Abstract
The antiviral drug oseltamivir (Tamiflu®) is a cornerstone in influenza pandemic preparedness plans worldwide. However, resistance to the drug is a growing concern. The active metabolite oseltamivir carboxylate (OC) is not degraded in surface water or sewage treatment plants and has been detected in river water during seasonal influenza outbreaks. The natural influenza reservoir, dabbling ducks, can thus be exposed to OC in aquatic environments. Environmental-like levels of OC induce resistance development in influenza A/H1N1 virus in mallards. There is a risk of resistance accumulation in influenza viruses circulating among wild birds when oseltamivir is used extensively. By reassortment or direct transmission, oseltamivir resistance can be transmitted to humans potentially causing a resistant pandemic or human-adapted highly-pathogenic avian influenza virus. There is a need for more research on resistance development in the natural influenza reservoir and for a prudent use of antivirals.
Influenza A is a major health concern in human and veterinary medicine alike. Viruses of human and avian origin are linked through the exchange of genetic material, e.g. the reassortment process forming pandemic viruses in humans. There is growing evidence that the active substance antiviral drug oseltamivir (Tamiflu®) is stable and that it can be detected in aquatic environments when oseltamivir is used widely. Low levels of the active substance can induce resistance in influenza A virus in mallards, the natural influenza reservoir. If resistance is established in viruses circulating among wild birds, there is a risk of an oseltamivir-resistant pandemic or highly-pathogenic avian influenza (HPAI) virus through reassortment or direct transmission (). This paper aims to review the current knowledge in this field, to identify gaps in the knowledge and to point out important future research areas.
Pandemics
During the last century, influenza A viruses have caused four pandemic outbreaks. In 1918–1920, the ‘Spanish Flu’ H1N1 pandemic killed at least 50 million, perhaps up to 100 million people Citation1. There has been a controversy regarding the genetic origin of the Spanish Flu, with some authors claiming a direct transmission of an avian virus Citation2 Citation3. However, phylogenetic evidence strongly suggests that the 1918 pandemic strain was the result of a reassortment of human, avian and possibly swine viruses Citation4. The 1957 H2N2 ‘Asian Flu’ and the 1968 H3N2 ‘Hong Kong Flu’ pandemics were both milder than the 1918 pandemic and caused much less mortality. They were the result of a reassortment of human and avian viruses. Little is known about the sequences of swine viruses at that time and thus the involvement of swine viruses in the reassortant cannot be precluded Citation5. The recent A/H1N1 ‘Swine Flu’ pandemic was a result of a reassortment of three different strains from swine, but all three had genetic elements recently derived from avian viruses Citation5.
Influenza in birds
Influenza A virus is a zoonosis, the natural hosts are wetland birds, mostly Anseriformes (ducks, geese, swans) and Charadriiformes (gulls, terns, waders) Citation6 Citation7. Dabbling ducks such as the mallard (Anas platyrhyncos), are particularly well suited for the perpetuation of influenza viruses in several aspects. They feed in shallow water which facilitates spread via the fecal – oral route Citation6. Furthermore, they congregate in flocks, have a large population size, migrate to interact with new individuals and every year new, immunologically naïve juveniles are added to the population. Influenza A viruses are well adapted to aquatic environments; some viral strains can remain infective for well over a year at 4°C but only for days at 37°C Citation8. Dabbling ducks are considered the major natural reservoir of influenza A viruses Citation7.
Most viruses circulating among wild birds are low-pathogenic (low-pathogenic avian influenza, LPAI). The prevalence of LPAI viruses among dabbling ducks vary with season with a higher percentage of birds infected during fall migration. One contributing factor to this variation is the high proportion of non-immune juveniles in the fall. There are also geographic differences; a study from Sweden found 15% infected mallards during fall and 4% in the springtime Citation9, whereas data from North America show a higher fall prevalence but a lower spring prevalence Citation6. All 16 HA and 9 NA variants of influenza A viruses discovered so far have been found in birds and most of them in dabbling ducks. Exceptions are H13 and H16 that are predominantly isolated from gulls and terns Citation10. Most subtypes of LPAI viruses have a large geographical spread, possibly through migration. H14 and H15 are exceptions, being mostly isolated in Russia and Australia Citation11 Citation12. Phylogenetically, LPAI viruses studied so far belong to either the Eurasian or the North American lineage Citation13. However, several findings of viruses with a lineage-mixed genome have been reported Citation14–Citation16 indicating that the separation is not complete. Hence, the naturally circulating gene pool of LPAI viruses in wild birds can be considered large and variable in several aspects.
