Abstract
Objective
Verbal autopsies (VAs) are interviews with a relative or friend of the deceased; VAs are a technique used in surveillance sites in many countries with incomplete death certification. The goal of this study was to assess the accuracy and validity of data on HIV status and antiretroviral therapy (ART) usage reported in VAs and their influence on physician attribution of cause of death.
Design
This was a prospective cohort study.
Methods
The Karonga Health and Demographic Surveillance Site monitors demographic events in a population in a rural area of northern Malawi; a VA is attempted on all deaths reported. VAs are reviewed by clinicians, who, with additional HIV test information collected pre-mortem, assign a cause of death. We linked HIV/ART information reported by respondents during adult VAs to database information on HIV testing and ART use and analysed agreement using chi-square and kappa statistics. We used multivariable logistic regression to analyse factors associated with agreement.
Results
From 2003 to 2014, out of a total of 1,952 VAs, 80% of respondents reported the HIV status of the deceased. In 2013–2014, this figure was 99%. Of those with an HIV status known to the study, there was 89% agreement on HIV status between the VA and pre-mortem data, higher for HIV-negative people (92%) than HIV-positive people (83%). There was 84% agreement on whether the deceased had started ART, and 72% of ART initiation dates matched within 1 year.
Conclusions
In this population, HIV/ART information was often disclosed during a VA and matched well with other data sources. Reported HIV/ART status appears to be a reliable source of information to help classification of cause of death.
Introduction
The WHO recommends collecting data on HIV/AIDS history in verbal autopsies (VAs) (Citation1), but there have been few published studies of validation of HIV status reported by a proxy after the person has died. VAs are used to assign likely cause of death (Citation2) for programmatic purposes in surveillance sites in many low-income settings where most deaths are not registered or attended by a medical professional. In some research settings, such as our own, the VA can provide an additional source of information on HIV and antiretroviral therapy (ART) in the community for use in analyses of demography and mortality. The HIV prevalence in Malawi was estimated to be 10.6% in 2010 (Citation3), and several sites in the country have used VAs (Citation4–Citation6).
A VA is an interview with a relative or friend who nursed the deceased (or was present) during most of their final illness, with open-ended and structured questions about signs and symptoms, pre-existing conditions, and treatments. Likely causes of death are assigned by physicians (Citation7) or computer models (e.g. InterVA or InSilicoVA) (Citation8). As may be expected, VAs compare well to hospital diagnoses (Citation9) but less well with physical autopsies (Citation10). VAs have been validated to identify HIV/AIDS deaths using symptoms alone (Citation11), but assigning cause of death in HIV-positive people is becoming more complex. Increasingly widespread ART usage and prolonged duration on treatment is changing the course of the illness and increasing the range of non-specific symptoms relating to side-effects and acceleration of degenerative conditions, which are also seen in HIV-negative people. In addition to the respondent's account of the deceased's illness, any information from the deceased's medical records, including HIV status and use of ART, is considered useful to physicians when assigning the cause of death; HIV diagnosis is also a variable used to assign cause of death in computer models (Citation12). In many settings where VAs are used, obtaining official medical information/history may be difficult due to lack of resources to attend clinics and search through the records, as well as poor record-keeping (Citation13). In our setting in Malawi, personal health records are held by the families, though in 2014 the deceased's health record was only available in 41% of VAs. Often the only information available is from the VA respondent.
In many communities, despite rapidly increasing availability of ART, HIV is still surrounded by stigma (Citation14), making disclosure of a positive HIV status challenging (Citation15). Predictors of disclosure do not follow clear trends (Citation15). People are more likely to disclose a negative status than a positive one (Citation16) but are more likely to disclose their positive status to their families when they are ill and indeed may not have any choice in the matter during a severe or terminal illness (Citation17). Relatives or friends may also make assumptions about a person's HIV status, based on their appearance, clinic attendance, past perceived sexual behaviour, or knowledge of the HIV status of a living or deceased spouse (Citation18). Onward disclosure outside the family, even after the person has died and during a confidential interview, may not be socially or culturally acceptable.
