Abstract
The dengue viruses (DENV) are endemic in the tropical and sub-tropical countries and cause the most common arthropod-borne viral disease in humans. Travelers visiting endemic areas may both acquire and spread DENV infections, and this is the reason why prevention of mosquito bites is of crucial importance. Dengue fever (DF) has become the most common cause for tropical fever in Swedish tourists. Swedish data from 1995 to 2010 show that the number of DF cases has increased since the beginning of 2000; partly due to improved diagnostics based on IgM detection, and partly due to an increase in the number of tourists traveling to, and between, endemic areas. Young adults aged 20–29 are mostly affected, and epidemiological data indicate increased incidence rates from 2008 onwards. Our data pose a call for attention when traveling to DENV endemic areas as well as an increased awareness among physicians when treating returning travelers.
The dengue viruses (DENV) are flaviviruses, including four antigenetically distinct serotypes, and are the cause of the most common arthropod-borne viral disease in humans. The DENV is endemic in tropical and subtropical regions and 2.5 billion people live in affected areas. Since neither vaccine nor effective antiviral drugs exist, only vector control of the DENV transmitting Aedes mosquitoes remains as a measure of DENV control. The WHO estimates that there are up to 100 million infections annually; 500,000 of which are classified as dengue hemorrhagic fever (DHF), with a yearly mortality rate of 25,000 Citation1. The geographical distribution of both DENV and its vector is expanding, and multiple DENV serotypes are co-circulating resulting in hyperendemic areas. An increased frequency of epidemics, and the emergence of dengue fever (DF) in new areas, emphasizes the global burden of dengue both by its toll in lives and by its economical impact Citation2 Citation3.
DENV infections are second only to malaria among the most common reasons for tropical fever in travelers Citation4 Citation5 Citation6 and occur more frequently than malaria in certain regions of South America and South East Asia (SEA) Citation7. The classic form of DENV infection, DF is self-limiting and rarely fatal, but can be incapacitating for the patient, and may require hospitalization and even evacuation and a return home for travelers. Common symptoms include high fever, myalgia, arthralgia, maculopapular rash, and retro orbital pain. The convalescence period may last for weeks because of asthenia and depression Citation8.
The Aedes aegypti and Aedes albopictus mosquitoes are well adapted to humans and transmit DENV easily because of their preference for human blood and by being intermittent feeders. The mosquitoes are well adapted to urban settings and breed in tires and artificial water jars near human dwellings Citation9. The principal vector Ae. aegypti is highly susceptible to DENV and can be found on every continent except Europe and the Antarctica Citation10. The secondary vector Ae. albopictus is also widely spread and is since the beginning of this century found also in Southern Europe. The natural home of Ae. albopictus ranges from temperate to tropical areas, and mosquitoes have been found in several states in the USA with a climate resembling that in Northern Europe, indicating that there are no major territorial or climate-related barriers to prevent the mosquito to be established in Northern Europe.
Changes in human demography and behavior have accelerated the emergence of DENV. Increased air travel, which facilitates rapid dissemination of pathogens between populations, constitutes one of the major risk factors for spreading DENV. A traveler to DENV endemic areas has the potential to acquire an infection and also to spread it to other parts of the world, thus functioning as a Trojan horse. One of the largest dengue epidemics known in history occurred in Greece during 1927–1928 with approximately 100,000 cases, of which 1000 were fatal Citation11. The first European autochthonous dengue cases in the 21st century were reported in September 2010 in Nice, France; an event that deserves serious attention Citation12.
Imported DENV infected cases have become more frequent in non-endemic European countries Citation13 Citation14 Citation15 . Swedes are frequent travelers, and some tropical destinations like Thailand and other countries in SEA, are among the most popular during the winter months. This has led to an increased number of imported dengue cases in Sweden. The knowledge about travelers’ risk of acquiring DF, however, is limited, as DF is usually not reported in most European countries, and the number of conducted studies is small. DF became a notifiable disease in Sweden in 2004, and there is a trend of an increasing number of DF cases among tourists returning from SEA. Data from the last 15 years show that there is a substantial risk of acquiring DF when visiting endemic areas. An increased awareness of the risk for DENV transmission is required among travelers as well as physicians in non-endemic countries.