LPAI in dabbling ducks has a mild clinical course but smaller ‘costs’ for the host are difficult to study. For example, in an infection experiment a slight, short-lasting increase in body temperature was observed Citation17 and in another experiment mallard hens had a transient decrease in egg production Citation18. Interestingly, a recent study found mallards in normal body condition more sensitive to LPAI infection when compared to mallards –10% and –20% in body weight Citation19. This is contradictory to the hypothesis that birds in worse body condition would be more sensitive to infection. LPAI infection in ducks is located mainly in the gastrointestinal tract Citation20.
Treatment and prophylaxis of influenza
There are two different strategies regularly used in the treatment and prophylaxis of influenza A: antiviral drugs and vaccines.
The admantanes, amantadine and rimantadine, block the M2 protein and thus inhibit viral replication at an early stage Citation21. However, due to a massive development of resistance both in human and avian strains Citation22 Citation23 the clinical use of admantanes has virtually stopped. Another disadvantage of admantanes is their high rate of adverse events such as nausea, insomnia and hallucinations Citation24.
The neuramidase inhibitors (NAIs) inhibit the viral enzyme neuraminidase (NA). NA is needed for the release of newly formed virions from the infected host cell Citation25 but also for the process of viral entry through the airways of the host by cutting mucoproteins Citation26. The possibility to decrease viral entry in the airway epithelium through hindering of NA – mucoprotein interaction may contribute to the successfulness of NAI prophylaxis Citation27. There are two commercially available NAIs worldwide: oseltamivir (Tamiflu®) and zanamivir (Relenza®). Zanamivir cannot be administered orally but is inhaled or rarely used in an intravenous formulation.
Vaccines are an effective and safe way to prevent influenza. A trivalent vaccine containing antigens from A/H1N1, A/H3N2 and B strains is the standard preventive measure for seasonal influenza Citation28. In a pandemic scenario, the rapid mass-production of vaccines is problematic as most production techniques still depend on embryonated hen eggs. According to the Global Action Plan developed by WHO, the goal in a pandemic situation is to have produced 2 billion doses 6 months after a vaccine candidate is available. However, in a WHO study evaluating the 2009 pandemic it was demonstrated that in 6 months, only 534 million doses had been produced and that it took 5 months from the identification of the pandemic A/H1N1 virus strain until the first vaccines were available. Furthermore, the supply of vaccines to developing countries is especially hard to accomplish; this is problematic as a severe pandemic is expected to hit particularly hard in those countries Citation29.
Oseltamivir
Oseltamivir is administered orally as a prodrug, oseltamivir phosphate (OP) due to the poor bioavailability of the active substance oseltamivir carboxylate (OC). OP is readily absorbed and rapidly converted to OC by esterases, mainly in the liver. More than 75% of an oral dose reaches the circulation as OC. The active metabolite is then excreted from the body in unchanged form predominantly via the urine Citation30. OC is likely as poorly absorbed in the intestine of ducks as in the intestine of humans. However, as the LPAI infection in ducks takes place in the intestine, replicating virus and OC co-exist potentially enabling resistance to develop. Oseltamivir has been extensively stockpiled; e.g. the US had 40 million treatment regimens in stock as of April 2009 Citation31. Worldwide, more than 220 million treatment courses have been stockpiled, and the shelf life has been extended to 7 years Citation32. As the mass-production of vaccines is a process of several months, antiviral drugs are the only option in the early phase of a pandemic. Thus, oseltamivir is a cornerstone of pandemic preparedness plans all over the world.
OC is stable in the aqueous phase and is not removed or degraded in normal sewage treatment plants (STPs) Citation33. Persistence of oseltamivir in surface water ranged from non-detectable degradation to a half-life of 53 days in another study Citation34. Thus, there is reason to believe that OC is present in the aquatic environment near STPs when oseltamivir is used extensively. Japan has had the highest per-capita consumption of oseltamivir during several seasonal influenza outbreaks. In one study, it was estimated that more than 10 million treatment courses – corresponding to almost 10% of the population – were used during the 2004–2005 season Citation35. The manufacturer Roche estimated that 6 million people out of 16 million infected with influenza used the drug during the same season Citation36.
A study on water from the Yodo River system in Japan during the influenza season 2007–2008 demonstrated OC levels of up to 58 ng/L Citation37. This correlated well with levels estimated from data on oseltamivir consumption. During the influenza season 2008–2009, another study in the same area measured levels of OC in river water up to 190 ng/L and in outgoing water from STPs up to 293 ng/L Citation38. Very limited sampling for OC has been performed in aquatic environments, thus higher levels certainly exist under circumstances yet to be examined. Furthermore, both studies were performed during seasonal influenza outbreaks; during a pandemic, usage and thus environmental levels of OC are expected to be considerably higher, reaching µg/L-levels.