Given these issues, it is important to know whether HIV/ART information reported in a VA can be relied upon to assist in assigning likely cause of death, evaluating ART programme success, and contributing data to other community-level analyses.
In the Karonga Health and Demographic Surveillance Site (HDSS) in Malawi, data on HIV status (actual test records and self-report) and data on use of ART (clinic records and self-report) can be linked, through personal identifiers that have been assigned to consenting individuals, to VA data, which have been systematically collected since 2003. We compared data from these pre-mortem sources to estimate the reliability of VA respondent reports of HIV status and ART usage, as well as their influence on cause of death assignment.
Methods
Population and data
Population
The Karonga HDSS is well established (Citation19). In brief, a population of about 39,000 people living in rural villages in northern Malawi has been under continuous surveillance since 2002. Births and deaths are captured monthly, and in- and out-migrations annually. The HDSS is run by the Karonga Prevention Study (KPS), which conducts many multi-round surveys (including on socio-economic status and HIV sero-surveys) and evaluates the impact of interventions (e.g. ART), using the HDSS as a sampling frame. The Karonga HDSS is a member of the ALPHA network for analysis of longitudinal, population-based HIV/AIDS data in Africa (alpha.lshtm.ac.uk) and the INDEPTH network (indepth-network.org).
VA data
Following the report of a death, a VA is carried out by a KPS medical assistant, usually between 2 and 6 weeks after the death. The questionnaire, which has closed and open-ended questions, has changed over time but is based on the WHO standard VA questionnaire (Citation1). Since 2003, respondents have been asked about the presence of disease symptoms and medical history, including whether the deceased had ever been diagnosed with HIV/AIDS; in 2009 more detailed questions were added about dates of HIV testing and ART usage.
Each completed VA questionnaire is reviewed by two clinicians, who independently assign a likely cause of death; if they do not agree, a third reviewer (a physician) makes the decision. A three-level hierarchical system of coding is used. As it is frequently not possible to distinguish between AIDS and TB deaths, such deaths are coded as follows: Level 1 (broad group), communicable disease; Level 2 (sub-group), AIDS/TB; Level 3 (single specific categories), one of unspecified, pulmonary TB, AIDS, or other specific unlisted. The HIV status of the deceased may be available to the reviewer within the VA questionnaire (through specific questions or in the free-text narrative or summary of their medical record) or through access to the HIV test data (see below).
HIV test data
A household-based adult HIV sero-survey using rapid tests was carried out in selected clusters in the HDSS in 2005–2006, followed by four adult HIV sero-surveys across the HDSS population from 2007 to 2011. In the first round, 83% of the population consented to testing, but this figure decreased to 73% by the fourth round. Rapid tests were used, and the vast majority of people consented to be informed of the result. HIV testing with disclosure to participants has also been offered during other studies from 1988 onwards. HIV testing is otherwise available from several providers in the area (and testing has rapidly increased during the period covered by this analysis), and self-reported data on previous test history in any setting and HIV status (which could be reported as positive, negative, or unknown) are collected at the time of HIV testing and in other studies.
ART use data
ART was available at the main hospital (which is 70 km from the HDSS area) beginning in June 2005, at the rural hospital within the HDSS area from September 2006 onward, and at smaller clinics in the HDSS area starting in October 2010. KPS identified and tracked cohorts of consenting people initiated on ART in all clinics, capturing current and previous ART usage (Citation20).
Socio-economic data
Data on each individual's schooling and employment status are collected during annual HDSS surveys. As indicators of socio-economic status, we used the highest education level and an employment score based on occupation and the reliability of the income, where low is the least skilled and reliable (e.g. piecework) and high is the most skilled and reliable (e.g. a government worker paid monthly). The majority of the population are self-employed subsistence farmers and were classified as medium.
