Abstract
Background: β-Lactamases are the main cause of bacterial resistance to penicillin, cephalosporins, and related β-lactam compounds. The presence of the novel penicillin-binding protein (pbp) Tp47 in Treponema pallidum has been reported to be a well-known mechanism for turnover of b-lactam antibiotics. Although, T. pallidum remains sensitive to penicillin, clinically significant resistance to macrolides has emerged in many developing countries. The genome sequence of T. pallidum has shown the presence of genes encoding pbp, but there are no current reports of the presence of mobile plasmids.
Methods: The phylogenetic analysis is used to study the diversity of chromosomal pbp genes and its relatedness to Tp47 in Treponema species.
Results: In our study, genes encoding penicillin-binding proteins that showed significant similarity to each other appeared in separate clusters.
Conclusion: Tp47 showed no substantial similarity to other β-lactamases in treponemes. The relatedness of Treponema denticola to other treponemes, including T. pallidum, and the reported presence of natural mobile antibiotic determinants highlight the importance of investigating the diversity of pbp genes in Treponema species. This will lead to a greater understanding of its potential to develop additional antibiotic resistance via horizontal gene transfer that could seriously compromise the treatment and control of syphilis.
The spirochaetes belong to the phylum of gram-negative bacteria—long, 0.1–0.5 µm in diameter, helically coiled, axial flagella that run lengthwise between the bacterial inner membrane and an outer membrane in the periplasmic space Citation1. Spirochetes are widely distributed in nature, typically anaerobic and free living, though many are notorious parasites. They are commonly isolated from the human mouth, marine environment, ulcerative lesion of a bovine foot, and the surfaces of protozoa that live in termite guts. Treponema pallidum, the most investigated treponemes, causes the sexually transmitted disease syphilis. The non-venereal treponemal infections include yaws, bejel, and pinta—endemic to remote regions of Africa, Southeast Asia, and South America Citation2.
Table 1. Summary of pathogenic and saprophytic Treponemes
The genus Treponema has further expanded to include treponemes with new ecological niches. Their role in the ecosystem is not completely understood, as three-fourths of the treponemes have not been sequenced. This limitation lasts as treponemes have largely proven difficult to culture. The 16S rRNA gene sequences have been the only way to identify oral Treponema species Citation3 Citation4 Citation5 Citation6 Citation7 . The recent advancement in culture and sequencing techniques has helped to identify and characterize several Treponema species. Treponema paraluiscuniculi are known to infect rabbits; Treponema denticola is associated with periodontal disease; Treponema bryantii are isolated from bovine rumen; Treponema primitia and Treponema azotonutricum have been isolated from the termite hindgut. T. bryantii and Treponema saccharophilum have been isolated from the rumen of cows, while Treponema succinifaciens have been isolated from swine. In the gastrointestinal tract of termites, comparative 16S rRNA gene sequence analyses have revealed more than 67 different treponemal phylotypes Citation3 Citation4 Citation8. The research study by Lilburn et al. reported that spirochetes from termite hindguts and freshwater sediments possess homolog of a nitrogenase gene (nifH) and exhibit nitrogenase activity Citation8 Citation9. This observation suggests that the spirochete symbionts have probably coevolved within specific termite species. The ectosymbiotic and free-swimming spirochetes play an important role in metabolic interactions within the host and benefit each other. The motility of spirochetes allows them to occupy unique ecological niches, such as the guts of certain arthropods and the rumen of cows and sheep Citation4 Citation10 Citation11.
Table 2. Strains with accession numbers used in this study
During the past three decades, and especially since 2004, there have been many reports of antibiotic resistance in treponemes, especially T. pallidum. Research studies have confirmed antibiotic resistance associated with macrolides such as erythromycin and azithromycin Citation12 Citation13 Citation14 Citation15 Citation16 Citation17 Citation18 Citation19 . The oral treponemes, T. denticola, primarily associated with periodontitis, have shown resistance to tetracycline. The research has also shown that tetB and ermF genes are now extensively distributed in the T. denticola population Citation20. The most important observation was that three of the T. denticola isolates were able to transfer their ermF determinants to Enterococcus faecalis recipients Citation20 Citation21 Citation22 . The presence of a natural mobile antibiotic resistance determinant in the genus Treponema is very alarming as it could move antibiotic resistant genes between different treponema species. In another study, with Treponema hyodysenteriae, intestinal treponemes, 4 of 32 isolates were shown to be resistant to penicillin and produced β-lactamases Citation23. Mobashery et al. reported penicillin-binding protein Tp47 in T. pallidum with an ability of this protein to turn over β-lactam antibiotics. Tp47 is strongly inhibited by products of the β-lactamase reaction, and, therefore, T. pallidum remains sensitive to penicillin Citation24. As the number of cases increase, there could be a potential for mutations in Tp47 or the presence of a mobile element that can cause multidrug resistance. The present state of knowledge on the diversity of pbp genes among clinical and ecological groups of treponemes has not been studied. Examining the natural patterns of occurrence of pbp genes in treponemes is an important starting point in understanding how these genes are related to each other and will help to uncover the ecological and evolutionary relationships existing between them.