Interestingly, in a study from Germany a high OP/OC ratio was found in river water at the German–Swiss border. The high ratio suggested outlet from a pharmaceutical factory in the vicinity as the origin of OP. Sectional sampling of the river showed higher ratios on the side of the river where the factory is situated and no OP was detected upstream of the factory Citation39. As OP can be converted to OC by naturally occurring esterases, the findings highlight that manufacturing of oseltamivir is also a potential source of environmental OC.
A promising means to reduce OC in the environment is to increase the degradation through ozonization. A study from Japan demonstrated that the addition of ozonization as a tertiary treatment in an STP increased the removal of OC to >90% Citation40. Another strategy to lower environmental levels of OC is to improve sewage treatment through bioremeditative measures. When granules with a bioplastic formulation of the fungus Phanerochaete chrysosporium was added to wastewater samples, the removal of OC was approximately doubled compared to controls Citation41. Furthermore, two bacterial strains growing on OC as the sole carbon source has been isolated from the sediment of Japanese rivers Citation34.
Resistance to neuraminidase inhibitors
When the NAIs were introduced, resistance development was not considered a practical problem. Resistant viruses were observed after drug pressure assays, e.g. in cell lines and in 4% of volunteers in an early study of oseltamivir Citation42, but the mutants had severely reduced viral fitness. Therefore, it was deduced that resistance development to NAIs interfered too much with the key function of NA to be a problem in vivo. However, resistance was observed also in clinical isolates and in some settings it reached considerable levels as in the study by Kiso et al. where 14% of Japanese children carried resistant viruses after oseltamivir treatment Citation43.
There are 19 amino-acid residues that are well conserved among NAs of all subtypes. They are divided into catalytic residues involved in the interaction of the substrate and the active site of NA (R118, D151, R152, R224, E276, R292, R371 and Y406) and framework residues important for the structure and stabilization of the active site (E119, R156, W178, S179, D198, I222, E227, H274, E277, N294 and E425) Citation44. Resistance could potentially arise from a mutation at or near any of those residues and many are previously described Citation45.
The mutation H274Y, conferring resistance to oseltamivir, was rarely seen in clinical practice until the season 2007–2008. That season, H274Y was observed in seasonal H1N1 viruses, first in Norway and then in the rest of Europe Citation46. Low percentages of H274Y was reported from the rest of the world 2007–2008, but in the next season 2008–2009, resistant viruses constituted the absolute majority world-wide Citation47. There was no correlation to the use of oseltamivir Citation47 Citation48.
A recent study has demonstrated that an influenza A/H1N1 virus in mallards exposed to of OC in their sole water source developed resistance through acquisition of the mutation H274Y Citation49. H274Y occurred at 1 µg/L of OC and quickly outnumbered the wild-type virus at 80 µg/L. The IC50 difference between wild-type isolates (2–4 nM) and H274Y isolates (400–700 nM) from the experiment is consistent with findings in human clinical isolates Citation50. As µg/L-levels of OC are expected in the environment, the experimental conditions correspond to a realistic scenario. This means that oseltamivir resistance could be induced in influenza A viruses of wild ducks when the drug is widely used, but this is only true for limited periods of time during pandemic or seasonal influenza outbreaks. As earlier in vitro studies have indicated a decreased viral fitness in strains with NAI resistance mutations the question arises: Will the resistance prevail when OC disappears from the environment?
In this sense, it is interesting to study the results from isolations in embryonated hen eggs of a sample with mixed genotype (i.e. a virus population consisting of both wild-type and H274Y) from the mallard study Citation49. During the replication process in the eggs, no OC is present and hence there is no drug pressure. Two different isolations gave rise to one wild-type and one H274Y-positive isolate which demonstrates that either genotype can dominate the replication and outcompete the other. Although not being a fitness test in a true sense, this is still a good indication that the fitness of the wild-type and the mutant are not dramatically different when H274Y is induced in a randomly chosen virus from a wild mallard in Sweden. Sweden uses oseltamivir conservatively and the sampling of the wild mallard was performed in a rural area on an island by the seaside. Therefore, this strain cannot possibly have been exposed to any drug pressure around the time of sampling. Another interesting fact is the accumulation of H274Y in seasonal influenza A/H1N1 in the seasons 2007–2008 and 2008–2009. As there was no correlation between the spread of resistance and the use of oseltamivir Citation47 Citation48, i.e. the drug pressure, the H274Y mutant must have been fit enough to outcompete the wild-type strain(s). It has been demonstrated that this is probably due to compensatory, ‘permissive’ mutations (V234M and R222Q) which restore the decreased surface expression of NA caused by H274Y Citation51. Another study has also demonstrated a compensatory effect on NA activity in H274Y mutants by D344N Citation52. Thus, it seems that the genetic makeup of the virus strain where resistance mutations such as H274Y develop will determine whether the mutation results in a decreased viral fitness or not.