Statistical methods
We restricted the analysis to adults aged 15 years and over, as HIV test data were only available for this group. We compared known HIV/ART status and date of ART initiation (according to test/clinic records or self-report) with information reported at VA and looked at factors associated with agreement. We used chi-square tests and kappa statistics and developed logistic regression models using forwards stepwise techniques – starting with a basic model including the deceased's sex, age, and year of death and keeping additional variables in the model if a likelihood ratio test gave a p-value smaller than 0.1. Available variables included time between date of death and VA, place of death, socio-economic status of deceased, type of respondent, number of HIV tests known to KPS, number of AIDS signs and symptoms that have been shown to have moderate to high specificity for predicting HIV/AIDS deaths in VAs (Citation11) (oral candidiasis, jaundice, herpes zoster, weight loss, wasting, ulcers/sores, diarrhoea for more than 1 month or cough for more than 3 weeks), and HIV and ART status captured by KPS pre-mortem. We also looked at the correlation between HIV status reported in the VA and physician coding of deaths as HIV/AIDS for those without HIV status available in the KPS database (no negative HIV test within 3 years and no positive tests). We used Stata 13 for all analyses.
Ethics
Ethical approval was obtained from the National Health Sciences Research Committee of Malawi (protocols 419, 424, and 448), and the Ethics Committee of the London School of Hygiene and Tropical Medicine (protocols 5081, 5067, and 5214).
Results
From 2003 to 2014, 2,023 adult (15 years and older) VAs were conducted. HIV/AIDS diagnosis was asked about in all but 71 of the VAs (3.5%). Additional questions on HIV test and ART start dates were added in 2009, so were only available for 772 (38.2%) VAs; 588 (76.2%) of these reported a latest HIV test date, and 151 (19.6%) an ART start date.
VA respondent reporting knowing HIV status
Of 1,952 VA respondents asked, 79.9% (1,560) reported that they knew the deceased's HIV status; this proportion increased over time, from 144/300 (48.0%) in 2003/04 to 299/303 (98.7%) in 2013/14 ().
Table 1 Characteristics of deceased according to whether respondents reported HIV statusa
In a multivariable analysis, the VA respondent reporting that they knew the deceased's HIV status was associated with the sex of the deceased (adjusted odds ratio [aOR] for female vs. male=0.7, p=0.011), age of the deceased (aOR for 20–29 years vs. 15–19 years=0.5, p=0.066), year of death (aOR for each increase of 1 year=1.3, p<0.001), and number of HIV tests captured pre-mortem by KPS (aOR for each additional test=1.4, p<0.001) ().
Agreement on HIV status
There were 959 VAs for which the VA respondent was asked about the deceased's HIV status and there was a linked HIV status (positive, negative, or unknown) captured by KPS pre-mortem; 993 had no linked pre-mortem data. According to the VA respondents, 251 of the 959 (26.2%) were HIV positive, while 279/959 (29.1%) were HIV positive according to pre-mortem data ().
Table 2 Comparing HIV status reported to KPS prior to death with HIV status reported by respondent in the VAa
Overall there was 79.1% agreement (kappa statistic=0.6, p<0.001). In a multivariable analysis of the 959, agreement was associated with the age of the deceased (aOR for increase=1.2, p=0.047), type of respondent (aOR for partner/sibling/child vs. parent=0.3, p=0.005 and aOR for other relative vs. parent=0.3, p=0.004), number of HIV tests reported to KPS (aOR for increase=1.3, p=0.005) and the nature of HIV status reported pre-mortem to KPS (aOR for positive vs. negative=0.6, p=0.03 and aOR for unknown vs. negative=0.02, p<0.001) ().
Table 3 Characteristics of deceased according to whether HIV status reported by respondent agrees with other information known to KPSa
Excluding those VAs where the informant reported to KPS that they didn't know the deceased's status left 842 people; agreement was then 88.7% (kappa=0.5, p<0.001). Of the 842, 563 were negative in KPS or self-reported tests, and 515 were also reported negative by the respondent (specificity of respondent report=91.4%). Two hundred seventy-nine were HIV positive according to KPS or self-reported HIV test, and 232 were also reported positive in the VA (sensitivity of respondent report=83.2%).