Material and methods
Patient data
Patient sera were analyzed according to the routine procedure for DF diagnostics at the Swedish Institute for Communicable Disease Control (SMI). An in-house immunofluorescence assay was used essentially as previously described Citation16. Briefly, serum dilutions were applied to spot slides with acetone-fixed Vero cells infected with DENV-2 strain New Guinea C. Following incubations and washes, bound antibodies were visualized by fluorescein-labeled goat anti-human IgG (Jackson Immuno Research Laboratories; West Grove, PA, USA). Starting in 2002, IgM-testing was introduced as a complement to the IFA, by the use of Dengue IgM capture ELISA (Panbio diagnostics, Sinnamon Park, QLD, Australia) according to the manufacturer's instructions. Since July 1, 2004, when DF became notifiable according to the Swedish Communicable Disease Act, all reported cases were included in the study. A minor part of these cases was only reported by the responsible physician and had not been verified at the SMI laboratory.
Travel data
The incidence of imported DF cases to Sweden was calculated using travel data from the Swedish Travel and Tourist Database (TDB) (Resurs AB, Sweden) as a denominator. The travel database is based on the answers from 2000 Swedish residents who are randomly selected each month and interviewed on the telephone about their travels and destinations during the previous month. Travel data were weighted based on Swedish demographic population data to estimate the total number of journeys from Sweden to Thailand, with at least one overnight stay during the period 2000–2009. Both leisure and business travels were included. If no respondents had been to Thailand during a specific month, the number of travels during that period was estimated to lie in-between the numbers of the preceding and subsequent months, respectively.
Statistical method
Data from 2000 to 2009 were analyzed using two separate quasi Poisson models for calculating adjusted risk ratios (RR) for DF among Swedish tourists traveling to Thailand. In the first model, year, age group and sex were included as covariates and in the second model, year and season. In both models, the number of travels from the TDB was used as offset. The parameter with the lowest RR in each category was used as a reference in the models. A chi-square test was used to determine which factors in each model had a significant association with the incidence of DF cases among travelers to Thailand. The effect of different levels of the factors on the model was evaluated by examining the separate beta estimations. The results were presented with 95% confidence intervals.
Results
The distribution of travel-acquired DENV infections by region of traveling reflects the global DENV activity, which is known to be highest in SEA, followed by South and Central America, and the Caribbean islands. During the 10-year study period, the number of travels from Sweden to Thailand varied between 190,000 and 450,000 trips a year, and there was an overall increasing trend. When the whole period was examined, half of the travels were made during the dry winter months (January to March). During an average year, there were more men than women traveling. The dominating age group among travelers varied between years, but travelers aged between 20 and 69 years were evenly distributed during the whole study period, with some predominance in the older half of the age span.
Dengue cases
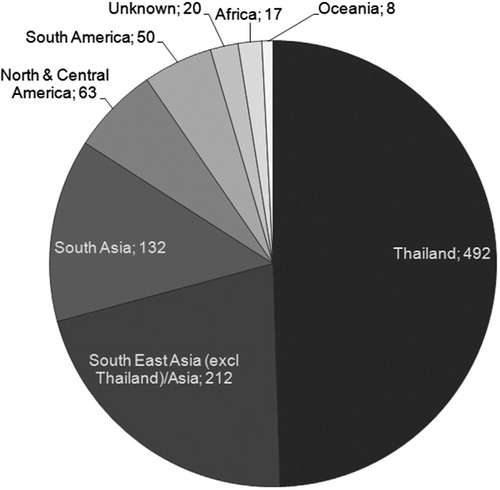
More than 70% of the Swedish DF cases from 1995 to 2010 originated from SEA, and particularly from Thailand (49%) (). In total, there were 309 notified DF cases imported from Thailand during the period 2000–2009, and there seems to be an increasing trend. Seventy five percent of the cases were either diagnosed or fell ill during the seven first months of the year. Fifty two percent of the cases were men, and the most affected age group was the 20–29 years (119 cases). There were only three cases reported in the age group 0–9 years and three in the age group 70–79. The youngest reported case was two years and the oldest 76 years, respectively.
Incidence
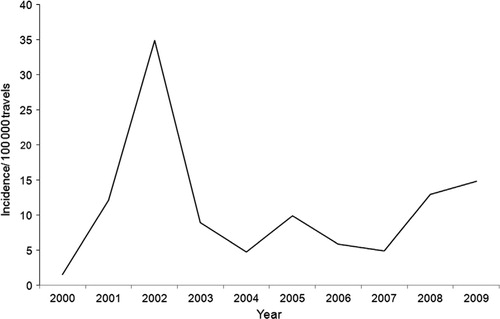
There was a peak in incidence in 2002, with a 25 times higher risk to be notified as a DF case in the Swedish surveillance system in comparison to the reference year 2000 (95% CI 6.58–94.89) (). During the five following years, there was a more stable incidence varying between about 5 and 10 cases per 100,000 travelers. During the two last years of the decade, 2008 and 2009, there was an increase in the incidence to 13–15 cases per 100,000 travelers in the respective years.
