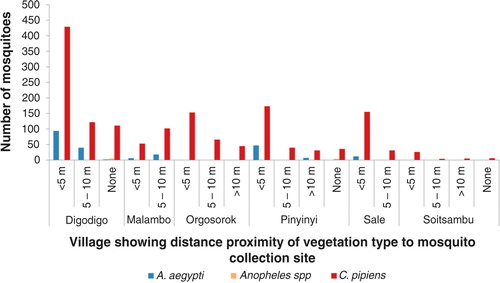
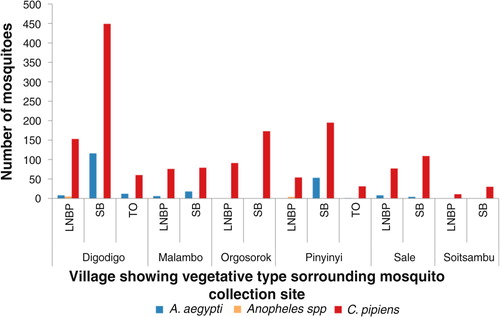
Fig. 4 Mosquito abundance in villages according to vegetation type in proximity with mosquito sampling sites.
LNBP =little or no present; SB=shrubs/bushes; TO=trees overhanging.
Mosquito distribution
Variations in abundance of C. pipiens complex and A. aegypti among different ecological and vegetation habitats were observed. Over three quarters (78%; N=1,399) of C. pipiens complex were trapped in banana and maize farms and areas without visible breeding sites in all villages. Most (85%; N=191) of A. aegypti were mainly trapped in banana and maize farms in Digodigo, Malambo, and Sale villages. About two-thirds (65%; N=1,035) of C. pipiens complex and 70% (N=159) of A. aegypti were trapped in areas with shrubs and bushes, regardless of the proximity of livestock within the areas (– ). Anopheles species were trapped in areas without visible breeding sites or without clear vegetation type but indoor in proximity to human hosts.
Large numbers of A. aegypti were collected in semi-arid thorn bushes and lower Afro-montane forests of Digodigo, Malambo, and Pinyinyi villages than in arid thorn bushes areas. These three villages were the most affected areas during the 2006–2007 RVF outbreak in Ngorongoro district ( and ). No statistically significant difference in abundance (p>0.05) was found between types of vegetation for C. pipiens complex.
RVF virus detection
We tested a total of 150 pools of C. pipiens complex and 45 pools of A. aegypti organised by village and site of collection. No RVF virus was detected in any of the pools.
Discussion
RVF epidemics have been correlated with complex processes including roles of known and unknown mosquito vectors, habitat characteristics, ecological and topographical features of vector communities, and seasonal- or climate-related vector abundances (Citation8, Citation15) (Citation29, Citation30) (Citation39). In this study, the relationship between abundance of potential mosquito vectors across habitat and vegetation types experiencing different levels of RVF epidemic in Ngorongoro district was examined. Most of the recent field-based studies on RVF in Tanzania during IEP focussed mainly on the role of virus antibodies within humans, livestock, and wildlife populations (Citation5, Citation40–Citation44) . Findings of the current study show that close examination of areas favouring the emergence of massive mosquito abundance can significantly influence the emergence of disease epidemics.
Banana plantation habitats located close to human premises or animal sleeping areas were the most suitable areas for effective outdoor sampling of large numbers of mosquitoes. This distribution pattern indicates some habitat characteristic implications related to the epidemiological impact because they express the proximity of these species to humans and animals in order to effectively transmit the virus. These findings provide an important step in identifying potential areas which might influence host–vector interactions and ultimately the emergence of RVF epidemics. As data for this study were conducted during the dry season, these areas are likely to be the areas with favourable conditions for the breeding of mosquitoes especially A. aegypti. However, other studies (Citation45) have reported that A. aegypti does not exclusively depend on the breeding grounds that emerge during the rainy period, thereby enabling this mosquito to maintain its life cycle during the dry period. Results from this study confirm findings from our previous study in the area (Citation46) and other studies in Brazil and Italy (Citation39, Citation47).