Agreement on ART usage
There were 154 VAs on HIV-positive people with information on ART usage that could be linked to self-reports or clinic reports of ART initiation: overall there was 83.8% agreement (kappa statistic=0.4, p<0.001). In a multivariable analysis of the 154, agreement was associated with the presence of fewer AIDS symptoms (aOR for two or more vs. one or fewer=0.1, p=0.004), number of HIV tests reported to KPS (aOR for increase=1.6, p=0.069), and ART usage reported pre-mortem to KPS (OR for started vs. not=31.3, p<0.001) ().
Table 4 Characteristics of deceased according to whether ART usage reported by respondent agrees with other information known to KPSa
According to clinic reports or self-reports, 28 people had not started ART, and 13 were also reported as such in the VA (specificity of respondent report=46.4%). In all, 126 people had started ART; 116 were also reported as such in the VA (sensitivity of respondent report=92.1%). In addition, 31 VAs provided information on whether the deceased had started ART that could not be linked to any pre-mortem data source, and 10 were reported to have started ART.
ART initiation dates reported
An ART initiation date was reported in 151 VAs, of which 115 (76.2%) could be linked to a clinic or self-reported start date: 83 (72.2%) of these matched within 1 year, 20 (17.4%) were more than 1 year after, and 12 (10.4%) were more than 1 year before.
Influence of VA HIV status on cause of death assignment
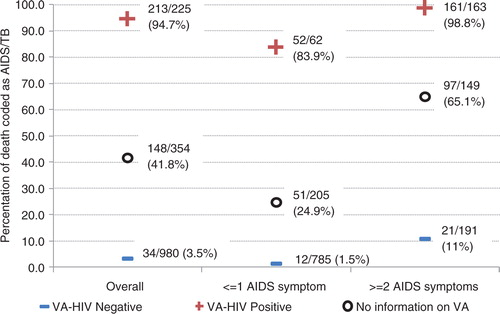
Of the 1,944 VAs with a likely cause of death assigned (79 [3.9%] did not have enough information to be able to assign a cause), 594 (30.6%) were coded as HIV/AIDS deaths. There were 1,559 VAs for which the reviewer had no KPS data on the deceased's HIV status (no negative HIV test within 3 years, and no positive tests), so cause of death would only be based on information from the VA questionnaire. In 354 cases there was no HIV status reported during the VA either, and 148 (41.8%) were coded as HIV/AIDS. In 225 cases, the respondent reported that the deceased was HIV positive and the death was coded as HIV/AIDS in 213 (94.7%), whereas in 980 cases a negative HIV status was reported and the death was coded as HIV/AIDS in only 34 (3.5%). This effect was also seen after stratifying the 1,559 according to whether they had AIDS symptoms: with one or fewer symptoms, a positive report from the VA increased the chance of a death to be coded as HIV/AIDS from 24.9 to 83.9%, and with two or more symptoms a negative report decreased this chance from 65.1 to 11.0% ().
Discussion
Our study confirms the importance of HIV data reported by the VA respondent, showing how the respondent's report of the deceased's HIV status influences the way the physician reviewer assigns cause of death irrespective of symptoms: a death in a person with a given set of symptoms consistent with AIDS was much more likely to be coded as HIV/AIDS when the respondent said the deceased was HIV positive than when he or she was reported to be HIV negative or when the status was missing. HIV results (particularly positive) are also weighted heavily in computer models (Citation12). VAs have often been shown to be fairly reliable compared to hospital notes (Citation9), though a study in Ethiopia found that for HIV/AIDS and TB diagnoses the sensitivity was high but specificity lower and a number of false positives could be expected (Citation21). This influence of VA HIV status on cause of death is likely to be found in other settings, which may have less complete or differential reporting of HIV/ART information in VAs, so it is important to know whether the HIV status reported by the VA respondent can be relied on.