Resistance in wild birds
The probability of a virus strain with a permissive genetic makeup appears to be higher in LPAI viruses of wild birds, and especially dabbling ducks, as they are the natural reservoir of influenza A viruses Citation10. This means that many more strains co-circulate in the wild bird population at a given time and that there is a larger genetic variation. An example of the variation is that the sensitivity to oseltamivir in avian A/H1N1 viruses showed a much larger variation when compared to mammalian viruses Citation53. The analysis of sequences from the NCBI database in the mallard study Citation49 revealed that H274Y has been reported in wild birds, though rarely. H274Y has been found both in H5N1 and H1N1 – interestingly, the H1N1 isolate originated from a duck in Minto Flats in Interior Alaska, a habitat with high densities of nesting ducks. The interior of Alaska is scarcely populated and oseltamivir use is negligible, thus there is no drug pressure. The occurrence of H274Y under these circumstances further supports the idea that H274Y does not require drug pressure to prevail when present in a virus with a suitable genetic makeup.
Spread of influenza and resistance from birds to humans
Influenza A viruses can spread from birds to humans in two distinctly different ways. Two or more strains from birds, humans and/or other mammals like swine can form a human-adapted virus with pandemic potential through reassortment. Another way of transfer is through a direct transmission of an avian virus to humans – also termed de novo introduction. HPAI can be transmitted directly from birds to humans but so far a very high infectious dose has been required and transmission has mostly been observed in people in close contact with infected birds. The mortality in human HPAI H5N1 infection is 59% according to the cumulative number of cases and deaths reported to WHO since 2003 Citation54. There have been no unequivocal reports of human-to-human transmission of HPAI.
An oseltamivir-resistant pandemic where an NA gene containing resistance mutation(s) has been recruited from an avian virus is an alarming example of the reassortment route. It would render stockpiles of oseltamivir useless and make the treatment and prophylaxis parts of pandemic preparedness plans very difficult to accomplish.
Another possibility is that oseltamivir resistance is established in the pool of circulating HPAI viruses with the risk that such a virus acquires human-to-human transmissibility while retaining high pathogenicity. This possibility is highlighted by the recent demonstration from two independent research groups that a modified HPAI H5N1 virus can be transmissible between ferrets. A group led by Kawaoka combined the HA gene from a HPAI H5N1 virus with the remaining genome from a human pandemic A/H1N1 virus which resulted in a ferret-transmissible virus, although it caused a less severe disease in the animals. Fouchier et al. introduced five mutations in a HPAI H5N1 virus and the resulting virus was transmissible between ferrets and seemed to retain pathogenicity. The results have been accepted for publication in Nature and Science, respectively, but the dual-use principle has sparked an intense debate on the bio-security aspects leading up to a 60-day moratorium on HPAI H5N1 transmission research and pleas for full publication from both groups Citation55 Citation56. Regardless of the final fate of the publications, the results demonstrate the limited genetic barrier for direct transmission and therefore stress the risk associated to resistance development in HPAI viruses circulating among wild birds.
To study resistance development in influenza viruses of treated patients is important. However, there are good reasons to complement these studies by investigating the role of antivirals in the environment and resistance development in wild birds:
The size and diversity of the influenza gene pool among wild birds is overwhelming compared to the pool of circulating human influenza viruses. At any given time, more or less only three different strains circulate among humans. In wild birds, all subtypes described to date have been found. Furthermore, there are two distinct genetic lineages and there is a constant circulation of virus year-round and a large proportion of the population is exchanged each year, adding non-immune juveniles. Thus, it is perhaps more common that resistance develops in treated humans, but it is more probable in the bird population that a resistance mutation occurs in a virus with a suitable genetic makeup.
If resistance spreads to humans via a reassortment event causing a pandemic or a human-adapted HPAI virus, the consequences are far worse than if it arises in a strain already circulating in the human population. In the latter case there is already some immunity in the population and the resistant virus is probably one of the more harmless circulating seasonal strains (like the development of H274Y in the pre-pandemic seasonal A/H1N1 virus). In the former case, preparedness plans rely on oseltamivir both as an attempt to blanket the outbreak and as treatment and prophylaxis especially during the first wave.
Strategies to lower environmental levels of OC
Measures to lower the environmental levels of OC and thus the drug pressure include bioremeditative and degradation-enhancing efforts as described above. Of these efforts, the addition of ozonization in STPs appears to be the most attractive measure. Apart from increasing the degradation of OC, the ozonization process has the potential to reduce the outlet of antibiotics and other pharmaceuticals.