Findings from this study show that there are associations between villages that experienced previous RVF epidemics with abundance and distribution of potential mosquito vectors. Malambo and Pinyinyi villages were the most hit by the 2006–2007 RVF epidemics followed by Digodigo (Citation5, Citation42). Based on our field-based observations, these villages have shown characteristic vegetation types that could predispose environmental conditions favouring major RVF epidemics and as a source of spread of infection to different areas (Citation48). This abundance–distribution phenomenon could also explain that the disease does not spread like other classical contagious diseases but with some spreading mechanism involving animal movements locally or from neighbouring endemic countries. Since the first virus description in sheep in 1930 (Citation1), RVF epidemics have been occurring in geographically limited areas with characteristic features and vegetation types (Citation46). This study emphasizes further efforts to consider local-based studies of the role of mosquito abundances as a risk for disease epidemics.
In this study, we were unable to demonstrate virus activity within mosquitoes collected despite sampling being done immediately before the short rainy season. Sampling during this dry season led to a relatively small number of mosquitoes collected compared to the rainy season. Detection of RVF virus activity in mosquitoes and animals is a challenge as it requires the presence of large numbers of vectors, and the potential for this to occur is clearly limited (Citation11, Citation18). It has been difficult to detect a virus in mosquitoes during IEP in Tanzania despite detection of a virus antibody among livestock and wildlife populations (Citation4). Interestingly, it has been possible to detect the virus during IEP in A. mcintoshi after artificial flooding in Kenya (Citation18). Despite unsuccessful detection of a virus, this attempt to examine virus infectivity with mosquito vectors could still add value to understanding the role of mosquitoes in the maintenance and persistence of the disease during IEP in relation to suitable sites.
Studies on vectors of RVF virus during IEP face many challenges and limitations. Lack of persistent water flooded areas during dry seasons may be an important factor in limiting the number and composition of potential RVF vectors. Absence of virus activity in this period is one of the limitations to conduct studies. Our sampling approach mainly focussed on potential vectors for RVF and on specific habitats such as banana plantations. Literature reviews suggest that attempted flooding can reveal more diversity of mosquito species relative to their abundance in disease epidemics hotspots such as Ngorongoro district (Citation11, Citation18).
Conclusions
To our knowledge, results presented here provide insights into unique habitat characterisation relating vector abundances and distribution in Ngorongoro district of northern Tanzania. Future studies should investigate long-term seasonal vector abundance in other locally known disease epidemic sites by taking into consideration the structure of the ecology and habitat distribution. Such studies could add value to strategic surveillance and control of Rift Valley fever in endemic areas.
Conflicts of interest and funding
The authors are not aware of any commercial associations or biases that might be perceived as affecting the objectivity of this research work.
Acknowledgements
We are very grateful to all the people who helped us with mosquito collection in the field especially Fransis Mwakyoma. Thanks to Joseph Myamba and Bernard Batengana of Amani Research Centre for their assistance in mosquito identification. We would like to thank the Executive Director, Veterinary Officer, and Medical officer for Ngorongoro district for logistic support. This study received financial assistance from Health Research Users Trust Fund of the National Institute for Medical Research.
References
- Daubney R, Hudson JR, Garnham PC. Enzootic hepatitis or Rift Valley fever: an undescribed disease of sheep, cattle and man from east Africa. J Pathol Bacteriol. 1931; 34: 545–79.
- Davies FG. Observations on the epidemiology of Rift Valley fever in Kenya. J Hyg (Lond). 1975; 75: 219–30. [PubMed Abstract] [PubMed CentralFull Text].
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002; 33: 330–42. [PubMed Abstract].
- Chengula AA, Mdegela RH, Kasanga CJ. Socio-economic impact of Rift Valley fever to pastoralists and agro pastoralists in Arusha, Manyara and Morogoro regions in Tanzania. Springerplus. 2013; 2: 549. [PubMed Abstract] [PubMed CentralFull Text].