In our study, the proportion of VA respondents reporting that they knew the deceased's HIV status was high and increased over time to 99%. The increase coincides with increased HIV testing (including increase of provider-initiated testing and counselling) and the rolling-out of ART in the area. Access to ART has been shown in other settings to normalise HIV and reduce stigma (Citation22) so may make people more likely to discuss test results within families or to disclose HIV results to interviewers.
At 89%, overall agreement on HIV status between the VA respondent's report and pre-mortem KPS data sources was high, implying that, at least in this rural area of Malawi among those for whom we have a result, data on HIV status from VAs are reliable enough to be useful in interpreting VA. However, this must also be interpreted in the context of only a moderate kappa statistic of 0.5. Agreement was higher in HIV negatives than positives: disclosure (to or by the VA respondent) could be more likely when the result is negative, which has been found in other settings (Citation15, Citation16) (Citation23, Citation24), or the respondent could be less willing to report the deceased as HIV positive in a VA. A small number of people (14, 2.5%) were reported to have HIV/AIDS in the VA but only had records of negative HIV tests in the KPS data: these are likely to be true positives that had not yet been captured by KPS, as the most recent full sero-survey was in 2011.
Agreement on ART usage was also fairly high at 84% (though the kappa statistic was only moderate at 0.4), and 72% of ART initiation dates matched within 1 year. Some studies suggest that having started ART was associated with disclosure (Citation25, Citation26), due to needing support during illness (Citation27) and treatment but others have found the opposite effect (Citation28). In Malawi it is recommended that people starting ART have a ‘treatment guardian’ (who attends the pre-ART counselling with the patient and is allowed to collect the medication on their behalf if required) (Citation29), so it is to be expected that agreement would be high.
Agreement on HIV status was more likely if the deceased was male. Evidence on the association between gender and disclosure of HIV status is mixed, with many studies reporting no association (Citation15, Citation25) (Citation30, Citation31) and some reporting that women are less likely to disclose their status to anyone (Citation24, Citation32). Unfortunately, the sex of the respondent was not recorded in our data.
In our data, increasing number of HIV tests reported by the deceased to KPS was associated with increased likelihood of the respondent reporting knowing the HIV status, as well as agreement on the status and on ART usage. Repeated voluntary HIV testing has been found to be associated with greater HIV knowledge, better health (Citation33), and less risky sexual behaviour (Citation34), which may also be linked to greater willingness to discuss HIV within the family.
We did not find any evidence that socio-economic status had any effect on reporting HIV/ART data in VAs; however the majority of the population are subsistence farmers, so there may not have been enough variation in socio-economic status to see an effect. The relationship between disclosure to a relative and socio-economic status is not consistent in the literature. One study in South Africa found that poorer women were less likely to disclose; however the opposite effect was seen in men (Citation30). Some studies found no association between disclosure and education (Citation25, Citation26), but others found that increased education was a predictor of disclosure (Citation31, Citation35).
There was some evidence that if the respondent was the parent of the deceased, agreement on HIV status was more likely than if they were any other type of respondent. It has been reported that some people find it easier to disclose to female relatives (Citation27) and that disclosing to the father is difficult (Citation36) and less common than to the mother (Citation26). A study in Ethiopia found that people were more likely to disclose to their partners but not to their children (Citation35). We could not explore this as our respondent data are only available by the broad categories shown in : partner is combined with child and sibling and age and sex of the respondent were not collected.