Results and discussion
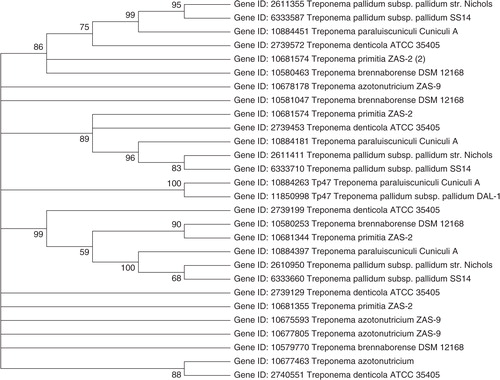
Treponemes are difficult to culture in vitro, a hindrance to experimental approaches such as mutational analysis to identify antibiotic resistance determinants. The report of azithromycin resistance in penicillin allergic patients has emerged as a clinical and public health challenge worldwide. T. pallidum continues to be one of the most penicillin-susceptible microorganisms, but mutation in Tp47 or presence of mobile plasmids with genes encoding β-lactamases could be one of the contributing factors leading to future penicillin resistance in T. pallidum. The genome of treponemes, Treponema azotonutricium ZAS-9 Citation25; Treponema brennaborense DD5/3, DSM 12168; T. denticola ATTC 35405 Citation26; T. pallidum (pallidum Nichols) Citation27; Treponema pallidum pallidum SS14 Citation28; Treponema paraluiscuniculi Cuniculi A Citation29; and T. primitia ZAS-2 Citation25, are analyzed for the presence of diversity of pbp genes. Phylogenetic calculations using maximum likelihood (ML) methods Citation30 are shown in . The novel penicillin-binding protein gene Tp47, Treponeme species (Gene ID 11850998, Gene ID 10884263), forms a separate cluster and does not show substantial similarity to any other pbp genes among the Treponema species used in this study. The analysis of genome sequence of T. pallidum suggests that it lacks genetic elements such as plasmids, bacteriophage, and transposons that are commonly associated with horizontal gene transfer mechanisms. The pbp gene for T. denticola ATTC 35405 (Gene ID 2739572) has shown 68% similarity to T. paraluiscuniculi cuniculi A (Gene ID 10884451) and 65% to T. brennaborense DD5/3, DSM 12168 (Gene ID 10580463). T. paraluiscuniculi is the causative agent of rabbit venereal spirochetosis Citation29. T. denticola, an oral spirochete associated with periodontal disease, has been reported for the presence of natural mobile antibiotic resistance determinant Citation20. T. brennaborense DD5/3, DSM 12168 (Gene ID 10580463) have shown 68% similarity to T. pallidum (pallidum Nichols) (Gene ID 2611355). T. brennaborense has been isolated from a cow suffering from digital dermatitis. T. denticola ATTC 35405 (Gene ID 2739453) has shown 66% similarity to T. primitia ZAS-2 (Gene ID 10681574) and T. paraluiscuniculi Cuniculi A (Gene ID 10884181). T. primitia has been isolated from termite hindguts. T. azotonutricium ZAS-9 does not show substantial similarity to any pbp genes among treponemes used in this study.
Fig. 1. (a) Phylogenetic analysis based on β-lactamase gene sequences from NCBI database. (b) Phylogenetic analysis based on β-lactamase gene sequences constructed after multiple alignment data by CLUSTAL W. (c) The clustering was performed with the maximum likelihood method and Kimura 2-parameter model using the software package MEGA version 5.05 (bootstrap confidence levels are shown as percentages of nodes, consensus tree with values above 50% are shown).