However, the most important measure remains a prudent use of antiviral drugs. The effect of NAIs in healthy people suffering a seasonal influenza infection is limited; oseltamivir 75 mg twice daily or a corresponding dose in children shortens duration of symptoms with approximately 1 day Citation57. On the contrary, in immunosuppressed patients there is growing evidence that a combination therapy is favorable. In vitro and in vivo experimental data suggest an additive or synergistic effect and a decrease in resistance development and therefore the use of combination therapy is increasingly advocated Citation58–Citation61. To strictly limit the use in the young and healthy in non-pandemic periods and to consider combination therapy for those with a suppressed immune defense appears to be a reasonable strategy.
Clinical consequences of resistance
Generally, resistance in influenza is a problem to physicians especially when treating immunosuppressed patients. Numerous reports exist on resistant viruses recovered from such individuals. An interesting example is the finding of I222R in a pandemic A/H1N1 virus isolated from an immunocompromised Dutch patient Citation62. This mutation caused resistance to all available NAIs and as the circulating pandemic A/H1N1 virus is already resistant to admantanes, there are no treatment options left in this case.
In the event of an oseltamivir-resistant pandemic with morbidity in the same magnitude as the Spanish Flu, the consequences are almost unimaginable – e.g. ventilator capacity in intensive care units would quickly be outnumbered. The same goes for the scenario of a human-adapted HPAI virus capable of human-to-human spread still retaining (some of) its pathogenicity.
Apart from limiting the use of NAIs, the development of new anti-influenza drugs is essential. However, it is crucial to consider the risk of resistance development – including what happens in the environment – and to limit the use of new drugs from the beginning. Each new antiviral, or antibiotic, has a limited life span which is heavily dependent on its use.
Future research
There are several aspects discussed in this review that deserve further attention. The following seem particularly important:
To learn more about OC in the environment. Studies performed so far are few and small. A global approach including pandemic periods is desirable. The environmental perspective discussed here needs to be applied on other NAIs and antivirals including those in the pipeline of drug development.
To extend the knowledge of the influenza situation among wild birds. This concerns influenza ecology in general, but the resistance situation in particular. An extensive screening for influenza viruses and resistance mutations among wild birds in different parts of the world is important, also to follow the development over time. Genotypic analysis might not always be sufficient; to some extent the screening should be complemented with functional methods such as the NA inhibition assay to detect decreased sensitivity to NAIs that does not correlate with previously described resistance mutations.
To broaden the understanding of influenza resistance development in dabbling ducks exposed to OC. It is crucial to study if, and how, resistance mutations are affected by decreasing levels of OC and thus how resistance prevails in between influenza outbreaks. Furthermore, to date only one subtype of influenza A virus is examined in the sense of resistance development under low drug pressure of OC. It is still unknown how environmental levels of OC affect the large and important phylogenetic N2-group of NAs that has distinct resistance mutations.
To further assess the genetic barrier to human-to-human transmission of HPAI viruses. To estimate the risk of human adaptation of such a virus is important in the sense of pandemic preparedness in general, but it also has implications for the potential spread of a resistant HPAI virus.
Summary
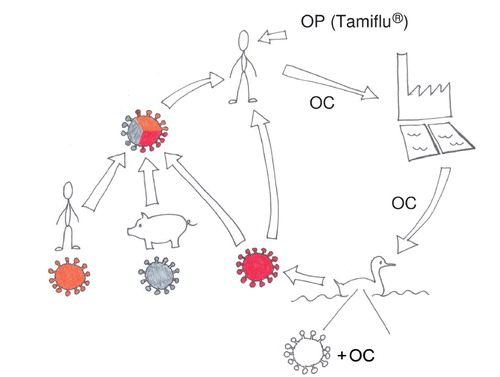
OC is present in the aquatic environment during a seasonal influenza outbreak. Thus, the natural influenza reservoir, dabbling ducks, can be exposed to the substance. Furthermore, an influenza A/H1N1 virus in mallards subjected to low, environmental-like, concentrations of OC developed oseltamivir resistance through acquisition of the resistance mutation H274Y. Therefore, there is reason to believe that resistance development occurs in influenza A viruses of wild ducks when oseltamivir is used widely. The occurrence of H274Y in influenza viruses isolated from wild birds and the fact that H274Y became established in the pre-pandemic seasonal human H1N1 virus support the thought that once induced, oseltamivir resistance can prevail if present in a virus with a suitable, permissive genetic makeup. Through reassortment or direct transmission, the NA gene conferring oseltamivir resistance can spread to a human-adapted influenza virus with pandemic potential, threatening to disable oseltamivir, a cornerstone in pandemic preparedness planning. See for a graphic display of the summary. In a bigger perspective, the material in this review exemplifies that antiviral drugs, like antibiotics, have a limited life span due to resistance development. The substances can have complex environmental effects that include humans, necessitating a broad, multi-disciplinary research approach.