The strength of our study of having a high number of VAs reporting HIV/ART information and being able to compare this information to other sources of pre-mortem data may make our results difficult to generalise to other settings, as regular sero-surveys and other studies create an artificial environment where more people know their status than would usually be the case and people are more used to taking part in interviews than in other areas. People who have taken part in more surveys will also have had more opportunity to report more HIV tests. About half of the VA records with HIV/ART information reported did not have any pre-mortem data to compare to, which made the numbers in some categories quite small, especially when looking at data on those who started ART, causing some difficulties in interpreting some of the results. People with no pre-mortem HIV/ART information may also have been different to those with information available, which could have introduced bias: deaths prior to 2007 (the first full sero-survey) would be less likely to have pre-mortem data, and pre-mortem data after 2011 (the final sero-survey) would come from smaller studies or ART clinics only, making them more likely to be HIV positive and seeking care. In addition, we allowed a person to be HIV negative for up to 3 years after a report of a negative test; however, they could have tested positive in that period prior to death so our pre-mortem data cannot be seen as infallible.
In conclusion, in this population, HIV/ART information was often reported during a VA in recent years and matched relatively well with other sources of data. Asking about HIV status and ART use is an important and, in this setting, reliable component of the VA. In other settings where reporting of HIV/ART information during a VA may be less common and more associated with background factors, bias might be introduced in assigning cause of death, which may affect the proportion of deaths attributed to HIV/AIDS in the community.
Authors' contributions
The idea for the analysis was provided by BZ and the analysis was designed and carried out by EM. MN had overall oversight of the research site, and the fieldwork and VA review processes were designed and implemented by AC, with OK, MC, and TM. The VAs were carried out by LK and reviewed by (among others) AC and OK. The manuscript was written by EM, with AC, JG, and OK. All authors commented on the manuscript.
Conflicts of interest and funding
This study was funded by the Wellcome Trust. This analysis was funded by the Bill and Melinda Gates Foundation through the ALPHA network for analysis of longitudinal, population-based HIV/AIDS data in Africa.
Paper context
Verbal autopsies (VA) are commonly used to assign cause of death in low-resource settings for surveillance purposes. In areas affected by the AIDS pandemic, information on the deceased's HIV status and ART usage is vital in accurately assigning cause of death. Medical records are not always available so the VA-respondent's report maybe the only source of information. This paper assesses the reliability of VA-respondent reports of HIV/ART information in a rural African setting.
References
- Leitao J, Chandramohan D, Byass P, Jakob R, Bundhamcharoen K, Choprapawon C, etal. Revising the WHO verbal autopsy instrument to facilitate routine cause-of-death monitoring. Glob Health Action. 2013; 6 21518, doi: http://dx.doi.org/10.3402/gha.v6i0.21518.
- Garenne M, Fauveau V. Potential and limits of verbal autopsies. Bull World Health Organ. 2006; 84: 164. [PubMed Abstract] [PubMed CentralFull Text].
- Malawi AIDS response progress report 2015. 2015. Available from: http://www.unaids.org/sites/default/files/country/documents/MWI_narrative_report_2015.pdf [cited 24 August 2015]..
- Qureshi JS, Samuel JC, Mulima G, Kakoulides S, Cairns B, Charles AG. Validating a verbal autopsy tool to assess pre-hospital trauma mortality burden in a resource-poor setting. Trop Med Int Health. 2014; 19: 407–12. [PubMed Abstract].
- Fottrell E, Osrin D, Alcock G, Azad K, Bapat U, Beard J, etal. Cause-specific neonatal mortality: analysis of 3772 neonatal deaths in Nepal, Bangladesh, Malawi and India. Arch Dis Child Fetal Neonatal Ed. 2015; 100: F439–47. [PubMed Abstract] [PubMed CentralFull Text].
- Bayley O, Chapota H, Kainja E, Phiri T, Gondwe C, King C, etal. Community-linked maternal death review (CLMDR) to measure and prevent maternal mortality: a pilot study in rural Malawi. BMJ Open. 2015; 5: e007753. [PubMed Abstract] [PubMed CentralFull Text].
- Soleman N, Chandramohan D, Shibuya K. Verbal autopsy: current practices and challenges. Bull World Health Organ. 2006; 84: 239–45. [PubMed Abstract] [PubMed CentralFull Text].