Experimental section
The list of pbp genes for treponemes with complete sequenced genomes was obtained from the NCBI (National Center for Biotechnology Information) database as listed in and . The Molecular Evolutionary Genetics Analysis version 5.05 (MEGA5) software program was used for the statistical analyses Citation30. The BLAST (basic local alignment search tool) algorithm was used to calculate the percentage of similarity between known sequences. The phylogenetic tree was constructed via the ML method, using the Kimura 2-parameter model and a discrete gamma distribution with five categories for capturing non-uniformity of evolutionary rates (K2+G). The K2+G model was selected by virtue of the fact that it had the lowest value of BIC (Bayesian information criterion) Citation30 Citation31. One thousand bootstrap trees were generated to determine bootstrap confidence levels Citation32. The resulting (bootstrap) consensus tree was condensed with values >50% as shown in . The gene sequences used in the study are available for electronic retrieval from the Gene Bank nucleotide sequence database Citation30.
Conclusions
The bifunctional pbp Tp47 had been known for the mechanism of turnover for β-lactam antibiotics in T. palladum Citation24 Citation33 Citation34 . Tp47 has showed no substantial similarity to other pbp genes in treponemes. Analysis of the T. pallidum genome sequence predicted the presence of pbp genes. Cha et al. Citation24 proposed that if a mutant variant of Tp47 emerges that overcomes the product inhibition of its β-lactamase activity, resistance to penicillin will emerge in T. pallidum. However, this requires a multistep mutational process, which is rarer than the single point mutations observed with macrolide resistance. There are no reports of the presence of mobile plasmids in T. pallidum although the emergence of a natural mobile antibiotic resistant in T. denticola provides no guarantee that it will not move to other Treponema species, including T. pallidum. The diversity among pbp genes across the phylogenetic tree is evident among treponemes that may be representative of their ecological niches. Interestingly, pbp gene for T. denticola (Gene ID 2739572) has shown relatedness to other treponemes, including T. paraluiscuniculi Cuniculi A (Gene ID 10884451); T. brennaborense DD5/3, DSM 12168 (Gene ID 10580463); T. pallidum (Gene ID 2611355); and T. primitia (Gene ID 10681574). Thus, the reported presence of a mobile element in T. denticola and the possibility of transfer of antibiotic resistant plasmid may be potentially dangerous, as it could provide a first step toward the acquisition of multidrug resistance. The pbp genes for treponemes investigated here could be used for further studies as Treponema species are strongly implicated in disease progression. The phylogenetic analysis data presented in this study demonstrate that this organism may possess the potential to acquire antibiotic resistance in the future.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Acknowledgements
The sequence data were obtained from National Center for Biotechnology Information database website (http://www.ncbi.nlm.nih.gov/).
References
- Charon NW, Goldstein SF. Genetics of motility and chemotaxis of a fascinating group of bacteria: the spirochetes. Annu Rev Genet. 2002; 36: 47–73.
- Antal GM, Lukehart SA, Meheus AZ. The endemic treponematoses. Microbes Infect. 2002; 4: 83–94.
- Nordhoff M, Taras D, Macha M, Tedin K, Busse HJ, Wieler LH. Treponema berlinense sp. nov. and Treponema porcinum sp. nov., novel spirochaetes isolated from porcine faeces. Int J Syst Evol Microbiol. 2005; 55: 1675–80.
- Berlanga M, Paster BJ, Guerrero R. Coevolution of symbiotic spirochete diversity in lower termites. Int Microbiol. 2007; 10: 133–9.
- Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci U S A. 2004; 101: 11030–5.
- Levy D, Yoshida R, Pachter L. Beyond pairwise distances: neighbor-joining with phylogenetic diversity estimates. Mol Biol Evol. 2006; 23: 491–8.
- de Melo FL, de Mello JC, Fraga AM, Nunes K, Eggers S. Syphilis at the crossroad of phylogenetics and paleopathology. PLoS Negl Trop Dis. 2010; 4: e575.
- Lilburn TG, Schmidt TM, Breznak JA. Phylogenetic diversity of termite gut spirochaetes. Environ Microbiol. 1999; 1: 331–45.
- Lilburn TG, Kim KS, Ostrom NE, Byzek KR, Leadbetter JR, Breznak JA. Nitrogen fixation by symbiotic and free-living spirochetes. Science. 2001; 292: 2495–8.
- Lo N, Evans TA. Phylogenetic diversity of the intracellular symbiont Wolbachia in termites. Mol Phylogenet Evol. 2007; 44: 461–6.