- Mohamed M, Mosha F, Mghamba J, Zaki SR, Shieh WJ, Paweska J, etal. Epidemiologic and clinical aspects of a Rift Valley fever outbreak in humans in Tanzania, 2007. Am J Trop Med Hyg. 2010; 83: 22–7. [PubMed Abstract] [PubMed CentralFull Text].
- EFSA. Scientific opinion on Rift Valley fever; EFSA Panel on Animal Health and Welfare (AHAW). EFSA J. 2013; 11: 3180.
- Rolin AI, Berrang-Ford L, Kulkarni MA. The risk of Rift Valley fever virus introduction and establishment in the United States and European Union. Emerg Microbes Infect. 2013; 2: 81.
- Lutomiah J, Bast J, Clark J, Richardson J, Yalwala S, Oullo D, etal. Abundance, diversity, and distribution of mosquito vectors in selected ecological regions of Kenya: public health implications. J Vector Ecol. 2013; 38: 134–42. [PubMed Abstract].
- Ochieng C, Lutomiah J, Makio A, Koka H, Chepkorir E, Yalwala S, etal. Mosquito-borne arbovirus surveillance at selected sites in diverse ecological zones of Kenya; 2007–2012. Virol J. 2013; 10: 140. [PubMed Abstract] [PubMed CentralFull Text].
- Sang R, Kioko E, Lutomiah J, Warigia M, Ochieng C, O'Guinn M, etal. Rift Valley fever virus epidemic in Kenya, 2006/2007: the entomologic investigations. Am J Trop Med Hyg. 2010; 83: 28–37. [PubMed Abstract] [PubMed CentralFull Text].
- LaBeaud AD, Sutherland LJ, Muiruri S, Muchiri EM, Gray LR, Zimmerman PA, etal. Arbovirus prevalence in mosquitoes, Kenya. Emerg Infect Dis. 2011; 17: 233–41. [PubMed Abstract] [PubMed CentralFull Text].
- Sang RC, Dunster LM. The growing threat of arbovirus transmission and outbreaks in Kenya: a review. East Afr Med J. 2001; 78: 655–61. [PubMed Abstract].
- Turell MJ, Lee JS, Richardson JH, Sang RC, Kioko EN, Agawo MO, etal. Vector competence of Kenyan Culex zombaensis and Culex quinquefasciatus mosquitoes for Rift Valley fever virus. J Am Mosq Control Assoc. 2007; 23: 378–82. [PubMed Abstract].
- Turell MJ, Linthicum KJ, Patrican LA, Davies FG, Kairo A, Bailey CL. Vector competence of selected African mosquito (Diptera: Culicidae) species for Rift Valley fever virus. J Med Entomol. 2008; 45: 102–8. [PubMed Abstract].
- Wilson ML. Rift Valley fever virus ecology and the epidemiology of disease emergence. Ann N Y Acad Sci. 1994; 740: 169–80. [PubMed Abstract].
- Wilson ML, Chapman LE, Hall DB, Dykstra EA, Ba K, Zeller HG, etal. Rift Valley fever in rural northern Senegal: human risk factors and potential vectors. Am J Trop Med Hyg. 1994; 50: 663–75. [PubMed Abstract].
- Worth CB, de Meillon. Culicine mosquitoes (Diptera: Culicidae) recorded from the province of Mozambique (Portuguese East Africa) and their relationship to arthropod-borne viruses. An Inst Med Trop (Lisb). 1960; 231–56.
- Linthicum KJ, Davies FG, Kairo A, Bailey CL. Rift Valley fever virus (family Bunyaviridae, genus Phlebovirus). Isolations from Diptera collected during an inter-epizootic period in Kenya. J Hyg (Lond). 1985; 95: 197–209. [PubMed Abstract] [PubMed CentralFull Text].