Conflict of interest and funding
The author is grateful to the Family Olinder-Nielsen's Foundation, the Swedish Research Council and the Swedish Research Council (FORMAS) for financial support. Roche supplied oseltamvir and deuterium-labeled oseltamivir to the studies (37) and (49) that the author took part in. Apart from that, no funding or benefits was recieved from industry or elsewhere to conduct this study.
References
- Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. 2002; 76: 105–15. 10.3402/iee.v2i0.18385.
- Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006; 12: 15–22. 10.3402/iee.v2i0.18385.
- Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG. Characterization of the 1918 influenza virus polymerase genes. Nature. 2005; 437: 889–93. 10.3402/iee.v2i0.18385.
- Smith GJ, Bahl J, Vijaykrishna D, Zhang J, Poon LL, Chen H, et al.. Dating the emergence of pandemic influenza viruses. Proc Natl Acad Sci U S A. 2009; 106: 11709–12. 10.3402/iee.v2i0.18385.
- Guan Y, Vijaykrishna D, Bahl J, Zhu H, Wang J, Smith GJ. The emergence of pandemic influenza viruses. Protein Cell. 2010; 1: 9–13. 10.3402/iee.v2i0.18385.
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992; 56: 152–79.
- Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus AD, Fouchier RA. Global patterns of influenza a virus in wild birds. Science. 2006; 312: 384–8. 10.3402/iee.v2i0.18385.
- Brown JD, Goekjian G, Poulson R, Valeika S, Stallknecht DE. Avian influenza virus in water: infectivity is dependent on pH, salinity and temperature. Vet Microbiol. 2009; 136: 20–6. 10.3402/iee.v2i0.18385.
- Wallensten A, Munster VJ, Latorre-Margalef N, Brytting M, Elmberg J, Fouchier RA, et al.. Surveillance of influenza A virus in migratory waterfowl in northern Europe. Emerg Infect Dis. 2007; 13: 404–11. 10.3402/iee.v2i0.18385.
- Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, et al.. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005; 79: 2814–22. 10.3402/iee.v2i0.18385.
- Kawaoka Y, Yamnikova S, Chambers TM, Lvov DK, Webster RG. Molecular characterization of a new hemagglutinin, subtype H14, of influenza A virus. Virology. 1990; 179: 759–67. 10.3402/iee.v2i0.18385.
- Rohm C, Zhou N, Suss J, Mackenzie J, Webster RG. Characterization of a novel influenza hemagglutinin, H15: criteria for determination of influenza A subtypes. Virology. 1996; 217: 508–16. 10.3402/iee.v2i0.18385.
- Schafer JR, Kawaoka Y, Bean WJ, Suss J, Senne D, Webster RG. Origin of the pandemic 1957 H2 influenza A virus and the persistence of its possible progenitors in the avian reservoir. Virology. 1993; 194: 781–8. 10.3402/iee.v2i0.18385.
- Makarova NV, Kaverin NV, Krauss S, Senne D, Webster RG. Transmission of Eurasian avian H2 influenza virus to shorebirds in North America. J Gen Virol. 1999; 80(Pt 12): 3167–71.
- Wahlgren J, Waldenstrom J, Sahlin S, Haemig PD, Fouchier RA, Osterhaus AD, et al.. Gene segment reassortment between American and Asian lineages of avian influenza virus from waterfowl in the Beringia area. Vector Borne Zoonotic Dis. 2008; 8: 783–90. 10.3402/iee.v2i0.18385.
- Wallensten A, Munster VJ, Elmberg J, Osterhaus AD, Fouchier RA, Olsen B. Multiple gene segment reassortment between Eurasian and American lineages of influenza A virus (H6N2) in Guillemot (Uria aalge). Arch Virol. 2005; 150: 1685–92. 10.3402/iee.v2i0.18385.
- Jourdain E, Gunnarsson G, Wahlgren J, Latorre-Margalef N, Brojer C, Sahlin S, et al.. Influenza virus in a natural host, the mallard: experimental infection data. PLoS One. 2010; 5: e8935. 10.3402/iee.v2i0.18385.
- Laudert EA, Sivanandan V, Halvorson DA. Effect of intravenous inoculation of avian influenza virus on reproduction and growth in mallard ducks. J Wildl Dis. 1993; 29: 523–6.
- Arsnoe DM, Ip HS, Owen JC. Influence of body condition on influenza A virus infection in mallard ducks: experimental infection data. PLoS One. 2011; 6: e22633. 10.3402/iee.v2i0.18385.
- Webster RG, Yakhno M, Hinshaw VS, Bean WJ, Murti KG. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology. 1978; 84: 268–78. 10.3402/iee.v2i0.18385.
- Wang C, Takeuchi K, Pinto LH, Lamb RA. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J Virol. 1993; 67: 5585–94.
- Ilyushina NA, Govorkova EA, Webster RG. Detection of amantadine-resistant variants among avian influenza viruses isolated in North America and Asia. Virology. 2005; 341: 102–6. 10.3402/iee.v2i0.18385.