- Lafond RE, Lukehart SA. Biological basis for syphilis. Clin Microbiol Rev. 2006; 19: 29–49.
- (CDC) CfDCaP. Azithromycin treatment failures in syphilis infections—San Francisco, California, 2002–2003. MMWR Morb Mortal Wkly Rep. 2004; 53: 197–8.
- Lukehart SA, Godornes C, Molini BJ, Sonnett P, Hopkins S, Mulcahy F, et al.. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004; 351: 154–8.
- Pandori MW, Gordones C, Castro L, Engelman J, Siedner M, Lukehart S, et al.. Detection of azithromycin resistance in Treponema pallidum by real-time PCR. Antimicrob Agents Chemother. 2007; 51: 3425–30.
- Katz KA, Klausner JD. Azithromycin resistance in Treponema pallidum. Curr Opin Infect Dis. 2008; 21: 83–91.
- Mabey D. Azithromycin resistance in Treponema pallidum. Sex Transm Dis. 2009; 36: 777–8.
- Van Damme K, Behets F, Ravelomanana N, Godornes C, Khan M, Randrianasolo B, et al.. Evaluation of azithromycin resistance in Treponema pallidum specimens from Madagascar. Sex Transm Dis. 2009; 36: 775–6.
- Zhou P, Li K, Lu H, Qian Y, Gu X, Gong W, et al.. Azithromycin treatment failure among primary and secondary syphilis patients in Shanghai. Sex Transm Dis. 2010; 37: 726–9.
- Ison CA. Antimicrobial resistance in sexually transmitted infections in the developed world: implications for rational treatment. Curr Opin Infect Dis. 2012; 25: 73–8.
- Roberts MC, Chung WO, Roe DE. Characterization of tetracycline and erythromycin resistance determinants in Treponema denticola. Antimicrob Agents Chemother. 1996; 40: 1690–4.
- Simonson LG, Goodman CH, Bial JJ, Morton HE. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect Immun. 1988; 56: 726–8.
- Fenno JC. Treponema denticola interactions with host proteins. J Oral Microbiol. 2012; 4: 9929.
- Tompkins DS, Millar MR, Heritage J, West AP. beta-Lactamase production by intestinal spirochaetes. J Gen Microbiol. 1987; 133: 761–5.
- Cha JY, Ishiwata A, Mobashery S. A novel beta-lactamase activity from a penicillin-binding protein of Treponema pallidum and why syphilis is still treatable with penicillin. J Biol Chem. 2004; 279: 14917–21.
- Rosenthal AZ, Matson EG, Eldar A, Leadbetter JR. RNA-seq reveals cooperative metabolic interactions between two termite-gut spirochete species in co-culture. ISME J. 2011; 5: 1133–42.
- Seshadri R, Myers GS, Tettelin H, Eisen JA, Heidelberg JF, Dodson RJ, et al.. Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc Natl Acad Sci U S A. 2004; 101: 5646–51.
- Fraser CM, Norris SJ, Weinstock GM, White O, Sutton GG, Dodson R, et al.. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998; 281: 375–88.
- Matejková P, Strouhal M, Smajs D, Norris SJ, Palzkill T, Petrosino JF, et al.. Complete genome sequence of Treponema pallidum ssp. pallidum strain SS14 determined with oligonucleotide arrays. BMC Microbiol. 2008; 8: 76.
- Šmajs D, Zobaníková M, Strouhal M, Čejková D, Dugan-Rocha S, Pospíšilová P, et al.. Complete genome sequence of Treponema paraluiscuniculi, strain Cuniculi A: the loss of infectivity to humans is associated with genome decay. PLoS One. 2011; 6: 20415.
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28: 2731–9.
- Nei M, Kumar S. Molecular evolution and phylogenetics. Oxford University Press. New york, 2000; 333.
- Trindade A, Chadha T. Phylogenetic Analysis of Genetic Diversity of Hemolysins in Leptospira. J Proteomics Bioinform. 2012; 5(7): 152–4.
- Houston S, Hof R, Francescutti T, Hawkes A, Boulanger MJ, Cameron CE. Bifunctional role of the Treponema pallidum extracellular matrix-binding adhesin Tp0751. Infect Immun. 2011; 79: 1386–98.
- Zhang W, Fisher JF, Mobashery S. The bifunctional enzymes of antibiotic resistance. Curr Opin Microbiol. 2009; 12: 505–511.