Fig. 2. Incidence of diagnosed DF cases per 100,000 travels in Swedish travelers returning from Thailand during 2000–2009.
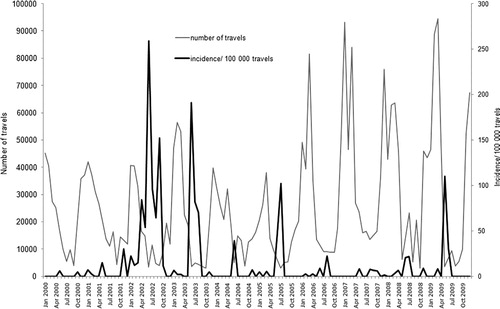
There was a 4.3 times increased risk if traveling during the wet summer months, June to August (95% CI 2.65–7.08), with obvious peaks in the incidence of DF in-between the peaks in traveling during the winter months (). There was no significant difference in the incidence between male and female travelers.
Fig. 3. Incidence of diagnosed DF cases per 100,000 travels in Swedish travelers returning from Thailand in 2000–2009 (black line) and the estimated number of travels to Thailand during the same time period (gray line).
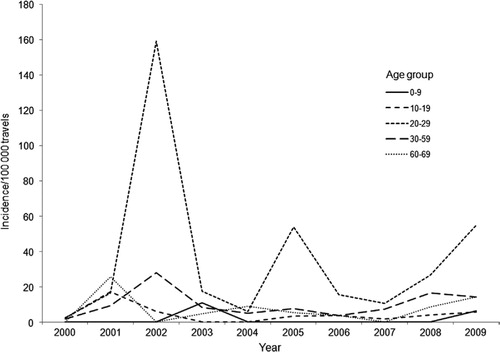
The risk of being diagnosed as a DF case when returning home to Sweden was considerably and significantly higher (30 times) in travelers aged 20–29 years (95% CI 6.60–140.16) (). People in the age span 30–59 years also had a significantly higher risk of being reported as a DF case, the risk being less pronounced with older age.
Discussion
DF is an important health issue in several tropical areas in the world with around 100 million infections annually. The global DENV burden is among the highest in SEA, followed by India and neighboring countries, Central America, and the Caribbean islands. Several countries in SEA are in addition hyperendemic, which contributes to the shorter intervals between the high epidemic peaks observed during the last years.
The number of reported DF cases has increased in Sweden during the last decade. We have analyzed Swedish travelers returning from Thailand, as these constitute the majority of reported DF cases in Sweden. Apart from reflecting the high endemicity in Thailand, the distribution of cases also reflects the popularity of the country as tourist destination. Thailand is among the most popular tourist destinations in Sweden, both among families with children and young travelers. Other issues, which could influence the epidemiology of travel-acquired DF are, for example, Citation1 the irregular occurrence of epidemics, Citation2 high versus low traveling season, Citation3 vector activity (which depends on season and local climate fluctuations), and Citation4 variations in the virulence of different DENV strains.
In our material, the DF incidence in travelers returning from Thailand peaked during the Swedish summer, i.e. the rainy season in Thailand. The peak periods of DENV transmission coincide with the rainy season when vector density increases. The risk of being reported as a DF case was incomparably highest in the age group 20–29 years, which could be interpreted as caused by common behavioral patterns in this group, i.e. traveling more in a back-packer style, using simpler accommodations and being more exposed to mosquitoes. In addition, travelers in the age group 20–29 probably stay away for a longer time period, thus having a prolonged exposure risk. For the majority of the cases, we had no information on the length of stay in DF endemic countries and could not analyze the influence of the time factor in our results. We cannot find any reason to believe that the DENV infections would be more severe in the age group 20–29, i.e. more of them seeking medical care. The incidence of DF cases peaked in the year 2002 reaching 250/100,000 travels, and coinciding with a DENV outbreak in Thailand Citation17. It has to be emphasized that the travel data from the TDB are an estimate of the true number of travels to Thailand. In our statistical analyses, this fact has not been taken into account, which may have contributed to the introduction of a bias in our results.