- Clark SJ, McCormick T, Li Z, Wakefield J. InSilicoVA: a method to automate cause of death assignment for verbal autopsy. 2013. Available from: https://www.csss.washington.edu/Papers/2013/wp133.pdf [cited 9 September 2015]..
- Chandramohan D, Maude GH, Rodrigues LC, Hayes RJ. Verbal autopsies for adult deaths: their development and validation in a multicentre study. Trop Med Int Health. 1998; 3: 436–46. [PubMed Abstract].
- Fligner CL, Murray J, Roberts DJ. Synergism of verbal autopsy and diagnostic pathology autopsy for improved accuracy of mortality data. Popul Health Metr. 2011; 9: 25. [PubMed Abstract] [PubMed CentralFull Text].
- Lopman B, Cook A, Smith J, Chawira G, Urassa M, Kumogola Y, etal. Verbal autopsy can consistently measure AIDS mortality: a validation study in Tanzania and Zimbabwe. J Epidemiol Community Health. 2010; 64: 330–4. [PubMed Abstract].
- Byass P, Calvert C, Miiro-Nakiyingi J, Lutalo T, Michael D, Crampin A, etal. InterVA-4 as a public health tool for measuring HIV/AIDS mortality: a validation study from five African countries. Glob Health Action. 2013; 6 22448, doi: http://dx.doi.org/10.3402/gha.v6i0.22448.
- Combs Thorsen V, Sundby J, Meguid T, Malata A. Easier said than done!: methodological challenges with conducting maternal death review research in Malawi. BMC Med Res Methodol. 2014; 14: 29. [PubMed Abstract] [PubMed CentralFull Text].
- Chan BT, Weiser SD, Boum Y, Siedner MJ, Mocello AR, Haberer JE, etal. Persistent HIV-related stigma in rural Uganda during a period of increasing HIV incidence despite treatment expansion. AIDS. 2015; 29: 83–90. [PubMed Abstract] [PubMed CentralFull Text].
- Bott S, Obermeyer CM. The social and gender context of HIV disclosure in sub-Saharan Africa: a review of policies and practices. SAHARA J. 2013; 10(Suppl 1):S5–16. [PubMed Abstract] [PubMed CentralFull Text].
- Rujumba J, Neema S, Byamugisha R, Tylleskär T, Tumwine JK, Heggenhougen HK. ‘Telling my husband I have HIV is too heavy to come out of my mouth’: pregnant women's disclosure experiences and support needs following antenatal HIV testing in eastern Uganda. J Int AIDS Soc. 2012; 15: 17429. [PubMed Abstract] [PubMed CentralFull Text].
- Deribe K, Woldemichael K, Wondafrash M, Haile A, Amberbir A. Disclosure experience and associated factors among HIV positive men and women clinical service users in southwest Ethiopia. BMC Public Health. 2008; 8: 81. [PubMed Abstract] [PubMed CentralFull Text].
- Conroy A, Yeatman S, Dovel K. The social construction of AIDS during a time of evolving access to antiretroviral therapy in rural Malawi. Cult Health Sex. 2013; 15: 924–37. [PubMed Abstract].
- Crampin AC, Dube A, Mboma S, Price A, Chihana M, Jahn A, etal. Profile: the Karonga health and demographic surveillance system. Int J Epidemiol. 2012; 41: 676–85. [PubMed Abstract] [PubMed CentralFull Text].
- Koole O, Houben RM, Mzembe T, Van Boeckel TP, Kayange M, Jahn A, etal. Improved retention of patients starting antiretroviral treatment in Karonga District, northern Malawi, 2005–2012. J Acquir Immune Defic Syndr. 2014; 67: e27–33. [PubMed Abstract] [PubMed CentralFull Text].
- Misganaw A, Mariam DH, Araya T, Aneneh A. Validity of verbal autopsy method to determine causes of death among adults in the urban setting of Ethiopia. BMC Med Res Methodol. 2012; 12: 130. [PubMed Abstract] [PubMed CentralFull Text].