- Fontenille D, Traore-Lamizana M, Diallo M, Thonnon J, Digoutte JP, Zeller HG. New vectors of Rift Valley fever in West Africa. Emerg Infect Dis. 1998; 4: 289–93. [PubMed Abstract] [PubMed CentralFull Text].
- Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010; 41: 61. [PubMed Abstract] [PubMed CentralFull Text].
- Hassan OA, Ahlm C, Sang R, Evander M. The 2007 Rift Valley fever outbreak in Sudan. PLoS Negl Trop Dis. 2011; 5: e1229. [PubMed Abstract] [PubMed CentralFull Text].
- Jupp PG, Cornel AJ. Vector competence tests with Rift Valley fever virus and five South African species of mosquito. J Am Mosq Control Assoc. 1988; 4: 4–8. [PubMed Abstract].
- Kreher F, Tamietti C, Gommet C, Guillemot L, Ermonval M, Failloux AB, etal. The Rift Valley fever accessory proteins NSm and P78/NSm-GN are distinct determinants of virus propagation in vertebrate and invertebrate hosts. Emerg Microbes Infect. 2014; 3: e71. [PubMed CentralFull Text].
- Le CA, Babin D, Fiette L, Jouvion G, Ave P, Misse D, etal. Aedes mosquito saliva modulates Rift Valley fever virus pathogenicity. PLoS Negl Trop Dis. 2013; 7: e2237.
- Meegan JM, Khalil GM, Hoogstraal H, Adham FK. Experimental transmission and field isolation studies implicating Culex pipiens as a vector of Rift Valley fever virus in Egypt. Am J Trop Med Hyg. 1980; 29: 1405–10. [PubMed Abstract].
- Moutailler S, Krida G, Schaffner F, Vazeille M, Failloux AB. Potential vectors of Rift Valley fever virus in the Mediterranean region. Vector Borne Zoonotic Dis. 2008; 8: 749–53. [PubMed Abstract].
- WHO. Growing threat of viral hemorrhagic fevers in the Eastern Mediterranean Region: a call for action. 2007. Regional Committee for the Eastern Mediterranean September 2007. EM/RC54/5. Available from: http://apps.who.int/iris/handle/10665/122585 [cited 8 November 2014]..
- FAO. Rift Valley fever. Vigilance needed in the coming months. Food and Agriculture Organization of the United Nations (FAO). 2012. Rome: EMPRES WATCH.
- Mboera LEG, Mayala BK, Kweka EJ, Mazigo HD. Impact of climate change on human health and health systems in Tanzania: a review. Tanzan J Health Res. 2011; 1–23.
- Linthicum KJ, Anyamba A, Tucker CJ, Kelley PW, Myers MF, Peters CJ. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science. 1999; 285: 397–400. [PubMed Abstract].
- Hightower A, Kinkade C, Nguku PM, Anyangu A, Mutonga D, Omolo J, etal. Relationship of climate, geography, and geology to the incidence of Rift Valley fever in Kenya during the 2006–2007 outbreak. Am J Trop Med Hyg. 2012; 86: 373–80. [PubMed Abstract] [PubMed CentralFull Text].
- Mweya CN, Kimera SI, Karimuribo ED, Mboera LEG. Comparison of sampling techniques for Rift Valley Fever virus potential vectors, Aedes aegypti and Culex pipiens complex, in Ngorongoro District in northern Tanzania. Tanzan J Health Res. 2013; 15: 1–7.
- Kitau J, Pates H, Rwegoshora TR, Rwegoshora D, Matowo J, Kweka EJ, etal. The effect of Mosquito Magnet Liberty Plus trap on the human mosquito biting rate under semi-field conditions. J Am Mosq Control Assoc. 2010; 26: 287–94. [PubMed Abstract].
- Mboera LEG, Kihonda J, Braks MA, Knols BG. Short report: influence of centers for disease control light trap position, relative to a human-baited bed net, on catches of Anopheles gambiae and Culex quinquefasciatus in Tanzania. Am J Trop Med Hyg. 1998; 59: 595–6. [PubMed Abstract].