Although numerous cases of DF in travelers returning from Thailand are reported annually in Sweden, the true incidence of DENV infection is underestimated due to several reasons. Firstly, patients diagnosed in Thailand, and who did not contact the Swedish healthcare system were not included in the study. In calculations from the 2010 surveillance data, it was observed that 10% of the cases which had been diagnosed clinically by a Swedish physician had not been sampled in Sweden, indicating that the original diagnosis had already been made abroad (author's unpublished observations). Secondly, two prospective studies on DENV seroconversion in travelers have also shown a high percentage of asymptomatic infections, indicating that the reported number of cases constitutes only the tip of the iceberg Citation18 Citation19.
Many travelers who fall ill during travel and recover abroad never get diagnosed in their home countries upon return. It would be of interest to conduct a prospective study in travelers to SEA by collecting serum samples from them before and after travel. The incidence risk for acquiring a DENV infection would be estimated by the number of seroconversions in all travelers. The clinical-to-subclinical infection ratio would also be possible to calculate in such a study.
The increased number of annual DF cases in Sweden and the tendency of risk behavior in the most affected age group (20–29 years old) emphasize the importance of tourists seeking information before traveling to DENV endemic areas. Information concerning DENV infections in travelers has been poor in Sweden, and very few travelers are aware of the risks Citation20. Pre-travel advice should include information about destinations associated with high risk; how to protect oneself from mosquito bites as well as signs and symptoms of a DENV infection. It should be emphasized that transmission also occurs between epidemics (e.g. during the dry season), and that the main risk of exposure among travelers is in urban areas. As a vaccine is still lacking, travelers ought to avoid mosquito bites by using insect repellents and protective clothing. This is the single-most effective measure of DF prevention, since the Aedes mosquitoes are day-active, and thus bed nets are of restricted use, except in the case of avoiding further transmission from a viremic individual to noninfected mosquitoes.
DF is an important differential diagnosis in travelers returning from DENV endemic areas with fever, and there is a need for healthcare providers in non-endemic countries to understand the clinical spectrum, diagnosis, epidemiology and prevention of DF among travelers. This is true also for travelers returning from parts of the world, e.g. Africa, where the endemicity of DENV is poorly monitored, but the few but nevertheless reported cases of DENV infections reported in travelers returning from Africa underline the requirement that DF should also be included as a differential diagnosis for fever in those travelers.
In the Swedish records, DF infections are reported without further characterization of the clinical picture. It is therefore impossible to calculate the incidence of DHF, even though DHF has been occasionally described in Swedish travelers Citation21. The majority of imported DENV infected cases are, however, known to be classical DF, which could be due to the fact that they are usually primary infections. The enhancing antibodies persist between 2 and 12 months post-onset and pose an increased risk for the patient to develop DHF if infected by a heterotypic DENV serotype Citation22. It should not be ignored, however, that DHF does sometimes occur during a primary infection caused by a particularly virulent DENV strain Citation23 Citation24 Citation25 .
In addition to being infected by DENV, international travelers could also spread the infection to new areas. In combination with lack of vector control, virus, and vector evolution, international travel poses a real threat to the emergence of DENV worldwide, including countries not yet affected. In September 2010, France reported two autochthonous cases of DENV infections, connected to the presence of the mosquito Ae. albopictus in Southern Europe [12]. Two DF cases were also found to have been infected in Croatia Citation26. Chikungunya virus also caused an outbreak in Italy in 2007, with 281 confirmed cases indicating that the establishment of Aedes mosquitoes in Europe poses a real threat for epidemics Citation27. A comprehensive registration of European DF and DHF cases would be useful in order to better estimate the true risk of acquiring a DENV infection as well as to monitor the DENV burden in non-endemic countries. Further knowledge concerning arthropod-borne diseases is needed to better understand transmission mediated by tourists visiting endemic areas as well as DENV-carrying visitors coming to hitherto non-endemic countries where the vector is present.
In conclusion, it is not enough to follow the development of global emerging diseases solely in the endemic countries. From the Swedish perspective, as both the number of travels and the incidence of DF have increased, there is an obvious need for better and more comprehensive information regarding which precautionary measures to take when traveling to DENV endemic countries.
Conflict of interest and funding
The authors declare that they have no conflict of interest.
Acknowledgements
We wish to thank Anna-Maria Kling who provided invaluable help in the statistical analysis.
References
- Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002; 2: 33–42.