- Roura M, Wringe A, Busza J, Nhandi B, Mbata D, Zaba B, etal. ‘Just like fever’: a qualitative study on the impact of antiretroviral provision on the normalisation of HIV in rural Tanzania and its implications for prevention. BMC Int Health Hum Rights. 2009; 9: 22. [PubMed Abstract] [PubMed CentralFull Text].
- Bedell RA, van Lettow M, Landes M. Women's choices regarding HIV testing, disclosure and partner involvement in infant feeding and care in a rural district of Malawi with high HIV prevalence. AIDS Care. 2014; 26: 483–6. [PubMed Abstract].
- Anglewicz P, Chintsanya J. Disclosure of HIV status between spouses in rural Malawi. AIDS Care. 2011; 23: 998–1005. [PubMed Abstract] [PubMed CentralFull Text].
- Kadowa I, Nuwaha F. Factors influencing disclosure of HIV positive status in Mityana district of Uganda. Afr Health Sci. 2009; 9: 26–33. [PubMed Abstract] [PubMed CentralFull Text].
- Suzan-Monti M, Kouanfack C, Boyer S, Blanche J, Bonono R-C, Delaporte E, etal. Impact of HIV comprehensive care and treatment on serostatus disclosure among Cameroonian patients in rural district hospitals. PLoS One. 2013; 8: e55225. [PubMed Abstract] [PubMed CentralFull Text].
- Maman S, van Rooyen H, Groves AK. HIV status disclosure to families for social support in South Africa (NIMH Project Accept/HPTN 043). AIDS Care. 2014; 26: 226–32. [PubMed Abstract].
- Osinde MO, Kakaire O, Kaye DK. Factors associated with disclosure of HIV serostatus to sexual partners of patients receiving HIV care in Kabale, Uganda. Int J Gynaecol Obstet. 2012; 118: 61–4. [PubMed Abstract].
- Ministry of Health. Clinical management of HIV in children and adults. 2014; Lilongwe, Malawi: Ministry of Health.
- Longinetti E, Santacatterina M, El-Khatib Z. Gender perspective of risk factors associated with disclosure of HIV status, a cross-sectional study in Soweto, South Africa. PLoS One. 2014; 9: e95440. [PubMed Abstract] [PubMed CentralFull Text].
- Amoran OE. Predictors of disclosure of sero-status to sexual partners among people living with HIV/AIDS in Ogun State, Nigeria. Niger J Clin Pract. 2012; 15: 385–90. [PubMed Abstract].
- Hodgson I, Plummer ML, Konopka SN, Colvin CJ, Jonas E, Albertini J, etal. A systematic review of individual and contextual factors affecting ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. PLoS One. 2014; 9: e111421. [PubMed Abstract] [PubMed CentralFull Text].
- Regan S, Losina E, Chetty S, Giddy J, Walensky RP, Ross D, etal. Factors associated with self-reported repeat HIV testing after a negative result in Durban, South Africa. PLoS One. 2013; 8: e62362. [PubMed Abstract] [PubMed CentralFull Text].
- Kabiru CW, Luke N, Izugbara CO, Zulu EM. The correlates of HIV testing and impacts on sexual behavior: evidence from a life history study of young people in Kisumu, Kenya. BMC Public Health. 2010; 10: 412. [PubMed Abstract] [PubMed CentralFull Text].
- Reda AA, Biadgilign S, Deribe K, Deribew A. HIV-positive status disclosure among men and women receiving antiretroviral treatment in eastern Ethiopia. AIDS Care. 2013; 25: 956–60. [PubMed Abstract].
- Iwelunmor J, Sofolahan-Oladeinde Y, Airhihenbuwa CO. Sociocultural factors influencing HIV disclosure among men in South Africa. Am J Mens Health. 2015; 9: 193–200. [PubMed Abstract].