- Nelson MI, Simonsen L, Viboud C, Miller MA, Holmes EC. The origin and global emergence of adamantane resistant A/H3N2 influenza viruses. Virology. 2009; 388: 270–8. 10.3402/iee.v2i0.18385.
- Jefferson T, Demicheli V, Rivetti D, Jones M, Di Pietrantonj C, Rivetti A. Antivirals for influenza in healthy adults: systematic review. Lancet. 2006; 367: 303–13. 10.3402/iee.v2i0.18385.
- Nayak DP, Hui EK, Barman S. Assembly and budding of influenza virus. Virus Res. 2004; 106: 147–65. 10.3402/iee.v2i0.18385.
- Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol. 2004; 78: 12665–7. 10.3402/iee.v2i0.18385.
- Hayden FG, Treanor JJ, Betts RF, Lobo M, Esinhart JD, Hussey EK. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA. 1996; 275: 295–9. 10.3402/iee.v2i0.18385.
- Centers For Disease Control (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). ; 2011. MMWR Morb Mortal Wkly Rep 60, pp. 1128–32.
- Partridge J, Kieny MP. Global production of seasonal and pandemic (H1N1) influenza vaccines in 2009–2010 and comparison with previous estimates and global action plan targets. Vaccine. 2010; 28: 4709–12. 10.3402/iee.v2i0.18385.
- Sweetman S. Martindale: the complete drug reference [online]. London: Pharmaceutical Press. Available from: http://www.medicinescomplete.com [cited 24 October 2011].
- Patel A, Gorman SE. Stockpiling antiviral drugs for the next influenza pandemic. Clin Pharmacol Ther. 2009; 86: 241–3. 10.3402/iee.v2i0.18385.
- Wan Po AL, Farndon P, Palmer N. Maximizing the value of drug stockpiles for pandemic influenza. Emerg Infect Dis. 2009; 15: 1686–7.
- Fick J, Lindberg RH, Tysklind M, Haemig PD, Waldenstrom J, Wallensten A, et al.. Antiviral oseltamivir is not removed or degraded in normal sewage water treatment: implications for development of resistance by influenza A virus. PLoS One. 2007; 2: e986. 10.3402/iee.v2i0.18385.
- Accinelli C, Sacca ML, Fick J, Mencarelli M, Lindberg R, Olsen B. Dissipation and removal of oseltamivir (Tamiflu) in different aquatic environments. Chemosphere. 2010; 79: 891–7. 10.3402/iee.v2i0.18385.
- Tashiro M, McKimm-Breschkin JL, Saito T, Klimov A, Macken C, Zambon M, et al.. Surveillance for neuraminidase-inhibitor-resistant influenza viruses in Japan, 1996–2007. Antivir Ther. 2009; 14: 751–61. 10.3402/iee.v2i0.18385.
- F. Hoffman – La Roche Ltd. Further expansion of Tamiflu manufacturing capacity. Available from: http://www.roche.com/med-cor-2005-10-18 [cited 19 March 2012].
- Söderstrom H, Järhult JD, Olsen B, Lindberg RH, Tanaka H, Fick J. Detection of the antiviral drug oseltamivir in aquatic environments. PLoS One. 2009; 4: e6064. 10.3402/iee.v2i0.18385.
- Ghosh GC, Nakada N, Yamashita N, Tanaka H. Oseltamivir carboxylate, the active metabolite of oseltamivir phosphate (Tamiflu), detected in sewage discharge and river water in Japan. Environ Health Perspect. 2010; 118: 103–7.
- Prasse C, Schlusener MP, Schulz R, Ternes TA. Antiviral drugs in wastewater and surface waters: a new pharmaceutical class of environmental relevance?. Environ Sci Technol. 2010; 44: 1728–35. 10.3402/iee.v2i0.18385.
- Ghosh GC, Nakada N, Yamashita N, Tanaka H. Occurrence and fate of oseltamivir carboxylate (Tamiflu) and amantadine in sewage treatment plants. Chemosphere. 2010; 81: 13–17. 10.3402/iee.v2i0.18385.
- Accinelli C, Sacca ML, Batisson I, Fick J, Mencarelli M, Grabic R. Removal of oseltamivir (Tamiflu) and other selected pharmaceuticals from wastewater using a granular bioplastic formulation entrapping propagules of Phanerochaete chrysosporium. Chemosphere. 2010; 81: 436–43. 10.3402/iee.v2i0.18385.
- Gubareva LV, Kaiser L, Matrosovich MN, Soo-Hoo Y, Hayden FG. Selection of influenza virus mutants in experimentally infected volunteers treated with oseltamivir. J Infect Dis. 2001; 183: 523–31. 10.3402/iee.v2i0.18385.
- Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, Kimura K, et al.. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004; 364: 759–65. 10.3402/iee.v2i0.18385.
- Colman PM, Varghese JN, Laver WG. Structure of the catalytic and antigenic sites in influenza virus neuraminidase. Nature. 1983; 303: 41–4. 10.3402/iee.v2i0.18385.
- Ferraris O, Lina B. Mutations of neuraminidase implicated in neuraminidase inhibitors resistance. J Clin Virol. 2008; 41: 13–9. 10.3402/iee.v2i0.18385.
- Meijer A, Lackenby A, Hungnes O, Lina B, van-der-Werf S, Schweiger B, et al.. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg Infect Dis. 2009; 15: 552–60. 10.3402/iee.v2i0.18385.
- Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med. 2009; 360: 953–6. 10.3402/iee.v2i0.18385.
- Kramarz P, Monnet D, Nicoll A, Yilmaz C, Ciancio B. Use of oseltamivir in 12 European countries between 2002 and 2007 – lack of association with the appearance of oseltamivir-resistant influenza A(H1N1) viruses. Euro Surveill. 2009; 14: 19112.
- Jarhult JD, Muradrasoli S, Wahlgren J, Soderstrom H, Orozovic G, Gunnarsson G, et al.. Environmental levels of the antiviral oseltamivir induce development of resistance mutation H274Y in influenza A/H1N1 virus in mallards. PLoS One. 2011; 6: e24742. 10.3402/iee.v2i0.18385.
- Rameix-Welti MA, Enouf V, Cuvelier F, Jeannin P, van der Werf S. Enzymatic properties of the neuraminidase of seasonal H1N1 influenza viruses provide insights for the emergence of natural resistance to oseltamivir. PLoS Pathog. 2008; 4: e1000103. 10.3402/iee.v2i0.18385.
- Bloom JD, Gong LI, Baltimore D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science. 2010; 328: 1272–5. 10.3402/iee.v2i0.18385.
- Collins PJ, Haire LF, Lin YP, Liu J, Russell RJ, Walker PA, et al.. Structural basis for oseltamivir resistance of influenza viruses. Vaccine. 2009; 27: 6317–23. 10.3402/iee.v2i0.18385.
- Stoner TD, Krauss S, DuBois RM, Negovetich NJ, Stallknecht DE, Senne DA, et al.. Antiviral susceptibility of avian and swine influenza virus of the N1 neuraminidase subtype. J Virol. 2010; 84: 9800–9. 10.3402/iee.v2i0.18385.
- WHO. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. Available from: http://www.who.int/influenza/human_animal_interface/EN_GIP_20111010CumulativeNumberH5N1cases.pdf [cited 26 October 2011].
- Fouchier RA, Herfst S, Osterhaus AD. Public health and biosecurity. Restricted data on influenza H5N1 virus transmission. Science. 2012; 335: 662–3. 10.3402/iee.v2i0.18385.
- Kawaoka Y. H5N1: flu transmission work is urgent. Nature. 2012; 482: 155.
- Jefferson T, Jones MA, Doshi P, Del Mar CB, Heneghan CJ, Hama R, et al.. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2012; 1: CD008965.
- Govorkova EA, Webster RG. Combination chemotherapy for influenza. Viruses. 2010; 2: 1510–29. 10.3402/iee.v2i0.18385.
- Hoopes JD, Driebe EM, Kelley E, Engelthaler DM, Keim PS, Perelson AS, et al.. Triple combination antiviral drug (TCAD) composed of amantadine, oseltamivir, and ribavirin impedes the selection of drug-resistant influenza A virus. PLoS One. 2011; 6: e29778. 10.3402/iee.v2i0.18385.
- Nguyen JT, Hoopes JD, Le MH, Smee DF, Patick AK, Faix DJ, et al.. Triple combination of amantadine, ribavirin, and oseltamivir is highly active and synergistic against drug resistant influenza virus strains in vitro. PLoS One. 2010; 5: e9332. 10.3402/iee.v2i0.18385.
- Nguyen JT, Smee DF, Barnard DL, Julander JG, Gross M, de Jong MD, et al.. Efficacy of combined therapy with amantadine, oseltamivir, and ribavirin in vivo against susceptible and amantadine-resistant influenza A viruses. PLoS One. 2012; 7: e31006. 10.3402/iee.v2i0.18385.
- van der Vries E, Veldhuis Kroeze EJ, Stittelaar KJ, Linster M, Van der Linden A, Schrauwen EJ, et al.. Multidrug resistant 2009 A/H1N1 influenza clinical isolate with a neuraminidase I223R mutation retains its virulence and transmissibility in ferrets. PLoS Pathog. 2011; 7: e1002276. 10.3402/iee.v2i0.18385.