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002; 33: 330–42.
- Gibbons RV, Vaughn DW. Dengue: an escalating problem. BMJ. 2002; 324: 1563–6.
- Suh KN, Kozarsky PE, Keystone JS. Evaluation of fever in the returned traveler. Med Clin North Am. 1999; 83: 997–1017.
- O'Brien D, Tobin S, Brown GV, Torresi J, et al.. Fever in returned travelers: review of hospital admissions for a 3-year period. Clin Infect Dis. 2001; 33: 603–9.
- Stephan C, Allwinn R, Brodt HR, Knupp B, Preiser W, Just-Nubling G, et al.. Travel-acquired dengue infection: clinical spectrum and diagnostic aspects. Infection. 2002; 30: 225–8.
- Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, Von Sonnenburg F, et al.. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006; 354: 119–30.
- Jelinek T. Dengue fever in international travelers. Clin Infect Dis. 2000; 31: 144–7.
- Lifson AR. Mosquitoes, models, and dengue. Lancet. 1996; 347: 1201–2.
- Pinheiro FP, Corber SJ. Global situation of dengue and dengue haemorrhagic fever, and its emergence in the Americas. World Health Stat Q. 1997; 50: 161–9.
- Chastel C. Lessons from the Greek dengue epidemic of 1927–1928. Bull Acad Natl Med. 2009; 193: 485–93.
- European Centre for Disease Prevention and Control (ECDC). Dengue fever in France 2010. Available from: http://www.ecdc.europa.eu/en/activities/sciadvice/Lists/ECDC%20Reviews/ECDC_DispForm.aspx?List = 512ff74f-77d4-4ad8-b6d6-bf0f23083f30&ID = 9462010 [cited 17 September 2010].
- Jelinek T, Muhlberger N, Harms G, Corachan M, Grobusch MP, Knobloch J, et al.. Epidemiology and clinical features of imported dengue fever in Europe: sentinel surveillance data from TropNetEurop. Clin Infect Dis. 2002; 35: 1047–52.
- Jelinek T. Dengue fever in international travelers. Clin Infect Dis. 2000; 31: 144–7.
- Wichmann O, Lauschke A, Frank C, Shu PY, Niedrig JH, et al.. Dengue antibody prevalence in German travelers. Emerg Infect Dis. 2005; 11: 762–5.
- Vene S, Mangiafico J, Niklasson B. Indirect immunofluorescence for serological diagnosis of dengue virus infections in Swedish patients. Clin Diagn Virol. 1995; 4: 43–50.
- WHO. Asia-Pacific Dengue Program Managers Meeting. W.P.R. World Health Organization., Singapore: WHO. 2008 p. 5.
- Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988; 38: 172–80.
- Guzman MG, Kouri GP, Bravo J, Soler M, Vazquez S, Morier L. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg. 1990; 42: 179–84.
- Lindback H, Lindback J, Tegnell A, Janzon R, Vene S, Ekdahl K, et al.. Dengue fever in travelers to the tropics, 1998 and 1999. Emerg Infect Dis. 2003; 9: 438–42.
- Wittesjo B, Eitrem R, Niklasson B. Dengue fever among Swedish tourists. Scand J Infect Dis. 1993; 25: 699–704.
- Halstead SB. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis. 1979; 140: 527–33.
- Barnes WJ, Rosen L. Fatal hemorrhagic disease and shock associated with primary dengue infection on a Pacific island. Am J Trop Med Hyg. 1974; 23: 495–506.
- Rosen L. The Emperor's new clothes revisited, or reflections on the pathogenesis of dengue hemorrhagic fever. Am J Trop Med Hyg. 1977; 26: 337–43.
- Gubler DJ, Suharyono W, Lubis I, Eram S, Gunarso S. Epidemic dengue 3 in central Java, associated with low viremia in man. Am J Trop Med Hyg. 1981; 30: 1094–9.
- Gjenero-Margan I, Aleraj B, Krajcar D, Lesnikar V, Klobucar A, Pem-Novosel I, et al.. Autochthonous dengue fever in Croatia, August-September 2010. Euro Surveill. 2011; 16.
- Fontenille D, Failloux AB, Romi R. Should we expect Chikungunya and dengue in Southern Europe?. In: Takken W, Knols BG, Ecology and control of vector-borne diseases. Emerging pests and vector-borne diseases in Europe. The Netherlands: Wageningen Academic Publishers. 2007, pp. 169–84.