- Huang YM. A pictorial key for the identification of the subfamilies of Culicidae, genera of Culicinae, and subgenera of Aedes mosquitoes of the Afrotropical Region (Diptera: Culicidae). Proc Entomol Soc Wash. 2001; 103: 1–53.
- Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region). 1987; Johannesburg, South Africa: South African Institute for Medical Research.
- Euler M, Wang Y, Nentwich O, Piepenburg O, Hufert FT, Weidmann M. Recombinase polymerase amplification assay for rapid detection of Rift Valley fever virus. J Clin Virol. 2012; 54: 308–12. [PubMed Abstract].
- Weidmann M, Sanchez-Seco MP, Sall AA, Ly PO, Thiongane Y, Lo MM, etal. Rapid detection of important human pathogenic Phleboviruses. J Clin Virol. 2008; 41: 138–42. [PubMed Abstract].
- Serpa LL, Marques GRM, de Lima AP, Voltolini JC, Arduino MB, Barbosa GL, etal. Study of the distribution and abundance of the eggs of Aedes aegypti and Aedes albopictus according to the habitat and meteorological variables, municipality of Sao Sebastiao, Sao Paulo State, Brazil. Parasit Vectors. 2013; 6: 321. [PubMed Abstract] [PubMed CentralFull Text].
- Chengula AA, Kasanga CJ, Mdegela RH, Sallu R, Yongolo M. Molecular detection of Rift Valley fever virus in serum samples from selected areas of Tanzania. Trop Anim Health Prod. 2014; 46: 629–34. [PubMed Abstract].
- Lagerqvist N, Moiane B, Mapaco L, Fafetine J, Vene S, Falk KI. Antibodies against Rift Valley fever virus in cattle, Mozambique. Emerg Infect Dis. 2013; 19: 1177–9. [PubMed Abstract] [PubMed CentralFull Text].
- Sindato C, Swai ES, Karimuribo ED, Dautu G, Pfeiffer DU, Mboera LEG, etal. Spatial distribution of non-clinical Rift Valley fever viral activity in domestic and wild ruminants in northern Tanzania. Tanzania Vet J. 2013; 28: 21–38.
- Sumaye RD, Geubbels E, Mbeyela E, Berkvens D. Inter-epidemic transmission of Rift Valley fever in livestock in the Kilombero River Valley, Tanzania: a cross-sectional survey. PLoS Negl Trop Dis. 2013; 7: e2356. [PubMed CentralFull Text].
- Swai ES, Schoonman L. Prevalence of Rift Valley fever immunoglobulin G antibody in various occupational groups before the 2007 outbreak in Tanzania. Vector Borne Zoonotic Dis. 2009; 9: 579–82. [PubMed Abstract].
- Souza SS, Silva IG, Silva HH. Association between dengue incidence, rainfall and larval density of Aedes aegypti, in the State of Goias]. Rev Soc Bras Med Trop. 2010; 43: 152–5. [PubMed Abstract].
- Mweya CN, Kimera SI, Kija JB, Mboera LEG. Predicting distribution of Aedes aegypti and Culex pipiens complex, potential vectors of Rift Valley fever virus in relation to disease epidemics in East Africa. Infect Ecol Epidemiol. 2013; 3: 1–7. http://dx.doi.org/10.3402/iee.v3i0.21748..
- Montarsi F, Martini S, Dal PM, Delai N, Ferro MN, Mazzucato M, etal. Distribution and habitat characterization of the recently introduced invasive mosquito Aedes koreicus [Hulecoeteomyia koreica], a new potential vector and pest in north-eastern Italy. Parasit Vectors. 2013; 6: 292. [PubMed Abstract] [PubMed CentralFull Text].
- Mweya CN, Holst N, Mboera LE, Kimera SI. Simulation modelling of population dynamics of mosquito vectors for Rift Valley fever virus in a disease epidemic setting. PLoS One. 2014; 9: e108430. [PubMed Abstract] [PubMed CentralFull Text].